Abstract
Background
Although existing literature demonstrates the association of attention-deficit/hyperactivity disorder (ADHD) with both substance use (SU) and autism spectrum disorder (ASD), few studies have examined rates of SU among adolescents with elevated ASD symptoms, with or without comorbid ADHD. Clinic-based studies suggest a possible protective effect of ASD against SU, but this has not been confirmed in population-based studies.
Objective
We examined alcohol, tobacco, and drug use in adolescents with either ADHD, elevated autistic traits, or both as compared with controls.
Methods
Subjects (N = 2937) who were 13 to 17 years old from a Missouri population-based large sibship sample were assessed for ADHD, autistic traits, and SU with the use of parent-report questionnaires. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition ADHD symptom criterion (Criterion A) was applied to the Strengths and Weaknesses of ADHD-symptoms and Normal-behavior (SWAN) questionnaire item responses to determine ADHD diagnosis. The presence of elevated autistic traits was defined as a raw Social Responsiveness Scale (SRS) score of 62 (95th percentile for this sample) or higher. SU was determined with the use of three items from the Child Behavior Checklist (CBCL). Statistical methods used included logistic and fractional polynomial regression.
Results
As compared with controls, adolescents with ADHD were at increased risk for alcohol, tobacco, and drug use whether or not they had elevated autistic traits. Adolescents with elevated autistic traits were at significantly increased risk for drug use other than alcohol and tobacco, even if they did not have ADHD. Among those with raw SRS scores in the range of about 20 (normal) to 80 (consistent with mild to moderate ASD), adolescents with ADHD had higher levels of SU than control individuals with similar levels of autistic traits. However, strong conclusions cannot be drawn regarding individuals with very low or very high SRS scores as a result of sparse data.
Conclusions
This study confirms previous research showing an increased risk of SU among adolescents with ADHD. It also provides new information indicating that adolescents with high levels of autistic traits are at elevated risk for alcohol and tobacco use if they have comorbid ADHD; in addition, they may be at high risk for other drug use, even if they do not have comorbid ADHD. Therefore, it should not be assumed that adolescents with mild to moderate ASD have a low risk of SU, especially if ADHD is also present.
Keywords: ADHD, autism, substance use, tobacco, alcohol
Introduction
Previous literature has strongly suggested that diagnosis of attention-deficit/hyperactivity disorder (ADHD) may place adolescents at elevated risk for the initiation of substance use (SU) and the development of substance use disorder (SUD) (1–3). For example, a recent meta-analysis of 27 prospective longitudinal studies that examined the association between childhood ADHD and lifetime SU and SUD found that a childhood diagnosis of ADHD was associated with a greater likelihood of using nicotine or another substance within one’s lifetime and also with developing a disorder of abuse or a dependence involving nicotine, alcohol, marijuana, cocaine, or another substance (4). Consistent with this view is the fact that epidemiological studies also suggest that ADHD and SUD frequently co-occur within the general population (5). Although the exact reason for this association remains unclear at the time of this writing, proposed explanatory mechanisms include psychosocial concerns (e.g., poor coping styles, inaccurate expectancies associated with use, limited knowledge of related health effects); cognitive or executive function issues (e.g., dysfunction of cognitive abilities related to impulsive behavior, such as reward delay aversion, reward/punishment sensitivity, inattention, and inhibitory control); and genetic or neurobiological concerns (e.g., genetically mediated monoaminergic neurotransmitter system dysfunction in frontostriatal brain regions) (6).
Although ADHD and autistic traits also co-occur in the general population at a rate greater than would be expected by chance (7), few studies have examined the risk for SU among adolescents with elevated levels of autistic traits. One study of children and adolescents (N = 414; mean age, 16.3 years; standard deviation, 4.6 years) who were identified through a Danish historic birth cohort reported that autism spectrum disorder (ASD) diagnosis may be associated with reduced risk for alcohol-related disorders as compared with those individuals without a diagnosis of ASD (8); however, the prevalence of SU (and non-alcohol-related SUDs) was not addressed. With the use of data obtained from a medical chart review, another study suggested that adolescents with ASD (N = 97; mean age, 14.3 years; standard deviation, 1.7 years) had a lower prevalence of using drugs, alcohol, or both as compared with patients with other psychiatric diagnoses (3% vs. 17%, respectively). When individuals with ASD did use drugs or alcohol, they co-presented with symptoms of ADHD (9). Although this study was among the first to discuss the co-presentation of ADHD, autistic traits, and the use of psychoactive substances, it did not comment on the extent to which ADHD and ASD traits were specifically related to the use of alcohol, tobacco, and other drugs among adolescents.
