Abstract
Background
The impact of screening mammography on breast cancer incidence is difficult to disentangle from cohort- and age-related effects on incidence.
Methods
We developed an age-period-cohort model of ductal carcinoma in situ (DCIS) and invasive breast cancer incidence in U.S. females using cancer registry data. Five functions were included in the model to estimate stage-specific effects for age, premenopausal birth cohorts, postmenopausal birth cohorts, period (for all years of diagnosis), and a mammography period effect limited to women aged ≥40 years after 1982. Incidence with and without the mammography period effect was calculated.
Results
More recent birth cohorts have elevated underlying risk compared to earlier cohorts for both pre- and postmenopausal women. Comparing models with and without the mammography period effect showed that overall breast cancer incidence would have been 23.1% lower in the absence of mammography in 2010 (95% CI 18.8, 27.4), including 14.7% (9.5, 19.3) lower for invasive breast cancer and 54.5% (47.4, 59.6) lower for DCIS. Incidence of distant-staged breast cancer in 2010 would have been 29.0% (13.1, 48.1) greater in the absence of mammography screening.
Conclusions
Mammography contributes to markedly elevated rates of DCIS and early stage invasive cancers, but also contributes to substantial reductions in the incidence of metastatic breast cancer.
Impact
Mammography is an important tool for reducing the burden of breast cancer, but future work is needed to identify risk factors accounting for increasing underlying incidence and to distinguish between indolent and potentially lethal early stage breast cancers that are detected via mammography.
Keywords: breast cancer, incidence, mammography screening, age-period-cohort, ductal carcinoma in situ
Introduction
Breast cancer incidence trends in the United States have changed dramatically over the past 30 years. For much of the 20th century, breast cancer incidence increased slowly, about 0.5 to 1.0% per year (1, 2). With the introduction of screening mammography, breast cancer incidence rose about 4% per year during 1982–1986 (3), plateaued through 1993, then increased to peak at an age-adjusted rate of 141.4 per 100,000 women in 1999. Rates subsequently declined abruptly during 1999–2003, stabilizing at about 127 per 100,000 women since 2003 (4).
These changes have been attributed to the utilization and performance of mammography, changes in risk factor prevalence—most notably reductions in postmenopausal hormone use (5)—and cohort differences among women born in eras as different as the Great Depression and the 1960s (6). Recently, attention has been given to the sustained burden of late-stage breast cancer despite widespread screening mammography (7). In contrast, incidence of early-stage breast cancer has dramatically increased, raising concerns that mammography screening leads to over-diagnosis without substantially reducing breast cancer mortality (8). However, interpretation of observed incidence trends is complicated by their dependence on many factors.
Age-period-cohort (APC) modeling is a statistical approach that can isolate the impact of mammography screening on breast cancer incidence, while accounting for the effects of variation in underlying incidence by age, year of diagnosis (period), and year of birth (cohort) (9). Previously, Holford et al (10) used an APC model to analyze breast cancer incidence rates through the year 2000 using data from the Surveillance, Epidemiology, and End Results (SEER) program. They estimated that screening mammography contributed to a 20% increase in overall breast cancer incidence in the United States. We modified and extended Holford’s approach to include the years 2000–2010 and evaluate the impacts of screening mammography on stage-specific incidence. Our objective was to quantify the impact of screening mammography on early and late stage incidence, accounting for influences of birth cohort and secular risk factor changes.
Materials and Methods
This study was determined to be exempt from human subjects review by the University of Wisconsin Health Sciences Institutional Review Board.
Data
Data on breast cancer incidence were obtained from the Surveillance, Epidemiology and End Results (SEER) registries (11). The numbers of ductal carcinoma in situ (DCIS) and malignant female breast cancer cases were tabulated by single years of age (20–84) and single-year periods (1935–2010). (Case definitions are provided in Supplemental Table S1.) Data from six registries were used since the population was consistent throughout the time period including Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, and Utah. Cases were diagnosed during 1973–2010. Data for three additional registries included cases diagnosed during different time frames: Seattle-Puget Sound (1974–2010), Atlanta (1975–2010), and Connecticut (1935–2010).
The denominators of the rates were estimates of the July 1 population for each year, derived from the decennial census. For Connecticut, population estimates by single years of age and period were obtained for 1935–2010 using cubic spline interpolation of population estimates for 5-year age groups by year provided by the Connecticut Tumor Registry. For the other SEER registries, population estimates by single years of age and period were obtained from SEER*Stat (Surveillance Research Program, National Cancer Institute SEER*Stat software, seer.cancer.gov/seerstat, version 8.0.4 released April 15, 2013).
