Abstract
The discovery of fibroblast growth factor 23 (FGF-23) has expanded our understanding of phosphate and vitamin D homeostasis and provided new insights into the pathogenesis of hereditary hypophosphatemic and hyperphosphatemic disorders, as well as acquired disorders of phosphate metabolism, such as chronic kidney disease. FGF-23 is secreted by osteoblasts and osteocytes in bone and principally targets the kidney to regulate the reabsorption of phosphate, the production and catabolism of 1,25-dihydroxyvitamin D and the expression of α-Klotho, an anti-ageing hormone. Secreted FGF-23 plays a central role in complex endocrine networks involving local bone-derived factors that regulate mineralization of extracellular matrix and systemic hormones involved in mineral metabolism. Inactivating mutations of PHEX, DMP1 and ENPP1, which cause hereditary hypophosphatemic disorders and primary defects in bone mineralization, stimulate FGF23 gene transcription in osteoblasts and osteocytes, at least in part, through canonical and intracrine FGF receptor pathways. These FGF-23 regulatory pathways may enable systemic phosphate and vitamin D homeostasis to be coordinated with bone mineralization. FGF-23 also functions as a counter-regulatory hormone for 1,25-dihydroxyvitamin D in a bone–kidney endocrine loop. FGF-23, through regulation of additional genes in the kidney and extrarenal tissues, probably has broader physiological functions beyond regulation of mineral metabolism that account for the association between FGF-23 and increased mortality and morbidity in chronic kidney disease.
Introduction
In the past decade, bone has been recognized to be an endocrine organ that releases at least two hormones into the circulation, osteocalcin and fibroblast growth factor 23 (FGF-23). Osteocalcin is proposed to participate in an endocrine axis whereby bone regulates energy metabolism. This axis is initiated by the systemic release of undercarboxylated osteocalcin from bone resorption, which regulates insulin secretion, insulin sensitivity and energy expenditure by the activation of GPRC6A, a G-protein coupled receptor located in β cells and other target tissues.1–3 A second endocrine axis, which involves the release of the hormone FGF-23 from osteoblasts and osteocytes, regulates systemic phosphate homeostasis and vitamin D metabolism.4 This Review focuses on the rapidly growing knowledge of this regulation by FGF-23.5
FGF-23, along with FGF-19 and FGF-21, is a member of the FGF family, which are released into the circulation owing to their low binding affinity for heparin. These proteins act as endocrine factors by virtue of an evolutionarily engineered C-terminus that permits interaction with FGF receptor–α-Klotho co-receptor complexes in cell membranes of target tissues. These hormonal FGFs differ from canonical FGFs that have high heparin affinity and act as extracellular autocrine or paracrine ligands for nearby cell-surface membrane FGF receptors.5 They also differ from intracellular, nuclear isoforms of FGFs, as exemplified by high-molecular weight (HMW)-FGF-2, which interact with intranuclear FGFR-1 to directly activate gene transcription.6 FGF-19 regulates bile acid metabolism in the liver, whereas FGF-21 regulates lipid metabolism in white adipose tissue.
Our understanding of the role of FGF-23 is changing the traditional view of the regulation of several physiological processes, including bone mineralization, phosphate homeostasis and vitamin D metabolism. Endocrine networks that have become apparent from the unveiling of FGF-23 regulation and functions are also modifying our categorization of hereditary hypophosphatemic and hyperphoshatemic disorders. Furthermore, this new knowledge is challenging how several diseases are conceptualized, including the definition of vitamin D deficiency, the pathogenesis of secondary hyperparathyroidism in chronic kidney disease (CKD) and the relationship between disordered mineral metabolism and cardiovascular mortality.
Overview of FGF-23
FGF-23 is a ~32 kDa protein with an N-terminal FGF homology domain and a novel 72-amino-acid C-terminus.7 Both osteoblasts and osteocytes produce and secrete FGF-23.7–10 Circulating FGF-23 binds to and activates receptor complexes consisting of FGFR-1, FGFR-3 or FGFR-4 and the transmembrane β glucuoronidase α-Klotho,11–14 which is located in target tissues. Excess FGF-23 in both humans and mouse models causes hypophosphatemia, suppression of 1,25-dihydroxyvitamin D levels and rickets or osteomalacia.15–19
The major target for FGF-23 is the kidney, where increments in FGF-23 inhibit renal phosphate reabsorption by decreasing the expression and membrane insertion of Na+-dependent co-transporters. In the kidney, excess FGF-23 also suppresses circulating levels of 1,25-dihydroxyvitamin D by inhibiting the enzyme CYP27B1 (which converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D) and possibly by stimulating the catabolism of 1,25-dihydroxyvitamin D by activating 24-hydroxylase (CYP24) in the proximal tubule.15–16,20–24 Elevated FGF-23 is associated with reduced α-Klotho expression and preliminary studies suggest that FGF-23 directly suppresses α-Klotho message expression in the distal tubule.25,26 Many unanswered questions exist regarding FGF-23 effects on specific tubular segments (proximal and/or distal), the specific FGFR receptors mediating its effects (that is, FGFR-1, FGFR-3 and/or FGFR-4) and the full complement of gene products directly and/or indirectly regulated by FGF-23 in the kidney,14,27 By contrast, reductions in FGF-23 in both humans and mouse genetic models are known to cause tumoral calcinosis, characterized by hyperphosphatemia, increased 1,25-dihydroxyvitamin D and soft tissue calcifications.9,20,28–32
Mutations that elevate FGF-23 levels
The study of hypophosphatemic disorders, which are caused by eight distinct gene mutations that result in a common phenotype,27 has contributed to our understanding of the regulation and function of FGF-23 (Table 1). Study of autosomal dominant hypophosphatemic rickets, which is caused by mutations in an RXXR site in FGF-23 that prevents its cleavage, helped to define the functional role of FGF-23 as an essential hormone that regulates phosphate and vitamin D metabolism. Comparative analysis of the other seven mutations associated with the hypophosphatemic disorders X-linked hypophosphatemic rickets, autosomal dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets 1, autosomal recessive hypophosphatemic rickets 2, osteoglophonic dysplasia, McCune–Albright syndrome and Jansen metaphyseal chondrodysplasia provided insights into local and systemic regulation of FGF23 gene transcription in osteoblasts and osteocytes (Table 1).4,27,33 In addition to these disorders, epidermal nevus, which can result from somatic mosaicism for mutations in the FGFR3 gene or the PIK3CA gene,34 is associated with increased circulating FGF-23 levels.35 Opsismodysplasia, a rare hereditary spondylo(epi) chondrodysplasia, is also characterized by hypophosphatemia, abnormal vitamin D metabolism and elevated FGF-23 levels, but the disease-causing gene mutation has not been identified.36 Elevated FGF-23 levels also cause acquired hypophosphatemic disorders, such as tumor-induced osteomalacia, and are an important adaptive response in CKD.37
Table 1.