The current study examined whether alcohol, tobacco, and drug use in adolescents with ADHD, autistic traits, or both was elevated as compared with controls in a non-clinical, population-based sample. It was hypothesized that adolescents with high levels of autistic traits would have relatively low levels of SU, likely because they have less opportunity for the initiation of SU as a result of having fewer social contacts and decreased access to drugs as compared with typically developing individuals (10). However, given past research, which indicates that individuals with the combination of ASD and ADHD may be at increased risk for SU (9), we expected that adolescents with ADHD would show increased rates of SU even if they presented with moderately elevated levels of autistic traits.
Methods
Sample
The study subjects were members of the Missouri large sibship sample (MO-BIGSIBS), which consists of families with four or more children born in the state of Missouri (as determined through a birth records database) (11). Because a later phase of the parent study would require DNA from the parents and their children, families were only screened if both biological parents were available (i.e., neither parent could be deceased or incarcerated). Families were excluded at the birth records review or tracking level if they were known to have twins (to ensure the availability of twins for other studies), if they had a level of difficulty with English that would otherwise prevent English-language telephone screening, or if one of the parents was deceased. Individual offspring were excluded during the parent-report telephone screening if the child was adopted or not a full sibling or if the parent reported that the child had a diagnosis of autism, major hearing impairment, or a significant medical illness such as cancer, Down’s syndrome, or intellectual disability. Because the original recruitment protocol did not allow interviewers to ask specifically about medical diagnoses, individual children were only excluded if the parent volunteered information about a child being diagnosed with any of these conditions (11).
Informed consent was obtained from respondent parents before the initial screening interview. Parents were informed that phase 1 of the study would include an initial telephone interview in addition to the completion of questionnaires regarding their children’s behaviors. They were additionally informed that they might be contacted in the future to participate in additional assessments, depending on the answers that they provided in response to the initial telephone screening. The results of the current article are based only on phase 1 parent-report screening data. The Washington University Human Research Protection Office reviewed and approved the study’s protocols, including the consenting procedures.
The total MO-BIGSIBS sample included 22,581 offspring with complete data regarding sex, age at screening, and ADHD screening interview items (11). Analyses for the current study made use of information obtained for an adolescent subset of the sample (i.e., those 13 to 17 years old) for whom we had complete data regarding their sex, age, and lifetime ADHD symptoms assessed by the screening interview plus questionnaire items required for this analysis (N = 2937). Of the 2937 subjects, 98% were Caucasian, and 51% were male.
Measures
The best informant parent, according to the parents’ opinions (usually the mother), completed behavioral rating questionnaires regarding offspring behavior, including the Strengths and Weaknesses of ADHD-symptoms and Normal-behavior (SWAN) scale, the Social Responsiveness Scale (SRS), and the Child Behavior Checklist (CBCL).
The SWAN scale contains 18 items that assess Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) ADHD Criterion A symptoms, each on the basis of a 7-point rating scale (i.e., −3 to +3) (12). Positive scores indicate problem behaviors, and negative scores suggest better-than-average behaviors. Items from the SWAN scale were used to define ADHD diagnosis at time of assessment based on the symptom criterion (Criterion A) of the DSM-IV (13), which lists the 18 symptoms of ADHD and describes the threshold number of symptoms required for diagnosis. SWAN-based ADHD symptoms were counted as present if the parent rated the child at a level of 1 or higher, which is a cutoff that has previously been shown to correspond with the 90th to 95th percentile for each item (11). Although the strict application of full DSM-IV criteria was not possible given a lack of information regarding age of onset (Criterion B), multiple settings (Criterion C), current impairment caused by ADHD symptoms (Criterion D), and disorders that exclude an ADHD diagnosis (Criterion E), our method of estimating DSM-IV ADHD diagnosis produced ADHD prevalence estimates for the state of Missouri that are similar to those of the Centers for Disease Control and Prevention (13).
