Introduction
Robert M. Sade, MD
One of the persistent problems in achieving informed consent for surgical patients is the question of what should be covered in the disclosure component of the consent process. Certainly, one need not tell patients about every conceivable complication or about every possible alternative therapy that might be available for their specific illnesses. So what must be included? In particular, what alternative therapies should be described or must be described to patients? The problem has become a bit more complicated in recent years as the competitive aspects of cardiothoracic surgical practice have become more prominent and as some new technologies have been used as much for marketing surgical programs as for addressing patients’ needs.
This issue was explored at the annual Ethics Forum session of the Southern Thoracic Surgical Association meeting in 2010. A case was built around an actual new device that was still investigational at the time of the meeting, but set in the future (November 2011) at a time when Food and Drug Administration (FDA) approval is presumed to have occurred.
Case
Richard Bishop, 60 years of age, has been known to have mitral insufficiency for 5 years, has recently developed shortness of breath, and his cardiologist at Lourdes Heart Center has found left ventricular dysfunction: ejection fraction 50%, end-systolic diameter 52 mm. He has referred Mr Bishop to his long-time colleague, Dr John Crowne, a cardiothoracic surgeon who has had excellent results with open mitral valve repair for more than 15 years.
A competing institution, St. Bernadette Medical Center, on the other side of town recently participated in a multicenter trial of a new device, the Evalve MitraClip, which is deployed percutaneously for mitral valve repair. It was approved for clinical use by the FDA 3 months ago, in August 2011. St. Bernadette has been using the device with considerable success for 3 years. Published studies indicate that the device is 98% successful in reducing moderately severe (3+) or severe (4+) regurgitation to mild or none at 30 days after than procedure [1], but there is no information about long-term durability. Many heart centers want to use MitraClip and are training personnel in the technique, but only the original investigating institutions are up and running with experience in use of the device, so Lourdes Heart Center does not yet have clinical access to it.
During his initial discussion with Mr Bishop, Dr Crowne realizes that no one in the cardiology department has mentioned the MitraClip to the patient, so he does not know what the patient’s preferences might be. He has read the limited number of published papers about the device and knows that its early success rate is excellent, no long-term results are available, and the FDA believes it is safe enough for clinical use. He wonders whether he is ethically obligated to tell the patient about the MitraClip and to offer referral to St. Bernadette as a treatment alternative.
Pro
Eric Skipper, MD
The surgeon should inform his patient and offer referral. The impact of health care technology has generally been positive, with new and simplified procedures entering the medical field on an almost daily basis. All innovations come with “issues [2].” Early adoption of new technologies can be a double-edged sword, with beneficence pitted against nonmaleficence in the name of progress, thus creating conflict between physicians’ most fundamental values [3].
This debate, however, is not about new technologies nor about their appropriateness. It is about full disclosure and, more generally, informed consent.
What exactly is informed consent? Certainly, it is more than a mere signed piece of paper. It is a process founded in ethical principles, and of paramount importance in the practice of medicine. In 1847, the American Medical Association (AMA) adopted the first national code of professional ethics in medicine. This was renamed “The Principles of Medical Ethics” in 1903. In 1985, the Judicial Council of the AMA became the Council on Ethical and Judicial Affairs (CEJA). The first Ethics Conference of the AMA was held in Philadelphia, the city of the AMA’s founding, in 1997, thus establishing the AMA’s Institute of Ethics [4].
The AMA’s Code of Medical Ethics describes informed consent as a patient-physician communication that results in the patient’s authorization or agreement to undergo a specific medical intervention. To obtain informed consent, the physician should disclose and discuss the diagnosis, nature, and purpose of a proposed treatment or procedure, risks and benefits thereof, alternatives, and risks/benefits of not receiving or undergoing a treatment or procedure [5]. This communication process is both an ethical obligation and a legal requirement spelled out in statutes and case law in all 50 states [6].
The AMA Council on Ethical and Judicial Affairs 2006 report states that with respect to informed consent, the physician’s obligation is to accurately present the medical facts, make recommendations for management in accordance with good medical practice, and help make choices from among the therapeutic alternatives consistent with good medical practice [7].
