Abstract
Objective
Evaluation of the long-term HPV-16/18 AS04-adjuvanted vaccine immunogenicity persistence in women.
Design
Multicentre, open-label, long-term follow-up (NCT00947115) of a primary phase–III study (NCT00196937).
Setting
Six centres in Germany and Poland.
Population
488 healthy women (aged 15–55 years, age-stratified into groups: 15–25, 26–45, and 46–55 years) who received three vaccine doses in the primary study.
Methods
Immune responses were evaluated in serum and cervicovaginal secretion (CVS) samples 6 years after dose 1. Anti-HPV-16/18 geometric mean titres (GMTs) were measured by enzyme-linked immunosorbent assay (ELISA), and were used to fit the modified power-law and piecewise models, predicting long-term immunogenicity. Serious adverse events (SAEs) were recorded.
Main outcome measures
Anti-HPV-16/18 seropositivity rates and GMTs 6 years after dose 1.
Results
At 6 years after dose 1, all women were seropositive for anti-HPV–16 and ≥97% were seropositive for anti-HPV–18 antibodies. GMTs ranged from 277.7 to 1344.6 EU/ml, and from 97.6 to 438.2 EU/ml, for anti-HPV–16 and anti-HPV–18, respectively. In all age groups, GMTs were higher (anti-HPV–16, 9.3–45.1-fold; anti-HPV–18, 4.3–19.4-fold) than levels associated with natural infection (29.8 EU/ml). A strong correlation between serum and CVS anti-HPV-16/18 levels was observed, with correlation coefficients of 0.81–0.96 (anti-HPV–16) and 0.69–0.84 (anti-HPV–18). Exploratory modelling based on the 6–year data predicted vaccine-induced anti-HPV-16/18 levels above natural infection levels for at least 20 years, except for anti-HPV–18 in the older age group (piecewise model). One vaccine-related and two fatal SAEs were reported.
Conclusions
At 6 years after vaccination, immune responses induced by the HPV-16/18 AS04-adjuvanted vaccine were sustained in all age groups.
Keywords: HPV-16/18 AS04-adjuvanted vaccine, human papillomavirus, persistence
Introduction
Persistent infection with oncogenic human papillomavirus (HPV) types is a necessary cause of cervical cancer, the third most common cancer in women worldwide and the fourth leading cause of cancer-related deaths in women.1–7 In Europe, cervical cancer is the second most frequent cancer among women aged 15–44 years.8
Approximately 10% of women worldwide with a normal cervical cytology carry a detectable cervical HPV infection.9,10 Although most HPV infections are transient, persistent infections may progress to pre-cancerous cervical intraepithelial neoplasia (CIN) and cervical cancer.11–14
Among approximately 15 high-risk (HR) oncogenic HPV types identified, HPV–16 and HPV–18 cause approximately 70% of invasive cervical cancer cases.1,3,12,15–18 About 100 million women worldwide carry HPV-16/18 DNA, and the detection of HPV-16/18 is associated with a five-fold greater risk of developing CIN than the detection of other HR HPV types.16,19
Cervical HPV infections are particularly common in young, sexually active women, but women remain at risk throughout their sexually active life, with another peak observed in women aged >45 years.10,12,20–25 Therefore, in addition to the current vaccination programmes targeting adolescent girls before their sexual debut, vaccination of women aged >25 years should be considered on an individual basis.26
The prophylactic HPV-16/18 AS04-adjuvanted vaccine (Cervarix®; GlaxoSmithKline, Rixensart, Belgium), which is licensed in over 100 countries worldwide,27–30 has been shown to be immunogenic and efficacious against HPV-16/18 infections in women aged 15–25 years.28,31–37 Recently, a high vaccine efficacy has also been demonstrated in women aged ≥26 years.38 The vaccine was also shown to have a clinically acceptable safety profile.27,39 In Europe, it is indicated for use in girls from 9 years of age.40
In a previously published primary open-label phase–III study, the HPV-16/18 AS04-adjuvanted vaccine was immunogenic and well tolerated in women aged 15–55 years; in addition, a high correlation between anti-HPV-16/18 antibody levels in sera and cervicovaginal secretion (CVS) samples was observed, regardless of age.41 In the first follow-up of the primary study (NCT00196937), immune responses to the vaccine were sustained up to 2 years after vaccination; CVS anti-HPV-16/18 antibodies were not assessed.30 The current extended follow-up evaluates the long-term persistence of antibody responses, up to 10 years after the first dose, in women vaccinated in the primary study. Here, we report persistence of the serum and CVS anti-HPV-16/18 antibodies, and vaccine safety, up to 6 years after vaccination. In addition, we present for the first time modelling data predicting long-term anti-HPV-16/18 antibody persistence in vaccinated women aged >25 years.
Methods
Study design
This study is a multicentre, open-label, age-stratified, long-term follow-up (LTFU) of the primary study (NCT00196937), conducted in six centres in Poland and Germany between October 2004 and July 2005, in which healthy women aged 15–55 years, stratified in three age groups (15–25, 26–45, and 46–55 years), received three doses of the HPV-16/18 AS04-adjuvanted vaccine at 0, 1, and 6 months. The immunogenicity persistence and vaccine safety will be evaluated up to 10 years after the first dose. Here, we present the results obtained up to 6 years after the first dose. The overall study design is summarised in Figure1.
Figure 1.
