Abstract
Injury- or disease-induced artemin (ARTN) signaling can sensitize primary afferents and contribute to persistent pain. We demonstrate that administration of an anti-artemin (α-ARTN) neutralizing antibody can block the development of, and reverse already established, bladder hyperalgesia associated with cyclophosphamide (CYP)-induced cystitis in mice. We further demonstrate that α-ARTN therapy blocks upregulation of TRPA1, an ion channel contributing to persistent bladder pain during CYP-induced cystitis, and decreases phospho-ERK1/2 (pERK) immunoreactivity in regions of the spinal cord receiving bladder afferent input. Thus, α-ARTN is a promising novel therapeutic approach for treatment of bladder hyperalgesia that may be associated with interstitial cystitis (IC)/painful bladder syndrome (PBS), as well as cystitis associated with anti-tumor or immunosuppressive CYP therapy.
INTRODUCTION
Pelvic/suprapubic pain is a cardinal symptom of interstitial cystitis/painful bladder syndrome (IC/PBS), a chronic condition affecting 2.7–6.5% of women in the U.S. 3. In contrast to bacterial cystitis, IC/PBS characteristically occurs in the absence of ongoing infection, but may be accompanied by varying degrees of inflammation 21. There is no single defining etiology or pathogenesis of IC/PBS, thus there is no consistently effective pain management strategy to improve quality of life in individuals with the disease.
Animal studies have shown that visceral injury and inflammation are accompanied by increased peripheral growth factor expression, which in turn can drive hyperalgesia 4, 12, 23, 31, 32. Studies of individuals with IC/PBS have reported increases in nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF) in urethral and bladder tissue 22, 29, 30, 41. We have previously shown in animal models of skin and colon inflammation that target-derived mRNA for artemin (ARTN), a member of the GDNF family, increases up to five-fold more than the mRNAs for either NGF or GDNF 31, 35. Moreover, a single injection of ARTN into uninflamed skin increases the duration of pain-like behavior in vivo and potentiates nociceptor function in vitro 35. ARTN sensitizes nociceptive afferents, in part, through upregulation and/or augmented function of the ion channels TRPV1 and TRPA1 31, 35. Recent studies from our labs revealed that bladder afferent TRPA1 contributes to persistent hyperalgesia in a mouse model of cyclophosphamide (CYP)-induced cystitis 16. Because of the previously demonstrated relationship between ARTN and TRPA1 expression, we hypothesized that treatment with an ARTN-neutralizing antibody (α-ARTN) might be effective in blocking bladder hyperalgesia.
To test our hypothesis, we used a mouse model of cystitis shown previously to elicit changes in TRPA1 expression and function 16 and quantified urinary bladder expression of growth factor mRNAs, bladder primary afferent TRPA1 expression and function, nociceptive behavior, and spinal pERK immunoreactivity. A single injection of α-ARTN, either at the initiation or the conclusion of CYP treatment, effectively blocked or reversed, respectively, bladder hyperalgesia. α-ARTN prevented CYP-induced upregulation of bladder afferent TRPA1 and reduced the number of activated second order neurons in the spinal cord that receive bladder primary afferent input. These results suggest that α-ARTN treatment could be effective for people experiencing bladder pain associated with IC/PBS.
MATERIALS AND METHODS
Animals
Experiments were performed on female, 8–12 wk old C57BL/6 mice (Jackson Laboratory) housed in the Division of Laboratory Animal Resources at the University of Pittsburgh Medical Center. Mice received food and water ad libitum. All procedures conformed to NIH guidelines and were in accordance with those of the University of Pittsburgh IACUC and the Committee for Research and Ethical Issues of the International Association for the Study of Pain.
Cyclophosphamide-induced cystitis and α-ARTN treatment
Cystitis was induced by intraperitoneal injection of cyclophosphamide (CYP, 100 mg/kg; Sigma-Aldrich) every other day for a period of five days (three injections total). A control group was administered intraperitoneal sterile saline injections on the same schedule. Some mice additionally received an intraperitoneal injection of α-ARTN (10 mg/kg; R&D Systems, Inc.) or IgG (10 mg/kg; R&D Systems, Inc.) 30 min prior to the first CYP or saline injection on day one; a final group received α-ARTN 30 min following the final CYP injection on day five (Figure 1). Mice were randomly assigned to all groups. Endpoint measurements were assessed at one or seven days following the final CYP injection.
Figure 1.