Genetic abnormalities that increase FGF-23 expression in bone
| Genetic abnormalities | Type of mutation | |||||||
|---|---|---|---|---|---|---|---|---|
| Inactivating | Activating | |||||||
| Hypophosphatemic disorder | ARHR1 | ARHR2 | None | XLH | OGD | None | MAS | JMC |
| OMIM# | 241520 | 613312 | None | 307800 | 166250 | None | 174800 | 168468 |
| Mutated gene | DMP1 | ENPP1 | ANK1 | PHEX | FGFR1 | FGF2-HMW | GNAS | PTH/PTHr receptor |
| Mouse model | Dmp1−/− | ;Enpp1−/− | AnkKI/KI | Hyp | None | Tg-FGF2-HMW | Tg-PTH/PTHr receptor | Tg-PTH/PTHr receptor |
Abbreviations: ARHR, autosomal recessive hypophosphatemic rickets; FGF2-HMW, high-molecular-weight FGF2; Hyp, mouse model of XLH; JMC, Jansen metaphyseal chondrodysplasia; MAS, McCune–Albright syndrome; OGD, osteoglophonic dysplasia; Tg, transgene; XLH, X-linked hypophosphatemic rickets.
Mutations causing impaired mineralization
Why is FGF-23 produced predominately by osteoblasts and osteocytes in bone? Studies of X-linked hypophosphatemia and autosomal recessive hypophosphatemic rickets in both humans and mouse models indicate that extracellular matrix mineralization in bone is linked to renal handling of phosphate through the release of FGF-23. X-linked hypophosphatemia in humans,27 and the corresponding hypophosphatemic (Hyp) mouse model, are caused by mutations of the PHEX gene, which encodes the endopeptidase PHEX (also known as HYP).9,38 The Phex gene in mice is highly expressed in differentiated osteoblasts and osteocytes, and conditional deletion of Phex in a mature osteoblast lineage in vivo in mice is sufficient to reproduce the Hyp phenotype.39 Although an initial study suggested that PHEX metabolizes FGF-23,40 subsequent studies failed to establish PHEX-dependent cleavage of FGF-23 in vitro.8,41,42 Rather, Phex mutations lead to elevations of FGF-23 levels owing to increased Fgf23 gene transcription in osteoblasts and osteocytes in mice.43 In this regard, increased Fgf23 gene transcription is present in isolated osteoblasts and bone marrow stromal cell cultures that differentiate into osteoblasts derived from Hyp mice and in Hyp bone explanted to wild-type mice.38
Autosomal recessive hypophosphatemic rickets 1, caused by inactivating mutations of DMP1 in humans44 and the corresponding Dmp1−/− mouse model,45 results in intrinsic stimulation of FGF-23 expression in osteoblasts and osteocytes. DMP-1 is a SIBLING (small integrin-binding ligand, N-linked glycoprotein) extracellular matrix protein that acts as a nucleator of mineralization and activates signaling pathways in osteoblasts and osteocytes via extracellular matrix–cell-surface interactions.46 DMP-1 is cleaved in vitro into 37 kDa and 57 kDa fragments by BMP-147 and MMP-2.48 The NH2-terminal fragment is a proteoglycan with a chondroitin sulfate chain attached at serine position 74 that binds to pro-MMP-9 and might sequester growth factors,49 whereas the C-terminal fragment has an RGD recognition sequence for binding to integrins and an ASARM motif that may mediate binding to PHEX. Phex and Dmp1 mutations in mice result in impaired mineralization that is independent of the concomitant hypophosphatemia.38,45 This impairment may be related to PHEX binding to ASARM peptides that are located within SIBLING proteins, such as MEPE44 and DMP-1, which act to inhibit mineralization.50 Nonadditive effects on FGF-23 expression are observed in compound mutant Hyp/Dmp1−/− mice, which suggests that common intrinsic abnormalities of mineralization and/or alterations of the extracellular matrix milieu are coupled to FGF-23 expression.4,51–53
Two additional genetic abnormalities regulating pyrophosphate metabolism also support a coupling of mineralization with FGF-23 expression in mouse models. Inorganic pyrophosphate, an inhibitor of calcification, is generated by the enzyme E-NPP1 and transported by ANK-1 into the extracellular matrix environment, where it is converted to the inorganic phosphate required for matrix mineralization by the alkaline phosphatase enzyme TNAP, which is expressed in osteoblasts. Inactivating mutations of ENPP1, which cause hereditary generalized arterial calcification of infancy, also cause autosomal recessive hypophosphatemic rickets 2, which is characterized by FGF-23-mediated hypophosphatemia.54,55 The mechanism whereby E-NPP1 increases FGF-23 expression is not known; however, the inactivation of E-NPP1 (which reduces pyrophosphate levels in soft tissues and causes ectopic calcification), also deprives TNAP of its substrate, which results in local phosphate depletion in bone that leads to osteomalacia and increased FGF-23 expression. In addition, inactivation of ANK-1, a pyrophosphate transporter located in osteoblasts, results in impaired mineralization of extracellular matrix and nearly a 10-fold increase in FGF-23 expression in bone in mice.56
In summary, mutations in the genes that encode DMP-1, PHEX, ANK-1 and E-NPP1, which block the mineralization of extracellular matrix, lead to increased FGF-23 production by osteoblasts and osteocytes.57,58 These observations suggest a physiological need to coordinate bone mineralization and kidney handling of phosphate and vitamin D metabolism via the release of FGF-23.
Mutations linking mineralization and FGF-23
A major knowledge gap exists for the mechanism whereby ‘intrinsic factors’ disrupting the process of bone mineralization (namely, by inhibiting E-NPP1, ANK-1, PHEX and DMP-1 functions) lead to the stimulation of FGF-23 gene expression in osteoblasts and osteocytes. However, study of additional single gene mutations in humans and mouse genetic models that cause FGF-23-mediated hypophosphatemia have identified an unexpected role of canonical and integrative nuclear FGFR-1 signaling in the regulation of FGF-23 expression in bone; both of these types of FGF receptor signaling have been linked to Phex and Dmp1 mutations and increased Fgf23 gene transcription in mice.
