Abstract
Maturation of social cognition and a gain in social proficiency are universal aspects of adolescent development that prepare individuals for adulthood. Social cognition involves the perception and interpretation of social cues, followed by the generation of a behavioral response. Social proficiency is acquired through the ability to make behavioral adaptations as one learns from social experience; increased social proficiency facilitates successful social interactions. In males, the neuroendocrine bases of these developmental changes involve both activational and organizational influences of testicular hormones. Using the male Syrian hamster as a model, this review provides evidence that social stimuli acquire rewarding properties during adolescence via activational effects of pubertal testosterone, whereas the adolescent gain in social proficiency depends on organizational actions of pubertal testosterone.
Keywords: puberty, adolescence, testosterone, social reward, social behavior
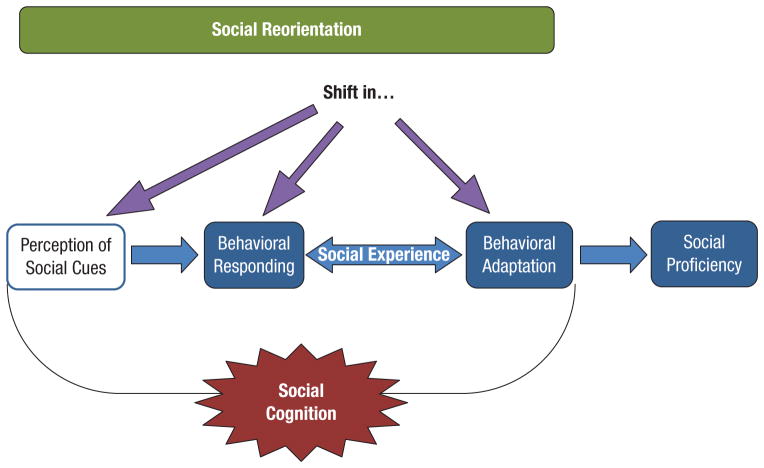
Teenagers may feel that their brains are going haywire, but really, their brains are being rewired to prepare them for the transition into adulthood. During adolescence, our bodies, minds, and social lives change dramatically, thanks largely to sex hormones, brain development, and a change in focus from family to peers, a process known as social reorientation (Nelson, Leibenluft, McClure, & Pine, 2005). Social reorientation introduces a new suite of social cues in novel contexts and requires social cognition to deal with them (Fig. 1). Social cognition includes perceiving and interpreting social cues and then responding appropriately (Adolphs, 2001). Many social cues are motivationally relevant—for instance, peer acceptance and early romantic experiences may lead to rewarding feelings, and being rejected by peers or one’s crush may lead to aversive feelings. By learning from social experience and adapting behavior accordingly, one gains social proficiency. Successful adolescent acquisition of social proficiency plays a key role in ensuring that individuals are able to become independent and appropriately interact with their peers. The adolescent maturation of social cognition coincides with numerous neural changes that have been reviewed extensively (Spear, 2000) and are described elsewhere in this special issue.
Fig. 1.
Adolescent maturation of social cognition and gain in social proficiency. The maturation of the perception of social cues that occurs in adolescence (represented by the white box) is the result of activational effects of testosterone; the maturation of behavioral responding, behavioral adaptation, and gains in social proficiency (represented by the blue boxes) are the result of organizational effects of pubertal testosterone.
The focus of our research is the interaction between pubertal gonadal hormones and the developing adolescent brain, using the male Syrian hamster as a model system. Although not all aspects of social reorientation and human social cognition can be studied using animal models (e.g., perspective taking), hamsters, like humans, undergo physiological and behavioral changes during adolescence that affect how they perceive, interpret, and respond to social stimuli in a species-appropriate manner. Social interactions in male hamsters depend on chemosensory social cues. Neural processing of pheromones present in vaginal secretions and flank-gland deposits (via flank marking) are required for sexual behavior and the maintenance of male-male relationships, respectively (Ferris, Axelson, Shinto, & Albers, 1987; Wood, 1998). Testosterone facilitates both mating and flank marking in adult, but not juvenile, hamsters, which indicates that both male-female and male-male social interactions are influenced by neuroendocrine events during adolescent maturation (Sisk, Schulz, & Zehr, 2003; Wood, 1998).
