Abstract
African Americans (AAs) have increased risk for cardiovascular, cerebral vascular and metabolic disease, including hypertension, stroke, coronary artery disease, metabolic syndrome and type II diabetes, relative to Caucasian Americans (CAs). While it is accepted that endothelial function is impaired in AAs, less is known regarding their cerebral vasodilatory capacity in response to hypercapnia. We hypothesized that AAs have a reduction in the total range of change in cerebral blood flow velocity (CBFV) measured in the middle cerebral artery and an index of cerebral vascular conductance (CVCI) in response to changes in the partial pressure of end-tidal carbon dioxide ( ) during rebreathing-induced hypercapnia when compared with CAs. Twenty-one healthy, college-aged AA (10 male) and 21 age- and sex-matched CA (10 male) subjects participated in this study. A four-parameter logistic regression was used for curve fitting the responses of CBFV and CVCI relative to changes in
) during rebreathing-induced hypercapnia when compared with CAs. Twenty-one healthy, college-aged AA (10 male) and 21 age- and sex-matched CA (10 male) subjects participated in this study. A four-parameter logistic regression was used for curve fitting the responses of CBFV and CVCI relative to changes in  . The total ranges of change in CBFV (101 ± 18 versus 69 ± 23%; P < 0.001) and CVCI (83 ± 21 versus 58 ± 21%; P < 0.001) as well as the maximal increase in CBFV (205 ± 24 versus 169 ± 24%; P < 0.001) and CVCI (188 ± 30 versus 154 ± 19%; P < 0.001) were reduced during hypercapnia in AAs relative to CAs despite a similar increase in
. The total ranges of change in CBFV (101 ± 18 versus 69 ± 23%; P < 0.001) and CVCI (83 ± 21 versus 58 ± 21%; P < 0.001) as well as the maximal increase in CBFV (205 ± 24 versus 169 ± 24%; P < 0.001) and CVCI (188 ± 30 versus 154 ± 19%; P < 0.001) were reduced during hypercapnia in AAs relative to CAs despite a similar increase in  (change, 15 ± 3 versus 15 ± 3 mmHg; P = 0.65). In conclusion, these data indicate that AAs have attenuated cerebral vascular capacity to respond to hypercapnia when compared with CAs.
(change, 15 ± 3 versus 15 ± 3 mmHg; P = 0.65). In conclusion, these data indicate that AAs have attenuated cerebral vascular capacity to respond to hypercapnia when compared with CAs.
New Findings
-
What is the central question of this study?
The main purpose of this investigation is to determine whether there is a difference in cerebral vasodilatory capacity in response to rebreathing-induced hypercapnia between African Americans and Caucasian Americans.
-
What is the main finding and its importance?
College-aged African Americans have reduced cerebral vasodilatory capacity during hypercapnia when compared with Caucasian counterparts, a finding that suggests cerebral vascular dysfunction in this population. These findings may contribute to the understanding of the greater prevalence of cerebral vascular disease in this population.
Introduction
African Americans (AAs) are at an increased risk for cardiovascular and metabolic disease, including hypertension, stroke, coronary artery disease, metabolic syndrome and type II diabetes, relative to Caucasian Americans (CAs). The underlying mechanisms for this increased risk remain unresolved; however, blunted endothelial function is a major contributing factor (Perregaux et al. 2000). Endothelial dysfunction results in impaired vasodilatation in the cerebral vasculature, and thus, leads to a dysfunction in cerebral blood flow (CBF) regulation (Zimmermann & Haberl, 2003; Lavi et al. 2006; Vicenzini et al. 2007). In this regard, AAs suffer from a greater prevalence of cerebral vascular disease, including stroke, relative to CAs (Roger et al. 2011).
The cerebral circulation is tightly regulated by arterial carbon dioxide tension ( ), with hypercapnia increasing and hypocapnia decreasing CBF (Ide et al. 2003; Willie et al. 2014). The relationship between changes in CBF and
), with hypercapnia increasing and hypocapnia decreasing CBF (Ide et al. 2003; Willie et al. 2014). The relationship between changes in CBF and  is often assessed to evaluate cerebral vascular function (Ringelstein et al. 1988; Kleiser & Widder, 1992). The cerebrovascular response to changes in CO2 is diminished in some clinical conditions, including diabetes (Kadoi et al. 2003), hypertension (Lavi et al. 2006) and carotid artery disease (Ringelstein et al. 1988), and is believed to be not only a predictor but also a contributor to stroke (Gur et al. 1996; Nur et al. 2009). The increase in CBF and/or cerebral vascular conductance during hypercapnia occurs primarily through modulation of the cerebral microcirculation, and thus, is a powerful tool that provides a non-invasive index of cerebral vascular health (Ringelstein et al. 1988; Lavi et al. 2003, 2006).
is often assessed to evaluate cerebral vascular function (Ringelstein et al. 1988; Kleiser & Widder, 1992). The cerebrovascular response to changes in CO2 is diminished in some clinical conditions, including diabetes (Kadoi et al. 2003), hypertension (Lavi et al. 2006) and carotid artery disease (Ringelstein et al. 1988), and is believed to be not only a predictor but also a contributor to stroke (Gur et al. 1996; Nur et al. 2009). The increase in CBF and/or cerebral vascular conductance during hypercapnia occurs primarily through modulation of the cerebral microcirculation, and thus, is a powerful tool that provides a non-invasive index of cerebral vascular health (Ringelstein et al. 1988; Lavi et al. 2003, 2006).