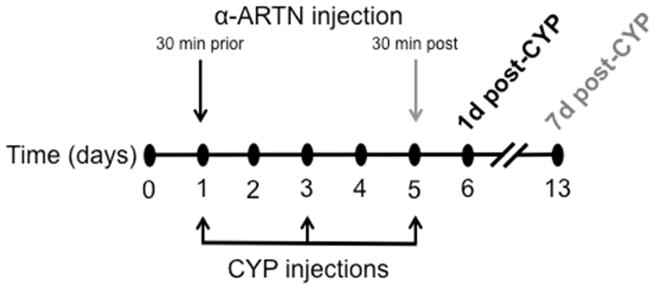
The experimental time line was as follows: cystitis was induced by injection of cyclophosphamide (CYP; 100 mg/kg, i.p.) on Days 1, 3, and 5; an ARTN neutralizing antibody (α-ARTN; 10 mg/kg, i.p.) was administered to one group of mice 30 min prior to CYP on day 1, and to another group of mice 30 min following CYP on day 5. Experimental endpoints were collected from these groups on Days 6 and 13, respectively.
Real-time RT-PCR
RNA isolation and real-time RT-PCR were performed as previously described 12, 35. Extracted RNA (5 μg) was treated with DNase (Invitrogen) to remove genomic DNA, then 1 μg was reverse transcribed using Superscript II (Invitrogen). SYBR Green PCR amplification was performed using a 7000 real-time thermal cycler (Applied Biosystems). Each sample was run in duplicate, and the threshold cycle (Ct) values were recorded as a measure of initial template concentration of NGF, GDNF, and ARTN, and were normalized to Ct values for β-actin. A relative change in mRNA expression was calculated as a ratio of the control group mean for each gene using the Pfaffl method 43. Primer sequences used were: NGF (F-TCCAATCCTGTTGAGAGTGG, R-CAGGCTGTGTCTATGCGGAT), GDNF (F-AAGGTCACCAGATAAACAAGCGG, R-TCACAGGAGCCGCTGCAATATC), ARTN (F-CTCAGTCTCCTCAGCCCG, R-TCCACGGTCCTCCAGGTG), β-actin (F-AGAGGGAAATCGTGCGTGAC, R-CAATAGTGATGACCTGGCCGT).
Retrograde labeling of bladder afferents
Mice were anesthetized using isoflurane and the urinary bladder was exposed via laparotomy. Three injections (12 μl total volume) of Alexa Fluor-conjugated cholera toxin-β (CTβ) were made into the bladder wall, and abdominal incisions were sutured. Mice were returned to their home cages and allowed to recover for at least three days prior to initiation of CYP injections.
Single-cell RT-PCR
Mice were deeply anesthetized with isoflurane and transcardially perfused with cold Ca2+/Mg2+-free Hank’s balanced salt solution (HBSS; Invitrogen). Bilateral L5-S1 DRG were dissected into cold HBSS and dissociated 34. Cells were plated in DMEM-F12 media (Invitrogen) containing 10% fetal bovine serum and antibiotics (penicillin/streptomycin, 50 U/ml). Coverslips were flooded with media 2 hrs after plating and used within 8–10 hrs.
Individual CTβ+ neurons were collected with large bore (50 μm) glass pipettes and expelled into microcentrifuge tubes containing reverse-transcriptase (RT) mix (Invitrogen). For each preparation of isolated cells on which single-cell PCR was performed, two negative controls were included: one omitting RT and one using a cell-free mix as template. The first-strand cDNA from CTβ+ neurons was used as template in a reaction containing 1× GoTaq reaction buffer (Promega), 20 μM external primers, 0.2 M deoxynucleoside triphosphates, and 0.2 μl GoTaq DNA polymerase (Promega). Each initial PCR product served as template in a subsequent reaction using a nested (internal) primer pair. Products were electrophoresed on 2% agarose-ethidium bromide gels and photographed. Only samples with detectable GAPDH were analyzed. Primer sequences used were: TRPA1 (EXT F-CTTCCTGGATTACAACAATGCTCTG, R-ATGTCCCCAACCGCCAAGC; INT F- CAGTGGCAATGTGGAGCAATAG, R-AAGGAAAGCAATGGGGTGC), TRPV1 (EXT F-GGGAAGAATAACTCACTGCCTGTG, R-TCATCCACCCTGAAGCACCAC; INT F-GGCGAGACTGTCAACAAGATTGC, R- TCATCCACCCTGAAGCACCAC), GAPDH (F- GCTGAGTATGTCGTGGAGTCTA, R- CATACTTGGCAGGTTTCTCCAG).