Statistical methods
An APC model was fit to the SEER data using a negative binomial log-linear regression model implemented in the R function glm.nb from the MASS package (12, 13). In this approach, we assume that the number of cases diagnosed in a given year follows a negative binomial (over-dispersed Poisson) distribution with mean λD and variance λD +(λD)2/θ, where λ is the incidence rate, D is the denominator for the rate and θ is a dispersion parameter. Note that smaller values of the dispersion parameter correspond to greater overdispersion relative to the Poisson distribution. The log rate is assumed to have additive contributions associated with age, period and cohort as well as SEER registry.
Identification of the APC effects (given the linear dependence of age, period and cohort) followed the approach of Carstensen (14). The age function represents the log age-specific rate for the 1920 cohort. The cohort function represents the log rate ratio relative to the 1920 cohort. Separate cohort functions are used for premenopausal (age 45 and below) and postmenopausal (age 55 and above) women to account for differences in the etiology of and risk factors for pre- and post-menopausal breast cancer; the cohort function for women age 46–54 is a weighted average of the premenopausal and postmenopausal functions. The period function represents the residual log rate ratio relative to the age-cohort prediction (constrained to be 0 on average with 0 slope). A second period function—referred to as the mammography function—represents the residual log rate ratio relative to the age-period-cohort prediction after 1982, reflecting the approximate beginning of widespread mammography screening in the United States, for women aged ≥40, since routine screening for women under 40 years of age was not recommended (3). The mammography function thus isolates the impact of screening mammography from other period effects affecting all women. Sub-models including all of the functions described here were developed for each stage of breast cancer at diagnosis (DCIS, local, regional, distant, and unknown).
We represented each of the components of the model (age, premenopausal cohort, postmenopausal cohort, period, and mammography) as natural cubic splines. The number of knots for the natural cubic splines was selected iteratively based on the Bayesian Information Criterion (BIC) (15) until convergence; the minimum number of knots for each term was 4, the maximum number of knots was one per 5 years for age and mammography effects, and 1 per 10 years for the remaining effects.
Breast cancer incidence in the absence of mammography is the sum of the age, premenopausal cohort, postmenopausal cohort and period effects as well as the SEER registry effects. Rates are calculated for each registry separately. Overall rates are calculated by summing the expected number of cases across all registries and dividing by the total population covered by the registries. Age-adjusted rates use the 2000 US standard population (11); 95% confidence intervals were calculated using a parametric bootstrap (16). Extrapolation beyond the range of the data uses the last value carried forward.
Results
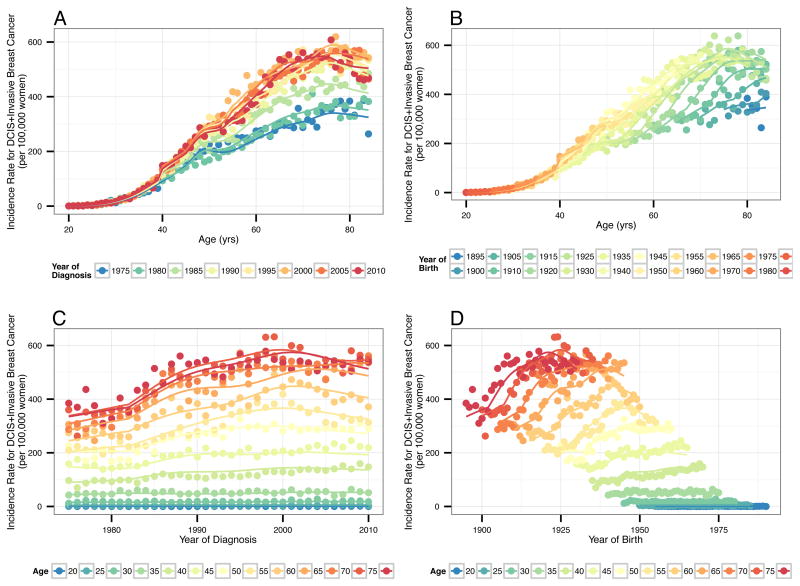
Age-specific incidence rates for all SEER registries combined are shown in Figure 1 along with the estimates from the APC model (see also Supplemental Movie). There is good agreement between fitted curves and observed rates. Differences between them are consistent with random variation rather than systematic modeling errors. These plots reflect higher breast cancer incidence in more recent years of diagnosis, more recent birth cohorts, and older ages.
Figure 1.
Observed and modeled overall breast cancer incidence rates for the 9 Surveillance, Epidemiology and End Results (SEER) registries, 1975–2010 by (A) age and period (year of diagnosis), (B) age and cohort (year of birth), (C) period (year of diagnosis) and age, and (D) cohort (year of birth) and age. Includes rates per 100,000 women for DCIS and invasive breast cancer combined. Observed crude rates shown with circles. Modeled rates shown with solid lines.
