Abstract
Aims
We studied the characteristics and clinical outcome related to diuretic response and the effects of serelaxin in patients hospitalized for acute heart failure (AHF).
Methods and results
RELAX-AHF was a double-blind, placebo-controlled trial, enrolling 1161 patients admitted to hospital for AHF who were randomized to 48 h i.v infusions of placebo or serelaxin (30 µg/kg per day) within 16 h from presentation. Diuretic response was defined as Δ weight kg/[(total i.v. dose)/40 mg] + [(total oral dose)/80 mg)] furosemide (or equivalent loop diuretic dose) up to day 5. Median diuretic response was −0.42 (−1.00, −0.14) kg/40 mg. A poor diuretic response was independently associated with Western-like region (Western Europe, North America, Israel, and Poland), lower diastolic blood pressure, the absence of oedema, higher blood urea nitrogen, and lower levels of aspartate aminotransferase and potassium (all P < 0.01). Randomization to serelaxin was associated with lower doses of i.v. loop diuretics and slightly less weight loss, resulting in a neutral effect on diuretic response. Worse diuretic response was independently associated both with less relief of dyspnoea, measured with a visual analogue scale (VAS) at day 5 (primary endpoint; P = 0.0002), and with a higher risk of cardiovascular death or rehospitalization for heart failure or renal failure through day 60 (secondary endpoint, P < 0.0001), but not with increased 180-day cardiovascular mortality (P = 0.507).
Conclusions
In patients hospitalized for AHF, a poor diuretic response was associated with a poor in-hospital and early post-discharge clinical outcome. Serelaxin had a neutral effect on diuretic response.
Trial registration: NCT00520806
Keywords: Heart failure, Renal function, Diuretic response
Introduction
Acute heart failure (AHF) is a life-threatening condition needing immediate medical attention and often requiring urgent hospital admission.1,2 Hospitalization for AHF is associated with a high risk of both in-hospital and post-discharge mortality and hospital readmission for heart failure.3–7 Several studies tried to identify patients at risk, but they could only moderately predict mortality and poorly predict readmission.8–11 In addition, most of the factors predicting outcome in these models are non-modifiable, such as age.
Treatment options in AHF are often limited to treatment with i.v. loop diuretics. However, not every patient responds well to their diuretic treatment, although our understanding of diuretic resistance is limited. Therefore, there is a need to better characterize and understand factors related to a poor diuretic response. Recently, we and others showed that a poor diuretic response, defined as weight loss per unit loop diuretic during the first 5 days after hospital admission, was strongly associated with in-hospital worsening heart failure, and predicted mortality and heart failure rehospitalization.12,13 In addition, rolofylline, an adenosine A1 antagonist, was shown to improve diuretic response, although the neutral findings of PROTECT halted the development of rolofylline. Serelaxin is a novel drug that holds promise for the treatment of patients with AHF.14,15 However, the effects of serelaxin on diuretic response have so far not been studied. In the present study, we aimed to describe patient characteristics, incidence of worsening renal function, and outcomes related to diuretic response in AHF patients. In addition, we studied the effects of serelaxin on diuretic response, both at the end of infusion of serelaxin at 48 h and at 5 days after hospital admission.
Methods
Study design
RELAX-AHF was a phase III randomized, double-blind, placebo-controlled, parallel-group, international trial comparing the i.v. administration of serelaxin for up to 48 h, started within 16 h of presentation, with placebo in patients hospitalized for AHF with dyspnoea, congestion on chest radiography, increased natriuretic peptide levels, mild to moderate renal insufficiency, and systolic blood pressure >125 mmHg. Patients were enrolled in the USA, Israel, Western Europe (France, Germany, Italy, The Netherlands, and Spain), Poland, Hungary, Romania, and Argentina. The background, design, and main results have been published elsewhere.14,15 This trial is registered at http://ClinicalTrials.gov (NCT00520806), complies with the Declaration of Helsinki, and a locally appointed ethics committee or institutional review board has approved the research protocol with written informed consent obtained from each patient.
Procedures
In the RELAX-AHF phase III study, blood samples, weight, and diuretic dose were collected in all patients at baseline and at 24 h (day 1); 48 h (day 2); on days 3 and 4, if in hospital; and on days 5, 14, and 60 for standard haematology and chemistry at a central laboratory using commercially available, validated assays. Samples for biomarkers including troponin T and NT-proBNP were collected at baseline and days 2, 5, and 14, and were analysed centrally. The endpoints of interest were (i) change in patient-reported dyspnoea as quantified by the area under the curve (AUC) of the change from baseline in visual analogue scale (VAS) scores (0–100 mm scale) from baseline to day 5 (a primary endpoint of RELAX-AHF); (ii) cardiovascular death or readmission to hospital for heart failure or renal failure through day 60 (a secondary endpoint of RELAX-AHF); and (iii) cardiovascular death through day 180. As specified in the analysis plan for the main study, the dyspnoea VAS score was imputed as the worst observed score following a death or worsening heart failure event when calculating the AUC of the change from baseline. All deaths and rehospitalizations were adjudicated by the clinical events committee.
Definitions
Diuretic response was defined as change in weight (kg) from baseline to day 5/[(total i.v. dose)/40 mg] + [(total oral dose)/80 mg] furosemide up to day 5. The last available weight was substituted for a missing day 5 weight. Additional comparative analyses were performed on diuretic response from baseline to 48 h. Loop diuretic doses considered equivalent to 40 mg of furosemide were 2 mg of bumetanide, 20 mg of torasemide, and 50 mg of ethacrynic acid. Worsening renal function was defined as an increase in serum creatinine of ≥0.3 mg/dL to day 5 (or day 4, if day 5 was missing)).