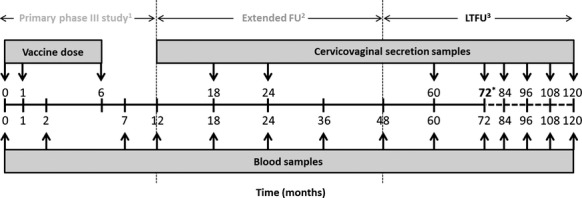
Study design.1NCT00196937.41 2Extended follow-up study (NCT00196937).30 3Long-term follow-up study (current study) NCT00947115. *Data presented here are for the year 6 (month 72) time point.
All women participating in the study provided written informed consent prior to enrolment. This study was conducted in accordance with the Good Clinical Practice guidelines and all applicable regulatory requirements, including the Declaration of Helsinki. This study has been registered at http://www.clinicaltrials.gov (NCT00947115).
Study objectives
The primary study objective was to evaluate the long-term immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in terms of anti-HPV-16 and anti-HPV-18 geometric mean antibody titres (GMTs) and seroconversion rates in serum, measured by enzyme-linked immunosorbent assay (ELISA) 6 years after the first dose.
The secondary objectives included: an evaluation of immune responses to the vaccine in serum compared with antibody levels achieved after natural infection,35,36 and compared with antibody levels achieved in other efficacy studies;28,31–33 a comparison of anti-HPV-16/18 levels in CVS and serum samples, and an evaluation of the safety of the vaccine up to 6 years following the first dose.
Study vaccine
Each dose of the HPV-16/18 AS04-adjuvanted vaccine contained 20 μg each of HPV–16 and HPV–18 L1 virus-like particles (VLPs) formulated with the AS04 adjuvant system, comprising 50 μg 3–O–desacyl-4'-monophosphoryl lipid A and 500 μg Al(OH)3. The vaccine was supplied in individual 0.5–ml pre-filled syringes and administered into the deltoid muscle.
Study population
Only women who completed the full vaccination course in the primary study were eligible for inclusion in this extended LTFU study. Inclusion and exclusion criteria for the primary study were previously described.41
Immunogenicity assessment
Blood samples for immunogenicity assessment were collected at 0, 2, 7, 12, 18, 24, 36, 48, 60 (year 5), and 72 months (year 6), and CVS samples (from women who volunteered) were collected at 18, 24, 60, and 72 months, as previously described.41 In this LTFU study, we assessed samples collected at year 5 and year 6 (Figure1). Anti-HPV–16 and anti-HPV–18 antibody titres in serum and CVS samples were measured by ELISA using type-specific VLPs as coating antigens (serum standardised protocol).5,32,33,41,42 Total immunoglobulin G (IgG) levels were measured to account for variation during the menstrual cycle. IgG titres were evaluated in both serum and CVS samples to standardise the immunogenicity results by dividing each anti-HPV–16 and anti-HPV–18 titre by the corresponding total IgG titre.
Seropositivity was defined as antibody titres ≥ 8 ELISA units (EU)/ml or ≥ 7 EU/ml for anti-HPV–16 and anti-HPV–18, respectively.30,41 Anti-HPV-16/18 GMTs resulting from natural infection used in this study were previously determined in women aged 15–25 years.35
Prediction of long-term persistence of anti-HPV-16/18 antibody responses
Modelling of the long-term persistence of anti-HPV-16/18 antibody responses was assessed as described previously.43 Briefly, two mixed-effects statistical models, the modified power-law model and the piecewise model, were fitted to the individual anti-HPV-16/18 titres measured at each time point up to year 6 in women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine in the primary study, and for whom post-vaccination results were available. To estimate post-vaccination antibody decay over time, the modified power-law model includes B–cell dynamics, in which two B–cell populations (activated and memory B–cells) are involved, accounting for the long-term persistence of a memory B–cell subpopulation and a long-term antibody plateau. The piecewise model fits the data on three non-overlapping time intervals, corresponding to the observed decay of humoral antibodies. Each piece of the model used a linear function; three break points (7, 12, and 21 months) were selected. The two model effects were fitted using an nlmixed sas procedure.
Safety assessment
Although no study vaccine was administered in this LTFU study, all serious adverse events (SAEs) related to vaccination, study participation, or concomitant GlaxoSmithKline Vaccines medication were recorded during the entire study period.
All women with SAEs or who were withdrawn from the study because of SAEs were followed until the SAE had resolved, subsided, stabilised, disappeared, or been otherwise explained.
Statistical analysis
All analyses were performed using sas 9.2 and Proc StatXact 8.1.
Study cohorts
The primary cohort for analysis of blood sample results at year 6 was the according-to-protocol (ATP) cohort for immunogenicity, which comprised all women included in the ATP immunogenicity analysis in the primary study, meeting all eligibility criteria, complying with procedures defined in the protocol, with no elimination criteria during the study, who attended the year–6 visit, and for whom serology results at year 6 were available.
The primary cohort for analysis of CVS sample results at year 6 was the total vaccinated cohort (TVC), comprising all women who volunteered for CVS sampling at year 6 and for whom CVS results were available at this time point.
The year–6 TVC for safety included all women participating in the current LTFU study. Two safety analyses were performed. The first was based on TVC data collected during this LTFU study from the last visit of the previous follow-up study (up to month 48) to the last visit in this follow-up study (year 6); the second analysis was based on the TVC data collected for the entire study period (from month 0 to year 6).