We hypothesized that the cerebral vasodilatory capacity, indexed by the range of change in cerebral blood flow velocity (CBFV) and an index of cerebral vascular conductance (CVCI), as well as the maximal value of CBFV and CVCI achieved during rebreathing-induced hypercapnia, are reduced in healthy college-aged AAs relative to CAs.
Methods
Ethical approval
The Institutional Review Board at The University of Texas at Austin approved all study procedures and the consent process used in the present study. Subjects were given a verbal description of all procedures and informed of the purpose and risks involved in the study before providing their informed, written consent. The study conformed to the provisions of the Declaration of Helsinki.
Subjects
Twenty-one Caucasian Americans (CAs; 10 males) and 21 African Americans (AAs; 10 males) participated in this study (Table 1). A subject's ethnicity was defined as AA or CA and was only accepted if both parents were AA or CA, respectively. Subjects were non-smokers, were not taking medications and were free from cardiovascular, neurological, metabolic or cognitive diseases. All studies were conducted in the morning following an overnight fast (>12 h). Subjects refrained from strenuous exercise and alcoholic beverages for 24 h prior to and refrained from consuming caffeine and food for 12 h prior to the experimental trial, which was conducted in a temperature-controlled laboratory (∼24°C and 40% relative humidity).
Table 1.
Subject characteristics
| Variable | Caucasian Americans (n = 21) | African Americans (n = 21) | P Value |
|---|---|---|---|
| Age (years) | 23 ± 3 | 23 ± 4 | 0.97 |
| Sex (male/female) | 10/11 | 10/11 | 1.00 |
| Body mass index (kg m−2) | 23 ± 3 | 24 ± 4 | 0.07 |
| Height (cm) | 174 ± 8 | 173 ± 9 | 0.85 |
| Weight (kg) | 68 ± 12 | 73 ± 14 | 0.24 |
Values are means ± SD.
Instrumentation and measurements
All data were collected in the supine position. Each subject was instrumented for continuous measurement of heart rate (HR) and cardiac rhythms from an electrocardiogram (HP Patient Monitor; Agilent, Santa Clara, CA, USA) interfaced with a cardiotachometer (CWE, Ardmore, PA, USA). Continuous mean arterial pressure (MAP) was recorded and monitored from a finger using the Penaz method (CNAP, Biopac Monitor 500, Bruck an der Mur, Austria). The CBFV was measured continuously using transcranial Doppler ultrasonography. The right middle cerebral artery was imaged through a 2 MHz Doppler probe (Multi-flow; DWL Elektronische Systeme, Singen, Germany) adjusted over the temporal window until a signal was identified. The probe was fixed securely in place using a head strap to prevent any slight movement of the Doppler probe. The CVCI was calculated from the ratio of CBFV to MAP. End-tidal carbon dioxide tension ( ) was measured continuously using a capnograph, through a mouthpiece, during all data collection periods and was used as an index of
) was measured continuously using a capnograph, through a mouthpiece, during all data collection periods and was used as an index of  (VitalCap Capnograph Monitor; Oridion, Needham, MA, USA).
(VitalCap Capnograph Monitor; Oridion, Needham, MA, USA).
Experimental protocol
Following instrumentation, subjects rested quietly in the supine position for 20 min. Following the resting period, subjects were fitted with a nose-clip and breathed through a mouthpiece attached to a Y-valve. One end of the Y-valve was connected to a 5 litre bag and the other end was open to room air. Subjects breathed room air for a 6 min period of baseline data collection while CBFV, MAP, HR and  data were collected. Subjects were then exposed to a rebreathing protocol. Subjects first performed a deep inspiration and exhaled into an empty rebreathing bag via the Y-valve. The subjects continued to rebreathe for ∼3 min before switching the valve for a recovery period. During the rebreathing period, arterial oxygen saturation was held constant (between 97 and 98%) by bleeding a small amount of oxygen into the rebreathing bag (Claassen et al. 2007).
data were collected. Subjects were then exposed to a rebreathing protocol. Subjects first performed a deep inspiration and exhaled into an empty rebreathing bag via the Y-valve. The subjects continued to rebreathe for ∼3 min before switching the valve for a recovery period. During the rebreathing period, arterial oxygen saturation was held constant (between 97 and 98%) by bleeding a small amount of oxygen into the rebreathing bag (Claassen et al. 2007).