In addition, osteoglophonic dysplasia, an autosomal dominant bone dysplastic disorder caused by activating mutations in FGFR-1, is associated with hypophosphatemia and elevated FGF-23 levels.59 Several pieces of data support a role of FGFR-1 in the regulation of FGF-23 in bone. First, FGFR-1 is expressed in osteoblasts and osteocytes.60 Second, both DMP-1 and PHEX act through common pathways regulating FGFR-1 activity to stimulate Fgf23 gene transcription in osteoblast and osteocytes in compound mutant mouse models.61 Third, pharmacological inhibition of FGFR-1 also inhibits Fgf23 transcription in bone in animal models.62 Fourth, the importance of integrative nuclear FGFR-1 signaling in activation of FGF-23 in bone is further supported by the observations that overexpression of HMW-FGF-2, the ligand for nuclear FGFR-1, in transgenic mice stimulates FGF-23 expression in bone and that HMW-FGF-2 is increased in the bone of adult Hyp mice.63 Integrative nuclear FGFR-1 signaling activates the transcription factor CREB, which is present in the proximal Fgf23 promoter, which suggests that the promoter contains a possible binding site for FGFR regulation of FGF23 gene transcription. Finally, FGF2 administration in vivo to mice also induces hypophosphatemia and impairs matrix mineralization in mice.64,65
Thus, both canonical and integrative nuclear FGFR-1 pathways appear to be involved in regulating FGF-23 expression in bone. These observations have lead to an organizing hypothesis that a physiological function of FGF-23 is to couple bone mineralization with FGF-23 production in osteoblasts and osteocytes via activation of FGFR-1 via yet-to-be defined mechanisms intrinsic to the bone milieu.27 Regardless, the regulation of FGF-23 by FGFR-1 suggests that the generation of a hormonal FGF-23 from the ancestral FGF gene is an evolutionary adaptation to provide a mechanism to link paracrine actions of canonical FGFs to systemic effects.
Whereas mutations in the genes that encode DMP-1, PHEX, ANK-1 and E-NPP1 block bone mineralization and lead to increased FGF-23 production by osteocytes, 57,58 nutritional osteomalacia is associated with decreased FGF-23 expression and, paradoxically, further suppression of FGF-23 after treatment with vitamin D and healing of rachitic bone disease.66 These findings suggest that defective mineralization per se (that is caused by vitamin D deficiency) is not sufficient to stimulate FGF-23 production in bone. Although the mechanisms of these disparate findings are not clear, differences in bone phosphate flux, which is increased during healing of vitamin D deficiency and decreased in defective mineralization caused by PHEX, DMP1, ANK1 and ENPP1 mutations, might account for these different responses. Also, bone remodeling might regulate FGF-23 expression. In this regard, inhibition of bone turnover mediated by osteoprotegerin or alendronate increases FGF-23 expression.67 The mechanism mediating the effects of bone remodeling on FGF-23 expression is also not clear, but alterations in phosphate influx into and out of bone could also be involved. Parathyroid hormone (PTH), leptin, estrogens and glucocorticoids also help to coordinate the regulation of FGF-23 and bone remodeling, 68 which suggests the possible presence of more-complex endocrine networks involving regulation of FGF-23 and energy metabolism (see below).
FGF-23 regulation by PTH signaling mutations
Jansen metaphyseal chondrodysplasia, which is caused by a mutation in the PTH1R gene that renders the corresponding PTH/PTHr receptor constitutively active, is associated with high FGF-23 concentrations in the circulation and low serum phosphate and inappropriately normal 1,25-dihydroxyvitamin D3 levels.69 Transgenic mice with constitutive activation of PTH receptor signaling in osteocytes exhibit increased bone mass and remodeling and increased circulating FGF-23 levels.70 McCune–Albright syndrome, which is caused by activating mutations of the GNAS gene (which encodes the Gαs subunit that is coupled to the PTH receptor), also results in increased FGF-23 expression in the fibrodysplastic lesions that are characteristic of the syndrome.71 Furthermore, serum levels of FGF-23 correlate with disease burden bone turnover markers in this syndrome. However, activating mutations of GNAS are necessary but not sufficient to increase FGF-23 expression, as evidenced by increased FGF23 transcripts in only a subset of the fibrodysplastic lesions showing GNAS mutations.72 Controversies regarding PTH regulation of FGF-23 are discussed below.
Integrative physiology of FGF-23
Knowledge of how the FGF-23 bone–kidney axis is integrated with other hormonal networks is increasing, but the information is incomplete, and many gaps in knowledge remain. At present, FGF-23 is believed to be involved in at least three endocrine axes and preliminary data is emerging for crosstalk between the FGF-23 endocrine axes and other endocrine networks that regulate energy metabolism and aging.
FGF-23–vitamin D axis
FGF-23 is involved in a FGF-23–vitamin D endocrine loop (Figure 1). In this loop, 1,25-dihydroxyvitamin D stimulates FGF-23 production by bone through VDR-dependent mechanisms and elevated FGF-23 levels exert a negative feedback effect on the kidney to suppress 1,25-dihydroxyvitamin D expression.73,74 Thus, FGF-23 acts as a counter-regulatory factor for 1,25-dihydroxyvitamin D.73 Although FGF-23 is a phosphate-regulating hormone, like PTH, tight coupling between changes in circulating phosphate and FGF-23 concentrations are not present.73,75 Some studies have failed to observe changes in FGF-23 levels in healthy individuals in response to either low or high phosphate diets.76,77 By contrast, other studies have shown that alterations in dietary phosphate lead to changes in FGF-23 levels, albeit after a lag time of up to 1 week.78–83 The effects of phosphate on FGF-23 levels are also modulated by vitamin D status, as increasing dietary phosphorus does not increase FGF-23 levels in the absence of the VDR in mouse models.15 The related observation that FGF-23 levels are elevated in primary disorders of bone pyrophosphate or phosphate metabolism in Enpp1 and Ank1 mutant mice, raises the possibility that phosphate may indirectly regulate FGF-23 by affecting bone mineralization.73,75
Figure 1.