Calcium imaging
Ca2+ imaging was performed on retrogradely labeled bladder afferent neurons that were isolated as described above. Neurons were incubated in the Ca2+ indicator Fura-2 AM ester (2 μM, Invitrogen) with 0.02% Pluronic F-127 (Invitrogen) in normal bath solution (in mM: 130 NaCl, 5 KCl, 1.5 CaCl2, 0.9 MgCl2, 20 HEPES, 5.5 glucose, 0.5 KH2PO4, 0.5 Na2HPO4, pH 7.4, osmolality 325 mOsm) containing 5 mg/ml BSA (Sigma-Aldrich) for 20 min at 37°C. Coverslips were mounted on an inverted microscope stage (Olympus) and continuously perfused with normal bath solution. Perfusion rate (5 ml/min) was controlled with a gravity flow and rapid-switching local perfusion system (Warner Instruments). Solutions were maintained at 30°C using a heated stage and in-line heating system (Warner Instruments). Firmly attached, refractile CTβ+ cells were identified as regions of interest. A ratio (R) of fluorescence emission at 510 nm in response to excitations at 340 and 380 nm was acquired at 1 Hz (Lambda DG-4 and 10-B SmartShutter, Sutter Instruments) via camera (ORCA-ER, Hamamatsu Corporation) and saved to a computer using HCImage (Hamamatsu Corporation). The intracellular concentration of calcium ([Ca2+]i) was determined following in situ calibration experiments according to the following equation:
where Kd is the dissociation constant for Fura-2, Sf2/Sb2 is the fluorescence ratio of the emission intensity excited by 380 nm signal in the absence of Ca2+, and Rmin and Rmax are the minimal and maximal fluorescence ratios, respectively. The procedure for determination of these variables has been described previously 26.
Only cells responsive to application of 50mM K+ were used. Following a 5 min recovery from 50 mM K+ stimulation, the percentage of cells exhibiting TRPA1-mediated Ca2+ influx was assessed by application of mustard oil (MO, 100 μM; Sigma). MO was applied one to three additional times (10 min interstimulus interval) to determine whether desensitization (tachyphylaxis) occurred. Then, ARTN was applied for a duration of seven min, and responses to MO were tested again. In neurons in which MO did not evoke Ca2+ influx, ARTN was applied for seven min and responses to MO were tested again. MO at 100 mM in 1-methyl-2-pyrrolidinone was used as a stock solution; 100 μM MO was made fresh daily in HBSS. Artemin (R&D Systems) was aliquoted at 10 μg/ml in HBSS, stored at −20°C, and diluted in HBSS to a final concentration of 100 ng/ml immediately before use. The magnitude of evoked Ca2+ transients was determined by peak evoked change in intracellular Ca2+ concentration (Δ[Ca2+]i).
Visceral nociceptive behavior
Mice were anesthetized via inhaled isoflurane in oxygen (4% induction, 2% maintenance) and a 24-gauge angiocatheter was passed through the urethra into the urinary bladder for delivery of compressed air. Silver wires were inserted into the left abdominal oblique musculature superior to the inguinal ligament for measurement of EMG responses. Anesthesia was reduced until flexion reflexes were present in the absence of spontaneous escape behavior (~1% isoflurane). Two 60 mmHg urinary bladder distensions were administered to overcome an initial period of sensitization 8 and were followed by graded distensions at pressures of 10–60 mmHg (10 sec duration; 2 min inter-stimulus interval). EMG data were recorded, rectified, and saved to computer using Spike2 software (Cambridge Electronic Design Ltd.). Distension-evoked EMG responses were normalized to baseline EMG as follows: EMGevoked − EMGbaseline/EMGbaseline. Normalized responses are presented as a percent of the maximal response of the sterile saline-treated control group.
Immunohistochemical analysis of pERK expression
Mice were deeply anesthetized with isoflurane and perfused with 4% paraformaldehyde. Lumbosacral spinal cord segments were dissected, embedded in gelatin (10% in 0.1M PB), post-fixed for 4 hrs in 4% paraformaldehyde, and cryoprotected overnight (30% sucrose) at 4°C. Transverse spinal cord sections (50 μm) were cut on a microtome. Following 3×5 min washes in 0.1M PB, floating spinal cord sections were incubated for 2 hrs at room temperature in a blocking reagent (5% normal horse serum and 0.1% Triton X-100 in 0.1M PB), then overnight at room temperature in rabbit anti-human phospho-ERK1/2 antibody (1:4000 in blocking reagent; Cell Signaling Technology, Inc). Sections were washed, incubated for 2 hrs at room temperature in Cy3-conjugated anti-rabbit IgG secondary antibody (1:500), washed, mounted and coverslipped. Photographs were taken at 20× using Leica Application Suite (Leica Microsystems Inc.) software. The total number of pERK-positive cells in regions receiving input from bladder afferent neurons (i.e., superficial dorsal horn, sacral parasympathetic nucleus, and dorsal commissure 5, 6, 36) was counted in 9–13 sections per mouse by an experimenter blinded to treatment. The average for each mouse was calculated and used for statistical analysis.
Statistical analysis
Statistical analyses were performed using Systat (Systat Software, Inc.). EMG responses to graded bladder distension were compared using repeated measures ANOVA followed by post hoc t-tests with Holm’s correction (Holm 1979). For real-time PCR data, expression ratios for each target gene in bladder tissue were compared between groups using t-tests. Single cell PCR data of bladder afferent gene expression were analyzed using χ2 tests and Fisher’s exact tests. The total number of cells expressing pERK was compared by ANOVA and post hoc t-tests. All data are expressed as mean ± SEM. P values of ≤0.05 were considered significant.
