Abstract
Background:
Abdominal tuberculosis (TB) is an uncommon condition in the United States (US) except for patients with human immunodeficiency virus (HIV). An increasing number of cases have been reported in western countries amongst immigrants. It is important to be aware of the data and clinical characteristics in the immigrant population.
Aims:
The purpose of this study is to determine the epidemiologic characteristics of abdominal TB among immigrants in the US and to review the clinical presentations of abdominal TB with a focus particularly on unusual features.
Materials and Methods:
In a community teaching hospital in New Jersey, patients diagnosed with abdominal TB were examined and included in this report. All nine patients were immigrants from countries with high prevalence of TB and a majority had resided in the US for at least 5 years. None had clinical evidence of HIV and those that were tested were not found to be positive for HIV. Initial examination, diagnostic workup, and response to therapy were all pertinent to the management and diagnosis of these patients.
Results:
Three patients had atypical clinical presentations with normal chest X-rays and either negative or unknown tuberculin tests leading to delayed diagnosis and inappropriate therapy in at least one patient. With antituberculous therapy, all except for one patient had satisfactory outcomes. Immigrant patients with a diagnosis of abdominal TB had no evidence of HIV infection or other associated conditions in contrast to native-born individuals.
Conclusion:
Atypical presentations may cause diagnostic difficulties. Failure to perform appropriate tests may lead to inappropriate therapy with adverse outcomes. Although there is a decline in the number of TB cases in the US and screening for latent pulmonary infection in foreigners has been implemented effectively, the diagnosis of abdominal TB continues to be under diagnosed.
Keywords: Abdominal tuberculosis, Diagnosis, Extrapulmonary, HIV, Immigrants, Tuberculosis
Introduction
In the last 2 decades, there has been a 19% decrease in tuberculosis (TB) cases in the United States (US). It has been reported that TB cases occurring in foreign-born individuals has continued to increase reaching 64.6% of the total documented cases.[1] The increase in TB incidence in this group was not limited to newly arrived persons; a similar trend was also observed among foreign-born, long-term residents.[2]
There has also been an increased incidence of extra pulmonary TB in US-born individuals, predominantly in the HIV infected population. Unlike the native-born Americans, most immigrants with extra pulmonary TB have no identifiable immunosuppression or risk factor for HIV infection.[2]
Through this report, nine patients diagnosed and managed for abdominal TB will be discussed. By reviewing their similarities and differences, it will give a better review of the characteristics or associations linked to abdominal TB. After such an analysis, a better understanding will be reached regarding an earlier diagnosis, method of diagnosis, associated complications, and solutions for treatment. By shedding light on the prevalence and significance of this form of extrapulmonary TB, specifically intra-abdominal TB, physicians will be more aware of its presentation and characteristics that may further improve its diagnosis and management.
Methods and Results
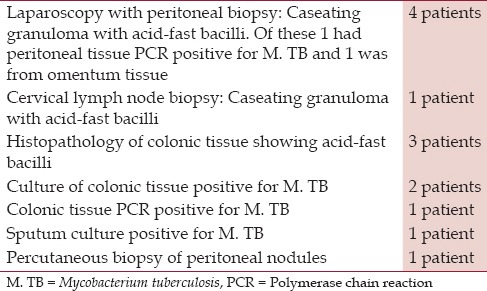
Nine patients with abdominal TB were diagnosed in a community teaching hospital in New Jersey with no evidence of human immunodeficiency virus (HIV); their demographic data, clinical features, some unusual manifestations, and a brief review of the current status of the disease entity are discussed. The nine patients being discussed in this study, six of which were males and three females, all had lived in the US for at least 5 years before the onset of illness. Their ages ranged from 27 to 73 years. Their countries of origin included five from India, two from Philippines, and one each from Pakistan and Peru [Table 1]. Clinical presentations, tuberculin status, and the chest X-ray findings are outlined in Tables 2 and 3. Three had peritoneal TB and four others had colonic involvement. Diagnostic workup confirming the diagnosis of abdominal TB is outlined in Table 4.
Table 1.
Abdominal tuberculosis; demographic data of seven patients
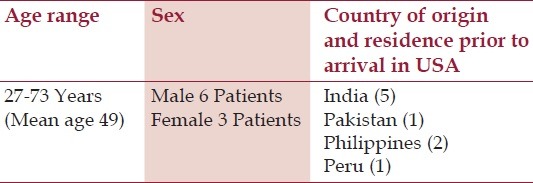
Table 2.