Statistical analyses
Mean and standard deviation, or geometric mean and 95% confidence interval, are presented for continuous variables, and absolute and relative frequencies for categorical variables. Because its distribution was highly skewed, and associations with outcomes non-linear, diuretic response was grouped by tertiles for analysis. Baseline characteristics were compared among diuretic response tertiles using the Jonckheere–Terpstra (J-T) trend test. Patients with missing values of diuretic response were deleted from the analyses in which diuretic response appears as an outcome variable. For analyses of clinical endpoints in which diuretic response appears as an explanatory factor, missing values of diuretic response were imputed so as to include those patients, thus preserving comparability with other such analyses. Sample sizes in the tables reflect these distinctions.
A multivariable cumulative logit model predicting diuretic response was developed using backwards elimination in the full study population, with P < 0.10 as the criterion for retention in the model. The treatment group-specific median or mode was imputed for missing continuous and categorical variables, respectively. A list of potential covariates was reduced by choosing one representative in certain groups of related covariates to represent the effect, e.g. alanine aminotransferase (ALT) or aspartate aminotransferase (AST), or haemoglobin or haematocrit, based on the lowest Akaike information criterion (AIC) in univariable analyses. The linearity of the association between the log cumulative odds and selected continuous candidate predictors was then assessed using restricted cubic splines, and, if significant non-linearity was found (at P < 0.10), we used the dichotomy or quadratic or cubic polynomial which had the lowest AIC. The interaction of each effect with serelaxin was then tested and, where significant at P < 0.10, the interaction was added to the effects to consider in the backwards elimination.
The associations of diuretic response with clinical outcomes (dyspnoea VAS AUC to day 5, cardiovascular death, or heart failure/renal failure rehospitalization through day 60, and cardiovascular mortality through day 180) were adjusted for covariates found to be associated with these outcomes in the placebo group only using multivariable linear regression and Cox regression models. Wald test χ2 statistics and P-values are reported. SAS release 9.3 (SAS Institute, Cary, NC, USA) was used for all analyses.
Role of the funding source
The study was designed by the members of the executive committee, which included two Corthera clinical scientists, and was part of a phase II/III trial design. Data collection and analysis were performed by contract research organizations. The study database was held both by the Sponsor and by Columbia University (New York, USA). The authors had access to tables and listings supplied by the sponsor and Columbia but did not have independent access to the study databases. The executive committee had full access to the final tables and figures. The authors not employed by the sponsor had ultimate editorial authority, with no interference by the sponsor in their final interpretation.
Results
The main results of RELAX-AHF have been published elsewhere.14,15 During the first 5 days of hospital admission, the median weight change was −2.2 kg, median total i.v. dose of furosemide was 80 mg, and median total oral dose of furosemide was 160 mg. The majority of patients received furosemide (92.2%); torsemide was administered to 9.0%, bumetanide to 1.3%, and ethacrynic acid to 0.95%. (A given patient may have received more than one type of loop diuretic.) Of the 1161 patients randomized, 7 died by day 5 and were excluded from the analyses. Fifty-seven patients were missing data such that the diuretic response could not be derived, and 70 patients were missing data on worsening renal failure. Daily weights and loop diuretic doses are provided in the Supplementary material online Figures S1 and S2.
Predictors of diuretic response
The median diuretic response was −0.42 kg/40 mg [interquartile range (IQR) –1.00, −0.14 kg/40 mg]. There were marked differences across the tertiles of diuretic response, as presented in Table 1. Poor responders (with higher diuretic response values) were more frequently found in Western-like countries (Western Europe, North America, Poland, and Israel). Also, poor diuretic responders were more likely to have ischaemic heart failure, a higher NYHA class 1 month prior to enrolment, lower systolic and diastolic blood pressures, fewer signs of congestion, and a poorer baseline renal function, and were more often on oral loop diuretics 1 month prior to enrolment. Other characteristics related to diuretic response are given in Table 1.