RESULTS
Bladder-derived ARTN and NGF and bladder afferent TRPA1 expression are increased following CYP
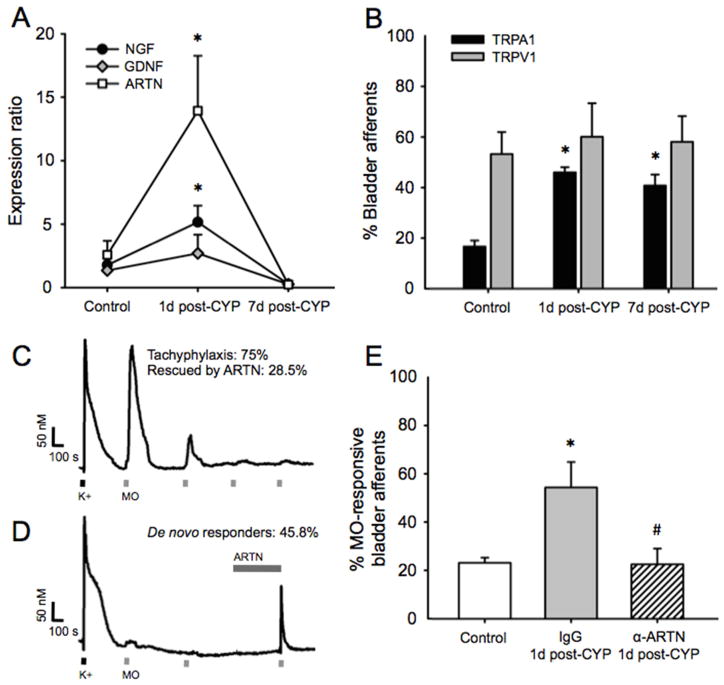
We used real-time PCR to determine growth factor mRNA expression and single-cell PCR to determine TRP channel mRNA expression in urinary bladder tissue and bladder afferent neurons, respectively. Expression of bladder-derived ARTN (F (2,18)=11.26, p=0.001) and NGF (F(2,18)=7.157, p=0.020) mRNAs were significantly upregulated 1d post-CYP, with peak expression at >5-fold (p<0.01) and 2.5-fold (p<0.05) above control levels, respectively (Figure 2A; N=7/group). ARTN and NGF returned to baseline levels by 7d post-CYP. GDNF expression was unchanged (F (2,18)=3.107, p=0.069).
Figure 2.
CYP upregulates bladder-derived ARTN mRNA concurrent with upregulation and sensitization of bladder afferent TRPA1. (A) Bladder-derived ARTN and NGF mRNAs were significantly upregulated 1d post-CYP. Expression peaked at >5-fold and 2.5-fold above control levels, respectively. Expression at 7d post-CYP did not differ from control. GDNF mRNA expression was unchanged. (B) Bladder afferent TRPA1 mRNA expression was significantly increased at 1d post-CYP and remained increased at 7d post-CYP. In contrast, CYP had no effect on TRPV1 mRNA expression at either time point. (C) Isolated bladder afferents from naive mice exhibited robust Ca2+ influx to application of 50 mM K+ (black dash). Approximately 15% of these responded to 100 μM MO (gray dash). 75% of MO-responsive afferents exhibited profound desensitization (tachyphylaxis) of TRPA1 channel with repeated MO application (10 min insterstimulus interval). Rescue from tachyphylaxis by ARTN was observed in 28.5% of these neurons (no example shown). (D) ARTN exposure (gray bar; 7 min) was efficacious in recruiting de novo responses to MO in 45.8% of bladder afferents that were initially insensitive to MO. (E) As predicted by afferent TRPA1 mRNA expression, the percentage of bladder afferents exhibiting MO-evoked Ca2+ transients was significantly increased 1d post-CYP relative to controls when mice were pre-treated with IgG (the control for α-ARTN). This CYP-induced increase in MO-responsive afferents was prevented in mice pre-treated with α-ARTN. * indicates p<0.05 versus control, # indicates p<0.05 α-ARTN versus IgG.
As previously reported 27, we found that bladder afferents arising from lumbosacral dorsal root ganglia express both TRPV1 and TRPA1 mRNA in normal conditions (Figure 2B; N=18–25/group). After a five day series of CYP injections, TRPA1 mRNA expression was significantly increased (χ2(2)=12.64, p<0.01) in both the 1d post-CYP (p<0.01) and 7d post-CYP (p<0.05) groups. However, in contrast to studies of the colon showing significant upregulation of TRPV1 with inflammation (e.g., 18), we observed no change in bladder afferent TRPV1 mRNA expression (χ2(2)=1.417, p=0.492), similar to what we have previously reported 16.