The SRS is a 65-item questionnaire that is sensitive to autistic traits (14–16). It shows moderate to high correlation with algorithm scores from the Autism Diagnostic Interview-Revised (14,16), a structured psychiatric interview designed to establish a diagnosis of ASD (17). It also shows moderate correlation with the Autism Diagnostic Observation Schedule (16), a semi-structured observational assessment instrument designed to elicit criterion symptoms related to autism (18). In the current study, the presence of a high level of autistic traits was defined by a raw SRS score of at least 62. This corresponded to an autistic trait score that is more severe than that of 95% of the present sample and to a clinically significant level of autistic traits associated with at least mild to moderate levels of interference with everyday social interactions in both male and female participants (male T-score, 61; female T-score, 64), according to the recently updated SRS-2 manual (15). Although clinically relevant SRS T-scores are calculated on the basis of comparison with same-sex peers, the use of an identical threshold for males and females may better estimate the absolute level of autistic traits (7,19) because DSM-IV criteria do not delineate sex-specific differences in ASD symptomatology. As such, we chose to use a single raw SRS score threshold for both sexes.
The CBCL is a well-validated measure of psychopathology that covers several problem behaviors of interest, including three items related to SU (20): “Drinks alcohol without parents’ approval,” “Smokes, chews, or sniffs tobacco,” and “Uses drugs for non-medical purposes (don’t include alcohol or tobacco).” In the current study, these items were used to measure alcohol, tobacco, and other drug use, respectively. These items were rated on a 3-point scale, with 0 meaning “Not True (as far as you know)”, 1 meaning “Somewhat or Sometimes True” (assumed to indicate “some” or moderate use), and 2 meaning “Very True or Often True” (assumed to indicate a frequent or “high” level of use).
Data analyses
The analyses were conducted with the use of STATA 12 data analysis and statistical software (21). In Table 1, the prevalences of “some,” “high,” and “any” (either “some” or “high”) use of alcohol, tobacco, and other drugs are reported for adolescents with ADHD only (ADHD+SRS−), high SRS score only (ADHD−SRS+), both ADHD and high SRS score (ADHD+SRS+), and controls (ADHD−SRS−). To address concerns regarding a lack of power to fully examine “some” versus “high” use, CBCL items were dichotomized to indicate “no” use versus “any” use for the main analysis. Logistic regression analyses were then applied to investigate whether the relevant diagnostic groups were at elevated risk for “any” use of alcohol, tobacco, or other drugs during adolescence (Table 2). Logistic regression models included diagnostic group (with ADHD−SRS− as the comparison class), sex, and age as independent variables. Standard errors were adjusted to account for family clustering by using the “Cluster” option available in STATA 12 (21). With the use of each of the three CBCL SU items, standardized scores (mean, 0; SD, 1) were then created to measure SU severity. The three standardized SU items were also combined to create a score that indicated overall SU severity. For this overall SU severity scale, the average inter-item correlation was 0.45, and the scale reliability coefficient (Cronbach’s alpha) was 0.71. Fractional polynomial regression plots were used to estimate the mean values of the standardized SU scores across a range of autistic trait severity as measured by the SRS (Figures 1 through 4). These analyses were performed separately for adolescents with and without ADHD.
TABLE 1.
