Abstract
Various stem cells and their progeny have been used therapeutically for vascular regeneration. One of the major hurdles for cell-based therapy is low cell retention in vivo, and to improve cell survival several biomaterials have been used to encapsulate cells before transplantation. Vascular regeneration involves new blood vessel formation which consists of two processes, vasculogenesis and angiogenesis. While embryonic stem cell (ESC)-derived endothelial cells (ESC-ECs) have clearer vasculogenic potency, adult cells exert their effects mainly through paracrine angiogenic activities. While these two cells have seemingly complementary advantages, there have not been any studies to date combining these two cell types for vascular regeneration. We have developed a novel chitosan-based hydrogel construct that encapsulates both CD31-expressing BM-mononuclear cells (BM-CD31+ cells) and ESC-ECs, and is loaded with VEGF-releasing microtubes. This cell construct showed high cell survival and minimal cytotoxicity in vitro. When implanted into a mouse model of hindlimb ischemia, it induced robust cell retention, neovascularization through vasculogenesis and angiogenesis, and efficiently induced recovery of blood flow in ischemic hindlimbs. This chitosan-based hydrogel encapsulating mixed adult and embryonic cell derivatives and containing VEGF can serve as a novel platform for treating various cardiovascular diseases.
Keywords: vascular regeneration, embryonic stem cells, CD31+ cells, chitosan hydrogel, lipid microtubes
1. Introduction
Tissue ischemia results from the loss of blood vessels. However, there are no effective ways to regrow blood vessels in human to repair this loss. Stem cell-based therapy has emerged as a new therapeutic option, and experimental and early clinical studies have shown promising outcomes [1-3]. However, several new hurdles to achieving successful therapeutic goals have come to light from these studies.
In the application of stem cell based therapy for neovascularization (new vessel formation), the selection of effective cells is of paramount importance. Neovascularization consists of two processes, angiogenesis and vasculogenesis. Angiogenesis refers to the growth of preexisting blood vessels, and vasculogenesis, the de novo development of vessels from endothelial progenitor or stem cells (reviewed in [4, 5]). Adult stem or progenitor cells have been reported to be effective for neovascularization in animal studies; however, the main mechanism by which this occurs is by paracrine angiogenic effects which are modest [6-11].
Recently, we identified a unique and effective angio-vasculogenic cell population in bone marrow and peripheral blood which expressed CD31 (PECAM-1) [12, 13]. These cells have high angiogenic activity, include stem cells and genuine endothelial progenitor cells (EPCs), and are more effective than other primary isolated BM-derived cells for regenerating ischemic tissues. These cells have many advantages for cell therapy due to their abundance, ease of isolation, and higher adhesion capacity, and they do not require cell culture. On the other hand, endothelial cells differentiated from pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) were found to be useful for neovascularization due to their strong vasculogenic potential [14-19]. While embryonic stem cells exhibit an efficacious regenerative potential [18], their use has been limited due to ethical issues, immunologic concerns, and the risk of tumorigenesis. Recent discovery of iPSCs, however, has evoked interest in the utility of similar PSCs for regenerative therapy by avoiding ethical and immunological issues [20, 21]. Along with the development of an in vitro endothelial cell differentiation system [22-24], studies have demonstrated the vasculogenic and therapeutic potential of pluripotent stem cell-derived endothelial cells (PSC-ECs) in ischemic hindlimb models [16, 17, 25].
The main obstacle to achieving optimal vascular regeneration by any modes of cell therapy is poor engraftment and survival of transplanted cells in the ischemic tissue [26-30]. Studies have shown that injected or transplanted cells remained at the site of treatment for a very short duration, leading to reduced therapeutic efficacy of the transplanted cells [31-34]. Due to the lack of supporting matrix and to an inflammatory cellular response, the injected cells easily die or are washed away. To prolong the cell retention and improve cell survival, several classes of biomaterials including natural and synthetic hydrogels have been successfully employed to serve as carriers for encapsulation. These biomaterials can provide matrix to support cell adhesion, and function as a barrier against inflammatory cell infiltration. Among natural biomaterials, chitosan, derived from crab shells, has been used in various forms including in situ gelling hydrogels. Recently, we developed a novel fabrication approach to generate in situ gelling hydrogels, with the ability to tailor mechanical properties and gelation kinetics. These hydrogels also showed enhanced neurite differentiation and extension in in vitro 3D cell culture models [35].