ARTN sensitizes MO-evoked Ca2+ signaling in bladder afferents in vitro
To determine the effect of ARTN on afferent TRPA1 function, we performed Ca2+ imaging experiments on dissociated bladder afferents (N=45) from naive mice. Progressive desensitization (i.e., tachyphylaxis) to repeated application of the TRPA1 agonist, MO, was observed in 75% of MO-responsive bladder afferents (Figure 2C). In 28.5% of these neurons, application of ARTN restored MO-evoked responses (data not shown). Furthermore, ARTN induced de novo MO-evoked responses in 45.8% of bladder afferents; that is, nearly half of the afferents that were initially unresponsive to MO became responsive following exposure to ARTN (Figure 2D). On average (including the neurons that responded only after ARTN exposure), the MO-evoked change in [Ca2+]i after ARTN exposure was ca. 4 times greater than that seen prior to ARTN exposure (data not shown).
α-ARTN reverses CYP-induced hyperalgesia in vivo and normalizes expression of bladder afferent TRPA1 and spinal pERK
We have previously shown that injections of CYP using the model described herein produces sustained bladder hyperalgesia 16. In the current experiments, we quantified abdominal EMG responses to bladder distension at two time points following CYP injections (1d post-CYP and 7d post-CYP). We additionally treated some mice with α-ARTN to determine whether it exerted an anti-nociceptive effect on CYP-induced hyperalgesia. A repeated measures ANOVA indicated a significant effect of treatment on abdominal EMG responses to bladder distension (F(5,32)=5.438, p=0.001). Similar to what we have previously reported, abdominal EMG responses to noxious bladder distension (40–60 mmHg) were significantly potentiated in CYP-treated mice at both 1d post-CYP and 7d post-CYP (all p values <0.05; Figure 3A; N=6–7/group). Remarkably, both preventive (pre-CYP) and palliative (post-CYP) treatment with α-ARTN blocked and reversed, respectively, CYP-induced potentiation of abdominal EMG responses (all p values >0.05; Figure 3B). Mice treated with IgG (the control for α-ARTN) immediately prior to the first CYP injection exhibited responses like those of mice that were treated with CYP alone and that were significantly greater than control mice (p values <0.05).
Figure 3.
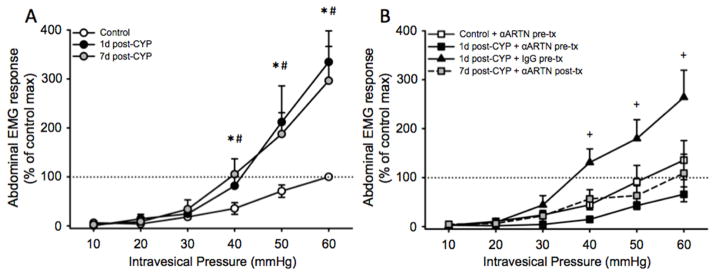
Treatment with α-ARTN before or after CYP injections prevents and reverses, respectively, CYP-induced bladder hyperalgesia. (A) Relative to controls (open circles), mice treated with CYP exhibited augmented abdominal EMG responses to noxious distension pressure at 1d post-CYP (black circles) that persisted at 7d post-CYP (gray circles). * p<0.05, 1d post-CYP versus control; # p<0.05, 7d post-CYP versus control. (B) At 1d post-CYP, mice pre-treated with IgG (black triangles) exhibited abdominal EMG responses like those of mice treated with CYP alone (panel A). Pre-treatment with α-ARTN prevented the development of hyperalgesia at 1d post-CYP (black squares). Post-treatment with α-ARTN after CYP, when hyperalgesia was already established, reversed CYP-induced hyperalgesia at 7d post-CYP (gray squares). + p<0.05, 1d post-CYP + IgG pre-tx versus control.
To determine whether bladder-derived ARTN regulates CYP-induced changes in afferent TRPA1 expression and function, we used Ca2+ imaging to measure TRPA1-mediated, MO-evoked Ca2+ influx. We quantified the proportion of retrogradely labeled bladder afferents from CYP-injected mice with and without α-ARTN treatment that were responsive to MO application (N=36–78/group). Again, similar to what we have previously reported 16, the percentage of MO-responsive afferents changed as a function of treatment group (χ2(2)=11.12, p=0.003). CYP administered in conjunction with IgG, the control for α-ARTN, significantly increased the percentage of MO-responsive afferents (p<0.01 versus control; Figure 4A). This effect of CYP was absent in mice in which α-ARTN was administered in conjunction with CYP (p<0.01 versus CYP+IgG).
Figure 4.