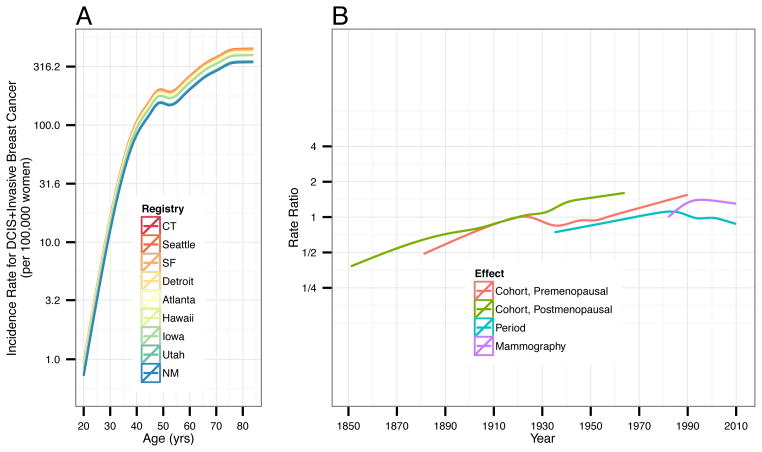
Estimated APC model components are presented in Figure 2; numerical values are given in Supplemental Table S2. The left panel of Figure 2 presents age-specific breast cancer incidence rates for each SEER registry for the 1920 cohort. The right panel of Figure 2 presents rate ratios for the cohort and period effects accounting for age and registry effects. The premenopausal birth cohort effect shows a steady increase in breast cancer rates for women born 1890 through 1990 except for a slightly reduction from 1930–1950. In contrast, rate ratios for postmenopausal birth cohorts increased steadily throughout.
Figure 2.
Estimated (A) age effect and (B) premenopausal cohort, postmenopausal cohort, overall period and mammography period effects from the APC model. Shading around lines indicates 95% confidence intervals. Rate ratios for pre- and postmenopausal cohort effects are shown for year of birth. Rate ratios for overall period and mammography period effects are shown for year of diagnosis.
After accounting for age, registry and cohort, the overall period effect shows a general increase in rate ratios for years of diagnosis from 1940 through 1980, with decreases thereafter to 2010 (Figure 2 and Supplemental Table S2). In contrast, the mammography effect in women age ≥40 increased over time with a peak rate ratio (compared with 1982) of 1.40 (95% CI 1.37–1.44) in 1995, and then decreased to 1.30 (95% CI 1.23–1.37) in 2010.
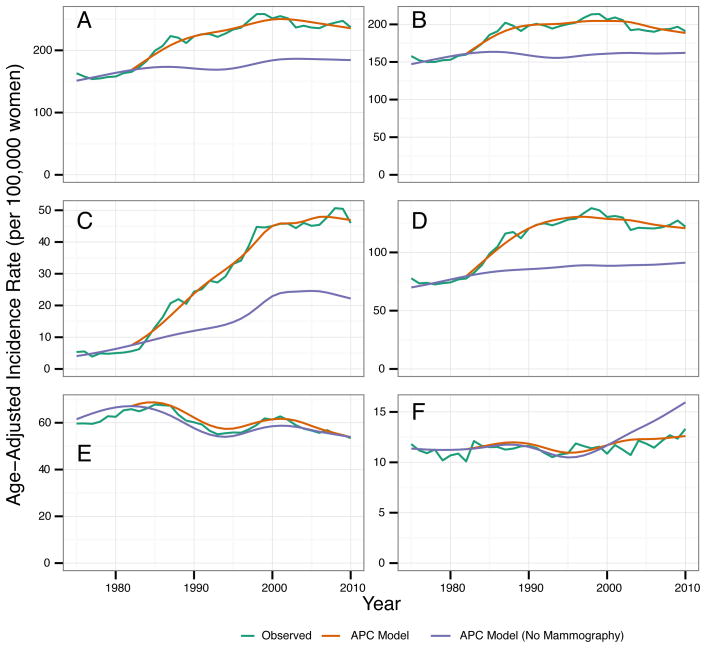
The APC model estimates that mammography has contributed to a substantial increase in breast cancer overall and in DCIS and localized invasive breast cancer individually (Figure 3). Conversely, the APC model estimates that screening mammography has reduced distant breast cancer incidence after the year 2000; regional breast cancer incidence was largely unaffected by screening mammography (Figure 3). In 1985, 11.5% (95% CI 10.3, 12.5) of DCIS and invasive breast cancer combined was attributable to screening mammography (Table 1). In 2010, total breast cancer incidence would have been approximately 23.1% (95% CI 18.8, 27.4) lower without mammography screening. While about 14.7% (95% CI 9.5, 19.3) of invasive breast cancer is attributable to screening in 2010, DCIS and localized invasive breast cancer incidence rates would have been 54% and 26% lower, respectively, in the absence of mammography screening (Table 1). The APC model predicts that incidence of distant-staged breast cancer would have been 29% higher in 2010 in the absence of mammography, with little difference in regional breast cancer incidence.
Figure 3.