Prevalence of reported alcohol, tobacco, and other drug use in adolescents between the ages of 13 and 17 years separated by attention-deficit/hyperactivity disorder diagnosis and Social Responsiveness Scale scores (N = 2937)
| Alcohol Use | “Some” Use | “High” Use | “Any” Use |
|---|---|---|---|
| ADHD−SRS− (n = 2586) | 5.5% (n = 142) | 0.5% (n = 13) | 6.0% (n = 155) |
| ADHD−SRS+ (n = 61) | 6.6% (n = 4) | 1.6% (n = 1) | 8.2% (n = 5) |
| ADHD+SRS− (n = 207) | 15.0% (n = 31) | 2.9% (n = 6) | 17.9% (n = 37) |
| ADHD+SRS+ (n = 83) | 10.8% (n = 9) | 3.6% (n = 3) | 14.4% (n = 12) |
| Tobacco Use | “Some” Use | “High” Use | “Any” Use |
|---|---|---|---|
| ADHD−SRS− (n = 2586) | 1.2% (n = 31) | 0.7% (n = 18) | 1.9% (n = 49) |
| ADHD−SRS+ (n = 61) | 3.3% (n = 2) | 3.3% (n = 2) | 6.6% (n = 4) |
| ADHD+SRS− (n = 207) | 6.8% (n = 14) | 4.3% (n = 9) | 11.1% (n = 23) |
| ADHD+SRS+ (n = 83) | 6.0% (n = 5) | 9.6% (n = 8) | 15.6% (n = 13) |
| Other Drug Use | “Some” Use | “High” Use | “Any” Use |
|---|---|---|---|
| ADHD−SRS− (n = 2586) | 0.9% (n = 23) | 0.2% (n = 5) | 1.1% (n = 28) |
| ADHD−SRS+ (n = 61) | 4.9% (n = 3) | 3.3% (n = 2) | 8.2% (n = 5) |
| ADHD+SRS− (n = 207) | 6.3% (n = 13) | 1.0% (n = 2) | 7.3% (n = 15) |
| ADHD+SRS+ (n = 83) | 9.6% (n = 8) | 2.4% (n = 2) | 12.0% (n = 10) |
ADHD, Attention-deficit/hyperactivity disorder diagnosis; SRS, Social Responsiveness Scale elevated score; +, present; −, absent
TABLE 2.
Summary of logistic regression analyses predicting alcohol, tobacco, and other drug use (N = 2937)
| Predictor of Alcohol Use | Odds Ratio | P Value | 95% Confidence Interval |
|---|---|---|---|
| ADHD−SRS+ | 1.06 | .912 | 0.38 to 2.92 |
| ADHD+SRS− | 4.37 | <.001 | 2.88 to 6.44 |
| ADHD+SRS+ | 2.44 | .010 | 1.24 to 4.81 |
| Male gender | 1.11 | .525 | 0.81 to 1.51 |
| Age | 2.38 | <.001 | 2.08 to 2.72 |
| Predictor of Alcohol Use | Odds Ratio | P Value | 95% Confidence Interval |
|---|---|---|---|
| ADHD−SRS+ | 2.70 | .090 | 0.86 to 8.55 |
| ADHD+SRS− | 6.47 | <.001 | 3.79 to 11.05 |
| ADHD+SRS+ | 8.35 | <.001 | 4.19 to 16.65 |
| Male gender | 1.98 | .007 | 1.20 to 3.26 |
| Age | 2.04 | <.001 | 1.68 to 2.47 |
| Predictor of Alcohol Use | Odds Ratio | P Value | 95% Confidence Interval |
|---|---|---|---|
| ADHD−SRS+ | 6.91 | <.001 | 2.41 to 19.84 |
| ADHD+SRS− | 8.07 | <.001 | 4.13 to 15.80 |
| ADHD+SRS+ | 11.96 | <.001 | 5.49 to 26.04 |
| Male gender | 1.28 | .422 | 0.70 to 2.35 |
| Age | 1.99 | <.001 | 1.59 to 2.49 |
ADHD, Attention-deficit/hyperactivity disorder diagnosis; SRS, Social Responsiveness Scale elevated score; +, present; −, absent. Bold type indicates statistical significance (p<.05)
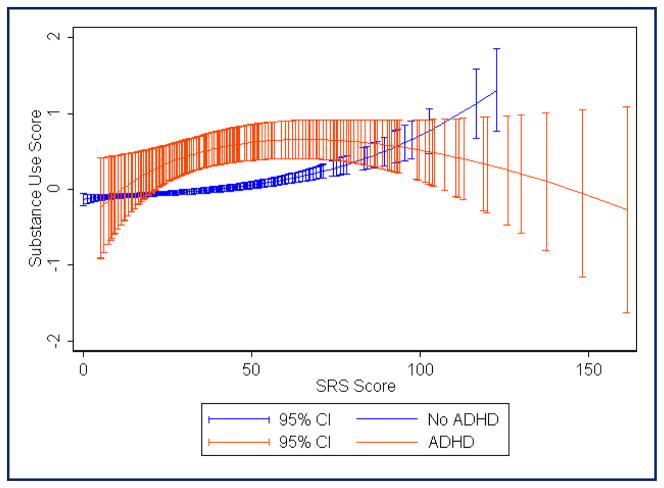
FIGURE 1.