Clinical presentations and final diagnosis of six cases of abdominal tuberculosis
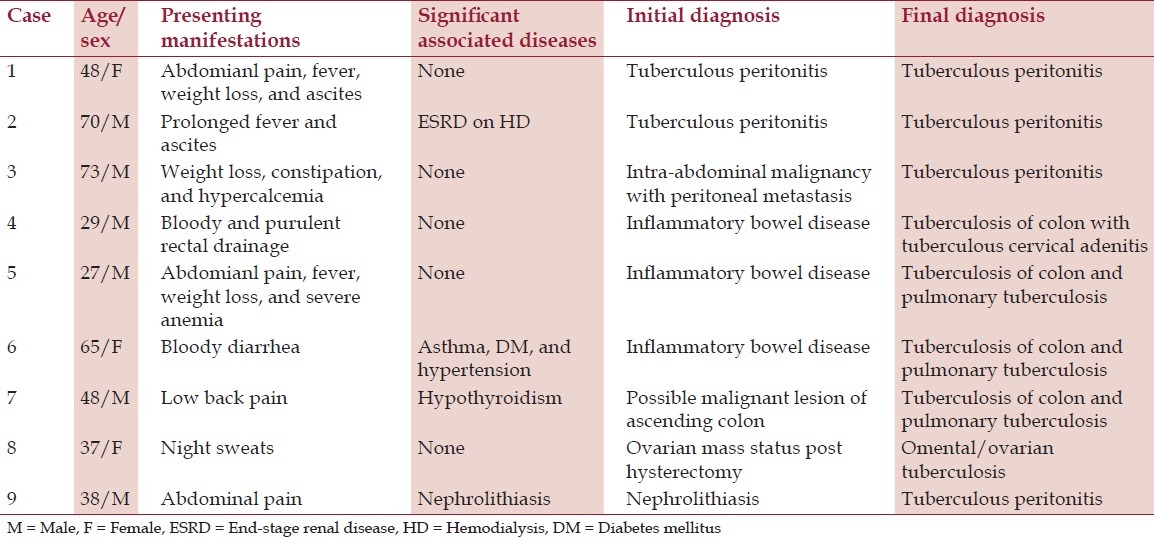
Table 3.
PPD and chest X-ray findings
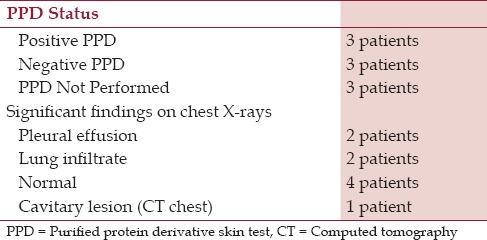
Table 4.
Diagnostic workup in nine patients with abdominal tuberculosis
Apart from countries of origin with high prevalence of TB, none had evidence of obvious risk factors for TB including history such as for HIV infection, alcoholism, incarceration, and homelessness or residence in a long-term care facility. One patient had history of end-stage renal disease (ESRD) on hemodialysis for 1 year prior to the diagnosis of peritoneal TB. He had also been treated with prednisone for 6 weeks for his kidney disease. Another patient had a history of bronchial asthma and diabetes mellitus. Three patients had peritoneal TB and the other four had colonic involvement. One patient with peritoneal TB and two others with colonic involvement had atypical presentation, which led to delayed diagnosis (discussed below).
Eight patients were treated with standard antituberculous drugs (isoniazid (INH), rifampin, and ethambutol with or without pyrazinamide) for a period of 9-12 months and did well. Patient number six received therapy for TB only for a short period of time, but died weeks after hospitalization from congestive heart failure.
