Summary
The tumor microenvironment plays an integral part in the biology of cancer, participating in tumor initiation, progression, and response to therapy. Factors released by tumor cells themselves contribute in creating an environment mostly favorable but sometimes detrimental to the tumor. Survivin, one of the key members of the inhibitor of apoptosis (IAP) family of proteins, has been shown in the cytoplasm, mitochondria, nucleus, and most recently in the extracellular space, transported via small membrane bound vesicles called exosomes. Exosomes are secreted from hematopoietic, non-hematopoietic, tumor, and nontumor cells, shuttling essential molecules such as proteins, RNAs, and microRNAs, all believed to be important for cell-cell and cell-extracellular communication. In this review, we discuss exosomal Survivin and its role in modifying the tumor microenvironment.
Keywords: Survivin, Tumor microenvironment, Exosomes
Introduction
Tumor growth requires the presence of a vascular network that supplies both oxygen and nutrients to the tumor cells as well as an avenue to dispose of waste. A tumor is composed of a mass of cancer cells, surrounded by fibroblasts, immune cells, and endothelial cells, which are constantly releasing factors that directly or indirectly modify the tumor microenvironment (TME). The composition and characteristics of the TME vary widely (Dvorak et al., 2011). The study of the TME, its cellular and molecular components and how they affect tumor initiation, progression, and advancement has become an imminent concern in cancer research. Importantly, events and molecules implicated in this cross talk within the TME have emerged as attractive targets in anticancer intervention (Swartz et al., 2012). Therefore, understanding and control of the TME is becoming as important as the understanding and control of the cancer cells themselves.
Various changes occur in the TME, some of which may affect the release of extracellular membrane vesicles called exosomes. Exosomes are 30-150 nm vesicles which have been shown to transport many types of proteins, RNAs, viruses, breakdown products, and most recently, miRNAs (Azmi et al., 2013). Extensive research over the last three decades has begun to reveal the role of exosomes in disease progression. In particular, human and mouse tumor cells secrete tumor cell-derived exosomes (TEX) with proven TME modulation capability (Wang et al., 2014b). Environmental changes such as stress, induced by chemotherapy and radiation, can also modulate TEX release and the biome they contain (Kucharzewska and Belting, 2013). Survivin, an important member of the inhibitor of apoptosis (IAP) protein family, and a known stress-activated protein, was found in exosomes and shown to increase after stress (Khan et al., 2009, 2011a). It is conceivable that like other stress proteins (heat shock proteins), Survivin becomes available to neighboring cells through its exosomal packaging and thus influences the TME. We have shown that Survivin protein can be released by cancer cells and taken up by surrounding cells, producing a field effect that confers a general stress-survival phenotype (Khan et al., 2009). Consistent with Survivin’s association with unfavorable clinicopathological parameters, extracellular trafficking of Survivin throughout the TME could be responsible for augmenting the aggressive status of a tumor while prohibiting or minimizing therapeutic results. In this review we will discuss: 1) exosomes in the tumor microenvironment; and 2) exosomal Survivin and its role in modulating the tumor microenvironment.
Exosomes in the tumor microenvironment (TME)
Tumors are comprised of both stroma and malignant cells (Zalatnai, 2006). Within the stroma, there are nutrient providing blood vessels that the tumor cells need to grow. This blood vasculature allows immune or inflammatory cells to gain access into the tumor and increases the tumor’s complexity (Swartz et al., 2012). Also, stroma contains the structural components comprised of proteins and strands of fibers that form the extracellular matrix (ECM). The TME also contains chemokines and cytokines, as well as oxygen and other chemicals that can change the acidity or the alkalinity of the tissue. In addition to these TME elements, cross-talk between the tumor cells and the surrounding cells can favor the process of tumor progression (Mueller and Fusenig, 2002). Transition from normal to benign, benign to malignant, and malignant to metastatic is primed not only by intracellular changes in the tumor cell itself but also by extracellular changes (Gould and Courtneidge, 2014; Tadeo et al., 2014; Zhang et al., 2014). Intracellular, intercellular, and distant cell interactions are maintained by constant trafficking of biological materials across nuclear and plasma membranes. However, one of the emerging mechanisms of tumor cell communication within the TME are the exosomes, which are recognized as active entities involved in regulating a variety of extracellular signals. Exosomes are extracellular membrane vesicles that are increasingly recognized in a variety of both normal and pathological cellular processes. These lipid bound vesicles are derived from late endosomes in multivesicular bodies, and are instrumental in cell-cell and cell-extracellular communication. Exosomes are heterogeneous in terms of size, protein content, RNA content, and origin. The presence of specific proteins within and on the vesicle suggests the existence of a protein sorting mechanism that may speak to their functional roles of communication (Khan et al., 2011b, Villarroya-Beltri et al., 2014). They may also provide a mechanism whereby tumor cells, quietly developing within the host, may be detected at an earlier time, allowing for a more effective therapy to be initiated.