Immunogenicity analyses
Immunogenicity analyses were age-stratified (15–25, 26–45, and 46–55 years at first vaccination).
Seroconversion was defined as the appearance of antibodies, with titres greater than or equal to the cut-off values, in sera of women who were seronegative before vaccination in the primary study. Conversion for CVS analyses was defined as the appearance of antibodies, with titres greater than or equal to the limits of quantification, in CVS samples of women who tested negative before vaccination in the primary study. Seropositivity rates and anti-HPV-16/18 GMTs were calculated with exact 95% confidence intervals (95% CIs). GMT calculations were performed by taking the antilog of the mean of the log titre transformations. In the absence of an accepted serological correlate of protection, we performed descriptive comparisons with anti-HPV-16/18 serology results from a previous efficacy study,32,33 and with anti-HPV-16/18 titres achieved after natural infection in another study.35,36
Safety analyses
Safety analyses were age-stratified (15–25, 26–45, and 46–55 years). Vaccine-, study participation- or concomitant GlaxoSmithKline Vaccines medication-related SAEs and fatal SAEs were described in detail. Vaccine-related SAEs were further evaluated for clinical relevance.
Results
Study population
In the primary study, 666 women received at least one dose of the HPV-16/18 AS04-adjuvanted vaccine. Of the 647 women who received three vaccine doses in the primary study, 159 did not show up for the follow-up visit after 6 years. At year 6, the TVC comprised 488 women, of whom 477 were included in the ATP cohort for immunogenicity (Figure2). Most women (99.8%) were white/caucasian.
Figure 2.
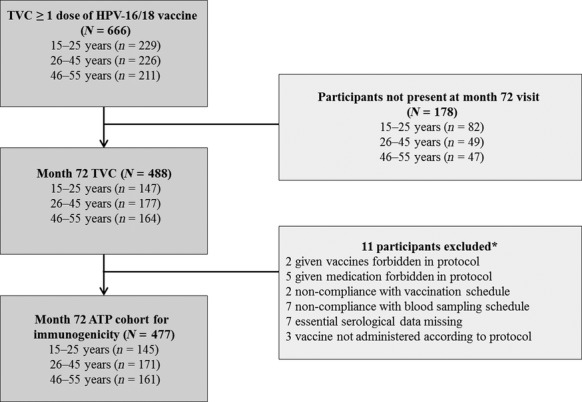
Flow chart of study participants. Key: 15–25 years, women aged 15–25 years at the time of first vaccine dose; 26–45 years, women aged 26–45 years at the time of first vaccine dose; 46–55 years, women aged 46–55 years at the time of first vaccine dose; ATP, according to protocol; N = total number of women; n = number of women in the age group; TVC, total vaccinated cohort. *Participants may have more than one reason for exclusion.
Immunogenicity
Serological immune response
Of the 644 women included in the ATP immunogenicity cohort in the primary study at baseline, over 70% were seronegative for both anti-HPV-16 and anti-HPV-18; among women in the age groups 15–25, 26–45, and 46–55 years, 82.5, 67.4, and 66.3% were seronegative for both HPV–16 and HPV–18, respectively, and only 6% (2.7, 6.9, and 9.0% in the age groups 15–25, 26–45, and 46–55 years, respectively) were seropositive for both HPV–16 and HPV–18.41 One month following the third vaccine dose (month 7), all initially seronegative women were seropositive for anti-HPV-16/18 antibodies.41
At year 6, all women remained seropositive for anti-HPV–16 antibodies and at least 97% were seropositive for anti-HPV–18 antibodies. Four women who were initially seronegative for anti-HPV–18 were still seronegative at year 6 (age group 46–55 years).
Antibody kinetics for both anti-HPV–16 and anti-HPV–18 showed a peak in antibody levels at month 7, followed by a gradual decline until month 18.35,36 This decline became less pronounced over time, suggesting that a plateau phase had been reached (Figure3). An age-dependent decrease in GMTs observed at the previous time points was also observed at year 6, as indicated by non-overlapping 95% CIs between the age groups (Figure3).
Figure 3.
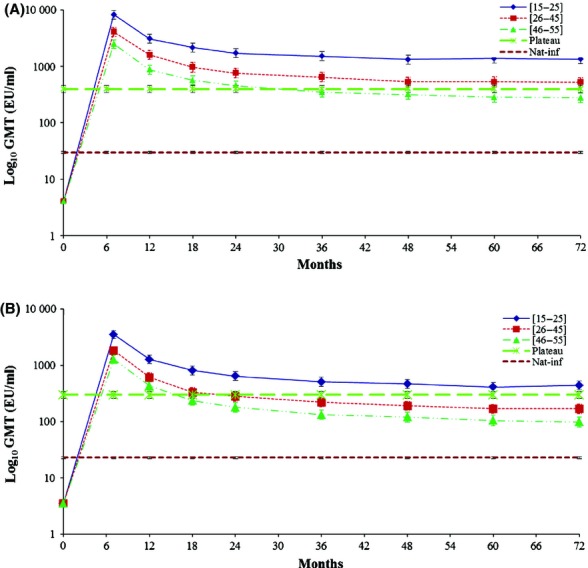
Anti-HPV–16 (A) and anti-HPV–18 (B) antibody levels in initially seronegative women aged 15–55 years (ATP cohort for immunogenicity at year 6). Key: [15–25], women aged 15–25 years at the time of first vaccine dose; [26–45], women aged 26–45 years at the time of first vaccine dose; [46–55], women aged 46–55 years at the time of first vaccine dose; GMT, geometric mean titre; Nat-inf, natural infection. GMTs of women who were (A) HPV–16 or (B) HPV–18 DNA-negative and seropositive at baseline (i.e. who had cleared a natural infection). GMTs were (A) 29.8 EU/ml and (B) 22.6 EU/ml.35,36 Plateau: GMTs of women aged 15–25 years at months 45–50 after the first vaccine dose (total vaccinated cohort). GMTs were (A) 397.8 EU/ml and (B) 297.3 EU/ml.33 The error bars represent 95% confidence intervals.