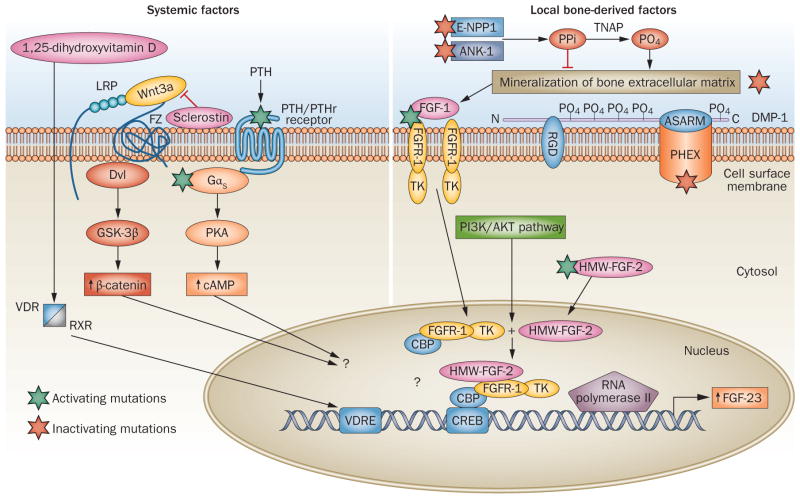
A speculative model of FGF23 gene transcriptional regulation. Four activating mutations or pathways (involving FGFR-1, Gαs encoded by GNAS, PTH/PTHr receptor and HMW-FGF-2) and four inactivating mutations (involving PHEX, DMP-1, E-NPP1 and ANK-1) are associated with increased FGF-23 expression in bone. Local bone-derived factors that are linked to mineralization are shown on the right-hand side. ANK-1 and E-NPP1 regulate the transport and biosynthesis of pyrophosphate, and TNAP regulates the conversion of pyrophosphate to phosphate in the extracellular matrix mineralization process, whereas both PHEX and DMP-1 regulate bone mineralization through mechanisms that remain to be fully elucidated. Evidence exists in osteoblasts derived from the Hyp mouse model that defective mineralization is linked to the activation of FGFR-1 as well as HMW-FGF-2 integrative nuclear signaling pathways. The left-hand side of the figure shows systemic factors involved in FGF-23 regulation. 1,25-dihydroxyvitamin D is an important regulator of FGF-23 expression, acting through the VDR and VDRE. PTH can also stimulate FGF-23 through a sclerostin-dependent mechanism involving the Wnt–β-catenin pathway, or through stimulation of GNAS and cAMP-dependent signaling pathways, as well as indirectly through stimulation of 1,25-dihydroxyvitamin D. Intrinsic and systemic factors are integrated at the levels of cis-acting elements in the proximal FGF23 promoter that remain to be elucidated. A question mark (?) indicates areas of uncertainty. Abbreviations: Hyp, mouse model of X-linked hypophosphatemic rickets; PO4, phosphate; PPi, pyrophosphate; TK, tyrosine kinase.
PTH–vitamin D axis
Prior to the discovery of FGF-23, regulation of phosphate and vitamin D metabolism was understood in the context of the PTH–vitamin D endocrine loop, wherein PTH stimulates 1,25-dihydroxyvitamin D production that feeds back to the parathyroid gland to suppress PTH secretion through direct and indirect mechanisms (Figure 1). The primary function of PTH is to maintain serum calcium levels in a narrow range. PTH secretion is stimulated by changes in serum calcium through activation of the calcium-sensing receptor in parathyroid chief cells. PTH, in turn, targets the kidney to decrease distal tubular calcium secretion and increase 1,25-dihydroxyvitamin D production by stimulating CYP27B1 activity and targets bone to increase calcium and phosphate efflux. PTH has phosphaturic actions that permit increments in serum calcium levels without elevations of serum phosphate levels, owing to 1,25-dihydroxyvitamin-D-mediated increases in calcium and phosphate absorption by the gastrointestinal tract.
PTH can indirectly stimulate FGF-23 serum levels by increasing 1,25-dihydroxyvitamin D synthesis, which in turn would directly stimulate FGF-23 production. The absence of parathyroid glands in Gcm2−/− mice results in low 1,25-dihydroxyvitamin D levels and decreased FGF-23 concentrations that are restored to normal by administration of 1,25-dihydroxyvitamin D supplements.73 The essential role of 1,25-dihydroxyvitamin D in the regulation of FGF-23 expression is supported by the study of Vdr−/− mice, which have markedly elevated PTH levels but low circulating levels of FGF-23 in the absence of VDR-mediated gene transcription.15
A PTH–FGF-23 axis?
Compelling but not incontrovertible evidence exists for a PTH–bone feedback loop, wherein PTH stimulates FGF-23 expression in bone and FGF-23 feeds back to the parathyroid gland to directly suppress PTH production.84 In support of the efferent limb of the PTH–FGF-23 endocrine loop, activating mutations of the PTH1R and GNAS genes result in increased FGF-23 expression (Table 1).69 FGF-23 concentration is also elevated in mice that overexpress a constitutively active PTH receptor in osteocytes and in some, but not all, animal models of excess PTH.85–87 A strong association also exists between elevated FGF-23 levels and the severity of hyperparathyroidism in CKD and other disorders.12,88,89 In addition, PTH increases FGF-23 expression and mediates the high FGF-23 levels observed in a CKD rat model fed a diet consisting of 0.75% adenine and 1.5% phosphate.86 In these studies, early parathyroidectomy to reduce circulating PTH levels prevented the increase in FGF-23 levels observed in rats with adenine-induced renal failure. These investigators also found that continuous PTH administration at high doses (50 μg/kg per day) stimulated FGF-23 production in mice and also stimulated FGF-23 expression in cultured UMR-106 osteoblasts, an osteosarcoma-derived cell line. Finally, PTH has been shown to directly stimulate FGF-23 in UMR-106 osteoblasts86 and primary osteoblasts, although the response in the later was transient.70,90 In osteoblast culture models, PTH-dependent stimulation of FGF-23 was blocked by sclerostin overexpression, which suggests the involvement of Wnt signaling and/or alterations in bone remodeling.