Data analysis
Data for MAP, HR, CBFV and  were collected at 125 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA, USA) during baseline conditions. Average cerebrovascular responses (CBFV and CVCI) during baseline and rebreathing conditions were assessed on a breath-by-breath basis. The percentage change in CBFV and CVCI was determined, while the absolute change in
were collected at 125 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA, USA) during baseline conditions. Average cerebrovascular responses (CBFV and CVCI) during baseline and rebreathing conditions were assessed on a breath-by-breath basis. The percentage change in CBFV and CVCI was determined, while the absolute change in  was determined. A four-parameter logistic regression was employed for sigmoidal curve fitting (Kent et al. 1972; Claassen et al. 2007, 2009), as follows:
was determined. A four-parameter logistic regression was employed for sigmoidal curve fitting (Kent et al. 1972; Claassen et al. 2007, 2009), as follows:
where a represents the range of change in the percentage of CBFV or CVCI, b is a constant that determines the overall sigmoidal property of the curve, x0 is the level of  where cerebral vasomotor reactivity for CBFV or CVCI is maximal, and y0 represents the maximal value of CBFV and CVCI during hypercapnia (Fig.1). Maximal cerebral vasomotor reactivity [CVMR (CVMRmax − CBFV and CVMRmax − CVCI)], which was obtained at
where cerebral vasomotor reactivity for CBFV or CVCI is maximal, and y0 represents the maximal value of CBFV and CVCI during hypercapnia (Fig.1). Maximal cerebral vasomotor reactivity [CVMR (CVMRmax − CBFV and CVMRmax − CVCI)], which was obtained at  , was calculated from the first-order derivative of the following logistic function:
, was calculated from the first-order derivative of the following logistic function:
Figure 1. Schematic representations of logistic regressions.

A logistic regression and four parameters of the velocity of blood in the middle cerebral artery (CBFV) and index of cerebral vascular conductance (CVCI) versus the partial pressure of end-tidal carbon dioxide ( ) are displayed in A and B, respectively. The parameter a represents the range of change in the percentage of CBFV or CVCI, b is a constant that determines the overall sigmoidal property of the curve, x0 is the level of
) are displayed in A and B, respectively. The parameter a represents the range of change in the percentage of CBFV or CVCI, b is a constant that determines the overall sigmoidal property of the curve, x0 is the level of  at which cerebral vasomotor reactivity for CBFV or CVCI is maximal, and y0 represents the maximal value of CBFV and CVCI during the hypercapnic rebreathing protocol.
at which cerebral vasomotor reactivity for CBFV or CVCI is maximal, and y0 represents the maximal value of CBFV and CVCI during the hypercapnic rebreathing protocol.
In order to compare with a four-parameter logistic curve model, CVMR using linear regression of changes in CBFV and CVCI (expressed as percentages) in response to a change (Δ) of 9 mmHg from its baseline 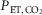 value was assessed. The Δ9 mmHg was chosen because it was the highest common change in
value was assessed. The Δ9 mmHg was chosen because it was the highest common change in  that all subjects achieved during hypercapnia.
that all subjects achieved during hypercapnia.
In order to assess cerebral vascular function further, indices of cerebral autoregulation were assessed by transfer function analysis over a specified frequency range (very low, 0.02–0.07 Hz; low, 0.07–0.20 Hz; and high, 0.20–0.35 Hz; Zhang et al. 1998). Transfer phase, gain and coherence functions were calculated using commercially available software (DADiSP; DSP Development, Cambridge, MA, USA).
Statistical analysis
Statistical analyses were performed using a statistical software package (SigmaPlot 12.5; Systat Software, Inc., San Jose, CA, USA). Subject characteristics, baseline haemodynamic data, transfer function analysis data, and all parameters and estimates of CVMR from a four-parameter logistic function and linear regression were compared using Student's two-tailed unpaired t tests. A χ2 test was used for difference in sex ratio between the two groups. All significance was set at P ≤ 0.05, and data are presented as means ± SD.
Results
Subject characteristics and haemodynamic values during baseline period
There were no differences between groups for age, sex, body mass index, height or weight (Table 1; P > 0.05 for all comparisons). During the eucapnic baseline period, there were no differences between groups for HR, MAP, CBFV,  and CVCI (Table 2; P > 0.05 for all comparisons).
and CVCI (Table 2; P > 0.05 for all comparisons).
Table 2.
Haemodynamic values during baseline period prior to rebreathing protocol
| Variable | Caucasian Americans (n = 21) | African Americans (n = 21) | P Value |
|---|---|---|---|
| HR (beats min−1) | 57 ± 10 | 61 ± 9 | 0.15 |
| MAP (mmHg) | 98 ± 15 | 99 ± 13 | 0.91 |
| CBFV (cm s−1) | 63 ± 13 | 65 ± 16 | 0.71 |
 (mmHg) (mmHg) |
38 ± 4 | 40 ± 5 | 0.29 |
| CVCI (cm s−1 mmHg−1) | 0.66 ± 0.2 | 0.67 ± 0.2 | 0.89 |
Values are means ± SD. Abbreviations: CBFV, velocity of blood in the middle cerebral artery; CVCI, index of cerebral vascular conductance; HR, heart rate; MAP, mean arterial pressure; and  , partial pressure of end-tidal carbon dioxide.
, partial pressure of end-tidal carbon dioxide.