Interpretive Guidelines from the Centers for Medicare and Medicaid Services (CMS) state that a well-designed informed consent process should include a description, indications, material risks and benefits of the proposed surgery, treatment alternatives, probable consequences of declining recommended or alternative therapies, who will conduct the surgery, and whether other physicians or physician-extenders will be involved [8]. The medical record should contain a statement that the procedure or treatment, including the anticipated benefits, material risks, and alternative therapies was explained to the patient [9].
The Code of Professional Conduct from the American College of Surgeons Statement on Principles states that informed consent is more than a legal requirement [10]. It is a standard of ethical surgical practice that enhances the surgeon-patient relationship, and a mechanism for presenting information fairly, clearly, accurately, and compassionately. The discussion conducted by the surgeon should include the nature of the illness and the natural consequences of no treatment. It should include the nature of the proposed operation, including estimated risks of mortality and morbidity. Common complications and benefits of the proposed operation should be disclosed. Alternative forms of treatment including nonoperative techniques should be divulged. A freedom of choice clause states that an ethical surgeon should not participate in a system that denies serving the best interest of the patient by refusing referral out of the system. Moreover, the doctor-patient relationship requires that the patient’s interests supersede all other interests, including the personal and financial interests of the surgeon.
The Hippocratic Oath states, “I will prescribe regimens for the good of my patients according to my ability and judgment and never do harm to anyone.” Historically the “Hippocratic tradition” fostered paternalism in which physicians were ethically obligated to promote the patient’s welfare by providing care in accordance with their own judgment regarding the most appropriate course of treatment. They could opt not to share medical information with the patient if they believed that disclosure might prove detrimental to the patient’s well-being [9–12]. Paternalism has long since been replaced with the contemporary concepts of patient autonomy and shared decision-making [13].
The lack of adequate information can preclude patients from receiving necessary medical attention or making optimal life decisions on the basis of their individual needs and personal values [14, 15]. The patient-physician relationship is founded on trust. The lack of candid disclosure can compromise this relationship. Thus, an act of deception intended to be benevolent can risk undermining both confidence and trust in the medical profession [16].
Conclusion
The Society of Thoracic Surgeons, in its position statement regarding the off-label use of coronary artery stents states, “…patients must be better informed of treatment options and the relative risks and benefits of medical therapy, percutaneous coronary interventions and coronary artery bypass grafting (CABG).” Their recommendations to the FDA included, “…adequate informed consent to be given to patients regarding treatment options … before catheter intervention is performed…” [17].
As surgeons, we cannot have it both ways. One can not argue successfully against full disclosure in this case; quite the contrary, full disclosure is the only right decision.
Con
Kevin Accola, MD
“Men in general are quick to believe that which they wish to be true.”
—Gaius Julius Caesar
“When you have a new hammer, choose your nails wisely.”
—Meredith L. Scott, MD
The surgeon has no obligation to inform or refer the patient. The simple and obvious answer to the central question of this debate is, no—Dr Crowne is not ethically required to discuss this new treatment option with the patient or to offer referral. Dr Crowne should proceed with conventional mitral valve repair surgery, as he is not obligated to discuss with the patient this new technology for mitral valve insufficiency, because the data are suspect, with few short- or long-term results upon which to make judgments, unless the patient is an inappropriate candidate for conventional surgery. Granted, if the patient asks Dr Crowne about alternative approaches, the surgeon is obligated to present the data that are available in an objective manner, before proceeding with open repair.
In my opinion, ethical concerns about new technologies should be more focused on the innovative institutions and individuals who pursue new treatment modalities than on the practicing surgeon. I do not wish to create some type of “Jerry McGuire mission statement” [18], but more often than not, individuals or institutions market themselves with new technologies in a gamesmanship manner. The ethical course of action should be evidence based, using data and results obtained from organized trials of a particular treatment modality or investigational device. Many of the procedures or technologies that have come and gone were marketed as procedures that would end conventional surgery as we know it, and now are merely curiosities that are of historical value only.