Table 1.
Baseline characteristics according to tertiles of diuretic response (defined as change in weight/[(total i.v. dose)/40 mg] + [(total oral dose)/80 mg)] furosemide up to day 5)
| ≤ −0.75 kg/40 mg (n = 365) | ≥ −0.75 to ≤ −0.22 kg/40 mg (n = 366) | > −0.22 kg/40 mg (n = 366) | P-valuea | |
|---|---|---|---|---|
| Demographics and heart failure characteristics | ||||
| Age (years) | 71.5 (11.5) | 72.7 (10.4) | 72.2 (11.6) | 0.4997 |
| Male | 220 (60.3) | 228 (62.3) | 237 (64.8) | 0.2112 |
| White/Caucasian | 353 (96.7) | 348 (95.1) | 343 (93.7) | 0.0590 |
| Geographic region | <0.0001 | |||
| Eastern EU | 227 (62.2) | 162 (44.3) | 142 (38.8) | |
| Western EU | 58 (15.9) | 57 (15.6) | 80 (21.9) | |
| South America | 27 (7.4) | 28 (7.7) | 14 (3.8) | |
| North America | 25 (6.8) | 35 (9.6) | 41 (11.2) | |
| Israel | 28 (7.7) | 84 (23.0) | 89 (24.3) | |
| LVEF | 39.2 (13.9) | 38.6 (14.8) | 37.9 (14.9) | 0.0906 |
| Ischaemic heart disease | 155 (42.5) | 215 (58.7) | 207 (56.6) | 0.0001 |
| Time from presentation to randomization (h) | 7.9 (4.5) | 7.7 (4.6) | 8.0 (4.8) | 0.9190 |
| CHF 1 month prior | 242 (66.3) | 293 (80.1) | 292 (79.8) | <0.0001 |
| NYHA class (I/II/III/IV) 30 days before admission | <0.0001 | |||
| I | 126 (34.6) | 82 (22.6) | 83 (23.1) | |
| II | 108 (29.7) | 112 (30.9) | 73 (20.3) | |
| III | 105 (28.8) | 117 (32.2) | 150 (41.7) | |
| IV | 25 (6.9) | 52 (14.3) | 54 (15.0) | |
| Clinical signs | ||||
| Body mass index, kg/m2 | 29.8 (6.0) | 29.4 (5.6) | 28.9 (5.4) | 0.1033 |
| Systolic blood pressure, mmHg | 145.0 (16.5) | 140.4 (15.3) | 140.7 (16.9) | <0.0001 |
| Diastolic blood pressure, mmHg | 81.9 (14.4) | 78.2 (13.7) | 76.4 (13.6) | <0.0001 |
| Heart rate, b.p.m. | 81.7 (15.4) | 78.5 (14.6) | 78.1 (14.6) | 0.0008 |
| Respiratory rate, breaths/min | 21.8 (4.4) | 21.8 (4.6) | 22.1 (5.0) | 0.8207 |
| HF hospitalization past year | 96 (26.3) | 131 (35.8) | 154 (42.1) | <0.0001 |
| Congestion at baseline | ||||
| Oedema | 305 (83.6) | 306 (83.8) | 265 (72.4) | <0.0001 |
| Orthopnoea | 350 (96.2) | 352 (96.2) | 350 (95.6) | 0.3781 |
| JVP | 283 (80.4) | 275 (76.8) | 252 (70.2) | 0.0031 |
| Dyspnoea on exertion | 361 (99.7) | 359 (99.7) | 359 (99.4) | 0.0907 |
| Dyspnoea by VAS | 44.6 (20.1) | 43.9 (20.8) | 43.5 (19.6) | 0.1714 |
| Rales | 138 (37.8) | 141 (38.5) | 156 (42.6) | 0.4716 |
| Co-morbidities | ||||
| Hypertension | 312 (85.5) | 323 (88.3) | 312 (85.2) | 0.9256 |
| Hyperlipidaemia | 158 (43.3) | 216 (59.0) | 209 (57.1) | 0.0002 |
| Diabetes mellitus | 151 (41.4) | 189 (51.6) | 183 (50.0) | 0.0197 |
| Cigarette smoking | 45 (12.3) | 45 (12.3) | 46 (12.6) | 0.9217 |
| Stroke or other cerebrovascular event | 44 (12.1) | 49 (13.4) | 58 (15.8) | 0.1369 |
| Peripheral vascular disease | 46 (12.6) | 53 (14.5) | 43 (11.7) | 0.7300 |
| Asthma, bronchitis, or COPD | 46 (12.6) | 54 (14.8) | 72 (19.7) | 0.0086 |
| AF at screening | 176 (48.2) | 147 (40.2) | 133 (36.5) | 0.0014 |
| History of AF or atrial flutter | 207 (56.7) | 187 (51.1) | 183 (50.0) | 0.0694 |
| History of CRT or ICD procedures | 63 (17.3) | 106 (29.0) | 113 (30.9) | <0.0001 |
| Myocardial infarction | 100 (27.4) | 136 (37.2) | 147 (40.2) | 0.0003 |
| Depression | 16 (4.4) | 16 (4.4) | 24 (6.6) | 0.1817 |
| Devices | ||||
| Pacemaker | 33 (9.0) | 41 (11.2) | 44 (12.0) | 0.1937 |
| ICD | 28 (7.7) | 56 (15.3) | 63 (17.2) | 0.0002 |
| Biventricular pacing | 17 (4.7) | 43 (11.7) | 46 (12.6) | 0.0003 |
| Medication (day 0, except nitrates and loop diuretics) | ||||
| ACE inhibitor | 202 (55.3) | 214 (58.5) | 187 (51.1) | 0.2476 |
| ACE inhitor or ARB | 245 (67.1) | 275 (75.1) | 234 (63.9) | 0.3507 |
| ARB | 56 (15.3) | 66 (18.0) | 58 (15.8) | 0.8549 |
| Beta-blocker | 227 (62.2) | 267 (73.0) | 263 (71.9) | 0.0048 |
| Aldosterone antagonist | 130 (35.6) | 116 (31.7) | 104 (28.4) | 0.0368 |
| I.v. loop diuretics | 365 (100.0) | 366 (100.0) | 366 (100.0) | NA |
| Digoxin | 85 (23.3) | 53 (14.5) | 74 (20.2) | 0.2952 |
| Nitrates at randomization | 35 (9.6) | 16 (4.4) | 26 (7.1) | 0.1897 |
| Oral loop diuretics 30 days prior to randomization | 25 (31.5) | 47 (52.1) | 67 (93.0) | <0.0001 |
| Baseline labs | ||||
| Sodium, mmol/L | 141.1 (3.6) | 141.0 (3.3) | 140.4 (3.8) | 0.0137 |
| Phosphate, mmol/L | 1.18 (0.45) | 1.19 (0.24) | 1.19 (0.24) | 0.4206 |