Fractional polynomial regression plot showing estimated mean substance use score (includes alcohol, tobacco, and other drugs; score standardized with mean = 0, standard deviation = 1) across the range of Social Responsiveness Scale raw scores. Red, Attention-deficit/hyperactivity disorder (ADHD) diagnosis; blue, no ADHD diagnosis. Total N = 2937 (290 with ADHD)
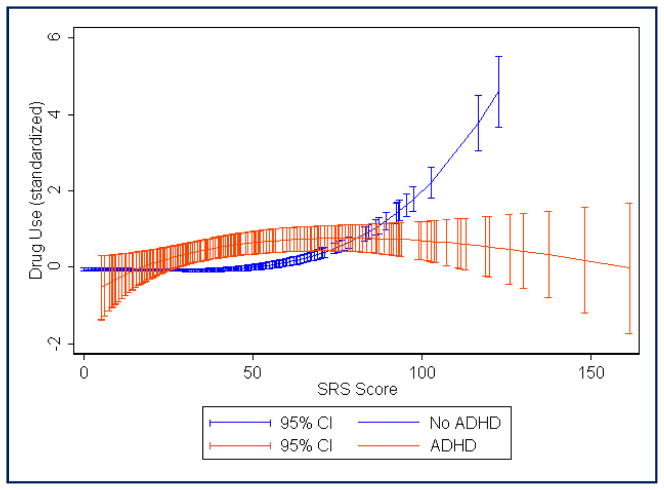
FIGURE 4.
Fractional polynomial regression plot showing estimated mean “other drug” use score (standardized with mean = 0, standard deviation = 1) across the range of Social Responsiveness Scale raw scores. Red, Attention-deficit/hyperactivity disorder (ADHD) diagnosis; blue, no ADHD diagnosis. Total N = 2937 (290 with ADHD)
Results
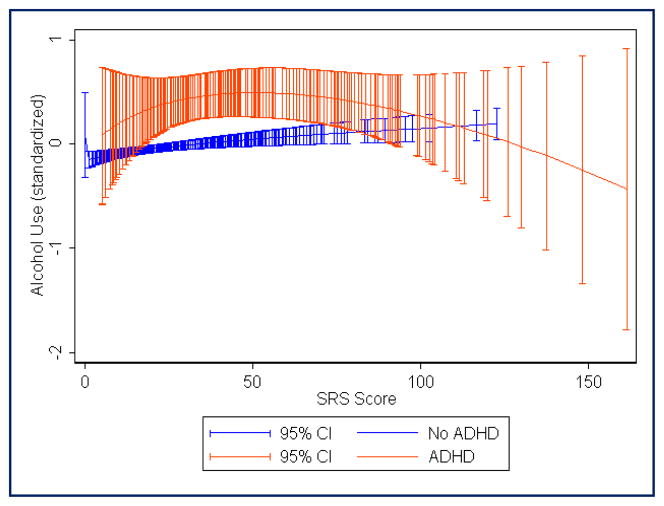
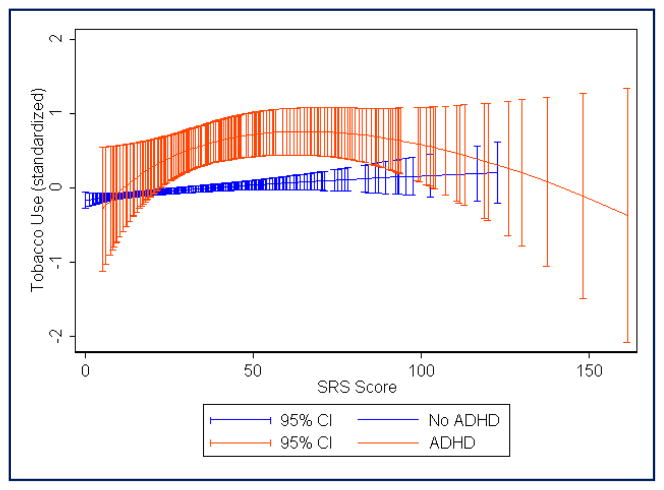
Table 1 shows the prevalence of alcohol, tobacco, and other drug use in each of the subject groups. Logistic regression analyses (see Table 2) showed that, as compared with the control group (ADHD−SRS−), adolescents with ADHD were at elevated risk for alcohol, tobacco, and drug use whether or not they had autistic traits above the 95th percentile. Adolescents with elevated autistic traits were not clearly at risk for alcohol or tobacco use unless they also had ADHD, but individuals with high levels of autistic traits were at significantly increased risk for “other drug” use as compared with controls, regardless of whether they had ADHD. For alcohol use, the highest odds ratio (0R, 4.37) was seen for the ADHD+SRS− group. However, for tobacco and other drugs, ORs were highest for the ADHD+SRS+ group (8.35 and 11.96, respectively). There were no clear differences in SU risk when comparing the ADHD+SRS+ group with the ADHD+SRS− and ADHD−SRS+ groups, because the odds ratio confidence intervals overlapped. Males were more likely to use tobacco, but sex effects were not statistically significant for alcohol and drug use. Trends observed in the fractional polynomial regression plots (see Figures 1 through 4) suggested that adolescents with the combination of ADHD and very high SRS scores (i.e., raw SRS scores of approximately ≥100) may have lower SU as compared with those with moderate SRS scores (i.e., raw scores in the range of 40 to 80). In those without ADHD, mean SU scores (especially in the case of “other drugs”) continued to trend upward with increasing SRS scores, even among individuals with the highest levels of autistic traits (see Figures 1 through 4). It is unclear whether this is partly an artifact related to the small number of individuals without ADHD who have very high SRS scores.