Several findings, however, do not support the PTH–FGF-23 endocrine loop. In this regard, FGF-23 effects on the PTH secretion by the parathyroid gland are controversial.88.89 FGFR-1 and α-Klotho are expressed in parathyroid chief cells, and FGF-23 directly suppresses PTH mRNA expression in vitro and decreases serum PTH in vivo in animal models,84 consistent with a negative feedback afferent limb of a PTH–FGF-23 loop. However, elevated FGF-23 concentrations do not prevent the development of hyperparathyroidism in various clinical disorders. Indeed, primary elevations in circulating FGF-23 levels in hereditary hypophosphatemic disorders as well as secondary increments in FGF-23 levels in CKD are both associated with increased, not decreased, PTH levels, suggesting that FGF-23 does not suppress PTH secretion.12 Rather, the positive association between FGF-23 and PTH suggests that FGF-23 might promote the development of hyperparathyroidism.12,88,89 The failure of FGF-23 to suppress PTH in CKD might also be explained by the resistance to FGF-23 in uremic parathyroid glands due to downregulation of α-Klotho and FGFR expression.91,92
Several additional observations also fail to support direct effects of PTH on FGF-23 production by bone. First, important limitations exist to the mouse model of ‘CKD’, which involves high dietary phosphate and adenine intake, which can confound establishing cause-and-effect relationships between PTH and FGF-23 expression. Second, many studies fail to show that PTH directly stimulates FGF-23 production or FGF-23 promoter activity in osteoblasts in vitro,73 or in calvarial cultures ex vivo.93 Third, PTH administration has also been shown to either suppress or have no effect on FGF-23 expression in wild-type mice.67,93 FGF-23 is also not elevated in patients with primary hyperparathyroidism.94 Fourth, in studies showing a relationship between PTH and FGF-23 levels, the increase in FGF-23 could be indirect, owing to the stimulatory effects of PTH on 1,25-dihydroxyvitamin D levels.85,95,96 Conversely, in settings of primary increments in FGF-23, such as occur in Hyp mice, elevations in PTH are likely to be mediated by FGF-23-mediated suppression of 1,25-dihydroxyvitamin D.96,97 The mechanism underlying the variable effects of PTH on FGF-23 expression is not known, but insights into potential crosstalk between pathways known to regulate Fgf23 gene transcription in vivo and integration of their downstream signaling pathways at the levels of the Fgf23 promoter may explain the context-dependent effects of PTH as well as the molecular mechanism whereby bone mineralization regulates FGF-23 levels (Figure 1).
Other possible FGF-23–hormonal axes
Given the importance of systemic phosphate homeostasis in intermediary metabolism, it is surprising that FGF-23 has not been linked to energy metabolism. Future elucidation of the novel bone–pancreas endocrine loop, wherein insulin receptors in osteoblasts stimulate bone turnover and release of undercarboxylated osteocalcin that regulates glucose homeostasis and energy balance, might identify new molecular pathways for FGF-23 regulation.3,97 The speculation of a role of FGF-23 in energy metabolism is supported by evidence that the insulin-responsive PI3K–Akt–Sgk3 pathway regulates FGF-23 expression in bone in mice, with Sgk3 knockout mice showing reductions in bone density, and 1,25-dihydroxyvitamin D and FGF-23 levels.98
Although many aspects of this bone–pancreas endocrine network remain controversial, peripheral actions of insulin to stimulate cellular phosphate uptake and bone actions to suppress FGF-23 might be of physiological importance. Indeed, insulin regulation of FGF-23 might lead to kidney retention of phosphate proportionate to the increased utilization of phosphate in peripheral tissues during enhanced energy metabolism. FGF-23 might also be linked to energy metabolism via its regulation of α-Klotho. Indeed, excess FGF-23 is associated with decreased α-Klotho expression in the kidney, either directly or indirectly through alterations of 1,25-dihydroxyvitamin D levels, which can also regulate α-Klotho gene transcription in distal tubular cells.14,99 In addition to its membrane functions, α-Klotho is also a circulating factor released by ecto-domain shedding and/or by transcription of an alternatively spliced isoform.100 Circulating α-Klotho has multiple functions, including the regulation of the activity of ion channels and growth factor receptors, such as insulin receptors and insulin-like growth factor-1 receptors.101 A complex network could be envisioned whereby insulin regulates FGF-23 through activation of insulin receptors in osteoblasts and FGF-23, in turn, regulates circulating levels of α-Klotho, which modulate insulin receptor function in multiple tissues. Other hormonal pathways might also be involved in FGF-23 regulation, including communication between adipose tissue and bone. For instance, leptin secreted by adipocytes has been shown to directly stimulate FGF-23 synthesis in bone cells.68
Phosphate regulation of FGF-23 conundrum
Given that FGF-23 regulates serum phosphate levels, it is logical that serum phosphate would feed back to regulate FGF-23 secretion. Phosphate regulation of FGF-23 is controversial. Serum phosphate levels positively correlate with elevations in FGF-23 levels in end-stage renal disease,102 but phosphate restriction failed to lower elevated FGF-23 levels in patients with CKD.103 A study in patients with CKD and elevated FGF-23 levels who were randomly allocated to either sevelamer (a binder known to provide an acidic load that may alter bone phosphate flux) or calcium carbonate found that urinary phosphorus excretion and PTH decreased in both treatment groups, but FGF-23 levels only decreased in the sevelamer arm.104
A small reduction in FGF-23 levels (84 pg/ml versus 61 pg/ml) has been reported in patients with CKD fed a low-phosphate, vegetarian diet.78 In an adenine-induced CKD rat model, sevelamer prevented the increase in PTH and FGF-23 levels, but the effects were delayed, requiring 2 weeks to achieve reductions in FGF-23 levels, whereas correction in hyperphosphatemia occurred rapidly.105 A high-phosphate diet was shown to enhance, and a low-phosphate diet to inhibit, the elevation of serum FGF-23 levels in five of six nephrectomized rats, but this result was obtained after 4 weeks of dietary treatment.106 At present, direct evidence that phosphate regulates Fgf23 gene transcription is lacking.73 Phosphate effects on FGF-23 might be indirectly mediated by bone mineralization, which could account for the delayed effects of phosphate on FGF-23 expression, and involve pathways similar to those affected by PHEX, DMP1, ENPP1 mutations (vide supra).
Hypothesis for FGF-23 regulation
Current knowledge about FGFR, PTH and 1,25-dihydroxyvitamin D signaling, as well as the function of genes in which mutations result in increased FGF-23 expression, can be used to formulate a speculative model of the regulation of FGF-23 gene expression (Figure 1). This model organizes existing data into a testable model for regulation of FGF23 gene transcription by local bone-derived and systemic factors under various pathological and physiological conditions.
This hypothetical model predicts that activation of FGFR-1, the predominant FGFR in osteocytes, is the common signaling pathway linking DMP-1, PHEX and mineralization to FGF-23 gene expression in osteocytes and osteoblasts. The role of FGFR-1 in the regulation of FGF-23 has been confirmed for Phex and Dmp1 mutations in mouse and cell culture modes.61 At this point, whether E-NPP1 and ANK-1 regulation of FGF-23 is through an FGFR-1-dependent pathway or other mechanisms is not known. The schema also shows that FGFR-1 activation can occur through both non-canonical and canonical pathways. The mechanisms linking PHEX and DMP-1 to FGFR-1 activation are not known. DMP-1 and PHEX are expressed in osteocytes, and DMP-1 potentially binds to PHEX via its ASARM motif and to integrins via its RGD sequence located in the 57 kDa C-terminal fragment, thereby providing a possible mechanism for FGFR-1 activation.107–110 Alternatively, a block in mineralization per se, resulting from PHEX, DMP1 or other mutations (such as ENPP1 or ANK1) might lead to canonical activation of FGFR-1 via increased FGF ligand expression and/or alterations of FGF bioactivity.110 The model also shows an intracrine effect of HMW-FGF-2, which increases FGF-23 expression in Hyp mice.63 In addition, FGFRs are shown coupled to additional signaling pathways (Figure 1), including those involving PI3K–AKT, Ras–Raf–MAPK, and PLCγ, which have not been investigated in regulation of FGF23 gene expression.