| Calcium, mmol/L | 2.27 (0.153) | 2.26 (0.159) | 2.27 (0.150) | 0.6419 |
| Haemoglobin, g/dL | 13.01 (1.79) | 12.62 (1.92) | 12.71 (1.90) | 0.0244 |
| White blood cell count, ×109/L | 8.168 (2.697) | 7.907 (2.654) | 8.222 (2.927) | 0.8668 |
| Lymphocytes, % | 18.39 (7.98) | 18.71 (8.19) | 17.41 (7.08) | 0.2053 |
| Potassium, mmol/L | 4.32 (0.66) | 4.26 (0.62) | 4.25 (0.63) | 0.2173 |
| Creatinine, µmol/L | 111.6 (32.2) | 116.6 (32.0) | 122.4 (33.9) | <0.0001 |
| Uric acid, µmol/L | 461.8 (135.9) | 475.5 (125.6) | 495.4 (144.8) | 0.0018 |
| Troponin T, µg/L | 0.035 (0.032, 0.038) | 0.034 (0.032, 0.038) | 0.036 (0.033, 0.039) | 0.2982 |
| BUN, mmol/L | 8.96 (3.36) | 9.78 (3.73) | 10.69 (4.54) | <0.0001 |
| Cystatin C, mg/L | 1.38(1.34, 1.42) | 1.47 (1.42, 1.51) | 1.54 (1.49, 1.58) | <0.0001 |
| Alanine aminotransferase, U/L | 25.3 (23.7, 27.1) | 21.6 (20.3, 22.9) | 23.1 (21.7, 24.6) | 0.0349 |
| NT-proBNP, ng/L | 5218 (4760, 5720) | 4861 (4417, 5349) | 5107 (4659, 5599) | 0.2983 |
| NT-proBNP, ng/L in patients with AF present at screening | 5566 (4979, 6223) | 5203 (4546, 5955) | 5086 (4455, 5806) | 0.1929 |
| NT-proBNP ng/L in patients with AF not present at screening | 4909 (4247, 5675) | 4645 (4070, 5302) | 5133 (4531, 5814) | 0.9200 |
| eGFR, mL/min/1.73 m2 | 55.36 (12.45) | 52.91 (13.32) | 51.52 (12.66) | <0.0001 |
| Total cholesterol, mmol/L | 4.20 (1.17) | 3.98 (1.17) | 4.06 (1.13) | 0.0624 |
| Glucose, mmol/L | 7.34 (3.51) | 7.91 (3.57) | 8.04 (3.71) | 0.0003 |
| Albumin, g/L | 39.99 (4.31) | 40.04 (4.72) | 40.58 (3.87) | 0.1819 |
BUN, blood urea nitrogen; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; JVP, jugular venous pressure; NA, not applicable; VAS, visual analogue scale.
P-values are for the Jonckheere–Terpstra trend test, except for geographic region for which the Kruskal–Wallis test P-value is presented.
Independent predictors of diuretic response are presented in Table 2. The most significant predictors of a poor diuretic response were Western-like region (Western Europe, North America, Israel, and Poland), lower diastolic blood pressure, the absence of oedema, higher blood urea nitrogen (BUN), and lower levels of AST and potassium (all P < 0.01). Independent predictors of diuretic response at 48 h are presented in Supplementary material online Table S1, and were largely similar to the predictors of diuretic response at day 5.
Table 2.
Multivariable predictors of poor diuretic responsea
| Covariate | OR | Lower 95% CI | Upper 95% CI | Wald χ2 | P-value |
|---|---|---|---|---|---|
| Region, Western-like | 2.514 | 1.889 | 3.347 | 39.90 | <0.0001 |
| Weight (kg) | 0.993 | 0.986 | 1.000 | 3.46 | 0.0630 |
| Diastolic blood pressure (mmHg) | 0.988 | 0.979 | 0.997 | 7.30 | 0.0069 |
| Body temperature (°C) | 0.654 | 0.471 | 0.910 | 6.35 | 0.0117 |
| Respiratory rate (breaths/min)b | 6.88 | 0.0321 | |||
| Median (21) vs. 25th percentile (19) | 0.941 | 0.878 | 1.008 | ||
| 75th percentile (24) vs. median (21) | 0.976 | 0.902 | 1.057 | ||
| HF hospitalization past year | 1.364 | 1.056 | 1.763 | 5.63 | 0.0176 |
| NYHA class 30 days prior | 6.80 | 0.0779 | |||
| NYHA II vs. I | 0.768 | 0.551 | 1.069 | ||
| NYHA III vs. I | 1.135 | 0.807 | 1.594 | ||
| NYHA IV vs. I | 1.107 | 0.711 | 1.722 | ||
| Oedema | 23.99 | <0.0001 | |||
| Oedema, 1 vs. 0 | 0.689 | 0.489 | 0.970 | ||
| Oedema, 2 vs. 0 | 0.531 | 0.374 | 0.753 | ||
| Oedema, 3 vs. 0 | 0.385 | 0.258 | 0.575 | ||
| Dyspnoea VAS (mm)c | 6.72 | 0.0814 | |||
| Median (45) vs. 25th percentile (30) | 0.867 | 0.751 | 1.001 | ||
| 75th percentile (57) vs. median (45) | 0.852 | 0.747 | 0.971 | ||
| Percutaneous intervention | 1.405 | 1.069 | 1.848 | 5.93 | 0.0149 |
| Peripheral vascular disease | 0.693 | 0.489 | 0.983 | 4.23 | 0.0397 |
| Hyperthyroid | 2.191 | 1.077 | 4.458 | 4.69 | 0.0304 |
| Atrial fibrillation/flutter at screening | 0.733 | 0.578 | 0.929 | 6.59 | 0.0103 |
| BUN (mmol/L) | 1.089 | 1.052 | 1.127 | 23.37 | <.0001 |
| Uric acid (µmol/L) | 1.001 | 1.000 | 1.002 | 3.75 | 0.0529 |
| Aspartate aminotransferase, log(U/L) | 0.686 | 0.527 | 0.893 | 7.86 | 0.0050 |
| Sodium (mmol/L) | 0.960 | 0.927 | 0.993 | 5.53 | 0.0187 |
| Potassium (mmol/L) | 0.706 | 0.577 | 0.864 | 11.36 | 0.0007 |
| Total protein (g/L) | 1.022 | 1.003 | 1.042 | 4.92 | 0.0265 |
| NT-proBNP, > median vs. ≤ median | 0.735 | 0.571 | 0.945 | 5.76 | 0.0164 |
| LVEF by serelaxin interaction | 4.26 | 0.0390 | |||
| LVEF (%), placebo | 0.991 | 0.979 | 1.003 | 0.1472 | |
| LVEF (%), serelaxin | 1.008 | 0.997 | 1.020 | 0.1634 |
Odds ratios (are for a one unit increase in the covariate unless otherwise specified.