Activational and Organizational Effects of Gonadal Hormones
The pubertal rise in testosterone secretion results in both activation and organization of the neural circuits underlying adult male social behaviors. Activational effects of testosterone facilitate the expression of appropriate behaviors in specific social contexts, and require the continued presence of the hormone to be observed. In contrast, organizational effects of testosterone create permanent alterations in the brain and behavior at particular points during development that persist even in the absence of the hormone later in life. Organizational effects of testosterone often program activational responses, usually by affecting sensitivity to the activating effects of hormones in adulthood (Wallen, 2009). For example, men who experience profound androgen deficiency during puberty show significant impairment in spatial ability in adulthood compared with controls, even after androgen-replacement therapy (Hier & Crowley, 1982). Additionally, there are correlations among pubertial timing, sex, and the development of certain psychological disorders (Conley & Rudolph, 2009; Zehr, Culbert, Sisk, & Klump, 2007). Therefore, determining which aspects of adolescent maturation are influenced by activational versus organizational effects of hormones provides insight into the neuroendocrine bases of human adolescent development.
To distinguish organizational from activational effects of pubertal testosterone on adolescent maturation of social cognition in hamsters, we systematically manipulate circulating testosterone concentrations during development and assess the effects on social behaviors. We first compare the behavior of juvenile males with that of adult males in social situations. If behavior differs between the two age groups, we then treat juvenile hamsters with adult concentrations of testosterone. If testosterone-treated juveniles show adult-like behavior, we conclude that activational effects of testosterone alone are sufficient for expression of the adult behavior; if not, we conclude that further neural maturation during adolescence is required before activational effects can occur. The expression of gonadal-steroid-hormone-receptor immunoreactivity within social-behavior neural circuits appears to be similar in juvenile and adult hamsters when examined in similar endocrine states (Meek, Romeo, Novak, & Sisk, 1997; Romeo, Diedrich, & Sisk, 1999; Romeo, Wagner, Jansen, Diedrich, & Sisk, 2002). Therefore, the failure of testosterone to activate behavior in juvenile males is unlikely to be related to low levels of hormone receptor.
To assess the organizational effects of pubertal testosterone (or a metabolite) on the maturation of social cognition, male hamsters are deprived of testosterone (via removal of the gonads) either during puberty (no testes/testosterone at puberty: “NoT@P”) or for an equivalent amount of time in adulthood (testes/testosterone at puberty: “T@P”) and then tested in a variety of behavioral paradigms after testosterone replacement in adulthood (Fig. 2). If the behavior of NoT@P and T@P males differs, then adult-typical expression of the behavior depends on organizational effects of pubertal testosterone. In this review, we provide evidence that the adolescent maturation of the perception of social rewards is due to activational effects of pubertal testosterone, whereas the adolescent maturation of social proficiency involves organizational effects of pubertal testosterone.
Fig. 2.
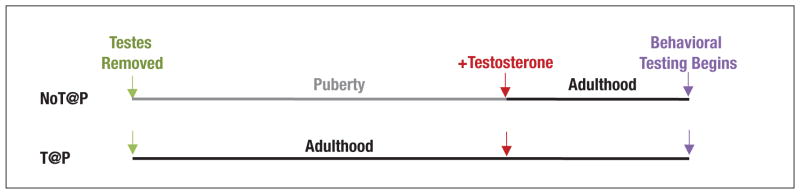
NoT@P/T@P experimental model. Male hamsters are castrated either before puberty or in adulthood to create groups of animals that go through adolescent development either without (NoT@P) or with (T@P) endogenous testosterone, respectively. Four weeks after castration, when all animals are adults, NoT@P and T@P males receive testosterone-filled capsules 2 weeks before behavior testing begins.
Social Reward
Almost all social cues are motivationally relevant, inducing an animal either to seek out potential rewards (e.g., a mate) or to avoid aversive stimuli (e.g., an angry neighbor). The Syrian hamster is an ideal model for studying the motivational properties of social cues because neural integration of external stimuli (pheromones contained in vaginal secretions) with internal stimuli (circulating hormones) are obligatory for expression of adult male sexual behavior (Wood, 1998). Unlike adult hamsters, juvenile hamsters are not attracted to female pheromones (Johnston & Coplin, 1979), nor do they show adult-typical neuroendocrine and neurochemical responses to pheromones (Romeo, Parfitt, Richardson, & Sisk, 1998; Schulz et al., 2003). Therefore, we hypothesized that an increase in the incentive salience (rewarding properties) of female pheromones occurs during male adolescence.
The rewarding properties of social cues can be assessed using an experimental paradigm that measures conditioned place preference (CPP), in which experimenters pair a potential reward with a specific environment and then determine if an animal increases its preference for that environment as a result of conditioning. We found that sexually naïve adult male hamsters form a CPP to female pheromones alone, which indicates that pheromones are an unconditioned reward in adulthood (Bell, Meerts, & Sisk, 2010). In contrast, juvenile male hamsters fail to form a CPP to female pheromones, which demonstrates that pheromones are not an unconditioned reward in preadolescent males, consistent with our hypothesis that the rewarding properties of female pheromones are acquired over adolescent development (Bell, De Lorme, Figueira, Kashy, & Sisk, 2012).