Observed SEER (green line) and modeled breast cancer incidence rates for the 9 Surveillance, Epidemiology and End Results (SEER) registries, 1973–2010. Age-adjusted breast cancer incidence rates per 100,000 women for ages 25–84 years, overall and by stage. Incidence rates from the age-period-cohort model estimated with (orange line) and without (blue line) the mammography screening period effect for (A) DCIS and invasive, (B) invasive, (C) DCIS, (D) localized, (E) regional, and (F) distant staged breast cancer.
Table 1.
Breast cancer incidence from the age-period-cohort model with and without the screening period effect, by stage and year of diagnosis
| Year and Stage of Diagnosis | Incidence per 100,000 womena | Percent of cases attributable to screening (95% CI) | ||
|---|---|---|---|---|
| Total | Without screening | Difference (95% CI) | ||
| All Cases | ||||
| 1985 | 274.8 | 243.1 | 31.6 (28.3, 34.3) | 11.5% (10.3, 12.5) |
| 1990 | 319.8 | 241.0 | 78.8 (72.2, 84.1) | 24.6% (22.7, 26.2) |
| 1995 | 338.4 | 241.1 | 97.3 (91.1, 102.9) | 28.8% (27.0, 30.4) |
| 2000 | 359.1 | 259.2 | 99.9 (91.6, 107.0) | 27.8% (25.7, 29.8) |
| 2005 | 352.1 | 262.0 | 90.1 (80.7, 100.8) | 25.6% (22.9, 28.5) |
| 2010 | 337.4 | 259.5 | 77.9 (63.3, 92.9) | 23.1% (18.8, 27.4) |
| Invasive | ||||
| 1985 | 257.0 | 230.2 | 26.8 (24.0, 29.2) | 10.4% (9.3, 11.3) |
| 1990 | 285.2 | 224.2 | 61.0 (55.4, 65.7) | 21.2% (19.3, 22.8) |
| 1995 | 289.7 | 220.5 | 69.3 (63.4, 74.6) | 23.6% (21.6, 25.4) |
| 2000 | 293.2 | 227.0 | 66.2 (57.5, 72.6) | 22.3% (19.3, 24.4) |
| 2005 | 282.9 | 227.4 | 55.5 (45.9, 64.5) | 19.3% (15.9, 22.5) |
| 2010 | 268.5 | 228.2 | 40.2 (26.5, 52.9) | 14.7% (9.5, 19.3) |
| DCIS | ||||
| 1985 | 17.8 | 12.9 | 4.8 (4.2, 5.2) | 27.1% (24.3, 29.6) |
| 1990 | 34.6 | 16.8 | 17.8 (16.3, 19.3) | 51.5% (47.5, 55.3) |
| 1995 | 48.7 | 20.6 | 28.1 (26.4, 29.8) | 57.5% (54.4, 61.0) |
| 2000 | 65.9 | 32.2 | 33.7 (32.0, 36.5) | 51.1% (48.2, 54.8) |
| 2005 | 69.2 | 34.6 | 34.6 (32.0, 37.6) | 49.9% (46.4, 54.3) |
| 2010 | 68.9 | 31.3 | 37.6 (33.0, 41.5) | 54.5% (47.4, 59.6) |
| Localized | ||||
| 1985 | 138.9 | 117.4 | 21.5 (19.8, 23.8) | 15.5% (14.2, 17.2) |
| 1990 | 174.8 | 121.6 | 53.2 (49.7, 57.8) | 30.5% (28.7, 33.0) |
| 1995 | 188.6 | 125.2 | 63.4 (59.3, 67.3) | 33.7% (31.5, 35.7) |
| 2000 | 187.1 | 125.9 | 61.2 (55.2, 65.9) | 33.0% (29.7, 35.5) |
| 2005 | 181.5 | 127.0 | 54.5 (47.5, 60.5) | 30.4% (26.6, 33.7) |
| 2010 | 174.8 | 129.7 | 45.1 (36.3, 54.0) | 26.2% (21.2, 31.4) |
| Regional | ||||
| 1985 | 96.1 | 91.4 | 4.7 (3.1, 6.2) | 4.9% (3.2, 6.4) |
| 1990 | 87.2 | 80.3 | 6.9 (4.0, 9.6) | 7.9% (4.7, 10.9) |
| 1995 | 80.3 | 75.5 | 4.8 (2.1, 7.3) | 6.1% (2.8, 9.1) |
| 2000 | 85.6 | 81.2 | 4.5 (0.5, 7.7) | 5.4% (0.9, 9.1) |
| 2005 | 81.4 | 78.7 | 2.7 (−1.5, 6.4) | 3.5% (−1.5, 7.9) |
| 2010 | 74.3 | 74.3 | 0.0 (−6.3, 5.4) | 0.1% (−8.2, 7.1) |
| Distant | ||||
| 1985 | 16.9 | 16.6 | 0.2 (−1.0, 1.0) | 1.3% (−6.0, 6.1) |
| 1990 | 17.0 | 16.5 | 0.5 (−1.6, 2.0) | 2.8% (−9.5, 11.3) |
| 1995 | 15.7 | 15.0 | 0.7 (−0.9, 2.3) | 4.7% (−5.5, 14.8) |
| 2000 | 16.7 | 16.6 | 0.0 (−1.8, 1.8) | 0.5% (−10.7, 10.9) |
| 2005 | 17.3 | 19.5 | −2.1 (−4.2, −0.4) | −12.0% (−24.3, −2.2) |
| 2010 | 17.5 | 22.5 | −5.1 (−8.2, −2.4) | −29.0% (−48.1, −13.1) |
Abbreviations: CI, confidence interval; DCIS, ductal carcinoma in situ.