BUN, blood urea nitrogen; CI, confidence interval; HF, heart failure; OR, odds ratio; VAS visual analogue scale.
Cumulative logit model of diuretic response tertile.
Non-linear association modelled as a quadratic polynomial.
Non-linear association modelled as a cubic polynomial.
Effects of serelaxin on diuretic response
The median (25th, 75th percentile) total i.v. loop diuretic dose before day 5 was 100 (20, 240) mg in the placebo group and 80 (0, 200) mg in the serelaxin group; median (25th, 75th percentile) total dose of oral loop diuretic dose through day 5 was 137.5 (40, 270) and 160 (80, 240), respectively. As defined for this analysis, median change in body weight at day 5 was −2.4 kg (IQR −4.8, −1.0) in the placebo group vs. –2.0 (IQR −4.0, −0.8) kg in the serelaxin group (P = 0.133). On univariable analysis, randomization to serelaxin was not associated with diuretic response: median of −0.42 (IQR −0.94, −0.15) kg/40 mg in the placebo group vs. –0.42 (IQR −1.09, −0.13) kg/40 mg in the serelaxin group (P = 0.644). Although a statistically significant serelaxin by EF interaction effect was included in the multivariable model, the slight worsening of diuretic response in the serelaxin group, and slight improvement in diuretic response in the placebo group, with increasing LVEF were small and possibly due to chance. In addition, we performed similar analyses on the effects of serelaxin on diuretic response at 48 h. As can be seen from Table S2 of the Supplementary material online, there was no significant difference in diuretic response at day 2 between treatment groups.
Worsening renal function and diuretic response
Worsening renal function, defined as an increase in serum creatinine of ≥0.3 mg/dL to day 5 (or day 4, if day 5 was missing), occurred in 280 of the 1084 patients (26%) with available data. Table 3 shows that patients with both worsening renal function and poor diuretic response (less than the median response) had the worst 60- and 180-day outcomes, but the risks were not more or less than the combined individual effects would predict (interaction P-values 0.72 and 0.53 for 60- and 180-day outcomes, respectively).
Table 3.
Clinical outcome according to worsening renal function and diuretic response
| Clinical endpoints | No WRF, good diuretic response (n = 389) | No WRF, poor diuretic response (n = 385) | WRF, good diuretic response (n = 136) | WRF, poor diuretic response (n = 138) | Interaction P-value, unadjusted | Interaction P-value, adjusteda |
|---|---|---|---|---|---|---|
| Dyspnoea improvement by VAS AUC to day 5, mean (SD) | 2788.1 (2600.4) | 2356.2 (2735.0) | 2889.2 (3187.7) | 2348.5 (3309.3) | 0.9988 | 0.2024 |
| Cardiovascular death or rehospitalization for HF or renal failure through day 60, n (Kaplan–Meier %) | 29 (7.49) | 66 (17.33) | 13 (9.56) | 26 (19.05) | 0.7362 | 0.7206 |
| Cardiovascular mortality through day 180, n (Kaplan–Meier %) | 23 (5.97) | 26 (6.86) | 10 (7.36) | 16 (11.79) | 0.7238 | 0.5262 |
A good diuretic response is defined as a response greater than the median.
AUC, area under the curve; HF, heart failure; SD, standard deviation; VAS, visual analogue scale; WRF, worsening renal function.
Each outcome is adjusted for the covariates given in the footnotes to Table 4.
Diuretic response and clinical outcomes
In univariable analyses, diuretic response was significantly associated with the dyspnoea VAS AUC to day 5 (P = 0.0015; Figure 1) and cardiovascular death or heart failure/renal failure rehospitalization through day 60 (P < 0.0001; Figure 2) but not with cardiovascular mortality through day 180 (P = 0.13; Table 4). After multivariable adjustment for baseline characteristics, worse diuretic response remained independently associated with a poorer dyspnoea relief to day 5 (P = 0.0002; Table 4) and with 60-day cardiovascular death or heart failure/renal failure rehospitalization (P < 0.0001). These associations were independent of worsening renal function (Figure 3). Further adjustment for changes to day 2 in BUN, creatinine, sodium, total protein, and log NT-proBNP did not change these associations significantly. However, diuretic response was not independently associated with mortality through day 180 (P = 0.507).
Figure 1.
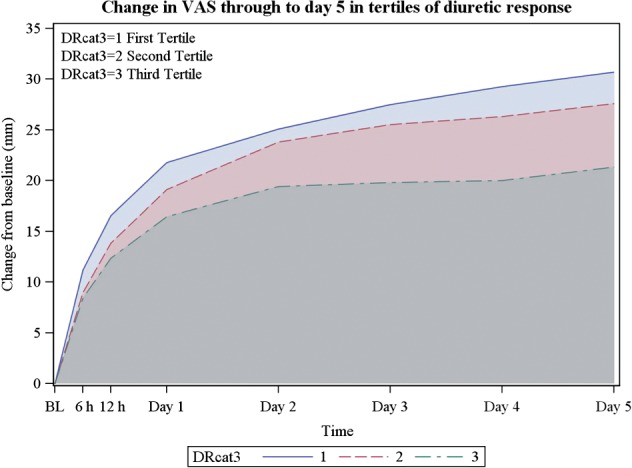
Changes in visual analogue scale through to day 5 in tertiles of diuretic response (DR).
Figure 2.