Cerebrovascular responses during hypercapnia
Representative examples of the relationship between CBFV versus
 and CVCI versus
and CVCI versus
 are illustrated in Fig.2A and B, respectively. Table 3 shows the group-averaged parameters and estimates of CVMR from the logistic and the linear regressions. Maximal CBF (CBFV, as a percentage of baseline) during hypercapnia was lower in AA relative to CA subjects (CA, 195 ± 17% versus AA, 170 ± 20%; P < 0.001). Likewise, maximal conductance was reduced in AAs (CA, 178 ± 17% versus AA, 153 ± 16%; P < 0.001). The magnitude of hypercapnia (
are illustrated in Fig.2A and B, respectively. Table 3 shows the group-averaged parameters and estimates of CVMR from the logistic and the linear regressions. Maximal CBF (CBFV, as a percentage of baseline) during hypercapnia was lower in AA relative to CA subjects (CA, 195 ± 17% versus AA, 170 ± 20%; P < 0.001). Likewise, maximal conductance was reduced in AAs (CA, 178 ± 17% versus AA, 153 ± 16%; P < 0.001). The magnitude of hypercapnia ( ) induced by rebreathing was similar between groups (CA, 15 ± 3 mmHg versus AA, 15 ± 3 mmHg; P = 0.65).
) induced by rebreathing was similar between groups (CA, 15 ± 3 mmHg versus AA, 15 ± 3 mmHg; P = 0.65).
Figure 2. Representative examples of cerebral vasodilatory response in African Americans (AAs) and Caucasian Americans (CAs).

Two examples of the changes in middle cerebral artery blood velocity (CBFV; A) and cerebral vascular conductance index (CVCI; B) with increase in 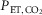 for an AA (filled circle) and a CA subject (open circle) are illustrated. The magnitude of increase in CBFV and CVCI was blunted in the AA relative to the CA.
for an AA (filled circle) and a CA subject (open circle) are illustrated. The magnitude of increase in CBFV and CVCI was blunted in the AA relative to the CA.
Table 3.
Cerebrovascular response to hypercapnia and parameters from logistic model
| Variable | Caucasian Americans | African Americans | P Value | |
|---|---|---|---|---|
| Hypercapnia | ||||
| Maximal flow (CBFV; % of baseline) | 195 (17) | 170 (20) | <0.001 | |
| Maximal conductance (CVCI; % of baseline) | 178 (17) | 153 (16) | <0.001 | |
Magnitude of hypercapnia achieved ( ; mmHg) ; mmHg) |
15 (3) | 15 (3) | 0.65 | |
| Parameters from logistic model | ||||
| Flow | a (%) | 101 (18) | 69 (23) | <0.001 |
| b | 0.34 (0.2) | 0.50 (0.3) | 0.06 | |
| x0 (mmHg) | 45 (5) | 44 (4) | 0.47 | |
| y0 (%) | 205 (24) | 169 (24) | <0.001 | |
| Conductance | a (%) | 83 (21) | 58 (21) | <0.001 |
| b | 0.34 (0.2) | 0.58 (0.4) | 0.018 | |
| x0 (mmHg) | 44 (6) | 43 (4) | 0.43 | |
| y0 (%) | 188 (28) | 154 (19) | <0.001 | |
| Estimates of CVMR | ||||
| Logistic regression | ||||
| CVMRmax − CBFV (% mmHg−1) | 8.3 (3.8) | 7.3 (2.8) | 0.35 | |
| CVMRmax − CVCI (% mmHg−1) | 6.8 (3.7) | 7.1 (2.9) | 0.82 | |
| Linear regression (Δ9 mmHg) | ||||
| CVMRmax − CBFV (% mmHg−1) | 6.6 (2) | 6.1 (2) | 0.40 | |
| CVMRmax − CVCI (% mmHg−1) | 5.6 (2) | 5.1 (2) | 0.39 | |
Values are means ± SD. Abbreviations: a, the range of change in percentage of CBFV or CVCI; b, a constant that determines the overall sigmoidal property of the curve; x0, the level of  at which cerebral vasomotor reactivity for CBFV or CVCI is maximal; and y0, the maximal value of CBFV and CVCI during the hypercapnic rebreathing protocol; CVMRmax, the maximal cerebral vasomotor reactivity using logistic regression analysis and linear regression analysis. Other abbreviations are as for Table 2.
at which cerebral vasomotor reactivity for CBFV or CVCI is maximal; and y0, the maximal value of CBFV and CVCI during the hypercapnic rebreathing protocol; CVMRmax, the maximal cerebral vasomotor reactivity using logistic regression analysis and linear regression analysis. Other abbreviations are as for Table 2.