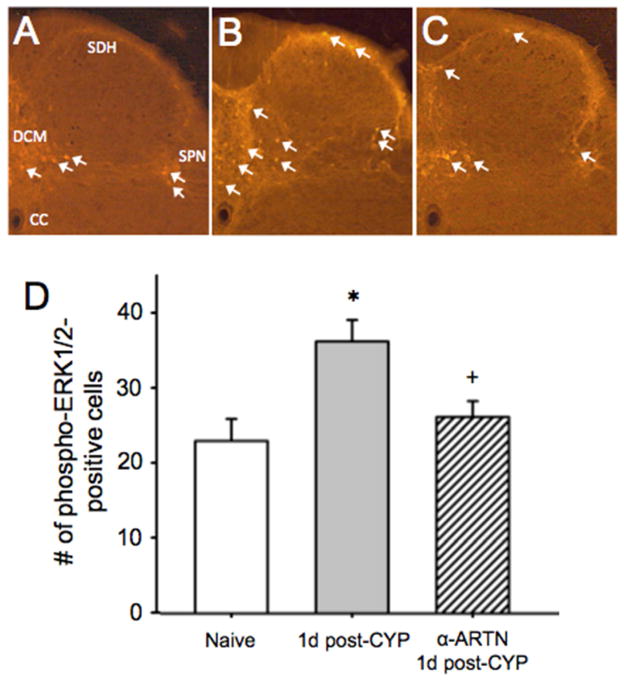
α-ARTN treatment normalizes spinal pERK expression after bladder stimulation and prevents CYP-induced upregulation of MO-responsive bladder afferents and. (A) Spinal neuronal pERK expression (white arrows) in response to noxious bladder distension was significantly increased 1d post-CYP in regions of the dorsal spinal cord receiving lumbosacral bladder afferent input. Pre-treatment with α-ARTN prior to CYP prevented upregulation of distension-evoked spinal pERK. SDH: superficial dorsal horn; DCM: dorsal commissure; CC: central canal; SPN: sacral parasympathetic nucleus. * p<0.05, 1d post-CYP versus naive; + p<0.05, α-ARTN versus 1d post-CYP.
Extracellular signal-regulated kinases regulate intracellular signal transduction, gene transcription, and post-translational protein modifications. Lai et al. 28 have shown that CYP-induced bladder hyperalgesia is positively correlated with increased neuronal pERK expression in regions of the dorsal horn receiving bladder sensory input, and that hyperalgesia can be reduced by spinal pERK inhibition. We, therefore, investigated whether α-ARTN treatment affected spinal pERK expression in CYP-treated mice (N=3/group). As we anticipated, pERK immunoreactivity was significantly greater in the CYP plus bladder distension condition compared to distension alone in portions of the spinal cord receiving lumbosacral bladder input (t(4)=−2.503, p=0.035; Figure 4B). In mice that were treated with α-ARTN prior to CYP, this effect was inhibited (t(4)=2.835, p=0.047; Figure 4C). As predicted by bladder afferent TRPA1 mRNA expression, the percentage of bladder afferents exhibiting MO-evoked Ca2+ transients were significantly different among mice treated with CYP with or without α-ARTN (χ2(2)=11.12, p=0.003). Injections of CYP + IgG increased the percentage of MO-sensitive bladder afferents (p<0.01 versus control), and this effect was prevented by α-ARTN (p<0.01 versus CYP + IgG).
DISCUSSION
Mature sensory neurons are sensitive to fluctuations in neurotrophic factor expression (e.g. ARTN, NGF); in response, they exhibit plasticity with respect to anatomy (e.g., sprouting 1, 15, 17, 25, 38), neurochemistry (e.g., upregulation of peptides, phosphorylation of channels such as TRPV1 24, 44), and function (e.g., increased sensitivity to mechanical/thermal stimuli 11, 17, 35). Such plasticity provides multiple ways in which neurotrophic factors can modulate the quality and quantity of sensory information transmitted from the periphery to the spinal cord. In humans and in animal models of neuropathic and inflammatory pain, dramatic changes in neurotrophic factors have been correlated with both the development and resolution of hypersensitivity 17, 31, 33. In our model, CYP produces mild bladder inflammation characterized by edema but without leukocyte infiltration 16, yet is sufficient to induce significant increases in NGF and ARTN mRNAs and alter sensory neuron function.