At year 6, in initially seronegative women, anti-HPV-16 GMTs were 1344.6 EU/ml (95% CI 1130.2–1599.6 EU/ml), 526.0 EU/ml (95% CI 434.7–636.4 EU/ml), and 277.7 EU/ml (95% CI 228.0–338.2 EU/ml) in the age groups 15–25, 26–45, and 46–55 years, respectively. Anti-HPV–18 GMTs were 438.2 EU/ml (95% CI 366.6–523.7 EU/ml), 167.5 EU/ml (95% CI 138.1–203.1 EU/ml), and 97.6 EU/ml (95% CI 79.2–120.3 EU/ml) in these age groups, respectively. Anti-HPV-16/18 titres were higher than those achieved after natural infection in all age groups: for initially seronegative women, anti-HPV–16 GMTs were approximately 45.1-, 17.7-, and 9.3-fold higher in the age groups 15–25, 26–45, and 46–55 years, respectively, than the natural infection level (29.8 EU/ml).35 Anti-HPV–18 GMTs were approximately 19.4-, 7.4-, and 4.3-fold higher in these age groups, respectively, than the natural infection level (22.6 EU/ml).35,36 At year 6, anti-HPV–16 GMTs were 3.4-fold higher (in the age group 15–25 years) or remained within a similar range (in the age group 26–45 years) when compared with the plateau level established in a previous efficacy study at 45–50 months (397.8 EU/ml).33 In the age group 46–55 years, anti-HPV–16 GMTs were below the plateau level (0.7–fold; non-overlapping 95% CIs). In the age group 15–25 years, anti-HPV–18 GMTs were also higher (1.5-fold) than the GMT plateau level observed in the previous efficacy study at 45–50 months (297.3 EU/ml).33 In the age groups 26–45 and 46–55 years, anti-HPV–18 GMTs were lower than this predefined GMT plateau level (0.6-fold and 0.3-fold, respectively), with non-overlapping 95% CIs.
CVS antibody levels (TVC)
At year 6, 190 women had their CVS samples tested, of whom 84 (29 each in the age groups 15–25 and 26–45 years, and 26 in the age group 46–55 years) had CVS samples considered suitable for HPV antibody testing (<200 erythrocytes/μl; samples with ≥200 erythrocytes/μl were considered to be contaminated with blood).
At year 6, anti-HPV–16 antibodies were detected in 72.4, 72.4, and 73.1% of CVS samples of women in the age groups 15–25, 26–45, 46–55 years, respectively. At the same time point, anti-HPV–18 antibodies were detected in 69.0, 62.1, and 53.8% of the CVS samples of women in these age groups, respectively. CVS anti-HPV–16 GMTs were 80.3, 43.8, and 37.1 EU/ml in the age groups 15–25, 26–45, and 46–55 years, respectively, and anti-HPV–18 GMTs were 22.9, 19.9, and 19.2 EU/ml.
At year 6, women with detectable anti-HPV-16/18 antibodies in their CVS samples had higher serum anti-HPV-16/18 GMTs than women with no antibodies detected in their CVS samples: for anti-HPV–16 they were between two- and five-fold higher and for anti-HPV–18 they were between one- and five-fold higher, depending on the age group (data not shown).
To account for the fluctuation in total IgG levels observed during the menstrual cycle, anti-HPV-16/18 antibody levels were standardised relative to the total IgG levels before evaluating the correlation. Correlations between the serum and CVS titres (standardised for total IgG) in the three age groups at year 6 are presented in Figure4. For anti-HPV–16, the correlation coefficients were high regardless of age, and ranged from 0.87 to 0.91 at year 5,44 and from 0.81 to 0.96 at year 6 (Figure4A); for anti-HPV-18, they ranged from 0.84 to 0.87 at year 5,44 and from 0.69 to 0.84 at year 6 (Figure4B).
Figure 4.
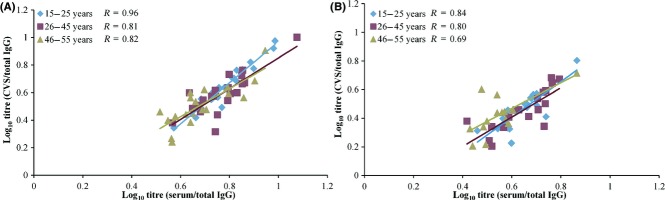
Correlation of ratio between cervicovaginal secretion and serum samples by age for anti-HPV–16 (A) and anti-HPV–18 (B) antibody levels at year 6 in women aged 15–55 years (total vaccinated cohort). Key: [15–25], women aged 15–25 years at the time of first vaccine dose; [26–45], women aged 26–45 years at the time of first vaccine dose; [46–55], women aged 46–55 years at the time of first vaccine dose; CVS, cervicovaginal secretion; R, correlation coefficient. Titres were measured using an ELISA assay for the detection of anti-HPV–16 and anti-HPV–18 antibodies both for cervicovaginal secretion and serum samples. The scatter plots show the ratio (specific IgG/total IgG) transformed to linear log10 values.