Changes in the TME have an effect on the release of exosomes. Accumulating evidence suggests that their bioactivity may be clinically applicable in cancer therapeutics. Regardless of their cell type of origin these membrane bound vesicles provide a protected and controlled internal microenvironment outside the cell for metabolic objectives of the host cell to be carried out at a distance by the recipient cell (Anderson et al., 2010). In one instance, exosomal release of matrix metalloproteinases and heat shock protein 90α from metastatic cancer cells is believed to control invasive cellular behavior by inducing changes in the extracellular matrix (ECM) and through modification of growth factor responses (Hendrix et al., 2010). Likewise, procoagulant exosomes from healthy host cells may facilitate tumor initiation, invasion, and dissemination by activating the clotting cascade extracellularly and coagulation-dependent growth signaling intracellularly (Milsom et al., 2007). These intricate interactions between tumor cells and their environment complicate therapeutic strategies for effectively treating the tumor. In addition, the complicated milieu of extracellular signals is increased by the sheer number of cells generating exosomes which reside in the TME.
B- and T-lymphocytes, dendritic cells, neurons, intestinal epithelial cells, and tumor cells all release exosomes (Denzer et al., 2000; Keller et al., 2006; Simpson et al., 2009). In particular, it has been shown that human tumor cells release TEX constitutively (Wolfers et al., 2001). Additionally, specific protein content found both on and within TEX gives an indication of not only their functional and biological roles, but also of their cell of origin, making TEX and their contents excellent biomarkers (Zitvogel et al., 1998; Andre et al., 2002; Wieckowski and Whiteside, 2006) or tools to detect malignant conditions. Serum taken from cancer patients has an increased level of TEX (Ginestra et al., 1998, 1999), and has a positive correlation with the progression of the tumor (Khan et al., 2012). TEX contents secreted from different cancer cells express diverse tumor antigens. In vitro studies show that TEX released from breast cancer cells contain HER2, carcinoembryonic antigen (CEA) from colon cancer cells, and MelanA/Mart-1 and gp100 from melanoma cells (Andre et al., 2002; Andreola et al., 2002). Exosomes obtained from plasma taken from cancer patients also showed similar characteristics of expressing tumor antigens of the tumor origin (Hegmans et al., 2004; Mears et al., 2004). The relative amounts of exosomal Survivin in prostate cancer (PCa) plasma was significantly higher than in those with pre-inflammatory BPH and control plasma. This differential expression of exosomal Survivin was seen with both newly diagnosed and advanced PCa subjects with high or low-grade cancers. Analysis of plasma exosomal Survivin levels may offer a convenient tool for diagnosing or monitoring PCa and may, as it is elevated in low as well as high Gleason scored samples, be used for early detection (Khan et al., 2012). Furthermore, exosome analysis may provide novel biomarkers to diagnose and/or monitor the treatment effectiveness in PCa and other cancers. Exosomes derived from breast cancer patients’ serum showed a differential expression of Survivin as well as its splice variants Survivin 2B and Survivin ΔEx3 (Khan et al., 2014). Differential expression of Survivin and its splice variants may serve as a diagnostic or prognostic marker in not only breast cancer but in cancer in general, something that we are referring to as a “liquid biopsy” (Khan et al., 2014). Progress has shown that tumor-derived exosomes play multiple roles in tumor growth and metastasis and may produce these functions via immune escape, tumor invasion and angiogenesis.
In addition to serum, TEX have been isolated from other fluids, such as urine, ascites (Adams et al., 2005), and pleural effusions (Andre et al., 2002). Malignant ascitic fluid derived exosomes contained higher levels of CD24 and epithelial cell adhesion molecule (EpCAM) indicating cellular progression to invasiveness (Runz et al., 2007). Urinary exosomes can be used as biomarkers for a variety of diseases including cancer (Pisitkun et al., 2004). Exosomes isolated from pleural effusions in mesothelioma and other cancer patients were useful to study the proteomic of different cancer types involving the pleura (Bard et al., 2004). MicroRNAs (miRNAs) identified in saliva and serum are primarily exosomal (Gallo et al., 2012). Therefore, using an exosomal fraction increases the sensitivity of detection of miRNA in order to improve the accuracy of diagnosis, predict prognosis and to monitor disease progression and responses to therapy.