In rodents, the majority of pelvic visceral afferents are peptidergic 11, 13, 14, 19, and expression of neuropeptides overlaps largely with expression of the receptors for ARTN (GFRα3) and NGF (TrkA) 2, 19, 42. Moreover, >90% of ARTN-responsive neurons express TRPV1 19, 42, a large portion of which co-express TRPA1 12, 27. TRPA1 and TRPV1 are requisite mediators of inflammatory hyperalgesia in somatic and visceral structures 7, 9, 11, 44. Using other models of inflammatory pain, we have shown that ARTN and NGF mRNAs increase in a coordinated fashion 31, 35. Thus, co-expression of the receptors for ARTN and NGF in the same neurons that express TRPA1 and/or TRPV1 makes these neurons particularly responsive to the inflammatory microenvironment. Interestingly, whereas a PubMed search of “Bladder AND NGF” yields almost 200 publications, there are no reports to our knowledge that examine the role of ARTN in bladder pain. Given that we observed an increase in ARTN mRNA that was more than twice greater than the increase in NGF mRNA, ARTN could be a very useful target for therapeutic interventions in bladder disease. It is worth noting that we conducted preliminary real-time PCR experiments using bladder tissue from CYP-treated mice to screen multiple reference genes. While we found that β-actin was most resistant to CYP-induced changes in expression, it was increased in bladder tissue from the 1d and 7d post-CYP groups relative to the control group (data not shown). Although the robust increases in growth factor mRNA expression at 1d post-CYP clearly overcome any confounding effect, it is likely that increased β-actin in the 7d post-CYP group contributes to the decrease in normalized growth factor expression in this group relative to controls.
We have previously demonstrated that CYP-induced bladder hyperalgesia correlates with increased TRPA1 expression and function in bladder afferents, and that a TRPA1 antagonist was effective at restoring normal sensitivity 16. Here, we demonstrate that one potential mechanism for these changes in TRPA1 is an increase in ARTN. In vitro application of ARTN to bladder afferents from naive (not treated with CYP) mice rescued nearly one-third of MO-sensitive, TRPA1-expressing neurons from tachyphylaxis upon repeated MO application. Application of ARTN also induced MO-evoked calcium transients in nearly half of bladder afferents that were initially not responsive to MO. Clearly, the latter must be the result of post-translational modification such as membrane insertion of TRPA1 protein. However, despite that the majority of peptidergic visceral afferents express GFRα3, an elegant study by Forrest et al.20 characterized a microanatomical network, including a GFRα3-expressing, non-neuronal component, by which ARTN could influence bladder sensation. Whether the mechanism of ARTN regulation of TRPA1 we observed in vivo is direct or indirect remains unknown and will be addressed by future studies. Future studies will also address the roles of ARTN and TRPA1 in bladder function, which may differ mechanistically from bladder pain. Interactions between ARTN and TRPA1 could also explain a clinical feature of bladder pain: in response to intravesical instillation of cold saline, individuals with bladder hypersensitivity report pain 37. Although it has previously been suggested that this cold-induced pain resulted from upregulation of the menthol receptor TRPM8, TRPA1 has also been linked to detection of noxious cold 10. We have previously shown that transgenic mice overexpressing ARTN exhibit upregulation of TRPA1 and enhanced cold sensitivity 17. We speculate that there is a potential link between cold-induced bladder pain in humans 37 and regulation of bladder afferent sensitivity by ARTN. Future studies aimed at empirically addressing the role of ARTN in regulation of TRPM8 will address this.
Behaviorally, CYP-induced cystitis facilitated responses to bladder stimulation within a noxious range of intravesical pressures (40–60 mmHg) 39, 40. Hyperalgesia was accompanied by phosphorylation of ERK1/2 in regions of the lumbosacral spinal cord receiving bladder afferent input (i.e., superficial dorsal horn, SPN, and DCM). Neural activation in these regions has been associated with noxious input (e.g., superficial dorsal horn, DCM) and/or organ distension (e.g., SPN, DCM) 5, 6. Specifically, ERK1/2 phosphorylation in these regions has previously been associated with the development of primary bladder hyperalgesia 28. While α-ARTN treatment effectively prevented the development of hyperalgesia and prevented spinal ERK1/2 phosphorylation, it is not clear what effect α-ARTN therapy would have on allodynia. Regardless, that α-ARTN was effective after bladder hyperalgesia was established is extremely encouraging in terms of its potential for treating ongoing disease, and underscores the translational importance of this approach in treating bladder pain in IC/PBS patients. Moreover, the effectiveness of α-ARTN administration prior to development of hyperalgesia suggests it could be used prophylactically in patients undergoing anti-tumor CYP therapy. A large number of biologics are currently used clinically, and most have a broader target profile than α-ARTN (e.g., infliximab that targets TNFα). The receptors for ARTN (GFRα3 and RET) have a relatively narrow distribution in the nervous system and other tissues that would limit off-target effects, potentially making α-ARTN a relatively safe biologic for use in humans.
Perspective.
α-ARTN therapy effectively prevented and reversed ongoing bladder hyperalgesia in an animal model of cystitis, indicating its potential as an efficacious treatment strategy for ongoing bladder pain associated with IC/PBS.
Highlights.
We determined whether treatment with an artemin-sequestering antibody (αARTN) affected cyclophosphamide-induced hyperalgesia and bladder primary afferent function.
αARTN effectively blocked the development of bladder hyperalgesia, and reversed hyperalgesia once it was established.
αARTN normalized noxious bladder distension-induced spinal expression of phosphoERK1/2 immunoreactivity.