Predicted long-term persistence of antibody responses
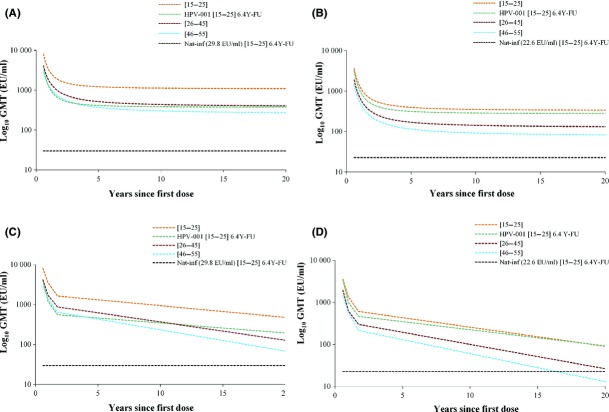
The long-term persistence of anti-HPV-16/18 antibody responses was predicted for the three age groups using the modified power-law and piecewise models, based on the 6–year immunogenicity data from the current study. Immunogenicity data obtained at 6.4 years after vaccination in a previous study in women aged 15–25 years were also included in the models (Figure5).28 The modified power-law model predicted that mean anti-HPV-16/18 levels would remain above those associated with natural infection for at least 20 years in all age groups. The piecewise model predicted that anti-HPV–16 GMTs would remain above GMTs associated with natural infection for all age groups, whereas anti-HPV–18 GMTs would remain above the natural infection levels for the age groups 15–25 and 26–45 years, but not for the age group 46–55 years (Table 1). Additional model-based estimations were performed to predict the duration ensuring that 95% of women would have anti-HPV-16/18 levels remaining above the natural infection level. The modified power-law model predicted lifelong anti-HPV–16 levels above the natural infection level for all women, and lifelong anti-HPV–18 levels above the natural infection level in the age groups 15–25 and 26–45 years; for the age group 46–55 years, the modified power-law model predicted that 95% of women would still have anti-HPV–18 levels above natural infection levels after 4.3 years. The piecewise model predicted durations of antibody levels remaining above natural infection levels of up to 40.1, 16.9, and 12.1 years for anti-HPV–16, and up to 19.2, 9.0, and 5.1 years for anti-HPV–18 for 95% of women in the age groups 15–25, 26–45, and 46–55 years, respectively (Table 2).
Figure 5.
Prediction of antibody responses against HPV–16 and HPV–18 over 20 years using the modified power-law and the piecewise models in women aged 15–55 years (TVC at year 6). (A) Anti-HPV–16 antibody levels prediction by the modified power-law model; (B) anti-HPV–18 antibody levels prediction by the modified power-law model; (C) anti-HPV–16 antibody levels prediction by modified piecewise model; (D) anti-HPV–18 antibody levels prediction by piecewise model. Key: [15–25], women aged 15–25 years at the time of first vaccine dose; [26–45], women aged 26–45 years at the time of first vaccine dose; [46–55], women aged 46–55 years at the time of first vaccine dose; HPV–001 [15–25] 6.4Y–FU, anti-HPV–16 or anti-HPV–18 antibody levels at 6.4 years in women aged 15–25 years from a previous HPV-001 study;28 Nat-inf, natural infection: geometric mean titres (GMTs) of women who were HPV–16 or HPV–18 DNA-negative and seropositive at baseline (i.e. who had cleared a natural infection). GMTs were 29.8 EU/ml for HPV–16 and 22.6 EU/ml for HPV–18.35,36
Table 1.
Predicted population GMTs for anti-HPV–16 and anti-HPV–18 at 20 years after the first vaccination in women aged 15–55 years
| Antigen | Model | Predicted GMTs 20 years after the first vaccination (EU/ml)* | ||
|---|---|---|---|---|
| 15–25 years** | 26–45 years*** | 46–55 years**** | ||
| HPV–16 | Modified power-law | 1092.3 | 404.2 | 272.4 |
| Piecewise | 474.9 | 127.1 | 67.8 | |
| HPV–18 | Modified power-law | 337.3 | 132.3 | 82.2 |
| Piecewise | 90.4 | 26.4 | 12.9 | |
GMTs, geometric mean antibody titres.
Women aged 15–25 years at the time of first vaccine dose.
Women aged 26–45 years at the time of first vaccine dose.
Women aged 46–55 years at the time of first vaccine dose.
Table 2.
Predicted duration ensuring that 95% of women would still have anti-HPV–16 and anti-HPV–18 antibody levels above natural infection levels in women aged 15–25, 26–45, and 46–55 years in the present study, and in women aged 15–25 years in a previous study (NCT00689741 and NCT00120848)41
| Antigen | Antibody levels induced by natural infection (EU/mL)* | Model | Duration ensuring antibody levels above those induced by natural infection in 95% participants (years) | |||
|---|---|---|---|---|---|---|
| Current study | Previous modelling study** | |||||
| 15–25 years old*** | 26–45 years old*** | 46–55 years old*** | 15–25 years old*** | |||
| HPV–16 | 29.8 | Modified power-law | Lifelong | Lifelong | Lifelong | Lifelong |
| Piecewise | 40.08 | 16.9 | 12.1 | 26.7 | ||
| HPV–18 | 22.6 | Modified power-law | Lifelong | Lifelong | 4.3 years | Lifelong |
| Piecewise | 19.2 | 9.0 | 5.1 | 19.9 | ||
Women aged 15–25 years in a previous study (NCT00689741 and NCT00120848).41
Age ranges indicate the ages of women at the time of the first vaccination dose.