Case 1
A 73-years-old Filipino man and currently a US resident for 14 years presented with a history of weight loss, progressive weakness, decreased appetite, and constipation for 2 months. Physical examination was unremarkable. Labs showed mild anemia, mild elevation of liver enzymes, and hypercalcemia with elevated ionized calcium. Studies for parathyroid hormones were normal. Chest X-ray was normal. CT scan of the abdomen was performed to rule out malignancy andshowed diffuse thickening of the mesenteric nodes with no neoplastic lesion. Exploratory laparotomy findings were consistent with peritoneal carcinomatosis [Figure 1]. Histopathologic examination revealed caseating granulomas with few acid-fast bacilli. Peritoneal tissue was positive for Mycobacterium tuberculosis by polymerase chain reaction (PCR). Lack of fever, ascites, and normal chest X-ray delayed the diagnosis of tuberculous peritonitis.
Figure 1.
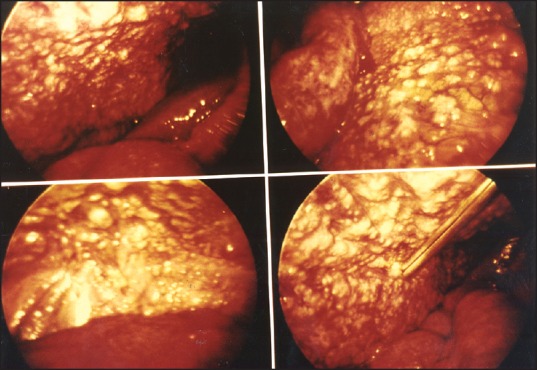
Laparotomy findings showing extensive peritoneal nodules due to granulomas
Case 2
A 29-year-old man, a native of Pakistan, presented with bloody and purulent rectal drainage for 6 weeks. Physical examination was unremarkable. A chest X-ray revealed a small left pleural effusion. Colonoscopic biopsy showed mucosal changes compatible with granulomatous colitis [Figure 2]. He was treated with systemic corticosteroids and sulfasalazine for presumed inflammatory bowel disease (IBD). Several weeks later, he developed massive bilateral cervical adenopathy and swelling of the abdomen. A follow-up chest X-ray showed worsening pleural effusion. CT of the abdomen showed fluid and induration in the retroperitoneum and peripancreatic areas. Biopsy of cervical lymph nodes showed caseating granulomas with acid-fast bacilli. With appropriate antituberculous therapy; his adenopathy, pleural effusion, and rectal drainage improved dramatically. This patient almost surely had tuberculous granulomatous colitis, but with corticosteroid therapy his gastrointestinal symptoms worsened and that treatment most probably resulted in dissemination of the disease. In patients with a clinical presentation of IBD who are from areas with high prevalence of TB, a tuberculin test should be performed prior to corticosteroid therapy and the possibility of TB should be strongly taken into consideration.
Figure 2.
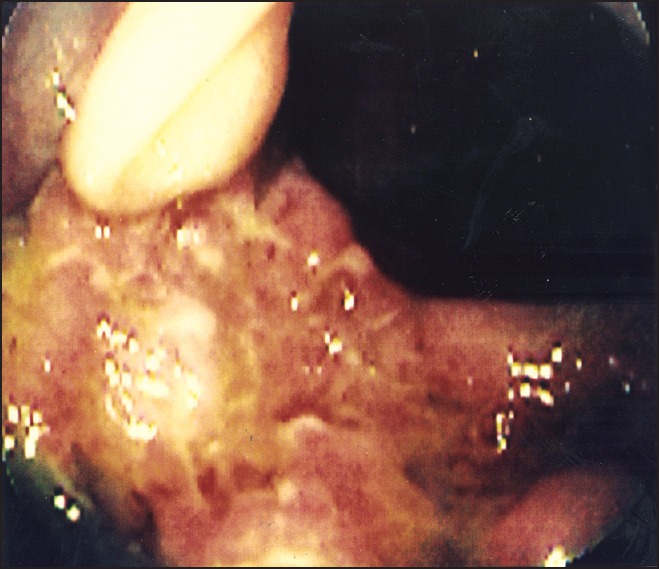
Colonoscopy findings showing acute inflammation of the ascending colon due to tuberculous colitis
Case 3
A 27-year-old man, native of Peru, presented with fever, abdominal pain, backache, and weight loss of 30 lbs over 4 months. Physical examination was unremarkable except forpallor, vitiligo, and a temperature of 38.8°C. Laboratory findings showed iron deficiency anemia. Chest X-ray showed increased markings in the right upper lobe. Barium enema examination revealed a short, spastic proximal colon, with an irregular appendiceal wall [Figure 3]. Colonoscopy revealed acute inflammation of the ascending colon, but no granuloma was found. Bronchoscopy was also performed for an unexplained right upper lobe lung lesion. Culture from both colonic tissue and bronchoscopy washings showed growth of M. tuberculosis. In this patient, the history was compatible with abdominal TB and although a negative tuberculin test due to its low sensitivity does not weaken the suspicion for colonic TB, the absence of granulomas in colonic tissue was significant.