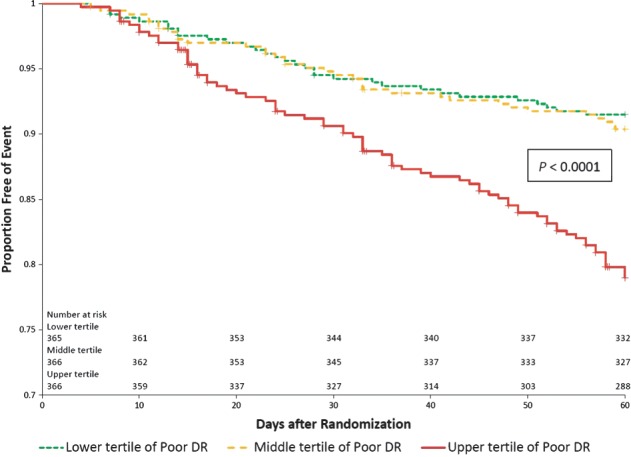
Kaplan–Meier survival curves presenting death or HF/RF readmission through day 60 according to tertiles of diuretic response. P < 0.0001 for log-rank test comparing diuretic response tertiles.
Table 4.
Associations of diuretic response with selected outcomes
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Outcome | Contrast | Measure of association (95% CI) | P-value | Measure of association (95% CI) | P-value |
| Dyspnoea VAS AUC to day 5a | 0.0002 | 0.0001 | |||
| T2 vs. T1 | −355.944 (−751.753, 39.866) | −319.370 (−645.481, 6.740) | |||
| T3 vs. T1 | −855.674 (−1265.072, −446.276) | −749.944 (−1096.009, −403.879) | |||
| CV death or HF/RF rehospitalization through day 60b | <0.0001 | 0.0005 | |||
| T2 vs. T1 | 1.122 (0.702, 1.794) | 0.919 (0.566, 1.494) | . | ||
| T3 vs. T1 | 2.735 (1.806, 4.142) | 1.855 (1.195, 2.879) | . | ||
| CV death through day 180c | 0.1323 | 0.5069 | |||
| T2 vs. T1 | 0.865 (0.502, 1.490) | 0.948 (0.533, 1.689) | . | ||
| T3 vs. T1 | 1.417 (0.856, 2.347) | 1.273 (0.722, 2.244) | . | ||
T1 = first tertile (≤ −0.75 kg/40 mg), T2 = second tertile (> − 0.75 to ≤ −0.22 kg/40 mg), T3 = third tertile (> −0.22 kg/40 mg).
AUC, area under the curve; CI, confidence interval; CV, cardiovascular; HF, heart failure; RF, renal failure; VAS, visual analogue scale.
Measure of association is mean change from linear regression model. Adjusted for age, weight, hypertension, mitral regurgitation, history of atrial fibrillation or flutter, USA-like region, dyspnoea on exertion, body temperature, troponin T, baseline dyspnoea VAS score, uric acid, alkaline phosphatase, and sodium.
Measure of association is hazard ratio from Cox regression model. Adjusted for white race; NYHA class 30 days prior; systolic blood pressure; respiratory rate; number of HF hospitalizations in past year; orthopnoea; asthma, bronchitis, or COPD; hyperthyroid; lymphocyte %; blood urea nitrogen; phosphate; sodium; and total protein.
Measure of association is hazard ratio from Cox regression model. Adjusted for USA-like region, systolic blood pressure, orthopnoea, angina, hyperthyroid, mitral regurgitation, atrial fibrillation or flutter at screening, white blood cell count, lymphocyte %, blood urea nitrogen, sodium, potassium, calcium, total protein, troponin T, and NT-proBNP.
Figure 3.
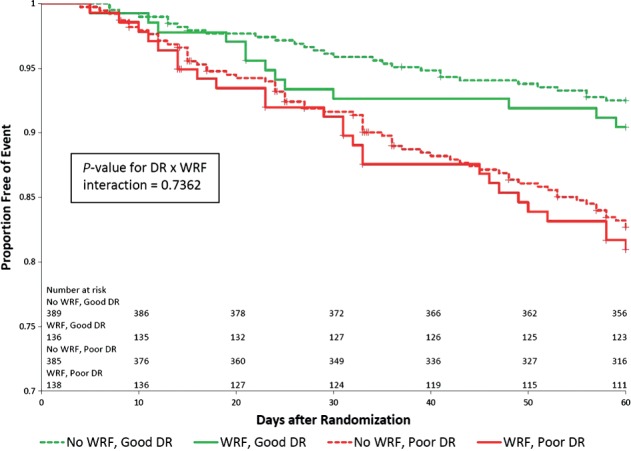
Kaplan–Meier survival curves presenting death or HF/RF readmission through day 60 in 1) patients with WRF and a poor diuretic response (<median); 2) patients with WRF and a good diuretic response (>median); 3) patients without WRF and a poor diuretic response (<median); 4) patients without WRF and a good diuretic response (>median). P = 0.7362 for Wald chi-square test of diuretic response- by-WRF interaction.
Discussion
The main finding of the present study is that a poor diuretic response is a strong and independent predictor of poorer dyspnoea relief and an increased risk of cardiovascular death or heart failure/renal failure hospital readmission through day 60 in patients admitted for AHF. Serelaxin treatment was associated with less diuretic use, but a neutral effect on diuretic response.
Recently, Valente et al. and Testani et al. presented a simple metric for diuretic responsiveness in patients admitted for AHF.12,13 The metric of weight loss/unit loop diuretic had additive prognostic value on top of weight loss or dose of loop diuretic alone. In both studies, diuretic response was related to clinical outcome. The present study confirms these findings, but adds information on the predictors of diuretic response and the absence of an effect of serelaxin on diuretic response. We additionally showed that predictors of diuretic response and the effects of serelaxin were similar between 48 h and day 5.