Discussion
Adolescents with ADHD are at elevated risk for alcohol, tobacco, and other drug use regardless of co-occurring autistic traits. Adolescents with autistic traits may have elevated risk for drug use (other than alcohol and tobacco), even if they do not have ADHD. However, these findings must be considered within the context of several limitations of this study.
Given the non-specificity of the wording regarding the “other drugs” item, it is unclear whether the reported use of “other drugs” refers to the use of street drugs, the non-medical use of prescription or over-the-counter pharmaceuticals, or some combination of these. Because this study is based on cross-sectional data, the direction of causality is also unclear. For example, the use of cannabis could actually cause social withdrawal symptoms that may increase the level of autistic traits as measured by the SRS.
Information regarding the age of onset of ADHD symptoms and the current functional impairments related to these ADHD symptoms was not available, thereby making it impossible to establish a strict DSM-IV diagnosis of ADHD. As such, it is possible that our estimate of the prevalence of a current diagnosis of ADHD in Missouri adolescents (9.9%) could have been higher than what would otherwise have been observed if strict DSM-IV ADHD criteria had been applied. Nevertheless, other studies that have recently attempted to estimate the prevalence of a current diagnosis of ADHD in adolescent samples from the United States have reported estimates somewhat consistent with our own. For example, in a parent-report–based national Centers for Disease Control and Prevention survey that examined the prevalence of ADHD among American children and adolescents between the ages of 4 and 17 years in 2007 (i.e., the National Survey of Children’s Health), 9.3% of the parents reported that their adolescent children had a current diagnosis of ADHD when their children were between the ages of 15 and 17 years, and 10.8% of parents in Missouri reported that their children had lifetime histories that included diagnosis with ADHD (22). Thus, as discussed in previous reports (11,13), it may be possible to generate reliable estimates of the prevalence of ADHD through the application of the DSM-IV symptom criterion (Criterion A) to SWAN items even when information regarding the age of onset or functional impairment is absent.