Figure 3.
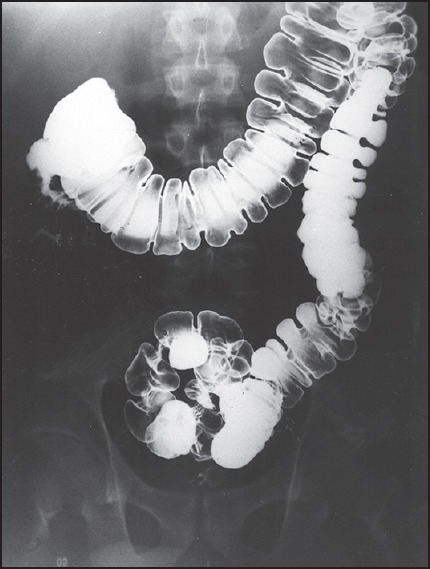
Barium enema examination revealing short, spastic proximal colon due to tuberculouscolitis
Discussion
Majority of TB cases in the US have been found in patients with HIV and foreign-born residents.[2] The total number of cases of TB and the percentage suffering from extrapulmonary disease has risen; this is in part due to the HIV epidemic.[3] Gastrointestinal TB is still a common disease in the developing world. In a series from India, abdominal TB constituted 17% of the total number of admissions for TB in a large urban hospital.[4]
The entity is still rare in industrialized countries, except in those with HIV, where abdominal TB is usually a manifestation of disseminated disease.[5] An increased number of patients with abdominal TB have been reported from England and US among non-HIV infected immigrants.[6] In the US, the disease is seen in foreign-born residents who left their native countries several years ago and is rarely seen in the offspring of immigrants born in western countries. Studies of phage types of mycobacteria isolates indicated that most of them acquired TB in their homeland with reactivation of the infection years later.[7]
Tuberculous peritonitis is the most common form, followed by involvement of small bowel, specifically the ileocecal area, jejunum, colon, esophagus, stomach, and duodenum which are rarely involved.[8] Most of the cases of tuberculous peritonitis in non-HIV patients are due to reactivation of latent peritoneal foci. Subsequently, peritonitis develops from contiguous spread of infection from the fallopian tubes or intestines.[7] CT evidence of omental infiltration has also been reported as a radiologic abnormality seen in peritoneal TB.[9] TB of the small bowel and colon may result from swallowing infected sputum in the presence of concomitant active pulmonary disease;[10] this may have been the sequence in two of our patients who had active pulmonary TB at the time of colonic involvement. Abdominal TB in foreign-born residents can also occur due to primary infection or reactivation of latent foci to keep in Mycobacterium bovis acquired by drinking unpasteurized milk. These individuals were either born in or traveled to countries where the prevalence of bovine TB is still high.[11]
TB was noted by culture or PCR in three of four patients. In the remaining cases, the diagnosis was suggested from histopathologic examination and demonstration of acid-fast bacilli in the biopsy specimen. In these cases the organism could be either M. tuberculosis or M. bovis. All of our patients, however, denied drinking unpasteurized milk. However, it is important to note that PCR is unable to discriminate the two as PCR cannot identify M. bovis; and furthermore, acid-fast bacilli is not able to differentiate between M. tuberculosis complex and non-TB mycobacteria. M. tuberculosis isolated from sputum in one patient was sensitive to all standard antituberculous drugs and in the other M. tuberculosis isolated from colonic tissue was INH resistant.