Association between poor diuretic response and poor clinical outcome
The relationship between diuretic response and early outcomes has many potential causes. First, higher doses of loop diuretic might have adversely influenced clinical outcome. However, the DOSE-AHF (Diuretic Optimization Strategies Evaluation in Acute Heart Failure) trial, which compared a higher vs. a lower dose of diuretics in AHF patients, found that the higher dose was associated with greater diuresis and transient worsening of renal function, but did not significantly affect patients' global assessment of symptoms.16 Secondly, patients with a poorer diuretic response might simply reflect sicker patients, and are therefore likely to have a poorer outcome. However, levels of NT-proBNP at admission were remarkably similar, and the predictive value of diuretic response remained remarkably consistent after adjusting for potential confounders. Thirdly, diuretic response might be a deleterious condition by itself, causing a poor clinical outcome. It is conceivable that a poor diuretic response results in less relief of dyspnoea, as was shown in the present study. Metra et al. previously demonstrated that less dyspnoea relief during hospitalization is independently related to a poorer clinical outcome.17 Also, the association between diuretic response and clinical outcome seems to be mainly driven by early discharge rehospitalization, and less by mortality. These findings support that a poor diuretic response might cause less relief of dyspnoea and congestion, potentially leading to a greater risk of rehospitalization.
This is of importance for two reasons. First, we are better able to predict those patients at higher risk for rehospitalization, which is a major problem in patients who are admitted for AHF. Secondly, and more importantly, strategies that improve diuretic response might lead to improved clinical outcome and fewer rehospitalizations in patients with AHF. However, potential strategies to improve diuretic response remain to be established. First, addition of thiazides/chlorothalidon and/or azetazolamide might improve diuretic response, although this should be proven by prospective randomized controlled trials. Ultrafiltration might be another option to improve diuretic response in diuretic-resistant patients. In UNLOAD, in AHF patients with signs of volume overload, ultrafiltration resulted in greater weight and fluid loss than i.v. diuretics, but had no effect on mortality.18 These beneficial effects could not be confirmed in a recent randomized trial, where loop diuretics were superior to ultrafiltration for the preservation of renal function, with a similar amount of weight loss.19 However, a good diuresis was observed in both arms, indicating that these patients were not diuretic resistant, although the results might be different in a population of patients selected based on a poor diuretic response.
Mechanisms behind a poor diuretic response
What are the potential mechanisms behind the diuretic response? Obviously, patients with a poor diuretic response have a poorer renal function. However, similar to the studies by Valente et al. and Testani et al., renal dysfunction explains only a part of poor diuretic response.12,13 There are a number of explanations for this. First, Valente et al. demonstrated that diuretic-resistant patients more often had heart failure of ischaemic origin and signs of atherosclerosis.12 A similar picture emerges in the present study. Patients with a poor diuretic response more often had ischaemic heart failure, a previous myocardial infarction, dyslipidaemia, and diabetes. A poorer diuretic response in atherosclerotic patients might be caused by atherosclerotic kidneys that are less likely to respond to diuretics. Alternatively, the presence of renal artery stenosis may be prevalent in patients with ischaemic heart failure20 and might also explain a poorer diuretic response, although we do not have any direct evidence for this. Secondly, patients with a poor diuretic response in the present study also had fewer signs of congestion. These patients might have AHF due to fluid redistribution, rather than fluid accumulation, and therefore they will not respond to diuretics. Loop diuretics might not be the best treatment option in these patients as they are not volume overloaded, and they might even be deleterious, causing relative dehydration and worsening renal function. So, it is reasonable to suggest that diuretic response is better in more congested patients with more peripheral oedema, but it should be noted that the poorer prognosis in diuretic-unresponsive patients was independent of the baseline level of congestion. Finally, we showed that worsening renal function (using the most commonly used definition of an increase in serum creatinine of ≥0.3 mg/dL to day 5) was not a predictor of clinical outcome. In addition, baseline BUN was an important predictor of diuretic response. This supports the concept that diuretic response is not primarily driven by glomerular filtration, but more by tubular function.21 Therefore, markers of tubular function and/or damage might better predict diuretic response than markers of glomerular function. Unfortunately, we did not collect urine in RELAX-AHF, and do not have data on plasma markers of tubular damage.
Effects of serelaxin on diuretic response
Despite a significant reduction in dyspnoea and lower 180-day mortality, serelaxin did not influence diuretic response in these patients. However, patients treated with serelaxin required lower doses of diuretics and had slightly less weight loss, which may have balanced the effects on diuretic response. Also, serelaxin was related to less deterioration of renal function. Therefore, the beneficial effects of serelaxin cannot be explained by improved diuretic response, and a potential explanation might be related to prevention of organ damage, as has been postulated previously.22
Limitations
The present study has obvious limitations that are related to the retrospective nature of the findings. Therefore, the suggestion that a poor diuretic response might cause a poor clinical outcome cannot be proven. Also, this study was not designed to examine the consequences of diuretic response, and therefore there are important data lacking, such as urinary collections and markers of tubular function/damage. Furthermore, it is well known that weight recordings are notoriously unreliable. In addition, diuretic doses might not always be properly recorded. Nevertheless, despite these limitation, the simple metric of weight loss divided by diuretic dose is clearly related to in-hospital and post-discharge outcome in patients admitted for AHF.
Conclusion and clinical implications
In a large cohort of patients admitted for AHF, a poor diuretic response was related to less improvement in dyspnoea through the first 5 days, and an increased risk of cardiovascular death or heart failure/renal failure rehospitalization through 60 days. Future studies should aim at predicting diuretic response in patients admitted with acute decompensated heart failure and should lead to prospective intervention studies, aiming to improve diuretic response, potentially leading to further improvement in dyspnoea, less early mortality, and fewer heart failure rehospitalizations.