Curve fitting and model parameters
The correlation coefficients (r2) for logistic regression were 0.95 and 0.94 for CBFV versus
 and CVCI versus
and CVCI versus
 , respectively. From Table 3, the total range of changes in CBFV (a; as a percentage) was attenuated in AAs (CA, 101 ± 18% versus AA, 69 ± 23%; P < 0.001). Likewise, the total range of changes in CVCI (a; as a percentage) was reduced in AAs (CA, 83 ± 21% versus AA, 58 ± 21%; P < 0.001). The maximal increase in CBFV (y0; as a percentage) was lower in AAs (CA, 205 ± 24% versus AA, 169 ± 24%; P < 0.001). Likewise, the maximal increase in CVCI (y0; as a percentage) was lower in AAs (CA, 188 ± 28% versus AA, 154 ± 19%; P < 0.001). The levels of
, respectively. From Table 3, the total range of changes in CBFV (a; as a percentage) was attenuated in AAs (CA, 101 ± 18% versus AA, 69 ± 23%; P < 0.001). Likewise, the total range of changes in CVCI (a; as a percentage) was reduced in AAs (CA, 83 ± 21% versus AA, 58 ± 21%; P < 0.001). The maximal increase in CBFV (y0; as a percentage) was lower in AAs (CA, 205 ± 24% versus AA, 169 ± 24%; P < 0.001). Likewise, the maximal increase in CVCI (y0; as a percentage) was lower in AAs (CA, 188 ± 28% versus AA, 154 ± 19%; P < 0.001). The levels of  that exhibited the highest CO2 sensitivity (x0; in millimetres of mercury) were not different between the two groups. Group differences for a and y0 are shown graphically in Fig.3A and B, respectively.
that exhibited the highest CO2 sensitivity (x0; in millimetres of mercury) were not different between the two groups. Group differences for a and y0 are shown graphically in Fig.3A and B, respectively.
Figure 3. Group differences for a and y0.

Total range of change in CVCI and CBFV (parameter a; A) as well as the maximal increase in CVCI and CBFV (parameter y0; B) during hypercapnic period were attenuated in AA relative to CA. The left Y axis in each figure represents the CVCI values while the right Y axis in each figure represents the CBFV values. Values are shown as means + SD. *P < 0.001.
Estimates of CVMR
No difference in maximal CVMR for CBFV and CVCI was observed between the two groups. In linear regression, there was no significant difference in the CVMR between the two groups (P = 0.35 and P = 0.82 for group differences of CBFV versus
 and CVCI versus
and CVCI versus
 , respectively).
, respectively).
Transfer function analysis
The estimates of transfer phase, gain and coherence functions are shown in Table 4. In the very low-frequency range, there was no difference in phase between CAs and AAs (CA, 0.038 ± 0.026 radians versus AA, 0.024 ± 0.030 radians; P = 0.18). In contrast, in the low-frequency and high-frequency ranges, the transfer phase function was lower in AAs relative to CAs (P = 0.04 and P = 0.02, respectively). Transfer gain and coherence functions in all frequency ranges were not significantly different between CAs and AAs (P > 0.05 for all comparisons).
Table 4.
Transfer function analysis
| Parameter | Caucasian Americans | African Americans | P Value | |
|---|---|---|---|---|
| Phase (radians) | Very low | 0.038 (0.026) | 0.024 (0.030) | 0.18 |
| Low | 0.056 (0.042) | 0.021 (0.071) | 0.04 | |
| High | 0.028 (0.099) | −0.045 (0.109) | 0.02 | |
| Gain (cm s−1 mmHg−1) | Very low | 0.83 (0.47) | 1.16 (0.76) | 0.17 |
| Low | 0.79 (0.34) | 0.86 (0.21) | 0.71 | |
| High | 0.61 (0.34) | 0.51 (0.15) | 0.20 | |
| Coherence | Very low | 0.37 (0.16) | 0.43 (0.19) | 0.39 |
| Low | 0.54 (0.15) | 0.47 (0.16) | 0.13 | |
| High | 0.42 (0.12) | 0.40 (0.11) | 0.51 |
Values are means ± SD. The following three different frequency ranges were used for the transfer function analysis: very low, 0.02–0.07 Hz; low, 0.07–0.20 Hz; and high, 0.20–0.35 Hz.
Discussion
The present study demonstrates that cerebral vasodilatation in response to rebreathing-induced hypercapnia is attenuated in young and healthy AAs compared with CAs. The lower cerebral vasodilatory response in AAs is a novel finding and may help to explain the greater prevalence of cerebral vascular disease in this population, although the underlying mechanisms need to be investigated further.
Carbon dioxide is a potent stimulus for changes in cerebral perfusion, and the sensitivity of this unique feature of the cerebral circulation is needed to ensure an adequate nutrient and oxygen supply as well as removal of metabolic waste products from brain tissue (Ringelstein et al. 1988; Kleiser & Widder, 1992). The ability of the brain to regulate its perfusion in response to changes in  is an indicator of cerebral vascular health (Ringelstein et al. 1988; Kleiser & Widder, 1992). A reduction in cerebral vasodilatation impairs the ability to provide adequate nutrient and oxygen supply to the cerebral microcirculation as well as to prevent fluctuations in pH status (Lavi et al. 2003, 2006). In this regard, the present findings are particularly important, given the high prevalence of a variety of diseases and conditions, including stroke, Alzheimer's disease and cognitive dysfunction, in the AA population (Mensah et al. 2005; Melikian et al. 2007).
is an indicator of cerebral vascular health (Ringelstein et al. 1988; Kleiser & Widder, 1992). A reduction in cerebral vasodilatation impairs the ability to provide adequate nutrient and oxygen supply to the cerebral microcirculation as well as to prevent fluctuations in pH status (Lavi et al. 2003, 2006). In this regard, the present findings are particularly important, given the high prevalence of a variety of diseases and conditions, including stroke, Alzheimer's disease and cognitive dysfunction, in the AA population (Mensah et al. 2005; Melikian et al. 2007).