Abdominal pain, fever, and ascites are the hallmarks of peritoneal TB.[12] Unusual manifestations include jaundice, hepatomegaly, and the absence of fever. In some rare cases, hypercalcemia is also a known but uncommon manifestation of TB which may be seen.[13] Ascites can frequently be present in upto 37% of cases.[5] A negative tuberculin test and normal chest X-ray findings are not uncommon[4,5] and add further to diagnostic confusion. Due to its low sensitivity and specificity, a tuberculin skin test can be further examined by culture, PCR, and at times interferon gamma release assay for both active and latent TB. Incorrect diagnoses include abdominal carcinomatosis, lymphoma, and cirrhosis of the liver with ascites. In one series of patients with peritoneal TB and concomitant cirrhosis of liver, an erroneous diagnosis was made in 100% of cases.[4] Patients with small intestinal TB characteristically present with abdominal pain, diarrhea, and weight loss; and occasionally with small bowel obstruction that mimics IBD. Manifestations of colon TB include diarrhea, bleeding, rectal polyps, fistulae, and perforation.[14] Infrequently, diffuse tuberculous colitis can mimic ulcerative colitis.
The clinical picture of small intestinal and colon TB can be confused with IBD, ileocecalactinomycosis, and malignancies; but in individuals from developing countries, chronic parasitic diseases including amebiasis, schistosomiasi, and strongyloidiasis are often considered the top priorities in the differential diagnosis.[15] Crohn's disease is uncommon in certain developing countries[16] and, therefore, in patients from those countries with the clinical picture of IBD, a tuberculous etiology should be the primary consideration.
Ascitic fluid analysis is often unrewarding. Frequency of positive cultures for M. tuberculosis varies from less than 20 to 83%.[4,12] Although an increased cell count with lymphocyte predominance is the expected finding in the peritoneal fluid, occasionally neutrophils may be predominant. This neutrophil response has specifically been noted in patients with peritoneal TB who have been on peritoneal dialysis.[17] CT findings are nonspecific, although CT evidence of omental infiltration or ileocecal thickening may be suggestive of the diagnosis in individuals from high prevalence areas.[18]
Peritoneal biopsy through laparoscopy is the preferred method for the diagnosis of peritoneal TB by identifying caseating granulomas in 85-90% of cases.[19] Ascitic fluid assay adenosine deaminase (ADA) has been emphasized as a diagnostic tool.[5] A high ADA level was found to be 100% sensitive and 95% specific. Patients with intestinal and colon TB require endoscopy and biopsy, with careful culture of the operative specimen for mycobacterial species. Most cases of abdominal TB respond to 6-9 months of antituberculous treatment.[4] The role of corticosteroids, particularly in tuberculous peritonitis, has appeared to reduce the frequency of complications.[20] None of our patients was treated with corticosteroids after the diagnosis was established and all except one did well with antituberculous therapy; the duration of therapy ranging from 9 to 12 months. Conservative surgical intervention may be necessary to manage complications such as perforations, fistula formation, or hemorrhage.[8]
Mortality in patients with abdominal TB without HIV infection remains high;[6] this is likely influenced by the compliance to treatment by different patient populations. In one series, higher mortality was observed in cirrhotic patients with tuberculous peritonitis.[4]
Despite the decline in the total number of TB cases in the US for the last 2 decades, the disease in the immigrant population from countries of high prevalence is continuously rising. Screening for latent pulmonary infection in foreign-born persons has been emphasized and implemented in the different countries as an effective tool for identifying person with TB before their arrival to US,[21] but may not be feasible for extrapulmonary TB including intestinal TB and also does not target active TB. The diagnosis of abdominal TB can be difficult as illustrated by our cases. Diagnosis is often delayed because physicians are not familiar with its clinical presentation. The lack of serious consideration made to the possibility of abdominal TB in turn may increase the risk for potential adverse outcomes.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Alami NN, Yuen CM, Miramontes R, Pratt R, Price SF, Navin TR, et al. Centers for Disease Control and Prevention (CDC).Trends in tuberculosis-United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:229–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Zuber PL, McKenna MT, Binkin NJ, Onrato IM, Castro KG. Long-term risk of tuberculosis among foreign-born persons in the United States. JAMA. 1997;278:304–7. [PubMed] [Google Scholar]
- 3.Shafer RW, Kim DS, Weiss JP, Quale JM. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine (Baltimore) 1991;70:384–97. doi: 10.1097/00005792-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Sircar S, Taneja VA, Kansra V. Epidemiology and clinical presentation of abdominal tuberculosis — A retrospective study. J Indian Med Assoc. 1996;94:342–4. [PubMed] [Google Scholar]
- 5.Bhargava D. Abdominal tuberculosis: Current status. Apollo Med. 2007;4:287–91. [Google Scholar]
- 6.Probert CSJ, Jayanthi V, Wicks AC, Carr-Locke DL, Garner P, Mayberry JF. Epidemiological study of abdominal tuberculosis among Indian migrants and the indigenous population of Leicester, 1972-1989. Gut. 1992;33:1085–8. doi: 10.1136/gut.33.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo J. Epidemiology and pathology of extrapulmonary and miliary tuberculosis. 2014. [Accessed June 28, 2014]. at http://www.uptodate.com/contents/epidemiology-and-pathology-of-extrapulmonary-and-miliary-tuberculosis?source=machineLearning&search=pathophysiology+of+abdminal+tb&selectedTitle=1%7E150§ionRank=1&anchor=H2#H7622739 .