Off-pump coronary artery surgery is an example of this phenomenon. It was once used in institutional advertising to increase market share, but although it remains a worthwhile treatment modality, it has not replaced conventional surgical procedures, which should remain the standard to which investigational devices are compared. Another example of the current prominence of marketing in cardiothoracic surgery is the widespread use of the term “minimally invasive surgery.” A plethora of surgeons and institutions market themselves as “minimally invasive” cardiac surgical centers, yet there are no broadly accepted inclusion criteria or even definitions of the term. Does it mean thoracotomy, partial or no sternotomy, alternative incision, small incision, port access, or robotics? Marketing of heart surgery programs featuring “minimally invasive cardiac surgery” has included all these.
These considerations should lead us to ask a series of questions. First, what is the surgeon’s responsibility regarding new technologies as they relate to clinical standards of care? If we surgeons do not responsibly monitor innovative procedures or technologies with strict oversight, someone else will, and we can almost guarantee it will not be a cardiothoracic surgeon. A second question is how surgeons adopt new procedures and technologies—when does an innovative procedure or technology become accepted as a standard practice? Interestingly, there is some science behind the questions about adoption or acceptance of new technologies: the Law of Diffusion of Innovation, which I discuss below [19]. A third question is about industry—what is industry’s perspective and behavior relative to new technologies, and how do companies interact with cardiothoracic surgeons? Finally, how do Internet-based information and televised infomercials influence surgeons’ responses to patient requests for “new technologies” in this Internet age? Patients are much better informed on medical matters than they were only a few years ago because of ready access to large amounts of information from self-help books, magazines, newspaper columns, and, most notably, the Internet. Much of this information, however, is not peer reviewed and is processed through an institutional marketing filter. Have we allowed patient-driven pursuit of new technology spawned by institutional marketing to displace our ethical responsibility to guide the patient through an evidence-based discussion of what procedure serves their best short-term and long-term interests?
Surgeons’ Responsibility
What is the surgeon’s responsibility to new technologies as these procedures or devices relate to clinical standards? Evidence-based medicine recommendations and protocols give us some guidance while these devices and procedures are becoming accepted. Evidence-based medicine seeks to assess the strength of evidence of the risks and benefits of treatments, including lack of treatment and diagnostic tests [20]. Evidence-based medicine aims to apply the best available evidence gained from the scientific method to diagnostic and therapeutic decisions [21]. Evaluation of evidence can be challenging, but it remains the foundation of clinical decision making.
Cardiothoracic surgeons have always been on the forefront of technological innovation. Before the Internet and pervasive institutional marketing, new procedures or technologies were critically and meticulously discussed and debated at national meetings; results, data, and experience fueled these discussions, and the information was published in peer-review journals. The trend in marketing seems to be using the Internet, YouTube, Twitter, infomercials, and medical health journalism seem to have become, for patients and often physicians, the primary source of news about new procedures.
Patients have become much better educated about health matters over the past 10 to 15 years, and rightly so. One major flaw and troublesome issue, however, is that this public information on technologies and procedures is not peer reviewed, has bias that may be subtle or obvious, and often is compromised by glaring conflicts of interest. Many cardiothoracic surgeons have been asked by patients if they perform procedures like “Institution X,” or ask why are they not candidates for particular treatment modalities or new technologies. Physicians come under significant pressure from hospital systems to provide new technologies to keep pace with the major institutions or with competing hospital systems. Where this will eventually lead is somewhat problematic. It is clear, however, that we surgeons must take the responsibility to do our best for our patients by making decisions based on clinical data and resisting institutional pressures. If we do not accept this responsibility, someone else will, and we will be held accountable. The paucity of evidence about the efficacy (and perhaps safety) of the Evalve MitraClip makes it optional for Dr Crowne to include it among the alternative therapies he describes to Mr Bishop.