The model also shows the effect of 1,25-dihydroxyvitamin D to stimulate FGF23 gene transcription by vitamin-D-receptor-mediated mechanisms (Figure 1). In addition, PTH could directly or indirectly regulate FGF23 gene transcription through possible involvement of sclerostin and the GSK-3β–β-catenin pathway. Also included is the potential crosstalk between FGFR-1 and PTH signaling pathways via the common role of CREB in mediating transcriptional regulation. Sclerostin, an osteocyte-derived inhibitor of Wnt signaling, is decreased in bone of Hyp mutant mice, and osteoblasts derived from Hyp mutant mice have increased expression of β-catenin.57 Moreover, regulation of FGF23 gene expression by PI3K, Akt, Foxo1 and Sgk3 is supported by data from global Sgk3-deficient mice98 and Foxo1-deficient111 mice, which have phenotypes that are consistent with alterations in FGF-23 expression, but in which alterations in FGF-23 expression remain to be confirmed.
Not shown in this model is a possible post-transcriptional mechanism to regulate FGF-23 independent of FGF23 gene transcription. As noted above, FGF23 has conserved sites for proprotein convertase cleavage into inactive fragments. The relevance of FGF-23 in metabolism is demonstrated by the fact that mutations lead to familial tumoral calcinosis, which has the opposite phenotype of disorders of FGF-23 excess, and is characterized by hyperphosphatemia, elevated 1,25-dihydroxyvitamin D levels and soft-tissue calcifications. Familial tumoral calcinosis is caused by inactivating mutations of FGF2328 or GALNT3,112 which protects FGF-23 from proteolytic processing by O-glycosylating the protein. Familial tumoral calcinosis can also be caused by inactivating mutations of α-Klotho, which result in loss of end-organ actions of FGF-23.113 The physiological role of FGF-23 processing is not clear, but under several circumstances discordance between FGF23 transcript levels and circulating FGF-23 concentrations exists that suggests an important role of post-translational regulation of FGF-23.
Clinical implications
FGF-23 in chronic kidney disease
The pathogenesis of CKD is traditionally viewed from the perspective of the PTH–vitamin D axis, and current treatments focus on suppressing PTH levels with active vitamin D analogues,114 which can raise serum calcium and phosphate concentrations115 and further stimulate FGF-23 production.116–119 Cross-sectional studies in patients with CKD show early FGF-23 elevations in relation to reductions in glomerular filtration rates,120 which are associated with reductions of levels of 1,25-dihydroxyvitamin D and increments in CYP24.121 FGF-23 is markedly elevated in end-stage renal disease,102,120,122 in which levels correlate with the degree of hyperphosphatemia,102,122 predict refractory hyperparathyroidism in some studies92 and are associated with increased mortality.123
Compelling data exists to support the scenario that FGF-23 is the initial event in CKD that leads to reductions in 1,25-dihydroxyvitamin D levels and secondary increments in PTH. Analysis of the expression of enzymes that regulate vitamin D metabolism, CYP27B1 and CYP24, suggest a pattern consistent with an effect of FGF-23 in CKD.96 In a rat model of anti-glomerular basement membrane (anti0GBM) nephritis, treatment with a neutralizing anti-FGF-23 antibody121 increased serum levels of 1,25-dihydroxyvitamin D and Cyp27b1, reduced Cyp24 levels and suppressed PTH levels. Treatment with paricalcitol further elevates FGF-23 and suppresses PTH levels in end-stage renal disease.124
In this conceptual framework that integrates knowledge of FGF-23 with the PTH and vitamin D endocrine networks, early CKD does not represent a reduction in 1,25-dihydroxyvitamin D levels due to impaired conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D caused by kidney disease per se; rather, FGF-23-mediated suppression of circulating 1,25-dihydroxyvitamin D levels is an active adaptive response, which protects against hyperphosphatemia through FGF-23-mediated reductions of CYP27B1 and increments in CYP24. Changes in the levels of these two enzymes lead to reduction in the levels of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and subsequent effects on gastrointestinal phosphate absorption. Moreover, these changes also increase levels of PTH, which acts in concert with FGF-23 to stimulate phosphaturia. On the other hand, in the late stages of CKD and end-stage renal disease, the dominant stimulus for FGF-23 elevations may be bone remodeling, due to elevated PTH or other factors stimulating FGF-23 expression in the setting of CKD. This hypothesis has been reviewed in great detail elsewhere.125
If this hypothesis is correct, FGF-23 might be an early biomarker for earlier interventions in CKD. In addition, treatment approaches to prevent elevated FGF-23 levels could become the initial therapeutic focus but may differ depending on the stage of CKD. As calcitriol increases FGF-23 levels, calcitriol-sparing therapies may be warranted, such as combined low-dose paricalcitol and calcimimetics, which can lower FGF-23 levels in patients with end-stage renal disease.114,126 Further studies that elucidate the interrelationships between FGF-23, PTH, vitamin D and bone metabolism are of fundamental importance in understanding the pathogenesis and treatment of CKD-mineral bone disorder.
Implications for low vitamin D levels
CKD is more prevalent in elderly adults than is detected by serum creatinine levels. FGF-23 elevations and increased CYP24 catabolism may contribute to the low levels of 25-hydroxyvitamin D in elderly patients. If so, future translational studies might assess FGF-23 and/or 24,25-dihydroxyvitamin D levels to interpret low 25-hydroxyvitamin D levels (for example, true deficiency or increased catabolism secondary to increased FGF-23 levels in subclinical CKD). This research would add a new perspective to the existing uncertainties regarding the definition of vitamin D insufficiency and effects of vitamin D supplementation.127
FGF-23 and mortality and morbidity
Elevated circulating FGF-23 concentrations are associated with increased mortality in end-stage renal disease119 and coronary artery disease,128 progression of renal disease129 and left ventricular hypertrophy, and increased fat mass and dyslipidemia in elderly patients.130 Additional knowledge of direct and indirect effects of FGF-23 are needed to understand if these associations are causal or an epiphenomena related to co-variance of circulating FGF-23 levels with the factors causing these associations.