Exosomes possess a capacity for immunostimulation. Dendritic cell (DC)-derived exosomes, also known as dexosomes (DEX), have been considered for use in cancer immunotherapy (Andre et al., 2004). Exosomes from mature DC and antigen presenting cells (APCs) carry co-stimulatory molecules such as MHC class II that are able to stimulate cognate T cells. However, exosomes can also have inhibiting effects on the immune system and promote tumor immunoevasion. A recent study on lymphoblastoid cell lines found exosomes carrying both MHCII and FasL, a death inducing ligand. These constitutively expressed exosomes had the capacity to cause autologous T cells to undergo apoptosis, and may be a means of immunosuppression (Klinker et al., 2014). The complex interaction of exosomes with immune cells have been recently reviewed by several groups (Altevogt et al., 2014; Gehrmann et al., 2014; Robbins and Morelli, 2014). Recent work suggests that the use of exosomes in immunotherapy may stimulate the immune system to recognize and kill cancer cells and thus could form an attainable basis for the development of novel cancer vaccines (Kovar et al., 2006; Tan et al., 2010; Wang et al., 2014a).
Various stresses can induce exosome release from tumor cells and stromal cells alike; a phenomenon which may induce the surrounding tissues to adapt to changes taking place in the microenvironment (Thery et al., 2009). Tumor cells that have undergone radiation or chemotherapy treatment have been shown to increase their release of TEX (Yu et al., 2006; Lehmann et al., 2008). Interestingly, when treated with chemotherapeutic agents, there is a significantly enhanced membrane vesicle secretion in chemoresistant cells compared to chemosensitive cells. This activity may be a factor leading to drug resistance (Shedden et al., 2003; Safaei et al., 2005). Radiation and chemotherapy treatments lead to DNA-damaging conditions. In this state, the p53 pathway is activated, leading to an induced expression of the transmembrane protein tumor suppressor activated pathway 6 (TSAP6) among various other physiological changes (Lespagnol et al., 2008; Yu et al., 2009). TSAP6 is an important cellular component because it regulates the secretion of protein via the non-classical pathway or the endoplasmic reticulum (ER)/Golgi-independent protein secretion pathway which is necessary for the enhanced release of exosomes (Nickel, 2003; Yu et al., 2006; Lespagnol et al., 2008).
Exosome-mediated release of various factors including tetraspanins, chemoattractants, adhesion molecules and proteases from cancer cells, platelets, and other cellular sources contributes to metastatic regulation in several experimental models (Janowska-Wieczorek et al., 2005; Jung et al., 2009). Exosomes may also act as important reservoirs of cytokines and mediators of inflammatory and immune responses (Bianco et al., 2009; Thery et al., 2009). For example, melanoma cells can be induced by Wnt5A to release exosomes containing IL-6, VEGF and MMP2 (Ekstrom et al., 2014). Proteomic analysis of exosomes has provided evidence of enrichment with heat shock protein family members (Iero et al., 2008). Mass spectrometry-based proteomic tools coupled with advanced purification methods for exosomes has allowed more in depth proteome analysis of molecular composition of exosomes. Exosomes carry an array of proteins that reflect the cells’ origin. Identification of a conserved set of common proteins that are essential for vesicle biogenesis, structure, and trafficking mechanisms can be determined. We can also detect cell-specific composition and abundance of proteins such as Survivin in exosomes that may be useful to reveal different cellular behaviors. Exosomes from various cancer cells express Fas ligand which induces T-cell apoptosis and abolishes the function of adaptive immune cells (Andreola et al., 2002; Huber et al., 2005). Alternatively, platelet-derived exosomes were shown to transfer integrins to breast and lung cancer cells (Janowska-Wieczorek et al., 2005). Thus, cancer cells can fuse with non-cancer cell-derived exosomes, thereby receiving specific proteins which may help them to escape immune surveillance and metastasize. Therefore, proteomic approaches show a great potential for future applications in diagnosis and prognosis.