Safety
The HPV-16/18 AS04-adjuvanted vaccine had a clinically acceptable safety profile in all age groups throughout the entire study duration. Twenty-eight women reported 32 SAEs. One woman (in the age group 26–45 years) reported one SAE (optic neuritis), considered by the investigators as causally related to the study vaccination; as a result of this SAE, the vaccination course of the women was discontinued, but she was not withdrawn from the study. This SAE occurred during the primary study and was resolved therein, as previously reported.41 Two fatal SAEs occurred during the entire study period: one woman committed suicide and one had a road accident. Both of these SAEs were reported in the age group 46–55 years, and both were considered unrelated to the vaccination.
Discussion
Main findings
Immune responses to the HPV-16/18 AS04-adjuvanted vaccine persisted up to 6 years after vaccination in women aged 15–55 years. At year 6, all women remained seropositive for anti-HPV-16 and ≥97% were seropositive for anti-HPV-18 antibodies, with anti-HPV-16/18 GMTs higher than levels associated with natural infection; anti-HPV–16 and anti-HPV–18 antibodies were detected in about 72 and 53.8–69.0% of CVS samples, respectively. A strong correlation between serum and CVS anti-HPV-16/18 GMTs was observed; serum anti-HPV-16/18 GMTs were higher in women with detectable CVS anti-HPV-16/18 antibodies. Exploratory modelling based on the 6–year data predicted that vaccine-induced population anti-HPV-16/18 GMTs would remain above those associated with natural infection for at least 20 years after vaccination. The vaccine had a clinically acceptable safety profile.
Strengths and weaknesses
This study included both young and mature women, allowing for a comparison of anti-HPV-16/18 levels between age groups. Although a sustained HPV-16/18 AS04-adjuvanted vaccine immunogenicity has been reported in women aged 15–25 years,34,37 to our knowledge this is the first study reporting such long-term (up to 6 years post-vaccination) persistence of antibody responses and correlation of the serum and CVS anti-HPV-16/18 antibodies in women aged >25 years. Using statistical models, we could for the first time predict the persistence of anti-HPV-16/18 responses for up to 20 years in mature women.
The main study limitations include open design, the lack of a direct control group, and antibody assessment only by ELISA. Assessment of neutralising antibodies, avidity, and cell-mediated immune responses for vaccine and non-vaccine HPV types would provide added value. Another limitation might be the lack of cervical screening data, which could help determine a clinical correlation with the immune data; however, the current study was designed to evaluate the HPV-16/18 AS04-adjuvanted vaccine immunogenicity and safety, and not its efficacy.
Interpretation
We previously showed that the HPV-16/18 AS04-adjuvanted vaccine was immunogenic in women aged 15–55 years for up to 48 months.30,41 Here, we report the immunogenicity persistence up to 6 years after vaccination. Anti-HPV-16/18 antibody kinetics were similar in all age groups and similar to those reported previously, with a peak response at month 7, followed by a gradual decline.30,33,41,45 The highest anti-HPV-16/18 levels were observed in the age group 15–25 years, in line with previous observations,30,41 and consistent with findings that immune responses decline with increasing age.46
Although an immunological correlate of protection has not yet been identified, high and sustained antibody titres obtained through vaccination are considered predictive of long-term protection against oncogenic HPV infections and CIN.47,48 We compared anti-HPV-16/18 levels with GMT plateaus associated with sustained protection at months 45–50 in a previous study on efficacy.33 In our study, anti-HPV-16/18 levels at year 6 remained above the predefined plateau levels of sustained efficacy in the age group 15–25 years,33 and in all age groups the levels were substantially higher than the natural infection levels.35
A previous modelling study showed the persistence of anti-HPV-16/18 antibody responses up to 20 years following vaccination with the HPV-16/18 AS04-adjuvanted vaccine in women aged 15–25 years.43 We report for the first time the modelling of antibody responses of up to 20 years after vaccination in older women. Based on our 6–year immunogenicity data, the modified power-law model predicted that post-vaccination anti-HPV-16/18 GMTs would remain substantially higher than levels associated with natural infection for at least 20 years in women aged 15–55 years. Anti-HPV-16/18 GMTs predicted using the more conservative piecewise model were lower than those predicted with the modified power-law model, but were higher than natural infection levels, except for the age group 46–55 years. Based on immunogenicity data from a previous study (HPV-001),28 or from the current study for the age groups 15–25 years, both models predicted similar anti-HPV–18 GMTs and lower anti-HPV–16 GMTs 20 years after vaccination. These different predictions may result from the different pre-established assumptions for each model. The modified power-law model is based on the assumption that B–cell memory does not decline over time, whereas the piece-wise model assumes that antibody responses follow a linear decrease over time, and takes into account only antibody levels obtained in a given study. Hence, the piecewise model is likely to yield a more conservative estimate of long-term protection.43 As the duration of protection associated with HPV vaccination is of crucial importance in cost-effectiveness, our modelling data could serve as a basis to assess the economic impact of cervical cancer vaccination programmes. Finally, a high HPV-16/18 AS04-adjuvanted vaccine efficacy (81.1%, 97.7% CI 52.1–94.0%) against HPV-16/18-related CIN1+ and 6–month persistent infection has been recently demonstrated in women aged ≥26 years.38 Altogether, these previous results and our immunogenicity data suggest that the HPV-16/18 AS04-adjuvanted vaccine provides a sustained protection against HPV-16/18 infection in women aged >25 years.