How Surgeons Come to Accept New Procedures or Technologies
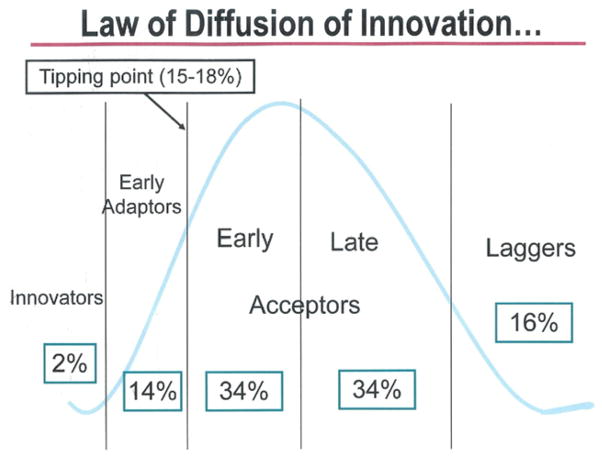
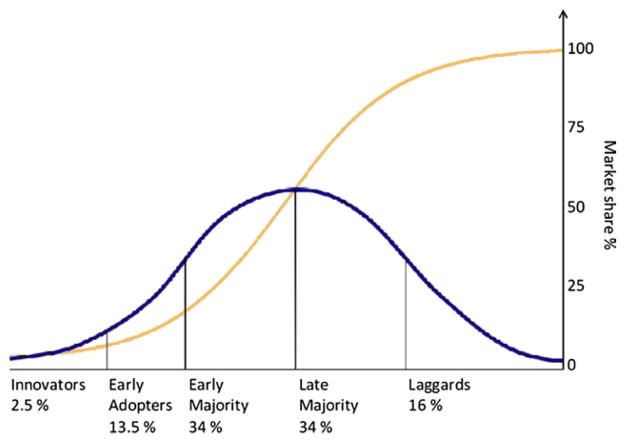
Most surgeons are cautiously optimistic in considering the uses of new technologies. The science behind behavioral patterns exhibited by purchasers of consumer products can be applied to the behavior of surgeons in using new technologies. The Law of Diffusion of Innovation describes a behavioral spectrum from “innovators/early adaptors” to “laggers. “[19] (Fig 1). This law applies to a wide range of consumer products, such as the cell phone, for example. The innovators stand in line waiting to be the first to have a cell phone, and the early adapters quickly follow because they simply must have one as well. Once a product reaches a “tipping point” of 15% to 18% market penetration, the early and late acceptors adopt it because they have seen data and now believe that cell phones work and are dependable. The laggers are the few who no longer use rotary phones only because they cannot find any to buy. Once the tipping point of consumer acceptance is reached, the product gains the interest and acceptance of the early and late acceptors, market penetration escalates, and it becomes a household product (Fig 2).
Fig 1.
Law of diffusion of innovation.
Fig 2.
Once the tipping point of consumer acceptance is reached, the product gains the interest and acceptance of the early and late acceptors, market penetration escalates, and it becomes a household product.
These behavior patterns can be applied to surgeons’ acceptance of new procedures or technologies. Fortunately, bright and innovative minds innovate, and early adapters are present to expand the new technologies, investigate them, and obtain data to demonstrate efficacy and safety. Most of us, though, want proof in the form of reliable data before we accept new technologies as part of our practice or accept them as a standard of care. Finally, the laggers include those who have been doing it a certain way for many years and see no reason to change.
Many cardiothoracic procedures and technologies illustrate the accuracy of the Law of Diffusion of Innovation. For instance, HeartPort was a good idea, but because of an inappropriate launch and other factors, it was unsuccessful in changing the way heart surgery is done—the tipping point was never reached [22]. Another example is off-pump coronary artery bypass surgery, which some suggested should be applied to all coronary revascularizations [23]. It nearly reached a tipping point, but the early and late Acceptors wanted more proof of the efficacy and advantages of the procedure, and the proof never appeared, so the rate of off-pump bypass surgery increased from 15% early after its introduction [24] to only 20% 10 years later [25].
An important point about the procedures mentioned above is that some are very beneficial for selected patients, but have not been widely accepted. That leads us to the next topic, the industry perspective.
Industry Perspective
Cardiothoracic surgeons’ collegial and collaborative relationship with industry has generated much industry funding and engineering expertise in support of research and development of new technologies. Without industry involvement, cardiothoracic surgical innovation could not occur at the levels achieved in the past. A corollary of this, however, is that marketing or promotion of these technologies is sometimes overwhelming and conflicting. Certainly, the need for promotion can be understood in the context of the huge economic impact on industry related to research and development, as well as launching new products.