Exosomal survivin
Recent work accomplished in our laboratory revealed the presence of Survivin in the extracellular space with further evidence of exosomal localization (Khan et al., 2009, 2011a). Survivin is an important member of the inhibitor of apoptosis (IAP) protein family because its tumor specific expression is unique out of all of the human gene products (Reed, 2001). Survivin expression is evident during embryonic and fetal development but not in terminally differentiated tissue (Li et al., 1998). It is expressed in virtually all types of human cancers, making Survivin an alluring protein in the study of carcinogenesis (Andersen et al., 2007). Survivin also has been shown to control diverse cellular functions, including surveillance checkpoints, suppression of cell death, the regulation of mitosis, and adaptation to unfavorable environments (Altieri, 2003, 2006), leading to it being intensely scrutinized in cancers. Protein compartmentalization is very important. Survivin has been localized in mitochondria, where it abolishes tumor cell apoptosis similar to the Bcl-2 family (Dohi et al., 2004) and previous work in our lab showed that stress can initialize a shift in Survivin localization to the mitochondria (Asumen et al., 2010). Its localization to the nucleus and cytosol confers roles in mitosis regulation and apoptosis inhibition, respectively (Fortugno et al., 2002). Unlike other IAP proteins which are predominantly cytosolic and have roles in caspase binding and inhibition, Survivin only indirectly inhibits caspases, is not restricted to the cytosol or nucleus (Fortugno et al., 2002), and has been recently found extracellularly (Khan et al., 2009) compartmentalized in the exosome (Khan et al., 2011a). While its mitochondrial role involves tumor growth in immunocompromised animals, and abolished tumor cell apoptosis in vivo (Dohi et al., 2004), its extracellular form has the ability to reenter cancer cells inducing increased proliferation, apoptosis resistance and invasion (Khan et al., 2009).
To test whether stress may induce increased release of Survivin along with exosomes, HeLa cells treated with sublethal doses of proton irradiation, showed a marked accumulation of Survivin in the exosomal fraction taken from the conditioned medium of these cells (Khan et al., 2011a). We also identified exosomes as mediators of basal and stress-induced Survivin secretion from HeLa cells (Khan et al., 2011a). Exosomes were purified and validated by acetylcholinesterase assays, an enzyme specific to exosome membranes (Johnstone, 2006), which showed significantly more activity in conditioned medium taken from Survivin-releasing cells compared to non-conditioned medium. The vesicular nature of these isolates was further confirmed by electron microscopy. Survivin has been shown to interact directly with intracellular Hsp60 (Ghosh et al., 2008), Hsp90 (Fortugno et al., 2003) and Hsp70 (unpublished data from our lab). The presence of exosomal Survivin and Hsp70 and their colocalization was confirmed using immunoelectron microscopy (Khan et al., 2011a). Signal sequences mediate classical protein secretion, and software programs are now being employed to more easily and accurately determine signal sequence existence (Bendtsen et al., 2004). Neither Survivin nor Hsp70 proteins can be predicted by these criteria to be secreted proteins. They are exported via exosomes (Khan et al., 2011a). Survivin’s interaction both intraand extracellularly with HSPs emphasizes the link between cellular stress responses, apoptosis, and cell proliferation occurring in the TME. Survivin-protein overexpression was found in conditioned medium from pancreatic and prostate cancer cell lines and not from the nontumor-derived cells, which indicates that Survivin’s localization in cancer cell line exosomes is a general finding for tumor cell-derived exosomes. Blocking of exosome release with cytochalasin D, which inhibits actin polymerization, blocked both exosome and Survivin release, clearly showing the intimate association of Survivin release with exosomes (Khan et al., 2011a).
Exosomal survivin in tumor microenvironment
Our recent discovery of exosomal Survivin in cancer cell lines demonstrates plausible oncogenic field effects in the TME. Clinically, serum/plasma-derived Survivin has been detected in many cancers (Tas et al., 2004; Guney et al., 2006; Goksel et al., 2007; Derin et al., 2008; Naumnik et al., 2009; Fawzy et al., 2012; Yahya et al., 2012), including breast and prostate cancers (Khan et al., 2012, 2014). In the last decade, serum/plasma levels of Survivin were detected in many cancers (Table 1) using commercially available enzyme linked immunosorbent assay (ELISA) kits. Tas et al, showed no significant difference in prognostic parameters to serum Survivin levels in forty-four melanoma patients. However, there was a significant increased Survivin level in ten patients who were treated with chemotherapy (Tas et al., 2004). This observation supports our in vitro study where we showed proton treated cancer cells increased the level of Survivin in the exosomes (Khan et al., 2011a). Guney et al, showed no significant changes in the level of Survivin in forty-three breast cancer patients’ sera or urine, however, they suggested that the serum level of Survivin can be used as a nodal metastasis marker in those patients (Guney et al., 2006). In another study, Goksel et al, found no differences in Survivin/Her2 level in early breast cancer patients compared to healthy controls (Goksel et al., 2007). This suggests that Survivin level may depend upon distant metastasis or tumor burden and the method applied for detection. We have recently shown that exosomal Survivin may be a useful tool for early detection, diagnosis, and even monitoring of prostate cancer progression. We also postulate that it could be used to monitor anticancer therapeutic effectiveness. Newly diagnosed and advanced prostate cancer patients with high or low-grade cancer had significantly higher levels of exosomal Survivin compared to control subjects or patients with pre-inflammatory benign prostatic hyperplasia (BPH) (Khan et al., 2012). In the breast cancer patients’ sera, Survivin was exosomally packaged (Khan et al., 2014). This observation corresponds with all of the breast cancer tissue expression patterns of the Survivin proteins evaluated in this study.