Conclusions
A key component of the capability of bone to participate in phosphate homeostasis resides in its endocrine function to produce the hormone FGF-23,4,27 a circulating factor produced predominately by osteoblasts and osteocytes that principally targets the kidney to regulate renal handling of phosphate and vitamin D metabolism. The current lack of understanding of the molecular mechanisms regulating FGF-23 release from osteoblasts and osteocytes is a critical barrier to fully understanding the physiological functions of FGF-23 and the pathogenesis of hereditary and acquired hypophosphatemic disorders. By the study of distinct, single gene mutations that lead to a common increase in FGF-23 production, several pathways for regulating FGF23 gene transcription have been discovered that link mineralization of bone, vitamin D receptor function and PTH-dependent signaling pathways in the control of circulating FGF-23 levels. The integrative physiology of FGF-23 is being uncovered, and this hormone appears to be involved in several endocrine loops.
Understanding the physiological regulation and function of FGF-23 is also providing new schemas for elucidating the pathogenesis of various disorders of bone and mineral metabolism. In addition to providing a molecular explanation for hereditary hypophosphatemic and hyperphosphatemic disorders, FGF-23 regulation and function is challenging our view of how to interpret circulating 25-hydroxyvitamin D levels and the pathogenesis of secondary hyperparathyroidism. Moreover, the yet-to-be determined functions of FGF-23 in the regulation of α-Klotho and other endocrine factors, as well as potential effects of FGF-23 on FGFR–α-Klotho complexes in tissues other than bone and kidneys may provide an explanation for the strong associations between elevated serum FGF-23 levels and increased mortality observed in patients with renal failure and in the general population.
Key points.
FGF-23 is a hormone produced by osteoblasts and osteocytes in bone that causes phosphaturia and inhibits 1,25-dihydroxyvitamin D production through its binding to FGFR–α-Klotho complexes in kidney tubules
Primary elevations of circulating FGF-23 concentrations cause hereditary hypophosphatemic disorders and acquired hypophosphatemic disorders, and reductions in circulating FGF-23 concentrations cause familial tumoral calcinosis
FGF-23 is regulated by local bone-derived factors through activation of FGFR-1 pathways and by systemic factors, including 1,25-dihydroxyvitamin D and parathyroid hormone (in a context-dependent fashion) in osteoblasts
FGF-23 participates in several physiologically relevant endocrine axes involving 1,25-dihydroxyvitamin D or parathyroid hormone, and possibly additional hormonal networks involving secreted α-Klotho and other kidney-derived factors
Elevations in serum FGF-23 is an early adaptive response in chronic kidney disease, leading to maintenance of phosphate balance and development of secondary hyperparathryroidism through reductions in 1,25-dihydroxyvitamin D levels
Elevations of circulating FGF-23 are an unexpectedly strong predictor of mortality in patients with renal failure, as a result of mechanisms that remain to be elucidated
Review criteria.
The articles upon which this Review was based were selected from a PubMed search using various combinations of keywords, including “hypophoshatemia”, “FGF-23”, “PTH”, “vitamin D”, “Cyp24”, “Klotho”, “bone”, “osteocalcin”, “hereditary hypophosphatemic disorders” for years ranging from 1953 to 2011. In addition, the Online Mendelian Inheritance in Man (OMIM) database was searched using the keyword “hypophosphatemia”. All papers included were full-text papers that were written in English, and data from primary sources in the reference lists of review papers were also confirmed.
Footnotes
Competing interests
The author declares associations with the following companies: Amgen, KAI Pharmaceuticals. See the article online for full details of the relationships.
References
- 1.Ferron M, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulzele K, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–1683. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quarles LD. Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest. 2003;112:642–646. doi: 10.1172/JCI19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 6.Stachowiak MK, et al. Integrative nuclear FGFR1 signaling (INFS) as a part of a universal “feed-forward-and-gate” signaling module that controls cell growth and differentiation. J Cell Biochem. 2003;90:662–691. doi: 10.1002/jcb.10606. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 10.Stubbs J, Liu S, Quarles LD. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dial. 2007;20:302–308. doi: 10.1111/j.1525-139X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 11.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Martin AC, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2010;300:E508–E517. doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada T, et al. Vitamin D receptorindependent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 16.Shimada T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 18.Larsson T, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 19.Fukumoto S, Yamashita T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr Opin Nephrol Hypertens. 2002;11:385–389. doi: 10.1097/00041552-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Shimada T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White KE, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 22.Tomiyama K, et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckman MJ, et al. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–8472. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 24.Hoenderop JG, et al. Regulation of gene expression by dietary Ca2+ in kidneys of 25-hydroxyvitamin D3–1 α-hydroxylase knockout mice. Kidney Int. 2004;65:531–539. doi: 10.1111/j.1523-1755.2004.00402.x. [DOI] [PubMed] [Google Scholar]
- 25.Meyer MH, Dulde E, Meyer RA., Jr The genomic response of the mouse kidney to low-phosphate diet is altered in X-linked hypophosphatemia. Physiol Genomics. 2004;18:4–11. doi: 10.1152/physiolgenomics.00210.2003. [DOI] [PubMed] [Google Scholar]
- 26.Marsell R, et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant. 2008;23:827–833. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]
- 27.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 29.Larsson T, et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146:3883–3891. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- 30.Sitara D, et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitara D, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 34.Brown JR, Auger KR. Phylogenomics of phosphoinositide lipid kinases: perspectives on the evolution of second messenger signaling and drug discovery. BMC Evol Biol. 2010;11:4. doi: 10.1186/1471-2148-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130:652–660. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeger MD, et al. Hypophosphatemic rickets in opsismodysplasia. J Pediatr Endocrinol Metab. 2007;20:79–86. doi: 10.1515/jpem.2007.20.1.79. [DOI] [PubMed] [Google Scholar]
- 37.Moe S, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab. 2007;293:E1636–E1644. doi: 10.1152/ajpendo.00396.2007. [DOI] [PubMed] [Google Scholar]
- 39.Yuan B, et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowe AE, et al. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 41.Benet-Pagès A, et al. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35:455–462. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Guo R, Liu S, Spurney RF, Quarles LD. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab. 2001;281:E837–E847. doi: 10.1152/ajpendo.2001.281.4.E837. [DOI] [PubMed] [Google Scholar]
- 43.Quarles LD, Drezner MK. Pathophysiology of X-linked hypophosphatemia, tumor-induced osteomalacia, and autosomal dominant hypophosphatemia: a perPHEXing problem. J Clin Endocrinol Metab. 2001;86:494–496. doi: 10.1210/jcem.86.2.7302. [DOI] [PubMed] [Google Scholar]
- 44.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, et al. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, et al. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J Biol Chem. 2011;286:29462–29469. doi: 10.1074/jbc.M110.194746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 48.Chaussain C, et al. MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell. Eur Cell Mater. 2009;18:84–95. doi: 10.22203/ecm.v018a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 50.David V, Quarles LD. ASARM mineralization hypothesis: a bridge too far? J Bone Miner Res. 2010;25:692–694. doi: 10.1002/jbmr.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai X, et al. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol Endocrinol. 2002;16:2913–2925. doi: 10.1210/me.2002-0113. [DOI] [PubMed] [Google Scholar]