We next asked whether organizational effects of pubertal testosterone are necessary for adult males to perceive female pheromones as rewarding by comparing the ability of NoT@P and T@P males to form a CPP to pheromones. Both groups of males showed a CPP to female pheromones; therefore, organizational effects of pubertal testosterone do not contribute to the adolescent gain in the incentive salience of female pheromones (De Lorme, Bell, & Sisk, 2012). Subsequent experiments demonstrated that activational effects of testosterone are both necessary and sufficient for pheromone reward in juvenile and adult male hamsters. First, when hamsters were tested several weeks after castration (in adulthood), they no longer showed a CPP to female pheromones (Bell & Sisk, 2013). Second, when juvenile hamsters were treated with testosterone, they did show a CPP to pheromones, whereas vehicle-treated (control) juveniles did not (Bell & Sisk, 2013). Thus, the perception of female pheromones as a rewarding social stimulus is the result of activational effects of pubertal testosterone and does not require organizational effects of testosterone. However, although testosterone can activate an increase in the incentive salience of pheromones in juvenile males, it does not activate sexual behavior in juveniles (Meek et al., 1997; Schulz et al., 2004; Schulz & Sisk, 2006). Thus, increasing the incentive salience of female pheromones may be necessary, but is not sufficient, for expression of male sexual behavior, which apparently requires additional, hormone-independent maturational processes during adolescence.
Neural mechanisms of sociosexual reward
Many rewarding stimuli, including drugs of abuse and sexual behavior, are associated with the release and actions of dopamine in several different neural circuits (Becker, 2009; Ikemoto, 2010). Some of these dopaminergic circuits are exquisitely sensitive to testosterone (Dominguez & Hull, 2005), and many are restructured during adolescence (Kuhn et al., 2010). Therefore, we determined whether dopamine-receptor activation is required for testosterone-facilitated CPP to female pheromones in juvenile hamsters. Indeed, a dopamine-receptor antagonist blocked the formation of a CPP to pheromones (Bell & Sisk, 2013).
We also investigated what brain regions are involved in the adolescent gain in the salience of social rewards by comparing neural responses to female pheromones in juvenile and adult male hamsters. We found that pheromones elicited an increase in dopaminergic activity in the hypothalamic preoptic area of adults, but not juveniles (Schulz et al., 2003). We also determined patterns of neural activity in response to pheromones, as indexed by expression of the immediate early gene protein Fos (Bell et al., 2012). In both juveniles and adults, exposure to female pheromones increased Fos expression in the medial amygdala, a brain region involved in the detection and initial evaluation of social stimuli, and in dopaminergic cells in the ventral tegmental area, the primary source of dopamine in the mesocorticolimbic reward circuit. Importantly, pheromones elicited a Fos response in the nucleus accumbens core and infralimbic prefrontal cortex (both recipients of midbrain dopamine projections) only in adult hamsters. Although hypothalamic dopaminergic circuits are testosterone sensitive, adolescent changes in corticolimbic dopaminergic receptors are hormone independent (Andersen, Thompson, Krenzel, & Teicher, 2002). Therefore, hypothalamic and corticolimbic regions may be involved in the hormone-dependent activation of social-reward perception and the hormone-independent maturation of social behavior, respectively, during puberty.
Social Proficiency
Once a social stimulus is evaluated and its motivational quality determined, a behavioral response follows. However, because most social interactions are not a one-time occurrence, behavioral responses in a particular social setting are generated in the context of prior social experience. Social experience may dictate a reevaluation of others’ behavior and adjustments of one’s own behavior in the current social situation. Being able to learn and adapt one’s behavior according to positive or negative social experiences—in other words, becoming socially proficient—may make or break one’s social life.
Sociosexual proficiency
Successful sexual encounters are essential for reproductive fitness, and social learning and behavioral adaptation gained during sexual experience generally increase sexual competence (Dewsbury, 1969; Schulz & Sisk, 2006). We found that the likelihood of making behavioral adaptations with sexual experience is dependent on the organizational effects of pubertal testosterone in male hamsters. Specifically, we found that NoT@P males continue to show high numbers of misdirected mounts even after several encounters with a receptive female, whereas misdirected mounts decrease with experience in T@P males (Schulz & Sisk, 2006).