Table 1.
Survivin levels in patient samples.
| Cancer Type | Samples | Methods | Authors |
|---|---|---|---|
| Breast cancer | Serum | ELISA (TiterZyme® EIA) | Goskel et al., (2007) |
| Breast cancer | Serum and urine | ELISA (TiterZyme® EIA) | Guney et al., (2006) |
| Lung cancer | Serum | ELISA | Derin et al., (2008) |
| Lung cancer | Serum | ELISA (R&D system) | Naumnik et al., (2009) |
| Lung cancer | Serum | ELISA (R&D system) | Fawzy et al., (2012) |
| Acute lymphoblastic leukemia (ALL) | Serum | ELISA (R&D system) | Yahya et al., (2012) |
| Prostate cancer | Plasma & serum | ELISA (R&D system) | Khan et al., (2012) |
| Breast cancer | Serum | ELISA (R&D system) | Khan et al., (2014) |
ELISA, Enzyme linked immunosorbent assay; EIA, Enzyme immunoassay
Utilization of exosomes to deliver anti-Survivin therapy
Thus far, there are numerous strategies to target Survivin from transcript to protein levels. A small molecule inhibitor, YM155, acts by inhibiting transcription of Survivin mRNA, while anti-sense oligonucleotides, hammerhead ribozymes and siRNA are designed to degrade Survivin mRNA and/or inhibit protein translation. Strategies to inhibit Survivin at the protein level include a small molecule antagonist named Sheperdin, which prevents Hsp90/Survivin interaction, as well as expression of two Survivin dominant negative mutants C84A and T34A into tumor cells introduced by plasmid or viral vectors (Pennati et al., 2007; Lladser et al., 2011). However, the therapeutic delivery was restricted to the periphery of the solid tumor thus limiting its effectiveness. Recently, an in vitro study showed that exosomal delivery of Survivin-T34A, resulted in the increased killing effect on pancreatic cancer cells (Aspe et al., 2014). Given the nature of T34A’s ability as a dominant negative-Survivin to kill its host, the one hurdle that will need to be overcome will be the method to produce large enough amounts of therapeutic T34A for delivery. In our lab and others, future studies are underway to exploit exosomes as shuttles in the delivery of anticancer therapeutics.
In recent years, many studies have been accomplished to determine whether downregulation of Survivin could reverse chemotherapy and radiotherapy resistance in cancer cells. Several groups have shown that inhibition of Survivin expression by shRNA, RNAi, and natural compounds like emodin can sensitize varieties of cancer cells, including squamous cell carcinoma of the tongue (Xu et al., 2010), osteosarcoma (Wang et al., 2010), breast cancer (Yang et al., 2011), and pancreatic cancer (Liu et al., 2008; Guo et al., 2009) to cisplatin, adriamycin, and gemcitabine. All the Survivin-based therapies mentioned previously have shown success in decreasing Survivin expression levels and thus we hypothesize that exosomal packaging and delivery can be utilized to inhibit further growth of malignant cells, increasing sensitivity to chemo- and radiotherapies. Exosomes derived from cells either engineered to produce an anti-Survivin moiety, such as T34A, or capable of packaging drugs into exosomes after treatment may provide useful tools for therapeutic use of exosomes. Exosomes, in addition to having low toxicity, are innately capable of fusion and uptake by other cells and thus advantageous for targeted delivery of therapeutic agents. Although potentially able to be produced with certain ligands or receptors for specific cell interactions, exosomal targeted delivery is still a confounding problem.