Funding
The Relaxin in Acute Heart Failure study is supported by Corthera, Inc. (a Novartis AG affiliate company).
Conflicts of interest: G.M.F. has received consulting income from Novartis, Medpace, Amgen, Otsuka, Trevena, Roche Diagnostics, Merck, BG Medicine, Medtronic, and St Jude, and grant funding from Amgen, Otsuka, Roche Diagnostics, and the NHLBI. G.F. is a consultant to Corthera, Bayer, and Cardiorentis, and has received research grants from Amgen, Nanosphere, and the European Union. B.H.G. served as a consultant for Corthera, Novartis, Celladon, Zensun, Teva, Janssen, and Mast, and receives honoraria from Novartis for this trial for the advisory panel and executive committee. M.M. has received consulting income from Abbott Vascular, Bayer, Corthera, and Novartis, and travel support and honoraria from Servier and Novartis. P.P. was a consultant for Corthera, Bayer, J&J, Novartis, Cardiorentis and Trevena, and has received honoraria from Bayer, Novartis, and Cardiorentis. J.R.T. has received research grants or consulting fees from Amgen, Bayer, Corthera, Cardio3 Bioscience, Cytokinetics, Merck, Novartis, Takeda, Teva, and Trevena. T.A.H. and T.S. are employees and shareholders of Novartis Pharmaceuticals Corporation. B.A.D., G.C. are employees of Momentum Research, which has provided consulting and trial management services to NovaCardia, Merck, Corthera, Novartis, Nile Therapeutics, Bioheart, Cardio3 Biosciences, Amgen, Celadon, Targegen, Trevena, Sorbent Therapeutics, and the NIH. P.S.P. has been a consultant and/or received honoraria or research support within the past 12 months from Janssen, Novartis, Medtronic, scPharmaceuticals, BG Medicine, Trevena, Cornerstone Therapeutics, Abbott, Alere, Medscape, and the NIH. A.A.V. has received consultancy fees and/or research grants from Alere, Bayer, Cardio3Biosciences, Celladon, Corthera, European Commission, Dutch Heart Foundation, Medscape, MSD/Merck, Novartis, Pfizer, Servier, Torrent, Trevena, and Vifor. B.L. receives partial salary support from Novartis for statistical analysis of the data for this trial.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Weight by treatment group and day.
Figure S2. Loop diuretic dose by treatment group and day.
Table S1. Multivariable predictors of poor diuretic response at 48 h.
Table S2. The effects of serelaxin on diuretic response at 48 h.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, ADHERE Scientific Advisory Committee and Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Alla F, Zannad F, Filippatos G. Epidemiology of acute heart failure syndromes. Heart Fail Rev. 2007;12:91–95. doi: 10.1007/s10741-007-9009-2. [DOI] [PubMed] [Google Scholar]
- 5.Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of ESC (HFA) EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2010;12:1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 6.Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of the European Society of Cardiology (HFA) EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2013;15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 7.Lee DS, Mamdani MM, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000. Am J Med. 2004;116:581–589. doi: 10.1016/j.amjmed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor CM, Mentz RJ, Cotter G, Metra M, Cleland JG, Davison BA, Givertz MM, Mansoor GA, Ponikowski P, Teerlink JR, Voors AA, Fiuzat M, Wojdyla D, Chiswell K, Massie BM. The protect in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14:605–612. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Chiswell K, Teerlink JR, Stevens S, Fiuzat M, Givertz MM, Davison BA, Mansoor GA, Ponikowski P, Voors AA, Cotter G, Metra M, Massie BM, O'Connor CM. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail. 2014;7:76–87. doi: 10.1161/CIRCHEARTFAILURE.113.000284. [DOI] [PubMed] [Google Scholar]
- 12.Valente MA, Voors AA, Damman K, van Veldhuisen DJ, Massie BM, O'Conner CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–1293. doi: 10.1093/eurheartj/ehu065. [DOI] [PubMed] [Google Scholar]
- 13.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WH. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–270. doi: 10.1161/CIRCHEARTFAILURE.113.000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponikowski P, Metra M, Teerlink JR, Unemori E, Felker GM, Voors AA, Filippatos G, Greenberg B, Teichman SL, Severin T, Mueller-Velten G, Cotter G, Davison BA. Design of the RELAXin in acute heart failure study. Am Heart J. 2012;163:149–155. doi: 10.1016/j.ahj.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M, RELAXin in Acute Heart Failure (RELAX-AHF) Investigators Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM, NHLBI Heart Failure Clinical Research Network Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metra M, O'Connor CM, Davison BA, Cleland JG, Ponikowski P, Teerlink JR, Voors AA, Givertz MM, Mansoor GA, Bloomfield DM, Jia G, DeLucca P, Massie B, Dittrich H, Cotter G. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J. 2011;32:1519–1534. doi: 10.1093/eurheartj/ehr042. [DOI] [PubMed] [Google Scholar]
- 18.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA, UNLOAD Trial Investigators Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 19.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E, Heart Failure Clinical Research Network Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Silva R, Loh H, Rigby AS, Nikitin NP, Witte KK, Goode K, Bhandari S, Nicholson A, Clark AL, Cleland JG. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol. 2007;100:273–279. doi: 10.1016/j.amjcard.2007.02.098. [DOI] [PubMed] [Google Scholar]
- 21.Cleland JG, Coletta A, Witte K. Practical applications of intravenous diuretic therapy in decompensated heart failure. Am J Med. 2006;119(12 Suppl 1):S26–S36. doi: 10.1016/j.amjmed.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR, RELAX-AHF Investigators Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Weight by treatment group and day.
Figure S2. Loop diuretic dose by treatment group and day.
Table S1. Multivariable predictors of poor diuretic response at 48 h.
Table S2. The effects of serelaxin on diuretic response at 48 h.