Age-adjusted to the 2000 U.S. female population for ages 40–84.
Discussion
The APC model demonstrates that screening mammography has contributed to increases in early stage breast cancer incidence and declines in distant stage incidence. The stable pattern in the observed SEER incidence rates of distant stage breast cancer should not be interpreted as evidence that screening has had no beneficial impact on reducing breast cancer mortality. Our analysis indicates that screening mammography has countered higher underlying risk of breast cancer for more recent birth cohorts, likely due to elevated risk factor profiles throughout their lifetimes.
For 2010, we estimate that 23% of DCIS and invasive breast cancer combined is attributable to mammography screening. As expected, mammography is responsible for detecting a greater percent of early stage breast cancer. This percentage of cases attributed to screening includes women that benefited from early detection and treatment as well as women over-diagnosed with tumors that never would have caused harm. Previous reviews suggest that 1–10% of breast cancer is over-diagnosed (17, 18); higher percentages, ranging from 5–75%, have been found in studies that do not adjust for lead-time or differences in underlying risk between screened and unscreened populations (19–21). Estimates of over-diagnosis are also sensitive to the age range included in the calculations (e.g., <40 or >80), whether in situ cases are included, and whether screening has reached a steady-state in the population (22). Our results cannot be used to estimate an over-diagnosis rate, but instead provide information regarding the impact of screening mammography on observed incidence rates.
The APC model reflects the long-term slow increase in rates during the 1940s through 1970 (1, 2); the brief increase in 1974 potentially due to greater awareness of breast cancer resulting from the publicized diagnoses of Happy Rockefeller and Betty Ford (23); the steep increase due to dissemination of mammography screening in the 1980s (3); another increase likely driven by expanded use of postmenopausal hormones in the 1990s (24); and the subsequent drop attributed to the decline in use of postmenopausal hormones after 2000 (5). The model also captures the increasing trend in breast cancer incidence with increasing age as well as the Clemmesen’s hook phenomenon (25), with the slope in the increase in rates with age changing around the time of menopause.
Our analysis differs in a number of ways from the previous APC model developed by Holford et al (10). The Holford et al model assumes no period effect prior to 1982 and two separate period effects after 1982, one for women 40 and over (the mammography effect) and one for women under 40. Our model assumes a general period effect for all women over the entire follow-up period and a separate period effect for women aged 40 and over after 1982 (the mammography effect). Our mammography effect is the contrast between the period effect for women 40 and over after 1982 and the period effect for women under 40 after 1982, while Holford et al define the mammography effect as the period effect for women ≥40 after 1982. As such, the estimated mammography effect from Holford et al is roughly equivalent to the sum of our estimated mammography effect and our estimated general period effect after 1982. Because the estimated period effect decreases from 1986–1995 and 1999–2005, our mammography effect is larger than the estimates from Holford et al (peak rate ratio 1.40 in 1995 compared with Holford’s peak of 1.25 in 1987 and 1.28 in 2000). Our mammography effect shows a rapid increase in breast cancer incidence associated with the introduction of mammography, which later peaks then declines slightly. The Holford et al estimate of the mammography effect shows a similar rapid increase, but it is followed by a decline and rebound to the same or possibly greater risk (10). In contrast to Holford et al, our model shows little, if any, rise in breast cancer incidence in the absence of screening after 1982. The two most important factors that inform our estimate of the incidence in the absence of screening are the observed change in breast cancer incidence before 1982 and the observed change in breast cancer incidence for women under 40 after 1982. Age-adjusted DCIS and invasive breast cancer incidence from SEER for women age 25–84 was 163.1 per 100,000 women in 1975 and 165.4 per 100,000 women in 1982, a rise of 0.4% per year; age-adjusted DCIS and invasive breast cancer incidence from SEER for women age 25–39 was 39.3 per 100,000 women in 1982, 39.3 per 100,000 women in 1987 and 40.6 per 100,000 women in 2007, a rise of 0.1% per year (11). Both of these trends are consistent with our APC model results.