In my experience, cardiothoracic surgeons are often approached with promotional pressures such as, “better do this before the other group in town or you will miss out,” and “this procedure is sure to increase your referrals.” Another inducement to use a new product is, “there is a CPT code for this, so you can bill for it.”
A number of well-organized controlled clinical trials investigating new technologies are scientifically designed to document efficacy and safety with reliable data. Trials such as these may be persuasive to early and late acceptors, allowing these technologies to reach a tipping point that will result in widespread use, expanding our armamentarium of treatment modalities. Again, we as surgeons must take a responsible role in this process with objective oversight.
Conclusion
In conclusion, Dr Crowne is acting ethically and responsibly by proceeding with conventional mitral valve repair surgery without mentioning the Evalve MitraClip to the patient. The limited amount of information about this device does not satisfy the standards of evidence-based medicine, and the lack of peer-reviewed data along with his personal experience and surgical results support such a decision. Because Dr Crowne is under no obligation to discuss with Mr Bishop a new technique with such a sparse track record, he is also not ethically or morally obligated to refer the patient to another institution for amelioration of his mitral valve insufficiency.
Concluding Remarks
Robert M. Sade, MD
The three informational requirements to ensure that consent is informed are these: preconditions (the patient’s competence and voluntariness), informational disclosure, and consent to undergo the chosen treatment. At issue in the case at hand is the second element, informational disclosure—how much information Dr Crowne is required to provide to the patient.
Dr Skipper has provided convincing evidence that providing information to the patient is necessary, but he has placed no upper or lower limit on the amount of information required. If Mr Bishop must be told about the Evalve MitraClip, must he also be told about each of the dozen or so rings and annulus suture techniques, the many distinct methods of mitral valve repair, and the dozen or more types of valve prostheses that are currently available to treat mitral valve insufficiency? What boundaries, if any, delimit the information required for adequately informed consent? Skipper has not made as strong a case as perhaps he might.
Dr Accola has provided persuasive reasons for the surgeon to discount the importance of disclosing the existence and availability of the Evalve MitraClip, but we may ask whether the patient is truly incapable of processing the available information himself. Is the surgeon’s skeptical interpretation of the data sufficient reason to withhold that information from the patient?
The informational requirements for informed consent are more complex than the discussants have appreciated. The concept of informed consent as we know it today has developed over more than 2 centuries. The evolution of informed consent, as is true of much else in the field of bioethics, has been driven more by lawyers and courts of law than by thoughtful analysis by philosophers. The first litigation in this area was recorded in 1767 when a physician was found liable for rebreaking and resetting a leg fracture without obtaining the patient’s consent [26]. A landmark decision came in 1914 when Justice Benjamin Cardozo famously recognized the right to bodily self-determination: “Every human being of adult years and sound mind has a right to determine what shall be done with his own body, and a surgeon who performs an operation without the patient’s consent commits an assault for which he is liable in damages” [27].
The law (and consequently ethics) has also seen evolution of the kinds of information that must be disclosed to the patient to ensure that consent is adequately informed. In 1957, the professional or “reasonable physician” standard of disclosure was established: a physician must disclose the amount of information that other reasonable physicians would disclose (also known as the Bolam principle, after the legal case) [28]. The “reasonable patient” standard was established in 1972, in yet another legal case: all the risks and alternatives that a reasonable person would want to know must be disclosed [29]. A 1980 court decision established that the risks of not accepting a recommended course of treatment also must be disclosed to the patient [30].
At this time, the predominant standard for informational disclosure is the reasonable patient standard, modified to include what the particular patient facing a decision would want to know. The latter, of course, requires that physicians gauge what they believe the patient wants to know, and also that they ask the patient whether he or she desires any additional information. The professional standard has not entirely disappeared; it is still used in some jurisdictions.
Applying Informational Standards to the Bishop Case
Making the case for or against requiring Dr Crowne to disclose to Mr Bishop the availability elsewhere of a less invasive procedure to treat his mitral valve insufficiency requires exploring the question of whether a reasonable person would want to have that information, and whether Mr Bishop in particular would want it. From the vignette as presented, we do not have enough information to know what the patient would want, information that might have been gleaned from the details of communication, spoken and unspoken, during the disclosure conversation, but those details were not given to us.