Agonistic proficiency
During male-male interactions, agonistic behaviors, including aggression, submission, and flank marking, are important for the establishment and maintenance of dominant-subordinate relationships. Male hamsters initially use aggression and submission to establish who is dominant and who is subordinate. In subsequent encounters, overt aggression and submission decline and are supplanted by flank marking, such that the dominant male marks significantly more often than the subordinate male does (Ferris et al., 1987). Thus, with social experience, both dominant and subordinate males make behavioral adaptations to peacefully maintain the status quo.
We asked whether organizational effects of pubertal testosterone program the ability to make these behavioral adaptations by comparing the behavior of NoT@P and T@P males during repeated male-male interactions in a neutral arena (De Lorme & Sisk, 2013). Pairs of males of the same hormonal history (i.e., NoT@P-NoT@P pairs and T@P-T@P pairs) were allowed to establish dominant-subordinate relationships during an initial social interaction; if the two males in a pair did not form a dominant-subordinate relationship, they were classified as no-status. After the establishment (or not) of a dominant-subordinate relationship, the same pairs were tested in several immediate follow-up trials and then again 24 hours later. T@P males displayed typical patterns of behavior, whereas NoT@P males did not. First, overall frequencies of flank marking did not differ among NoT@P males who had and had not formed dominant-subordinate relationships, which suggests that NoT@P males either inappropriately express flank marking or misinterpret their own status. Second, NoT@P males used overt aggression, not flank marking, to maintain the dominance hierarchy in the 24-hour follow-up trials, which suggests that NoT@P males either employ a different behavioral strategy for maintaining social status or have a deficit in social recognition or memory. Therefore, we concluded that pubertal testosterone organizes neural circuits underlying social proficiency by programming behavioral strategies used for maintaining social status during repeated male-male interactions.
Neural mechanisms of social proficiency
Using Fos expression as an index of neural activation, we compared neural activation patterns of T@P and NoT@P males immediately after their last social interaction in the above study (De Lorme & Sisk, 2013). Fos expression in the anterior cingulate cortex was higher in dominant T@P males than in no-status T@P males (subordinate T@P males did not significantly differ from either group); in contrast, this status-dependent difference in anterior cingulate Fos expression was not observed in NoT@P males. Furthermore, Fos expression was greater in the lateral septum in dominant T@P males compared with that of dominant NoT@P males. The anterior cingulate cortex is implicated in emotion regulation, response selection, and appropriate social behavior during social interactions (Devinsky, Morrell, & Vogt, 1995). The lateral septum suppresses aggression and reactivity (Albert & Walsh, 1984; David, Cervantes, Trosky, Salinas, & Delville, 2004) and is involved in regulating flank marking in hamsters (Ferris, Gold, De Vries, & Potegal, 1990; Irvin, Szot, Dorsa, Potegal, & Ferris, 1990). Interestingly, vasopressin-receptor binding in the lateral septum (which is linked to the expression of agonistic behaviors) was greater in NoT@P than in T@P males (Schulz, Menard, Smith, Albers, & Sisk, 2006). Additionally, both regions are hormone sensitive (Cyr, Landry, & Di Paolo, 2000; Fink, Sumner, Rosie, Grace, & Quinn, 1996; Scordalakes & Rissman, 2004). Therefore, organizational effects of pubertal testosterone may be required to engage the anterior cingulate cortex and lateral septum to promote socially proficient male-male interactions.
Conclusion
Experimental evidence from a tractable animal model indicates that the maturation of male social behavior in adolescence involves alterations in the processing of social rewards and in social proficiency, and that pubertal hormones influence the maturation of both components of social behavior. Social stimuli—in this case, female pheromones—become socially rewarding during adolescence via activational effects of testosterone that likely involve dopaminergic actions in the hypothalamus or corticolimbic circuits. In contrast, the behavioral adaptations and strategies that underlie social proficiency are programmed during adolescence by organizational effects of pubertal testosterone and are correlated with structural and functional organization of the anterior cingulate cortex and the lateral septum. Social reorientation in human adolescents may be similarly influenced by both activational and organizational effects of pubertal hormones, although the sites of action of hormonal influences may differ in rodents and humans because of differences between the two species’ socially salient sensory modalities, as well as their social structures.
Acknowledgments
Funding
This work was supported by National Institutes of Health Grants R01-MH068764 (C. L. S.), T32-NS44928 (M. R. B.), and T32-MH070343 (M. R. B. and K. C. D.).