Other investigators have used APC models to describe overall breast cancer incidence trends in numerous regions and countries, with different approaches for addressing the influence of population screening (26–41). Other than Holford et al (10), most previous APC models did not include a mammography period effect. In their APC models for several Nordic countries, Rostgaard et al (27) and Moller (34) corrected for the effects of systematic screening to isolate secular trends without mammography. The relative period effect for Finland showed an increasing trend perhaps reflecting Finland’s fast economic growth; period effects were more modest in other Nordic countries (27, 34). Furthermore, APC models of breast cancer in Taiwan, Mumbai and Japan found significant effects for both cohort and period in the absence of broad participation in population-based breast cancer screening programs (29, 31, 36). Other APC models (30, 35), such as the model describing breast cancer incidence rates in France (28), found that cohort effects were stronger than period effects, probably due to the absence or limited extent of population screening mammography.
Additional modeling is needed to examine the impact of multiple factors on past breast cancer rates as well as more recent trends. For example, several factors, beyond changes in detection by mammography screening, likely contributed to the increase in breast cancer incidence during the 1980s and more recent declines in incidence. A previous study suggested that the increase in the 1980s was entirely explained by mammography screening (42). However, use of combined estrogen-progestin hormones also increased in the 1980s (43–45), and several reports suggest that declines after 2000 in breast cancer incidence coincide with reductions in use of hormones (5, 6, 46). In Norway, investigators estimated that, for women aged 50–69, 23% of breast cancer cases diagnosed in 2002 were attributable to mammography whereas 27% of cases were attributable to hormone use (47). Notably, the prevalence of obesity also increased in the 1980s. Based on data from the National Health and Examination Survey (NHANES), the prevalence of overweight in U.S. females increased from 17% in 1976–80 to 27% in 1991–1994 to 34% by 2000 (48). Simulation modeling suggests that only about 5% of breast cancer is attributable to obesity, in part because of the differing effects of obesity on pre- and post-menopausal breast cancer (49). Additional studies are needed to disentangle the impact of concurrent changes in multiple risk factors from changes in screening. While individual level data from case-control or cohort studies is needed to definitively address these questions, in the absence of such data, simulation modeling can provide useful insights. For example, simulation modeling could remove the influence of mammography screening on breast cancer incidence to examine the role of risk factors in breast cancer development and, consequently, evaluate the relative benefits that primary and secondary prevention have on the breast cancer burden (50).
Studies based on APC models inherently include limitations, most notably the availability of cancer surveillance data. Projections of breast cancer incidence in the absence of screening depend on trends prior to the dissemination of mammography in 1982, but limited cancer registry data were available prior to 1982. The SEER Program began in 1973; only the Connecticut registry operated between 1935 and 1973 (51). Additionally, our APC model identifies the mammography period effect as the residual period effect for women ≥40 after 1982. This assumption was based on the limited availability of screening mammography prior to 1982 (52, 53) and general support for screening mammography for women ≥40, although recommendations for women in their forties has fluctuated.
In summary, our APC model describes the impact of mammography screening on breast cancer incidence in the U.S. over the past three decades. Consistent with prior studies, these results find greater changes in incidence attributable to birth cohort than according to period. In particular, recent pre- and postmenopausal birth cohorts continue to experience elevated risk of breast cancer, even after accounting for mammography screening and other period effects. Our results suggest that screening mammography is associated with elevated rates of early-stage breast cancer and concurrent reductions in late-stage breast cancer. These results suggest that mammography is an important tool for reducing the burden of breast cancer in the United States, but future work is needed to identify risk factors accounting for increasing underlying incidence and to distinguish between indolent and potentially lethal early stage breast cancers that are detected via mammography.
Supplementary Material
Acknowledgments
Funding: This study was supported by the National Cancer Institute to R. Gangnon, B. Sprague, N. Stout, O. Alagoz, and A. Trentham-Dietz (Grants U01 CA152958 and P50 CA014520).
The authors would like to express their gratitude to John Hampton, Alex Binder, and Patricia Jewett for assistance with data, and to the CISNET Breast Working Group especially Drs. Rocky Feuer, Kathy Cronin, Jeanne Mandelblatt, and Clyde Schechter for advice and support for this project.
Footnotes
The authors declare no conflict of interest.
Certain data used in this study were obtained from the Connecticut Department of Health. The authors assume full responsibility for analyses and interpretation of these data. This study was approved by the Connecticut Department of Health Human Investigators Committee.