We are left with the reasonable person standard—what information would a hypothetical reasonable person want? Would such a person want to know about a treatment for his problem that has the benefit of avoiding open-heart surgery, but also the uncertainties accompanying a sparse number of short- to medium-term studies and no long-term data? The surgeon could tell the patient that the device in question is safe and effective enough to have gained approval for clinical use by a federal regulatory agency, and provide such information as already exists about the results of the investigational studies, while carefully placing those results in the context of the well-established safety and effectiveness of the open procedure. It is perfectly acceptable, even laudable, for the surgeon to give the patient his opinion about the balance of benefits and harms associated with the two differing approaches, whether he believes that balance favors the open-heart operation or the newer transcatheter procedure. With all of the information laid out before him, the patient could then make an informed decision.
Certainly, the sheer volume of information might be confusing, but a carefully planned discussion, using language consistent with the patient’s level of education and capability of comprehension, can help the patient to make a decision that is consistent with his own values and beliefs.
What Is the Correct Choice for the Patient?
It would be wrong to assume that Mr Bishop will choose the less invasive procedure, no matter how appealing it seems at first glance. An open, honest relationship with a patient, reinforced by full disclosure of reasonable therapeutic alternatives in terms the patient can understand, establishes a level of trust that is not easily fractured. Most patients in a trusting relationship with their physicians will follow their doctors’ advice.
The open-heart techniques and the transcatheter device both seem to be reasonable options. The patient’s ultimate decision is likely to be substantially influenced by the degree of trust he has in his surgeon. If Mr Bishop had been referred to Dr Accola, after a frank and open discussion, the patient probably would choose open repair of his deformed valve. If he had been referred to Dr Skipper, he probably would choose the MitraClip and accept referral to another center that has access to that device. The patient will have made his decision based on his understanding of both options, and no matter which alternative he selects, it will have been the right choice for him.
Acknowledgments
Dr Sade’s role in this publication was supported by the South Carolina Clinical and Translational Research Institute, Medical University of South Carolina’s Clinical and Translational Science Award Number UL1RR029882. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Presented at the Fifty-seventh Annual Meeting of the Southern Thoracic Surgical Association, Orlando, FL, Nov 3–6, 2010.
References
- 1.Tamburino C, Ussia GP, Maisano F, Capodanno D. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J. 2010;31:1382–9. doi: 10.1093/eurheartj/ehq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon K, McCarthy J, Goodson R, Stein J, O’Donnell D, Zyck K. Health care technology’s impact on medical malpractice. Crittenden Medical Insurance Conference; May 5– 6, 2005; Available at: www.kfplegal.com/articles/InsuranceJournalArticle.pdf. [Google Scholar]
- 3.Frenkel C. From the Editor: responsible progress in surgical innovation. A balancing act. American Medical Association virtual mentor. 2010 Feb;12(2):69–72. doi: 10.1001/virtualmentor.2010.12.2.fred1-1002. Available at: http://virtualmentor.ama-assn.org/2010/02/fred1-1002.html. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed October 26, 2010];American Medical Association code of medical ethics. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/history-ama-ethics.shtml.
- 5.Council on Ethical and Judicial Affairs. E-8.08 informed consent. American Medical Association code of medical ethics: current opinions with annotations, 2010–2011. Chicago: American Medical Association; 2010. [Accessed January 8, 1011]. pp. 260–1. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion808.shtml. [Google Scholar]
- 6. [Accessed October 26, 2010];American Medical Association informed consent. Available at: http://www.ama-assn.org/ama/pub/physician-resources/legal-topics/patient-physician-relationship-topics/informed-consent.shtml.
- 7.American Medical Association report of the Council on Ethical and Judicial Affairs. [Accessed October 26, 2010];CEJA report 2-A-06. Available at: http://www.ama-assn.org/ama/pub/about-ama/our-people/ama-councils/council-ethical-judicial-affairs/ceja-reports.shtml. Cited with permission.