From the examples depicted in Fig.2A and B, an increase in CBFV and CVCI tends to be similar until a change of ∼10 mmHg of  , and this tendency is reflected in the CVMR results that were the same between the two groups (Table 3). Despite the similarity in this variable, AA subjects achieved maximal CBFV and CVCI prematurely, which resulted in a plateau as the hypercapnic period continued. In other words, the maximal increase and total range of increase in CBFV and CVCI were blunted in the AA subjects. In comparison, CAs exhibited a relatively stable elevation in CBFV and CVCI. Seven AA subjects showed a complete plateau similar to Fig.2A and B, whereas no CA subjects showed this plateau. This trend for a plateau in AAs was reflected in a higher value of b, which is the curvilinear property of the regression curve. Taken together, these findings further support the hypothesis of reduced cerebral vascular function in AAs. The underlying mechanisms for the attenuated cerebral vasodilatory response in AAs are multifactorial; however, several possibilities can be proposed, as outlined below.
, and this tendency is reflected in the CVMR results that were the same between the two groups (Table 3). Despite the similarity in this variable, AA subjects achieved maximal CBFV and CVCI prematurely, which resulted in a plateau as the hypercapnic period continued. In other words, the maximal increase and total range of increase in CBFV and CVCI were blunted in the AA subjects. In comparison, CAs exhibited a relatively stable elevation in CBFV and CVCI. Seven AA subjects showed a complete plateau similar to Fig.2A and B, whereas no CA subjects showed this plateau. This trend for a plateau in AAs was reflected in a higher value of b, which is the curvilinear property of the regression curve. Taken together, these findings further support the hypothesis of reduced cerebral vascular function in AAs. The underlying mechanisms for the attenuated cerebral vasodilatory response in AAs are multifactorial; however, several possibilities can be proposed, as outlined below.
Cerebral autoregulation
Damage to the cerebral microcirculation as a result of impaired cerebral autoregulation may explain why the capacity of the brain vasculature to dilate in response to changes in CO2 was reduced in AAs. Cerebral autoregulation is a mechanism to protect the cerebral vasculature against changes in blood pressure and to stabilize CBF (Meel-van den Abeelen et al. 2014; Willie et al. 2014). Impaired cerebral autoregulation is associated with increased morbidity and mortality (Hu et al. 2008) and is present in patients with hypertension, ischaemic stroke, severe head injury, Parkinson's disease and carotid artery disease (Czosnyka et al. 1996; Eames et al. 2002, 2003; Vokatch et al. 2007; Reinhard et al. 2008). Long-term dysfunction of cerebral autoregulation induces physiological alterations, including cerebral microvascular remodelling and rarefaction (Levy et al. 2008; Reinhard et al. 2008), which limits maximal cerebral vasodilatory capacity, and if present in AAs, could partly explain the present findings. The transfer estimates of phase (a reflection of the temporal shift required to align the MAP signal with the CBFV signal) were lower in AAs in the low- and high-frequency ranges (Table 4). These findings suggest impaired cerebral autoregulation (Blaber et al. 1997; Zhang et al. 1998). However, transfer estimates of gain (the relative amplitude between changes in MAP and CBFV) and coherence (the linear relationship between MAP and CBFV) were similar between AAs and CAs in all frequency ranges (Table 4). These findings suggest similar autoregulation between groups. Based on these findings, differences in cerebral autoregulation between AAs and CAs are inconclusive and warrant further investigation.
Endothelial dysfunction and CO2-mediated pathway in the brain
Cerebral blood flow is controlled by a CO2-mediated pathway and endothelial signals in response to sheer stress as well as changes in blood pressure. Thus, endothelial dysfunction or an impaired CO2-mediated pathway in the brain would restrict cerebral vasodilatory capacity. Nitric oxide synthase inhibition reduces basal CBF (Joshi et al. 2000) and blunts CBF in response to hypercapnia in rats (Buchanan & Phillis, 1993), primates (Thompson et al. 1996) and humans (Schmetterer et al. 1997), indicating that basal cerebral perfusion as well as the response to hypercapnia is regulated, in part, by NO. This response is reversed by internal carotid artery infusion of l-arginine, a precursor for NO synthesis, in primates (Thompson et al. 1996) and in individuals with elevated cerebral vascular risk (Zimmermann & Haberl, 2003). The effect of NO synthase inhibition is greater as  increases during hypercapnia (Buchanan & Phillis, 1993), suggesting a positive correlation. Furthermore, hypertensive and diabetic patients with impaired peripheral endothelial function have impaired cerebral vascular responses to hypercapnia, and this impairment was abolished following administration of the exogenous NO donor sodium nitroprusside (Lavi et al. 2006). African Americans have impaired endothelial function (Perregaux et al. 2000), reduced NO bioavailability (Melikian et al. 2007) and impaired NO-dependent vasodilatation (Perregaux et al. 2000). These impairments could contribute to the reduced cerebral vasodilatory capacity in this population.
increases during hypercapnia (Buchanan & Phillis, 1993), suggesting a positive correlation. Furthermore, hypertensive and diabetic patients with impaired peripheral endothelial function have impaired cerebral vascular responses to hypercapnia, and this impairment was abolished following administration of the exogenous NO donor sodium nitroprusside (Lavi et al. 2006). African Americans have impaired endothelial function (Perregaux et al. 2000), reduced NO bioavailability (Melikian et al. 2007) and impaired NO-dependent vasodilatation (Perregaux et al. 2000). These impairments could contribute to the reduced cerebral vasodilatory capacity in this population.