References
- 1.Sullivan PD, Christine B, Connelly R, Barrett H. Analysis of trends in age-adjusted incidence rates for 10 major sites of cancer. Am J Public Health. 1972;62:1065–71. doi: 10.2105/ajph.62.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devesa SS, Silverman DT, Young JL, Jr, Pollack ES, Brown CC, Horm JW, et al. Cancer incidence and mortality trends among whites in the United States, 1947–84. J Natl Cancer Inst. 1987;79:701–70. [PubMed] [Google Scholar]
- 3.Miller BA, Feuer EJ, Hankey BF. Recent incidence trends for breast cancer in women and the relevance of early detection: an update. CA Cancer J Clin. 1993;43:27–41. doi: 10.3322/canjclin.43.1.27. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) 2012 Apr; http://seercancergov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site.
- 5.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–4. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 6.Kerlikowske K, Miglioretti DL, Buist DS, Walker R, Carney PA National Cancer Institute-Sponsored Breast Cancer Surveillance C. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99:1335–9. doi: 10.1093/jnci/djm111. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309:800–5. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 8.Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA internal medicine. 2014;174:448–54. doi: 10.1001/jamainternmed.2013.13635. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20:1263–8. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006:19–25. doi: 10.1093/jncimonographs/lgj016. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance Epidemiology and End Results (SEER) Program [database on the Internet] Research Data (1973–2010) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; [released April 2013, based on the November 2012 submission]. Available from: www.seer.cancer.gov. [Google Scholar]
- 12.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/ [Google Scholar]
- 13.Venables WN, Ripley BD. Modern Applied Statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 14.Carstensen B. Age-Period-Cohort models for the Lexis Diagram. Stat Med. 2007;26:3018–45. doi: 10.1002/sim.2764. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–4. [Google Scholar]
- 16.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall; 1993. [Google Scholar]
- 17.Puliti D, Duffy SW, Miccinesi G, de Koning H, Lynge E, Zappa M, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen. 2012;19 (Suppl 1):42–56. doi: 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 18.Independent UK. Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 19.Biesheuvel C, Barratt A, Howard K, Houssami N, Irwig L. Effects of study methods and biases on estimates of invasive breast cancer overdetection with mammography screening: a systematic review. Lancet Oncol. 2007;8:1129–38. doi: 10.1016/S1470-2045(07)70380-7. [DOI] [PubMed] [Google Scholar]
- 20.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 21.Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013;158:831–8. doi: 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Gelder R, Heijnsdijk EA, van Ravesteyn NT, Fracheboud J, Draisma G, de Koning HJ. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev. 2011;33:111–21. doi: 10.1093/epirev/mxr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betsill WL, Jr, Byrd BF, Jr, Hartmann WH. Breast cancer report. Cancer. 1975;36:305–7. doi: 10.1002/1097-0142(197508)36:2<305::aid-cncr2820360202>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999–2010. Obstet Gynecol. 2012;120:595–603. doi: 10.1097/AOG.0b013e318265df42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemmensen J. Carcinoma of the breast: results from statistical research. Br J Radiology. 1948;21:583–90. doi: 10.1259/0007-1285-21-252-583. [DOI] [PubMed] [Google Scholar]
- 26.Wong IO, Cowling BJ, Schooling CM, Leung GM. Age-period-cohort projections of breast cancer incidence in a rapidly transitioning Chinese population. Int J Cancer. 2007;121:1556–63. doi: 10.1002/ijc.22731. [DOI] [PubMed] [Google Scholar]
- 27.Rostgaard K, Vaeth M, Holst H, Madsen M, Lynge E. Age-period-cohort modelling of breast cancer incidence in the Nordic countries. Stat Med. 2001;20:47–61. doi: 10.1002/1097-0258(20010115)20:1<47::aid-sim613>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Viel JF, Rymzhanova R, Fournier E, Danzon A. Trends in invasive breast cancer incidence among French women not exposed to organized mammography screening: an age-period-cohort analysis. Cancer epidemiology. 2011;35:521–5. doi: 10.1016/j.canep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Shen YC, Chang CJ, Hsu C, Cheng CC, Chiu CF, Cheng AL. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: implications from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1986–90. doi: 10.1158/1055-9965.EPI-04-0932. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Ioka A, Nakayama T, Tsukuma H, Nakamura T. Comparison of trends in cancer incidence and mortality in Osaka, Japan, using an age-period-cohort model. Asian Pacific journal of cancer prevention: APJCP. 2011;12:879–88. [PubMed] [Google Scholar]