- 8.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–99. [PubMed] [Google Scholar]
- 9.Jadvar H, Mindelzun RE, Olcott EW, Levitt DB. Still the great mimicker: Abdominal tuberculosis. AJR Am J Roentgenol. 1997;168:1455–60. doi: 10.2214/ajr.168.6.9168707. [DOI] [PubMed] [Google Scholar]
- 10.Nagahashi M, Aoyagi T, Yamada A, Rashid O, Adams B, Takabe K. Intestinal Co-infection of Tuberculosis and CMV can Cause Massive Lower GI Bleeding in a Patient with HIV. J Surg Sci. 2013;1:12–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Dankner W, Waecker N, Essey M, Moser K, Thompson M, Davis C. Mycobacterium bovis infections in San Diego: A clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen. Med. 1993;72:11–37. [PubMed] [Google Scholar]
- 12.Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: A retrospective review of 86 cases. QJM. 2008;101:189–95. doi: 10.1093/qjmed/hcm125. [DOI] [PubMed] [Google Scholar]
- 13.Shek CC, Natkunan A, Tsang V, Cochran CS, Swaminanthan R. Incidence, causes and mechanism of hypercalcemia in a hospital population in Hong Kong. Q J Med. 1990;77:1277–85. doi: 10.1093/qjmed/77.3.1277. [DOI] [PubMed] [Google Scholar]
- 14.Sibartie V, Kirwan WO, O’Mahony S, Stack W, Shanahan F. Intestinal tuberculosis mimicking Crohn's disease: Lessons relearned in a new era. Eur J Gastroenterol Hepatol. 2007;19:347–9. doi: 10.1097/MEG.0b013e328012122b. [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Thomas V, Mathan M, Chacko A, Chandy G, Ramakrishna BS, et al. Colonoscopic study of 50 patients with colonic tuberculosis. Gut. 1992;33:347–51. doi: 10.1136/gut.33.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.al Karawi MA, Mohamed AE, Yasawy MI, Graham DY, Shariq S, Ahmed AM, et al. Protean manifestation of gastrointestinal tuberculosis: Report of 130 patients. J Clin Gastroentrol. 1995;20:225–32. doi: 10.1097/00004836-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Lui S, Lo C, Choy B, Chan T, Lo W, Cheng I. Optimal treatment and long-term outcome of tuberculous peritonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1996;28:747–51. doi: 10.1016/s0272-6386(96)90259-0. [DOI] [PubMed] [Google Scholar]
- 18.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: The epidemiology and the response. Clin Infect Dis. 2010;50:S201–7. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 19.Das P, Shukla HS. Clinical diagnosis of abdominal tuberculosis. Br J Surg. 1976;63:941–6. doi: 10.1002/bjs.1800631213. [DOI] [PubMed] [Google Scholar]
- 20.Kadhiravan T, Deepanjali S. Role of corticosteroids in the treatment of tuberculosis: An evidence-based update. Indian J Chest Dis Allied Sci. 2010;52:153–8. [PubMed] [Google Scholar]
- 21.Posey DL, Naughton MP, Willacy EA, Russell M, Olson CK, Godwin CM, et al. Centers for Disease Control and Prevention (CDC). Implementation of new TB screening requirements for U. S. -bound immigrants and refugees - 2007-2014. MMWR Morb Mortal Wkly Rep. 2014;63:234–6. [PMC free article] [PubMed] [Google Scholar]