We thank past and present lab members who helped with the design and execution of the experiments discussed in this article.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Walsh ML. Neural systems and the inhibitory modulation of agonistic behavior: A comparison of mammalian species. Neuroscience & Biobehavioral Reviews. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroen-docrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: A novel mechanism? Hormones and Behavior. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, De Lorme KC, \ueira RJ, Kashy DA, Sisk CL. Adolescent gain in positive valence of a socially relevant stimulus: Engagement of the mesocorticolimbic reward circuitry. European Journal of Neuroscience. 2012;37:457–468. doi: 10.1111/ejn.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Meerts SH, Sisk CL. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Hormones and Behavior. 2010;58:410–414. doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Sisk CL. Dopamine mediates testosterone-induced social reward in juvenile male hamsters. Endocrinology. 2013 doi: 10.1210/en.2012-2042. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and Psychopathology. 2009;21:593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000;23:69–78. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- David JT, Cervantes MC, Trosky KA, Salinas JA, Delville Y. A neural network underlying individual differences in emotion and aggression in male golden hamsters. Neuroscience. 2004;126:567–578. doi: 10.1016/j.neuroscience.2004.04.031. [DOI] [PubMed] [Google Scholar]
- De Lorme KC, Bell MR, Sisk CL. Maturation of social reward in adult male Syrian hamsters does not depend on organizational effects of pubertal testosterone. Hormones and Behavior. 2012;62:180–185. doi: 10.1016/j.yhbeh.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorme KC, Sisk CL. Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian hamsters. Physiology & Behavior. 2013 doi: 10.1016/j.physbeh.2013.02.003. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Copulatory behaviour of rats (Rattus norvegicus) as a function of prior copulatory experience. Animal Behaviour. 1969;17:217–223. doi: 10.1016/0003-3472(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiology & Behavior. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Shinto LH, Albers HE. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiology & Behavior. 1987;40:661–664. doi: 10.1016/0031-9384(87)90114-4. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, Potegal M. Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in golden hamsters. Journal of Comparative Neurology. 1990;293:476–485. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: Effect on mood, mental state, and memory. Cellular and Molecular Neurobiology. 1996;16:325–344. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier DB, Crowley WF., Jr Spatial ability in androgen-deficient men. New England Journal of Medicine. 1982;306:1202–1205. doi: 10.1056/NEJM198205203062003. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neuroscience & Biobehavioral Reviews. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiology & Behavior. 1990;48:693–699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Coplin B. Development of responses to vaginal secretion and other substances in golden hamsters. Behavioral and Neural Biology. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Hormones and Behavior. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: Dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Hormones and Behavior. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Diedrich SL, Sisk CL. Estrogen receptor immunoreactivity in prepubertal and adult male Syrian hamsters. Neuroscience Letters. 1999;265:167–170. doi: 10.1016/s0304-3940(99)00233-5. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Parfitt DB, Richardson HN, Sisk CL. Pheromones elicit equivalent levels of Fos-immunoreactivity in prepubertal and adult male Syrian hamsters. Hormones and Behavior. 1998;34:48–55. doi: 10.1006/hbeh.1998.1463. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Wagner CK, Jansen HT, Diedrich SL, Sisk CL. Estradiol induces hypothalamic progester-one receptors but does not activate mating behavior in male hamsters (Mesocricetus auratus) before puberty. Behavioral Neuroscience. 2002;116:198–205. doi: 10.1037//0735-7044.116.2.198. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Hormones and Behavior. 2006;50:477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Romeo RD, Morris JA, Lookingland KJ, Sisk CL. Medial preoptic area dopaminergic responses to female pheromones develop during puberty in the male Syrian hamster. Brain Research. 2003;988:139–145. doi: 10.1016/s0006-8993(03)03358-4. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and Behavior. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes, Brain and Behavior. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: A finishing school for male social behavior. Annals of the New York Academy of Sciences. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Wallen K. The organizational hypothesis: Reflections on the 50th anniversary of the publication of Phoenix, Goy, Gerall, and Young (1959) Hormones and Behavior. 2009;55:561–565. doi: 10.1016/j.yhbeh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Annals of the New York Academy of Sciences. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and Behavior. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recommended Reading
- Adolphs R. The social brain: Neural basis of social knowledge. Annual Reviews in Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. A comprehensive review of the brain regions involved in social knowledge in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. An excellent review describing the shift in balance between frontal-cortical immaturity and social-reward seeking during adolescence. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. One of the first papers demonstrating developmental changes in grey and white matter during adolescence in humans. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and Behavior. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. A review that discusses organizational effects of pubertal gonadal hormones in more detail than the current paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. 2009 (See References). A well-written review of the organization/activation dichotomy of hormone effects, based on the seminal paper by Phoenix, Goy, Gerall, and Young (1959) [Google Scholar]