- 31.Dhillon PK, Yeole BB, Dikshit R, Kurkure AP, Bray F. Trends in breast, ovarian and cervical cancer incidence in Mumbai, India over a 30-year period, 1976–2005: an age-period-cohort analysis. Br J Cancer. 2011;105:723–30. doi: 10.1038/bjc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. Br J Cancer. 2011;105:1795–803. doi: 10.1038/bjc.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor R, Boyages J. Estimating risk of breast cancer from population incidence affected by widespread mammographic screening. J Med Screen. 2001;8:73–6. doi: 10.1136/jms.8.2.73. [DOI] [PubMed] [Google Scholar]
- 34.Moller B, Weedon-Fekjaer H, Hakulinen T, Tryggvadottir L, Storm HH, Talback M, et al. The influence of mammographic screening on national trends in breast cancer incidence. Eur J Cancer Prev. 2005;14:117–28. doi: 10.1097/00008469-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Persson I, Bergstrom R, Sparen P, Thorn M, Adami HO. Trends in breast cancer incidence in Sweden 1958–1988 by time period and birth cohort. Br J Cancer. 1993;68:1247–53. doi: 10.1038/bjc.1993.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minami Y, Tsubono Y, Nishino Y, Ohuchi N, Shibuya D, Hisamichi S. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer. 2004;108:901–6. doi: 10.1002/ijc.11661. [DOI] [PubMed] [Google Scholar]
- 37.Sim X, Ali RA, Wedren S, Goh DL, Tan CS, Reilly M, et al. Ethnic differences in the time trend of female breast cancer incidence: Singapore, 1968–2002. BMC Cancer. 2006;6:261. doi: 10.1186/1471-2407-6-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakai K, Suzuki S, Ohno Y, Kawamura T, Tamakoshi A, Aoki R. Epidemiology of breast cancer in Japan. Int J Epidemiol. 1995;24:285–91. doi: 10.1093/ije/24.2.285. [DOI] [PubMed] [Google Scholar]
- 39.Seow A, Duffy SW, McGee MA, Lee J, Lee HP. Breast cancer in Singapore: trends in incidence 1968–1992. Int J Epidemiol. 1996;25:40–5. doi: 10.1093/ije/25.1.40. [DOI] [PubMed] [Google Scholar]
- 40.Wang PP, Cao Y. Incidence trends of female breast cancer in Saskatchewan, 1932–1990. Breast Cancer Res Treat. 1996;37:197–207. doi: 10.1007/BF01806501. [DOI] [PubMed] [Google Scholar]
- 41.Chia KS, Reilly M, Tan CS, Lee J, Pawitan Y, Adami HO, et al. Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: a comparative population-based study in Singapore and Sweden. Int J Cancer. 2005;113:302–6. doi: 10.1002/ijc.20561. [DOI] [PubMed] [Google Scholar]
- 42.Wun LM, Feuer EJ, Miller BA. Are increases in mammographic screening still a valid explanation for trends in breast cancer incidence in the United States? Cancer Causes Control. 1995;6:135–44. doi: 10.1007/BF00052774. [DOI] [PubMed] [Google Scholar]
- 43.Wysowski DK, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982–1992. Obstet Gynecol. 1995;85:6–10. doi: 10.1016/0029-7844(94)00339-f. [DOI] [PubMed] [Google Scholar]
- 44.Hemminki E, Kennedy DL, Baum C, McKinlay SM. Prescribing of noncontraceptive estrogens and progestins in the United States, 1974–86. Am J Public Health. 1988;78:1479–81. doi: 10.2105/ajph.78.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy DL, Baum C, Forbes MB. Noncontraceptive estrogens and progestins: use patterns over time. Obstet Gynecol. 1985;65:441–6. [PubMed] [Google Scholar]
- 46.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 47.Weedon-Fekjaer H, Bakken K, Vatten LJ, Tretli S. Understanding recent trends in incidence of invasive breast cancer in Norway: age-period-cohort analysis based on registry data on mammography screening and hormone treatment use. BMJ. 2012;344:e299. doi: 10.1136/bmj.e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ljungvall A, Zimmerman FJ. Bigger bodies: long-term trends and disparities in obesity and body-mass index among U.S. adults, 1960–2008. Soc Sci Med. 2012;75:109–19. doi: 10.1016/j.socscimed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Chang Y, Schechter CB, van Ravesteyn NT, Near AM, Heijnsdijk EA, Adams-Campbell L, et al. Collaborative modeling of the impact of obesity on race-specific breast cancer incidence and mortality. Breast Cancer Res Treat. 2012;136:823–35. doi: 10.1007/s10549-012-2274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandelblatt J, van Ravesteyn N, Schechter C, Chang Y, Huang AT, Near AM, et al. Which strategies reduce breast cancer mortality most? Collaborative modeling of optimal screening, treatment, and obesity prevention. Cancer. 2013;119:2541–8. doi: 10.1002/cncr.28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Surveillance Epidemiology and End Results: Overview of the SEER Program. 2013 http://seer.cancer.gov/about/overview.html. [cited May 28, 2013]; Available from.
- 52.Cronin KA, Mariotto AB, Clarke LD, Feuer EJ. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr. 2006:26–9. doi: 10.1093/jncimonographs/lgj005. [DOI] [PubMed] [Google Scholar]
- 53.Lantz PM, Remington PL, Newcomb PA. Mammography screening and increased incidence of breast cancer in Wisconsin. J Natl Cancer Inst. 1991;83:1540–6. doi: 10.1093/jnci/83.21.1540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.