Limitations
The CBFV reflects CBF only if the diameter of the middle cerebral artery is constant. Previous studies (Huber & Handa, 1967; Bradac et al. 1976; Serrador et al. 2000) demonstrated that the diameter of the middle cerebral artery does not change significantly during moderate changes in arterial blood pressure and carbon dioxide tension in plasma (within the range in the present study); thus, changes in CBFV reflect changes in CBF and can be used as an index of cerebral perfusion.
We did not measure  . Instead,
. Instead,  was used as an index of
was used as an index of  . However, Phan et al. (1987) and others (Whitesell et al. 1981; Yosefy et al. 2004) have demonstrated that
. However, Phan et al. (1987) and others (Whitesell et al. 1981; Yosefy et al. 2004) have demonstrated that  is correlated well with
is correlated well with  at rest. Moreover, Xie et al. (2006) reported that there were no differences in CVMR values between
at rest. Moreover, Xie et al. (2006) reported that there were no differences in CVMR values between  and
and  during hypercapnia. Thus, we believe that
during hypercapnia. Thus, we believe that  is a valid index for representing
is a valid index for representing  during both rest and hypercapnia.
during both rest and hypercapnia.
Conclusions
In conclusion, we observed that young, otherwise healthy African Americans had reduced cerebral vasodilatory capacity in response to the same degree of elevation in  induced by hypercapnic rebreathing when compared with their Caucasian counterparts. This finding supports the hypothesis that the cerebral vascular response is reduced in relatively young, healthy African Americans. Given that African Americans are at higher risk of cardiovascular and cerebral vascular disease, the findings indicate that reduced cardiovascular and cerebrovascular function is present at an early age in African Americans, leaving a long period of time to become progressively worse and develop into overt cardiovascular and cerebral vascular diseases and their complications with advancing age.
induced by hypercapnic rebreathing when compared with their Caucasian counterparts. This finding supports the hypothesis that the cerebral vascular response is reduced in relatively young, healthy African Americans. Given that African Americans are at higher risk of cardiovascular and cerebral vascular disease, the findings indicate that reduced cardiovascular and cerebrovascular function is present at an early age in African Americans, leaving a long period of time to become progressively worse and develop into overt cardiovascular and cerebral vascular diseases and their complications with advancing age.
Acknowledgments
We would like to express our appreciation to all of our subjects for their participation in this study.
Additional information
Competing interests
None declared.
Author contributions
C.H. contributed to the study design, data analysis and data interpretation, and drafted this manuscript. K.K., M.L.H. and R.M.B. contributed to the study design, data collection, data analysis, data interpretation and editorial process of the manuscript. All authors approved the final version of this manuscript. All experiments of the present study were conducted in the Environmental and Autonomic Physiology Laboratory at The University of Texas at Austin.
Funding
This research was supported by start-up funds to R.M.B. from The University of Texas at Austin.
References
- Blaber AP, Bondar RL, Stein F, Dunphy PT, Moradshahi P, Kassam MS, Freeman R. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28:1686–1692. doi: 10.1161/01.str.28.9.1686. [DOI] [PubMed] [Google Scholar]
- Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence of different CO2 tensions. Neuroradiology. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- Buchanan JE, Phillis JW. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 1993;610:248–255. doi: 10.1016/0006-8993(93)91408-k. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Levine BD, Zhang R. Cerebral vasomotor reactivity before and after blood pressure reduction in hypertensive patients. Am J Hypertens. 2009;22:384–391. doi: 10.1038/ajh.2009.2. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Zhang R, Fu Q, Witkowski S, Levine BD. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol. 2007;102:870–877. doi: 10.1152/japplphysiol.00906.2006. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2002;72:467–472. doi: 10.1136/jnnp.72.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames PJ, Blake MJ, Panerai RB, Potter JF. Cerebral autoregulation indices are unimpaired by hypertension in middle aged and older people. Am J Hypertens. 2003;16:746–753. doi: 10.1016/s0895-7061(03)00947-6. [DOI] [PubMed] [Google Scholar]
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients. Stroke J Cereb Circ. 1996;27:2188–2190. doi: 10.1161/01.str.27.12.2188. [DOI] [PubMed] [Google Scholar]