- 8.Hamilton TE. Memorandum summary, April 13, 2007, revisions to hospital interpretive guidelines for informed consent. Department of Health and Human Services, Center for Medicare and Medicaid Services; Ref:S&C-07-17. [Google Scholar]
- 9.Hamilton TE. Memorandum summary, April 13, 2007, revisions to hospital interpretive guidelines for informed consent. Department of Health and Human Services, Centers for Medicare and Medicaid Services; Ref:S&C-07-17, Tag A-0238. [Google Scholar]
- 10.American College of Surgeons statements on principles. [Accessed October 26, 2010];Relation of the surgeon to the patient. Available at: http://www.facs.org/fellows_info/statements/stonprin.html#top.
- 11.DiMaio JM, Salerno TA, Bernstein R, Araujo K, Ricci M, Sade RM. Ethical obligation of surgeons to noncompliant patients: can a surgeon refuse to operate on an intravenous drug-abusing patient with recurrent aortic valve prosthesis infection? Ann Thorac Surg. 2009;88:1–8. doi: 10.1016/j.athoracsur.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 12.Meisel A. The “exceptions” to the informed consent doctrine: striking a balance between competing values in medical decision making. Wisc Law Rev. 1979;413:460. [PubMed] [Google Scholar]
- 13.American Medical Association report of the Council on Ethical and Judicial Affairs. [Accessed October 26, 2010];CEJA report 6-A-07. Available at: http://www.ama-assn.org/ama/pub/about-ama/our-people/ama-councils/council-ethical-judicial-affairs/ceja-reports.shtml. Cited with permission.
- 14.Herbert PC, Hoffmaster B, Glass KC, Singer PA. Bioethics for clinicians: 7. Truth telling. Can Med Assoc J. 1997;156:225–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Weeks JC, Cook F, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1708–14. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 16.Bok S. Lying: moral choice in public and private life. New York: Vintage Books; 1979. Lies to the sick and dying; p. 28. [Google Scholar]
- 17.Grover FL. The bright future of cardiothoracic surgery in the era of changing health care delivery: an update. Ann Thorac Surg. 2008;85:8–24. doi: 10.1016/j.athoracsur.2007.10.100. [DOI] [PubMed] [Google Scholar]
- 18.Jerry McGuire mission statement clip. [Accessed January 8, 2011];World News. Available at: http://wn.com/jerry_mcguire_mission_statement_clip [at 58 seconds]
- 19.Sinek SO. Start with why: how great leaders inspire everyone to take action. New York: Penguin Group; 2009. [Google Scholar]
- 20.Elstein AS. On the origins and development of evidence-based medicine and medical decision making. Inflam Res. 2004;53(Suppl 2):5184–189. doi: 10.1007/s00011-004-0357-2. [DOI] [PubMed] [Google Scholar]
- 21.Timmermans S, Mauck A. The promises and pitfalls of evidence-based medicine. Health Aff. 2005;24:18–24. doi: 10.1377/hlthaff.24.1.18. [DOI] [PubMed] [Google Scholar]
- 22.King RT. Keyhole heart surgery arrived with fanfare, but was it premature? Wall Street Journal. 1999 May 5;:A1. [Google Scholar]
- 23.Bergsland J, D’Ancona G, Karamanoukian H, et al. Technical tips and pitfalls in OPCAB surgery: the Buffalo experience. Heart Surg Forum. 2000;3:189–93. [PubMed] [Google Scholar]
- 24.Mack MJ. Coronary surgery: off-pump and port access. Surg Clin North Am. 2000;80:1575–91. doi: 10.1016/s0039-6109(05)70246-2. [DOI] [PubMed] [Google Scholar]
- 25.Sellke FW, Chu LM, Cohn WE. Current state of surgical myocardial revascularization. Circ J. 2010;74:1031–7. doi: 10.1253/circj.cj-10-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eng Rep. Slater v. Baker and Stapleton, 1767 (Slater v. Baker and Stapleton 95 Eng Rep. 860 [k.b. 1767]).
- 27.NE Schoendorff v. Society of New York Hospital, 1914 (106N.E. 93 [N.Y. 1914]).
- 28.WLR Bolam v. Friern Hospital Management Committee, 1957 (1 WLR 583).
- 29.F Canterbury v. Spence, 1972 (464 F.2d 772 [d.c. 1972]).
- 30.P Truman v. Thomas, 1980 (611 P.2d 902 [Cal 1980]).