- 52.Erben RG, et al. Overexpression of human PHEX under the human b-actin promoter does not fully rescue the Hyp mouse phenotype. J Bone Miner Res. 2005;20:1149–1160. doi: 10.1359/JBMR.050212. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, Guo R, Tu Q, Quarles LD. Overexpression of Phex in osteoblasts fails to rescue the Hyp mouse phenotype. J Biol Chem. 2002;277:3686–3697. doi: 10.1074/jbc.M107707200. [DOI] [PubMed] [Google Scholar]
- 54.Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy-Litan V, et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen IP, Wang L, Jiang X, Aguila HL, Reichenberger EJ. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD) Hum Mol Genet. 2011;20:948–961. doi: 10.1093/hmg/ddq541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S, et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–1518. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jean G, et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 59.White KE, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens DA, et al. Thyroid hormone activates fibroblast growth factor receptor-1 in bone. Mol Endocrinol. 2003;17:1751–1766. doi: 10.1210/me.2003-0137. [DOI] [PubMed] [Google Scholar]
- 61.Martin A, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wohrle S, et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF23 signaling and regulating FGF23 expression in bone. J Bone Miner Res. 2011;26:2486–2497. doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 63.Xiao L, et al. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J Biol Chem. 2010;285:2834–2846. doi: 10.1074/jbc.M109.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nauman EA, Sakata T, Keaveny TM, Halloran BP, Bikle DD. bFGF administration lowers the phosphate threshold for mineralization in bone marrow stromal cells. Calcif Tissue Int. 2003;73:147–152. doi: 10.1007/s00223-002-1033-6. [DOI] [PubMed] [Google Scholar]
- 65.Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5780–5788. doi: 10.1210/endo.140.12.7195. [DOI] [PubMed] [Google Scholar]
- 66.Uzum AK, et al. Effects of vitamin D replacement therapy on serum FGF23 concentrations in vitamin D-deficient women in short term. Eur J Endocrinol. 2010;163:825–831. doi: 10.1530/EJE-10-0591. [DOI] [PubMed] [Google Scholar]
- 67.Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150:4835–4845. doi: 10.1210/en.2009-0472. [DOI] [PubMed] [Google Scholar]
- 68.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 69.Brown WW, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhee Y, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–643. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riminucci M, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi K, et al. Expression of FGF23 is correlated with serum phosphate level in isolated fibrous dysplasia. Life Sci. 2006;78:2295–2301. doi: 10.1016/j.lfs.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 74.Kolek OI, et al. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 75.Mirams M, Robinson BG, Mason RS, Nelson AE. Bone as a source of FGF23: regulation by phosphate? Bone. 2004;35:1192–1199. doi: 10.1016/j.bone.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 76.Nishida Y, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 77.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 78.Moe SM, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 80.Burnett SA, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 81.Vervloet MG, et al. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6:383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 83.Perwad F, et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 84.Ben-Dov IZ, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawata T, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 86.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 87.Sato T, et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–487. [PubMed] [Google Scholar]
- 88.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 89.Li SA, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 90.Rhee Y, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res. 2011;26:1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 92.Komaba H, Fukagawa M. FGF23 -parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–298. doi: 10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 93.Saji F, et al. Fibroblast growth factor 23 production in bone is directly regulated by 1α,25-dihydroxyvitamin D, but not PTH. Am J Physiol Renal Physiol. 2010;299:F1212–F1217. doi: 10.1152/ajprenal.00169.2010. [DOI] [PubMed] [Google Scholar]
- 94.Tebben PJ, Singh RJ, Clarke BL, Kumar R. Fibroblast growth factor 23, parathyroid hormone, and 1α,25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. Mayo Clin Proc. 2004;79:1508–1513. doi: 10.4065/79.12.1508. [DOI] [PubMed] [Google Scholar]
- 95.Saji F, et al. Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron Physiol. 2009;111:59–66. doi: 10.1159/000210389. [DOI] [PubMed] [Google Scholar]
- 96.Helvig CF, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 97.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 98.Bhandaru M, et al. Decreased bone density and increased phosphaturia in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase 3. Kidney Int. 2011;80:61–67. doi: 10.1038/ki.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 100.Shiraki-Iida T, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 101.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 102.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 103.Isakova T, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oliveira RB, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagano N, et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531–537. doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- 106.Saito H, et al. Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 107.Mori S, et al. Direct binding of integrin αvβ3 to FGF1 plays a role in FGF1 signaling. J Biol Chem. 2008;283:18066–18075. doi: 10.1074/jbc.M801213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo R, et al. Inhibition of MEPE cleavage by Phex. Biochem Biophys Res Commun. 2002;297:38–45. doi: 10.1016/s0006-291x(02)02125-3. [DOI] [PubMed] [Google Scholar]
- 109.Rowe PS, et al. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP) Bone. 2005;36:33–46. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Y, et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007;303:191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rached MT, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11:147–160. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Topaz O, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 113.Ichikawa S, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7:318–319. [PubMed] [Google Scholar]
- 114.Wetmore JB, Quarles LD. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat Clin Pract Nephrol. 2009;5:24–33. doi: 10.1038/ncpneph0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tentori F, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 116.Young EW, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44 (Suppl):34–38. doi: 10.1053/j.ajkd.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 117.Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- 118.Block GA, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 119.Gutiérrez OM, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gutierrez O, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 121.Hasegawa H, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 122.Imanishi Y, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 123.Stubbs JR, Quarles LD. Fibroblast growth factor 23: uremic toxin or innocent bystander in chronic kidney disease? Nephrol News Issues. 2009;23:33–34. [PubMed] [Google Scholar]
- 124.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quarles LD. The bone and beyond: ‘Dem bones’ are made for more than walking. Nat Med. 2011;17:428–430. doi: 10.1038/nm0411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5:110–116. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 128.Parker BD, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fliser D, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 130.Mirza MA, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31:219–227. doi: 10.1161/ATVBAHA.110.214619. [DOI] [PubMed] [Google Scholar]