Our sample was composed of adolescents from stable families with four or more children. Families with parents who were incarcerated for criminal behavior were excluded from participation. Responses on CBCL items related to SU were based on parental report as opposed to self-report, and the prevalence of ADHD and high levels of autistic traits is expected to be low within a general population sample such as our own. All of these factors may have contributed to the small number of participants who were identified as using substances of abuse in our study. Other studies that have examined the prevalence of SU in Missouri-based samples of adolescents have reported a higher prevalence of SU. For example, in a national self-report–based Centers for Disease Control and Prevention survey of adolescents in the ninth through twelfth grades that was performed in the United States in 2009 (i.e., the High School Youth Risk Behavior Survey), 39.3% of Missouri-based adolescents reported that they had had at least one drink of alcohol on at least one day during the 30 days before the survey was administered, and 70.5% reported that they had ever had at least one drink of alcohol in their lifetime (23). In this same survey, 25.5% of the sample reported that they had either smoked cigarettes, cigars, or cigarillos (little cigars) or used chewing tobacco, snuff, or dip on at least 1 day during the 30 days before the survey, and 46.5% reported that they had ever tried cigarette smoking in their lifetime (even just one or two puffs). In addition, 20.6% of the sample reported having used marijuana one or more times during the 30 days before the survey, and 34.9% reported that they had ever used marijuana one or more times in their lifetime. In the present study, we observed that 7.1% of parents of stable families with four or more children in Missouri indicated that their children between the ages of 13 and 17 years had consumed alcohol without parental approval; 3.0% of the parents endorsed the item that asked whether their children had smoked, chewed, or sniffed tobacco, and 2.0% endorsed the item that asked whether their children used drugs other than alcohol or tobacco for non-medical purposes. The discrepancies between these estimates and those provided by other studies raise the concern that parents may not have been fully aware of the their children’s SU or that the prevalence of SU in stable Missouri families with four or more children that do not include parents who have been incarcerated for criminal behavior could be lower than what may be observed in the general population. Additional work that examines larger samples and that involves more detailed measures of SU behaviors across time will be needed to further explore the complex relationship between ADHD, autistic traits, and SU disorders.
Despite these limitations, it should be noted that the purpose of the present study was not to estimate the prevalence of ADHD or SU in Missouri adolescents but rather to determine whether the level of risk for SU was elevated by the presence of clinically relevant levels of ADHD symptoms and autistic traits. In this regard, it is remarkable that significant associations were found. In another recently published study that examined the risk for self-reported regular SU in a population-representative sample of adult Australian twins (N= 3080), clinically relevant levels of ADHD symptoms and autistic trait scores were not only associated with elevated levels of regular smoking and cannabis but were also associated with the risk of dependence on these substances. Although those individuals with high autistic trait scores were less likely to report drinking alcohol to intoxication, after the initiation of the regular use of alcohol, these individuals were at elevated risk for developing alcohol dependence, even when the influence of ADHD was controlled (24). This finding highlights the importance of examining the risk for SU in individuals with clinically relevant symptoms of ADHD and autistic traits. These individuals may not only be at risk for SU, but they may also be at risk for dependence on these substances after the initiation of their use.
FIGURE 2.
Fractional polynomial regression plot showing estimated mean alcohol use score (standardized with mean = 0, standard deviation = 1) across the range of Social Responsiveness Scale raw scores. Red, Attention-deficit/hyperactivity disorder (ADHD) diagnosis; blue, no ADHD diagnosis. Total N = 2937 (290 with ADHD)
FIGURE 3.
Fractional polynomial regression plot showing estimated mean tobacco use score (standardized with mean = 0, standard deviation = 1) across the range of Social Responsiveness Scale raw scores. Red, Attention-deficit/hyperactivity disorder (ADHD) diagnosis; blue, no ADHD diagnosis. Total N = 2937 (290 with ADHD)
Clinical Significance.