A significant relationship between naturally acquired anti-HPV–16 antibodies and the risk of new infection has been reported: the incidence of infection declined with increasing antibody titres.49 In another study, unvaccinated women with higher anti-HPV-16/18 levels had significantly lower risk of subsequent HPV-16/18 infection than seronegative women, suggesting that natural infection confers a certain degree of protection against new HPV-16/18 infections.50 Antibody levels after natural infection are much lower than those generated through vaccination, however, and thus natural infection confers only partial protection. The mechanism by which prophylactic HPV vaccines induce protection is assumed to be mediated by vaccine-induced neutralising IgG antibodies, which transudate across cervical epithelium to the HPV infection site and are likely to prevent HPV infection of the cervical basal cell layer at the transformation zone.47,49,51,52 We observed a strong correlation between the serum and CVS anti-HPV-16/18 levels that persisted up to 6 years after vaccination. To our knowledge, this is the first study showing such long persistence of anti-HPV-16/18 antibody transudation following vaccination with HPV-16/18 AS04-adjuvanted vaccine in women aged >25 years. Although anti-HPV-16/18 levels in serum decreased since the first assessment time point (month 24) in the primary study,41 correlation between the serum and CVS antibody levels at year 6 remained high in all age groups. A pooled analysis of data from four clinical trials assessing serum and CVS anti-HPV-16/18 correlation following administration of the HPV-16/18 AS04-adjuvanted vaccine has shown that higher antibody levels in serum resulted in higher antibody levels in CVS, supporting transudation of serum antibodies as the mechanism by which antibodies are introduced into CVS.51 Furthermore, in a phase–IV clinical trial in girls aged 12–15 years vaccinated with the HPV-16/18 AS04-adjuvanted vaccine, or Gardasil®, cross-neutralising antibodies were detected at the genital site of infection.53
The HPV-16/18 AS04-adjuvanted vaccine safety profile was consistent with that described in previous reports.27,28,31,36
Conclusion
The HPV-16/18 AS04-adjuvanted vaccine was well tolerated and induced sustained immune responses in women aged 15–55 years. Anti-HPV-16/18 antibody levels remained several-fold higher than levels associated with natural infection for at least 6 years after vaccination. Exploratory modelling of antibody persistence based on the 6–year data predicted that vaccine-induced anti-HPV-16/18 GMTs would remain above those induced by natural infection for at least 20 years, except for anti-HPV–18 levels in the age group 46–55 years (piecewise model). A strong correlation between anti-HPV-16/18 levels in serum and CVS samples 6 years after vaccination indicates a long-lasting transudation of serum antibodies across the cervical epithelium. These results suggest that in addition to the routine vaccination programmes in adolescents, sexually active mature women could potentially also benefit from vaccination with HPV-16/18 AS04-adjuvanted vaccine on an individual basis.
Acknowledgments
The authors thank study participants and their families and the following principal investigators/co-investigators: Günter Cichon, Beate Leifels, and Dominik Pruski. The authors also acknowledge the input and assistance of the following individuals at GlaxoSmithKline Vaccines: Ariane Meurée (ELISA serology) and Laurence Luyten (ELISA on CVS) for laboratory support; Amulya Jayadev and Grégory Catteau for statistical support; Lan Lin Cresens (XPE Pharma and Science for GlaxoSmithKline Vaccines), Kurt Dobbelaere, and Frank Struyf for the management of the clinical trial; Philippe Marius for clinical data management; Annelies Vanneuville and Geneviève Meiers for study coordination; Carys Calvert for regulatory aspects; Fernanda Tavares for safety aspects; and Nele Martens for protocol writing. Urszula Miecielica (XPE Pharma & Science) provided medical writing services. Mélanie Muylaert and Stéphanie Delval (XPE Pharma & Science) provided publication coordination for GlaxoSmithKline Vaccines. Cervarix® is a registered trademark of the GlaxosmithKline group of companies. Gardasil® is a registered trademark of Merck & Co. Inc.
Disclosure of interests
FT, DD, and PVS are employed by the GlaxoSmithKline (GSK) group of companies. FT and DD own stock options in the GSK group of companies. TFS, MS, and AMK have received support for travel to meetings for the study from the GSK group of companies. JW has received travel, accommodation, and meeting reimbursements for participation in international scientific conferences, board membership, lectures, and consultancy on results from clinical trials. TFS, AMK, and MS have received payment for lectures, including service on a speakers bureau, from the GSK group of companies. TFS was paid for the development of educational presentations from the GSK group of companies. AMK has received a research grant from the GSK group of companies. TFS was paid for board membership and consultancy from the GSK group of companies. Consulting fees or honoraria have been received by AMK for advisory board membership and presentations, by JW and his institution as payment for the clinical trial, and by MS. AG and KS declare that they have no conflicts of interest.