- Hu K, Peng CK, Czosnyka M, Zhao P, Novak V. Nonlinear assessment of cerebral autoregulation from spontaneous blood pressure and cerebral blood flow fluctuations. Cardiovasc Eng. 2008;8:60–71. doi: 10.1007/s10558-007-9045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P, Handa J. Effect of contrast material, hypercapnia, hyperventilation, hypertonic glucose and papaverine on the diameter of the cerebral arteries. Angiographic determination in man. Invest Radiol. 1967;2:17–32. doi: 10.1097/00004424-196701000-00016. [DOI] [PubMed] [Google Scholar]
- Ide K, Eliasziw M, Poulin MJ. The relationship between middle cerebral artery blood velocity and end-tidal Pco2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 2003;95:129–137. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- Joshi S, Young WL, Duong DH, Ostapkovich ND, Aagaard BD, Hashimoto T, Pile-Spellman J. Intracarotid infusion of the nitric oxide synthase inhibitor, l-NMMA, modestly decreases cerebral blood flow in human subjects. Anesthesiology. 2000;93:699–707. doi: 10.1097/00000542-200009000-00019. [DOI] [PubMed] [Google Scholar]
- Kadoi Y, Hinohara H, Kunimoto F, Saito S, Ide M, Hiraoka H, Kawahara F, Goto F. Diabetic patients have an impaired cerebral vasodilatory response to hypercapnia under propofol anesthesia. Stroke. 2003;34:2399–2403. doi: 10.1161/01.STR.0000090471.28672.65. [DOI] [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke. 1992;23:171–174. doi: 10.1161/01.str.23.2.171. [DOI] [PubMed] [Google Scholar]
- Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- Meel-van den Abeelen AS, van Beek AH, Slump CH, Panerai RB, Claassen JA. Transfer function analysis for the assessment of cerebral autoregulation using spontaneous oscillations in blood pressure and cerebral blood flow. Med Eng Phys. 2014;36:563–575. doi: 10.1016/j.medengphy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Melikian N, Wheatcroft SB, Ogah OS, Murphy C, Chowienczyk PJ, Wierzbicki AS, Sanders TA, Jiang B, Duncan ER, Shah AM, Kearney MT. Asymmetric dimethylarginine and reduced nitric oxide bioavailability in young Black African men. Hypertension. 2007;49:873–877. doi: 10.1161/01.HYP.0000258405.25330.80. [DOI] [PubMed] [Google Scholar]
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- Nur E, Kim YS, Truijen J, van Beers EJ, Davis SC, Brandjes DP, Biemond BJ, van Lieshout JJ. Cerebrovascular reserve capacity is impaired in patients with sickle cell disease. Blood. 2009;114:3473–3478. doi: 10.1182/blood-2009-05-223859. [DOI] [PubMed] [Google Scholar]
- Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, Dandona P. Brachial vascular reactivity in blacks. Hypertension. 2000;36:866–871. doi: 10.1161/01.hyp.36.5.866. [DOI] [PubMed] [Google Scholar]
- Phan CQ, Tremper KK, Lee SE, Barker SJ. Noninvasive monitoring of carbon dioxide: a comparison of the partial pressure of transcutaneous and end-tidal carbon dioxide with the partial pressure of arterial carbon dioxide. J Clin Monit. 1987;3:149–154. doi: 10.1007/BF01695936. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Gerds TA, Grabiak D, Zimmermann PR, Roth M, Guschlbauer B, Timmer J, Czosnyka M, Weiller C, Hetzel A. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol. 2008;255:1182–1189. doi: 10.1007/s00415-008-0865-z. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke. 1988;19:963–969. doi: 10.1161/01.str.19.8.963. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmetterer L, Findl O, Strenn K, Graselli U, Kastner J, Eichler HG, Wolzt M. Role of NO in the O2 and CO2 responsiveness of cerebral and ocular circulation in humans. Am J Physiol Regul Integr Comp Physiol. 1997;273:R2005–R2012. doi: 10.1152/ajpregu.1997.273.6.R2005. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Thompson BG, Pluta RM, Girton ME, Oldfield EH. Nitric oxide mediation of chemoregulation but not autoregulation of cerebral blood flow in primates. J Neurosurg. 1996;84:71–78. doi: 10.3171/jns.1996.84.1.0071. [DOI] [PubMed] [Google Scholar]
- Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi GL. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur Neurol. 2007;58:84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- Vokatch N, Grötzsch H, Mermillod B, Burkhard PR, Sztajzel R. Is cerebral autoregulation impaired in Parkinson's disease? A transcranial Doppler study. J Neurol Sci. 2007;254:49–53. doi: 10.1016/j.jns.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Whitesell R, Asiddao C, Gollman D, Jablonski J. Relationship between arterial and peak expired carbon dioxide pressure during anesthesia and factors influencing the difference. Anesth Analg. 1981;60:508–512. [PubMed] [Google Scholar]
- Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosefy C, Hay E, Nasri Y, Magen E, Reisin L. End tidal carbon dioxide as a predictor of the arterial Pco2 in the emergency department setting. Emerg Med J. 2004;21:557–559. doi: 10.1136/emj.2003.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Haberl RL. l-Arginine improves diminished cerebral CO2 reactivity in patients. Stroke. 2003;34:643–647. doi: 10.1161/01.STR.0000056526.35630.47. [DOI] [PubMed] [Google Scholar]