Calcium imaging experiments of bladder afferents using mustard oil indicate that ARTN may regulate neuronal function via TRPA1.
αARTN may be an effective therapeutic strategy for individuals suffering from interstitial cystitis-related bladder pain.
Acknowledgments
We would like to acknowledge the University of Pittsburgh Rodent Behavioral Analysis Core and our funding sources: NINDS NS0050758 (BMD), NIDDK DK094593 (JJD), NIDDK DK063922 (JJD), American Pain Society Future Leaders in Pain Research grant (JJD).
Footnotes
Disclosures: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neuroscience letters. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 3.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. The Journal of urology. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielefeldt K, Ozaki N, Gebhart GF. Role of nerve growth factor in modulation of gastric afferent neurons in the rat. Am J Physiol Gastrointest Liver Physiol. 2003;284:G499–507. doi: 10.1152/ajpgi.00356.2002. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:4878–4889. doi: 10.1523/JNEUROSCI.12-12-04878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:R326–333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 7.Blackshaw LA. Transient receptor potential cation channels in visceral sensory pathways. Br J Pharmacol. 2014;171:2528–2536. doi: 10.1111/bph.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neuroscience letters. 2001;306:97–100. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nature communications. 2013;4:2501. doi: 10.1038/ncomms3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B, Vergnolle N. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60:171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2006 doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 15.Davis BM, Albers KM, Seroogy KB, Katz DM. Overexpression of nerve growth factor in transgenic mice induces novel sympathetic projections to primary sensory neurons. J Comp Neurol. 1994;349:464–474. doi: 10.1002/cne.903490310. [DOI] [PubMed] [Google Scholar]
- 16.DeBerry JJ, Schwartz ES, Davis BM. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain. 2014;155:1280–1287. doi: 10.1016/j.pain.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel MA, Khalil M, Mueller-Tribbensee SM, Becker C, Neuhuber WL, Neurath MF, Reeh PW. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J Gastroenterol. 2012;47:256–265. doi: 10.1007/s00535-011-0495-6. [DOI] [PubMed] [Google Scholar]
- 19.Forrest SL, Osborne PB, Keast JR. Characterization of bladder sensory neurons in the context of myelination, receptors for pain modulators, and acute responses to bladder inflammation. Frontiers in neuroscience. 2013;7:206. doi: 10.3389/fnins.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest SL, Osborne PB, Keast JR. Characterization of axons expressing the artemin receptor in the female rat urinary bladder: a comparison with other major neuronal populations. The Journal of comparative neurology. 2014;522:3900–3927. doi: 10.1002/cne.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanno PM. Interstitial cystitis-epidemiology, diagnostic criteria, clinical markers. Rev Urol. 2002;4(Suppl 1):S3–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs BL, Smaldone MC, Tyagi V, Philips BJ, Jackman SV, Leng WW, Tyagi P. Increased nerve growth factor in neurogenic overactive bladder and interstitial cystitis patients. The Canadian journal of urology. 2010;17:4989–4994. [PubMed] [Google Scholar]
- 23.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 24.Jankowski MP, Koerber HR. Neurotrophic Factors and Nociceptor Sensitization. In: Translational Pain Research: From Mouse to Man. In: Kruger L, Light AR, editors. Frontiers in Neuroscience. Boca Raton, FL: 2010. [Google Scholar]
- 25.Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao JP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 27.La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai HH, Qiu CS, Crock LW, Morales ME, Ness TJ, Gereau RWt. Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain. 2011;152:2117–2124. doi: 10.1016/j.pain.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104:1476–1481. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- 30.Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- 31.Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:743–752. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malin SA, Christianson JA, Bielefeldt K, Davis BM. TRPV1 expression defines functionally distinct pelvic colon afferents. J Neurosci. 2009;29:743–752. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 35.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. The Journal of comparative neurology. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 37.Mukerji G, Waters J, Chessell IP, Bountra C, Agarwal SK, Anand P. Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol. 2006;6:31. doi: 10.1186/1471-2490-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murota H, Izumi M, Abd El-Latif MI, Nishioka M, Terao M, Tani M, Matsui S, Sano S, Katayama I. Artemin causes hypersensitivity to warm sensation, mimicking warmthprovoked pruritus in atopic dermatitis. J Allergy Clin Immunol. 2012;130:671–682. e674. doi: 10.1016/j.jaci.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain research. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 40.Ness TJ, Randich A, Gebhart GF. Further behavioral evidence that colorectal distension is a ‘noxious’ visceral stimulus in rats. Neuroscience letters. 1991;131:113–116. doi: 10.1016/0304-3940(91)90349-x. [DOI] [PubMed] [Google Scholar]
- 41.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. The Journal of urology. 1999;161:438–441. discussion 441–432. [PubMed] [Google Scholar]
- 42.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RTPCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]