Contribution to authorship
All authors participated in the design, implementation, or analysis and interpretation of the study, and in the development of this article. All authors had full access to the data and gave final approval before submission. The corresponding author was responsible for the submission of the publication.
Details of ethics approval
All study procedures, the protocol, any amendment, and the informed consent were reviewed and approved by the following national independent ethics committees: Ethik-Kommission der Universität Würzburg (EudraCT no. 2009-011357-41, 1 July 2009; on behalf of Ethik-Kommission der Bayerischen Landesärztekammer and Charité Universitätsmedizin Berlin Campus Benjamnim Franklin Frauenklinik und Hochschulambulanz) and Komisja Bioetyczna przy Uniwersytecie Medycznym im. Karola Marcinkowskiego w Poznaniu (resolution no. 833/07, 3 December 2009).
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the conduct of the study and the analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of this article.
References
- 1.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and orevalence in 2012. [ http://globocan.iarc.fr/Pages/fact_sheets_population.aspx ]. Accessed 7 January 2014.
- 3.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi f WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 8. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) 2010. Human Papillomavirus and Related Cancers. Summary Report Update. September 15, 2010.
- 9.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 10.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 11.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(Suppl 1):S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 13.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43(4 Suppl):S5–25. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 15.Bayas JM, Costas L, Munoz A. Cervical cancer vaccination indications, efficacy, and side effects. Gynecol Oncol. 2008;110(3 Suppl 2):S11–14. doi: 10.1016/j.ygyno.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 16.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 17.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 18.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97:1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 20.Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–92. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 22.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–5. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschi S, Herrero R, Clifford GM, Snijders PJ, Arslan A, Anh PT, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–84. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 24.Lawson MA. Human papillomavirus infection in adolescent and young women. Mo Med. 2008;105:42–6. [PubMed] [Google Scholar]
- 25.Moscicki AB. HPV infections in adolescents. Dis Markers. 2007;23:229–34. doi: 10.1155/2007/136906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellsague X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol. 2009;115(3 Suppl):S15–23. doi: 10.1016/j.ygyno.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009;5:332–40. doi: 10.4161/hv.5.5.7211. [DOI] [PubMed] [Google Scholar]
- 28.The GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz TF. Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine, Cervarix. Adv Ther. 2009;26:983–98. doi: 10.1007/s12325-009-0079-5. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Schulze K, et al. Persistence of immune response to HPV-16/18 AS04-adjuvanted cervical cancer vaccine in women aged 15-55 years. Hum Vaccin. 2011;7:958–65. doi: 10.4161/hv.7.9.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–55. doi: 10.1016/j.vaccine.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 33.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 34.Naud P, Roteli-Martins CM, De Carvalho N, Teixeira J, Borba P, Sanchez N, Zahaf T, et al. HPV-16/18 vaccine: sustained immunogenicity and efficacy up to 9.4 years (Conference abstract). Presented at: International Papillomavirus Conference; September 17-22, 2011; Berlin, Germany.
- 35.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 36.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 37.Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8:390–7. doi: 10.4161/hv.18865. [DOI] [PubMed] [Google Scholar]
- 38.Skinner SR, Szarewski A, Romanowski B, Garland S, Lazcano E, Salmerón J, et al. Efficacy, safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in women over 25 years: 4-year interim follow-up of the double-blind, randomised VIVIANE study. The Lancet. doi: 10.1016/S0140-6736(14)60920-X. 2014; doi: 10.1016/S0140-6736(14)60920-X [Epub ahead of Print] [DOI] [PubMed] [Google Scholar]
- 39.Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26:6630–8. doi: 10.1016/j.vaccine.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 40.European Medicines Agency. Cervarix human papillomavirus vaccine [types 16, 18] (recombinant, adjuvanted, adsorbed). Committee for Medicinal Products for Human Use (CHMP) 2011.
- 41.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;27:581–7. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 42.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 43.David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, et al. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol. 2009;115(3 Suppl):S1–6. doi: 10.1016/j.ygyno.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Schulze K, et al. Persistence of immune response 5 years after administration of HPV-16/18 AS04-adjuvanted vaccine to women aged 15–55 years. Asia-Oceania Research Organization in Genital Infection and Neoplasia – 2011 Interim Conference (AOGIN). March 17-19 2011, Bali, Indonesia.
- 45.Petäjä T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, et al. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129:2147–57. doi: 10.1002/ijc.25887. [DOI] [PubMed] [Google Scholar]
- 46.Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal. 2011;14:1551–85. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol. 2008;110(3 Suppl 1):S1–10. doi: 10.1016/j.ygyno.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 48.Viscidi RP, Schiffman M, Hildesheim A, Herrero R, Castle PE, Bratti MC, et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:324–7. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 49.Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–70. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–62. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz TF, Kocken M, Petaja T, Einstein MH, Spaczynski M, Louwers JA, et al. Correlation between levels of human papillomavirus (HPV)-16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine. Hum Vaccin. 2010;6:1054–61. doi: 10.4161/hv.6.12.13399. [DOI] [PubMed] [Google Scholar]
- 52.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(Suppl 3):106–13. doi: 10.1016/j.vaccine.2006.05.110. S3. [DOI] [PubMed] [Google Scholar]
- 53.Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12–15 year old girls. PLoS ONE. 2013;8:e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]