Conclusions
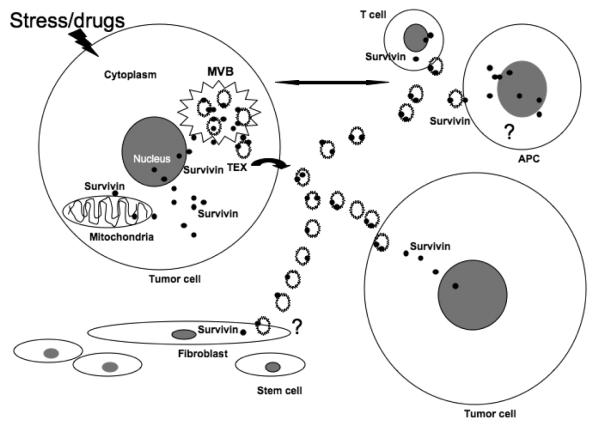
The TME is a critical component in determining the growth and metastasis of tumors. Exosomes are one of the major modalities for transporting molecules throughout the TME and influencing the behavior of surrounding cells (Fig. 1). Survivin is a unique inhibitor of apoptosis with described multifunctionality. Survivin’s upregulation in most cancers, in addition to its presence in serum exosomes, make it an important molecule both as a diagnostic as well as prognostic biomarker. It may also be a means for early detection as it localizes to the surface of the exosome. To validate its role, a large number of case-control studies need to be adapted. Subsequent studies are needed to explore whether exosomal anti-Survivin strategies can be used in cancer therapeutics.
Fig. 1.
Schematic presentation of plausible roles of Exosomal Survivin in tumor microenvironment. (MVB: multivesicular body; APC: antigen presenting cells).
Acknowledgements
Funding for our laboratory comes from grants for health disparity research: NIH-NCMHD Project EXPORT Program 5P20MD001631/Project 3 (NRW) and NIH-NIMHD P20-MD006988 subproject 2. Funding was also obtained from a National Merit Test Bed (NMTB) award sponsored by the Department of the Army under Cooperative Agreement Number DAMD17-97-2-7016 (NRW). The funders had no role in the decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest. The authors declare that they have no competing interests.
References
- Adams M, Navabi H, Croston D, Coleman S, Tabi Z, Clayton A, Jasani B, Mason MD. The rationale for combined chemo/immunotherapy using a toll-like receptor 3 (tlr3) agonist and tumour-derived exosomes in advanced ovarian cancer. Vaccine. 2005;23:2374–2378. doi: 10.1016/j.vaccine.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin. Cancer Biol. 2014 doi: 10.1016/j.semcancer.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Andersen MH, Svane IM, Becker JC, Straten PT. The universal character of the tumor-associated antigen survivin. Clin. Cancer Res. 2007;13:5991–5994. doi: 10.1158/1078-0432.CCR-07-0686. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab. Invest. 2010;90:1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- Andre F, Escudier B, Angevin E, Tursz T, Zitvogel L. Exosomes for cancer immunotherapy. Ann. Oncol. 2004;15:iv141–iv144. doi: 10.1093/annonc/mdh918. [DOI] [PubMed] [Google Scholar]
- Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of fasl-bearing microvesicles. J. Exp. Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspe JR, Diaz Osterman CJ, Jutzy JMS, Deshields S, Whang S, Wall NR. Enhancement of gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the survivin-t34a mutant. J. Extracell. Vesicles. 2014;3:23244. doi: 10.3402/jev.v3.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asumen MG, Ifeacho TV, Cockerham L, Pfandl C, Wall NR. Dynamic changes to survivin subcellular localization are initiated by DNA damage. Onco Targets Ther. 2010;3:129–137. doi: 10.2147/ott.s11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi A, Bao B, Sarkar F. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metast. Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen L-AA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 2004;31:114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Jensen LJ, Blom N, von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 2004;17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase–positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheumatism. 2009;60:380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell. Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Derin D, Soydinç HO, Guney N, Tas F, Çamlıca H, Duranyıldız D, Yasasever V, Topuz E. Serum levels of apoptosis biomarkers, survivin and tnf-alpha in nonsmall cell lung cancer. Lung Cancer. 2008;59:240–245. doi: 10.1016/j.lungcan.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Weaver VM, Tlsty TD, Bergers G. Tumor microenvironment and progression. J. Surg. Oncol. 2011;103:468–474. doi: 10.1002/jso.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom E, Bergenfelz C, von Bulow V, Serifler F, Carlemalm E, Jonsson G, Andersson T, Leandersson K. Wnt5a induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer. 2014;13:88. doi: 10.1186/1476-4598-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzy A, Gaafar R, Kasem F, Ali S, Elshafei M, Eldeib M. Importance of serum levels of angiopoietin-2 and survivin biomarkers in non-small cell lung cancer. J. Egypt Natl. Canc. Inst. 2012;24:41–45. doi: 10.1016/j.jnci.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Fortugno P, Wall NR, Giodini A, O’Connor DS, Plescia J, Padgett KM, Tognin S, Marchisio PC, Altieri DC. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J. Cell. Sci. 2002;115:575–585. doi: 10.1242/jcs.115.3.575. [DOI] [PubMed] [Google Scholar]
- Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC. Regulation of survivin function by hsp90. Proc. Natl. Acad. Sci. USA. 2003;100:13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS one. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann U, Näslund TI, Hiltbrunner S, Larssen P, Gabrielsson S. Harnessing the exosome-induced immune response for cancer immunotherapy. Semin. Cancer Biol. 2014;286:58–67. doi: 10.1016/j.semcancer.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- Ginestra A, La Placa MD, Saladino F, Cassara D, Nagase H, Vittorelli ML. The amount of proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18:3433–3437. [PubMed] [Google Scholar]
- Ginestra A, Miceli D, Dolo V, Romano FM, Vittorelli ML. Membrane vesicles in ovarian cancer fluids: A new potential marker. Anticancer Res. 1999;19:3439–3445. [PubMed] [Google Scholar]
- Goksel G, Taneli F, Uslu R, Ulman C, Dinc G, Coskun T, Kandiloglu A. Serum her-2/neu and survivin levels and their relationship to histological parameters in early-stage breast cancer. J. Int. Med. Res. 2007;35:165–172. doi: 10.1177/147323000703500201. [DOI] [PubMed] [Google Scholar]
- Gould CM, Courtneidge SA. Regulation of invadopodia by the tumor microenvironment. Cell Adhesion Migration. 2014;8:24–23. doi: 10.4161/cam.28346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guney N, Soydine H, Derin D, Tas F, Camlica H, Duranyildiz D, Yasasever V, Topuz E. Serum and urine survivin levels in breast cancer. Med. Oncol. 2006;23:335–339. doi: 10.1385/mo:23:3:335. [DOI] [PubMed] [Google Scholar]
- Guo Q, Chen Y, Zhang B, Kang M, Xie Q, Wu Y. Potentiation of the effect of gemcitabine by emodin in pancreatic cancer is associated with survivin inhibition. Biochem. Pharmacol. 2009;77:1674–1683. doi: 10.1016/j.bcp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hegmans JP, Bard MPL, Hemmes A, Luider TM, Kleijmeer MJ, Prins J, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am. J. Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, Westbroek W, Bracke M, De Wever O. An ex(o)citing machinery for invasive tumor growth. Cancer Res. 2010;70:9533–9537. doi: 10.1158/0008-5472.CAN-10-3248. [DOI] [PubMed] [Google Scholar]
- Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Human colorectal cancer cells induce t-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Different. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Molecules Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky D, Roffler S, Brodt P, Zöller M. Cd44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, De Leon M, Langridge WH, Wall NR. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br. J. Cancer. 2009;100:1073–1086. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Jutzy J, Aspe J, McGregor D, Neidigh J, Wall N. Survivin is released from cancer cells via exosomes. Apoptosis. 2011a;16:1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Jutzy JMS, Aspe JR, Valenzuela MMA, Park JS, Turay D, Wall NR. The application of membrane vesicles for cancer therapy. In: Gali-Muhtasib PH, editor. Advances in cancer therapy. InTech Publishing; 2011b. pp. 21–52. [Google Scholar]
- Khan S, Jutzy JMS, Valenzuela MMA, Turay D, Aspe JR, Ashok A, Mirshahidi S, Mercola D, Lilly MB, Wall NR. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS one. 2012;7:e46737. doi: 10.1371/journal.pone.0046737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Ferguson Bennit H, Turay D, Perez M, Mirshahidi S, Yuan Y, Wall NR. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer. 2014;14:175. doi: 10.1186/1471-2407-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinker MW, Lizzio V, Reed TJ, Fox DA, Lundy SK. Human b cell-derived lymphoblastoid cell lines constitutively produce fas ligand and secrete mhcii+fasl+ killer exosomes. Front. Immunol. 2014;5:144. doi: 10.3389/fimmu.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar M, Boyman O, Shen X, Hwang I, Kohler R, Sprent J. Direct stimulation of t cells by membrane vesicles from antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 2006;103:11671–11676. doi: 10.1073/pnas.0603466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzewska P, Belting M. Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J. Extracell. Vesicles. 2013;2:20304. doi: 10.3402/jev.v2i0.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine J-C, Vidal M, Amson R, Telerman A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in tsap6/steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- Liu W-S, Yan H-J, Qin R-Y, Tian R, Wang M, Jiang J-X, Shen M, Shi C-J. Sirna directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig. Dis. Sci. 2008;54:89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- Lladser A, Sanhueza C, Kiessling R, Quest AFG. Is survivin the potential achilles’ heel of cancer? Adv. Cancer Res. 2011;111:1–37. doi: 10.1016/B978-0-12-385524-4.00001-5. [DOI] [PubMed] [Google Scholar]
- Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- Milsom C, Yu J, May L, Meehan B, Magnus N, Al-Nedawi K, Luyendyk J, Weitz J, Klement P, Broze G, Mackman N, Rak J. The role of tumor-and host-related tissue factor pools in oncogene-driven tumor progression. Thromb. Res. 2007;120(Suppl. 2):S82–S91. doi: 10.1016/S0049-3848(07)70135-4. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70:486–497. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- Naumnik W, Nilklińska W, Ossolińska M, Chyczewska E. Serum levels of hmgb1, survivin, and vegf in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochem. Cytobiol. 2009;47:703–709. doi: 10.2478/v10042-009-0025-z. [DOI] [PubMed] [Google Scholar]
- Nickel W. The mystery of nonclassical protein secretion. Eur. J. Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: Fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. The survivin saga goes in vivo. J. Clin. Invest. 2001;108:965–969. doi: 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain cd24 and epcam. Gynecol. Oncol. 2007;107:563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Molecular Cancer Therap. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- Simpson RJ, Lim JWE, Moritz RL, Mathivanan S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteomic. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadeo I, Berbegall AP, Escudero LM, Álvaro T, Noguera R. Biotensegrity of the extracellular matrix: Physiology, dynamic mechanical balance and implications in oncology and mechanotherapy. Front. Oncol. 2014;4:39. doi: 10.3389/fonc.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, De La Peña H, Seifalian A. The application of exosomes as a nanoscale cancer vaccine. Int. J. Nanomed. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas F, Duranyildiz D, Argon A, Oguz H, Camlica H, Yasasever V, Topuz E. Serum bcl-2 and survivin levels in melanoma. Melanoma Res. 2004;14:543–546. doi: 10.1097/00008390-200412000-00017. [DOI] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014;286:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-W, Liu Y, Tian H.-m.Z., Wei Effect of survivinsiRNA on drug sensitivity of osterosarcoma cell line mg-63. Chinese J. Cancer Res. 2010;22:68–72. [Google Scholar]
- Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor t cell immunity by exosomes from cd40l gene-modified lung tumor cells. Mol. Med. Rep. 2014a;9:125–131. doi: 10.3892/mmr.2013.1759. [DOI] [PubMed] [Google Scholar]
- Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxiainducible factors and rab22a mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA. 2014b;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol. Res. 2006;36:247–254. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for ctl cross-priming. Nat. Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Xu J-H, Wang A.-x., Huang H-Z, Wang J-G, Pan C-B, Zhang B. Survivin shrna induces caspase-3-dependent apoptosis and enhances cisplatin sensitivity in squamous cell carcinoma of the tongue. Oncol. Res. 2010;18:377–385. doi: 10.3727/096504010x12644422320663. [DOI] [PubMed] [Google Scholar]
- Yahya R, Fouda M, El-Baz H, Mosa T, Elmaksoud M. Serum survivin and tp53 gene expression in children with acute lymphoblastic leukemia. Iranian J. Public Health. 2012;41:37–44. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gao Y, Chen L, Huang Y, Li Y. Downregulation of survivin expression and enhanced chemosensitvity of mcf-7 cells to adriamycin by pdmae/survivin shrna complex nanoparticles. Int. J. Pharmaceutics. 2011;405:188–195. doi: 10.1016/j.ijpharm.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: A novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- Yu X, Riley T, Levine AJ. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 2009;276:2201–2212. doi: 10.1111/j.1742-4658.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Zalatnai A. Molecular aspects of stromal-parenchymal interactions in malignant neoplasms. Curr. Mol. Med. 2006;6:685–693. doi: 10.2174/156652406778195053. [DOI] [PubMed] [Google Scholar]
- Zhang G, Miyake M, Lawton A, Goodison S, Rosser C. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14:310. doi: 10.1186/1471-2407-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]