Mental health providers should not assume that adolescents with mild to moderate ASD are at low risk for SU (25), especially if they have coexisting ADHD. Monitoring for SU is important for individuals with ADHD or ASD and particularly in those who exhibit symptoms of both disorders. At this time, is it unclear whether SU treatment should be modified in any way for individuals with ASD or autistic traits, so this topic is also worthy of further investigation.
Acknowledgments
This work was supported by grant numbers MH067921, MH080287, and MH083823 from the National Institute of Mental Health and by a grant from the McDonnell Center for Systems Neuroscience. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Disclosures
All authors have received research grant support from the National Institutes of Health. AMR has received grant support from the McDonnell Center for Systems Neuroscience and the McDonnell Center for Cellular and Molecular Neurobiology, travel support and speaker’s honorarium support from the American Academy of Child & Adolescent Psychiatry, and travel support from the Child and Adolescent Psychiatric Department, Region Zealand (a hospital in Denmark), to attend and speak at a scientific meeting. None of the authors have financial relationships with commercial industry.
References
- 1.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64(10):1145–52. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 2.Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 3.Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis. 1997;185(8):475–82. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31(3):328–41. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry. 2005;57(11):1442–51. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass K, Flory K. Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clin Child Fam Psychol Rev. 2010;13(3):291–313. doi: 10.1007/s10567-010-0070-3. [DOI] [PubMed] [Google Scholar]
- 7.Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48(5):464–72. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah MW, Greaves-Lord K, Grove J, Norgaard-Pedersen B, Hougaard DM, Mortensen EL. Psychiatric comorbidities in autism spectrum disorders: findings from a Danish historic birth cohort. Eur Child Adolesc Psychiatry. 2011;20(11–12):599–601. doi: 10.1007/s00787-011-0220-2. [DOI] [PubMed] [Google Scholar]
- 9.Santosh PJ, Mijovic A. Does pervasive developmental disorder protect children and adolescents against drug and alcohol use? Eur Child Adolesc Psychiatry. 2006;15(4):183–8. doi: 10.1007/s00787-005-0517-0. [DOI] [PubMed] [Google Scholar]
- 10.Ramos M, Boada L, Moreno C, Llorente C, Romo J, Parellada M. Attitude and risk of substance use in adolescents diagnosed with Asperger syndrome. Drug Alcohol Depend. 2013;133(2):535–40. doi: 10.1016/j.drugalcdep.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49(3):217–28. e1–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson J, Shnuck S, Mann M, Carlson C, Hartman K, Sergeant J, et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: The SNAP and the SWAN rating scales. Available from: www.adhd.net. [PMC free article] [PubMed]
- 13.Reiersen AM, Todorov AA. Exploration of ADHD subtype definitions and co-occurring psychopathology in a Missouri population-based large sibship sample. Scand J Child Adolesc Psychiatr Psychol. 2013;1(1):3–13. doi: 10.21307/sjcapp-2013-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–33. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 15.Constantino JN, Gruber CP. Social Responsiveness Scale. 2. Torrance, CA: Western Psychological Services; 2012. (SRS-2) [Google Scholar]
- 16.Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1668–76. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- 17.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 18.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Services; 2001. [Google Scholar]
- 19.Reiersen AM, Constantino JN, Todd RD. Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiary. 2008;47(6):662–72. doi: 10.1097/CHI.0b013e31816bff88. [DOI] [PubMed] [Google Scholar]
- 20.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 21.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children -- United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59(44):1439–43. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Youth risk behavior surveillance - United States, 2009. Surveillance Summaries. MMWR. 2010;59:SS–5. [PubMed] [Google Scholar]
- 24.De Alwis D, Agrawal A, Reiersen AM, Constantino JN, Henders A, Martin NG, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs. 2014;75(2):211–21. doi: 10.15288/jsad.2014.75.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmqvist M, Edman G, Bolte S. Screening for substance use disorders in neurodevelopmental disorders: a clinical routine? Eur Child Adolesc Psychiatry. 2013;23(5):365–68. doi: 10.1007/s00787-013-0459-x. [DOI] [PubMed] [Google Scholar]