VEGF is a well-known angiogenic growth factor and has been shown to enhance angiogenesis, endothelial cell survival and migration, and revascularization in vivo [36-38]. However, the protein form of VEGF acts only short-term and therefore has limited therapeutic utility. Therefore, a carrier for slow release of VEGF would be required to enhance its therapeutic efficacy. We previously demonstrated that lipid-based microtubes are efficient and useful vehicles to provide sustained delivery of protein factors such as BMP-2 and BDNF [39, 40]. Furthermore, when coupled with hydrogels, these systems further enhanced local delivery of the growth factors without inducing cytotoxicity or inflammatory responses [39-41]. With this technique, a therapeutic agent can potentially be released for longer periods of time than with microtubes alone.
Accordingly, in the present study, we investigated the effects of vasculogenic endothelial cells (ESC-ECs) and angiogenic effector cells (BM-CD31+ cells) on ischemic tissue repair by engineering the two complementary cells with chitosan hydrogel containing VEGF-loaded microtubes. Here, we show that these engineered hybrid cell constructs prolonged cell survival in vivo, and could effectively induce neovascularization and enhance vascular repair in a model of ischemic vascular disease.
2. Materials and Methods
2.1 Harvesting BM-CD31+ cells
Mouse bone marrow (BM) cells were harvested as previously described [12]. Briefly, mouse BM- mononuclear cells (MNCs) were isolated by density gradient centrifugation using Histopaque 1083 (Sigma, St. Louis, Missouri) according to the manufacturer's protocol. CD31+ cells were further isolated from BM-MNCs: mouse BMMNCs were stained with APC conjugated anti-mouse-CD31 monoclonal antibody (MEC 13.3, Cat.# 551262, BD Biosciences) for 30 min at 4°C, washed, and incubated with anti-APC magnetic beads (Miltenyi Biotec) for 30 min at 4°C. Mouse BM-CD31 + cells (mBM-CD31+) were isolated using magnetic columns (MACS®, Miltenyi Biotec) according to the manufacturer's protocol.
2.2 Endothelial cell differentiation
J1 mouse ES cells (ESCs) were cultured and maintained on Mitomycin C treated mouse embryonic fibroblasts (MEF) with DMEM (11965-092, Invitrogen) supplemented with 15% FBS, 2-Mercaptoethanol (100 μM) and LIF (1000 U/ml, ESG1107, Millipore). For differentiation into endothelial cells (ECs), embryoid bodies (EBs) of mouse ESCs were cultured for 3.5 days in alpha-MEM (12561-056, Invitrogen) supplemented with 15% FBS, glutamine (2 mM), and ascorbic acid (50 μg/ml). Mouse ESC-EBs were dissociated with Accutase Cell Detachment medium (00-4555-56, eBiosciences) for 30 min. with vortexing every 5 min. Flk1+ cells were isolated by MACS (Miltenyi Biotech) from the dissociated EBs to enrich mesodermal lineage cells. These Flk1+ cells were cultured on an OP9 feeder cell layer for five more days in alpha-MEM medium supplemented with 15% FBS, 2-Mercaptoethanol (100 μM), VEGF165 (10 ng/ml, 100-20, PeproTech), and FGF2 (20 ng/ml, 233-FB/CF, R&D Systems) for further differentiation. Differentiated CD31+ endothelial cells (mESC-ECs) were isolated by MACS for further experimentation.
2.3 Construction of cell-chitosan hydrogel patch
The photo-crosslinkable chitosan was prepared as previously described by dissolving it in PBS to prepare a 2% (w/v) chitosan stock solution and sterilizing it using UV light (365 nm)[35]. The 0.1% (w/v) photo-initiator (Irgacure® 2959, BASF) was dissolved in PBS and filter-sterilized via a 0.2 μm filter. The individual components were stored at 4°C until used. Lipid microtubes were fabricated as previously described [41, 42]. Dehydrated microtubes (5 mg) were loaded with 200 ng of VEGF165. mBM-CD31+ cells (1 × 105 cells) and mESC-ECs (1 × 105 cells) were mixed with chitosan (final 0.5%), photo-initiator (final 0.01%) and 1 mg of VEGF165-containing lipid microtubes in 200 μl. This solution was then transferred to a 48-well plate and exposed to UV light (365 nm) for 5 min for gelation.
For measuring release of VEGF from chitosan hydrogel, 20 ng VEGF165 loaded in 500 μg microtubes, or 20 ng VEGF165 without microtubes were encapsulated into 200 μl chitosan gel (0.5%). Triplicate constructs were prepared. Each construct was incubated in 1000 μl of sample buffer (2% BSA in PBS), which was collected and replaced with fresh sample buffer (1000 μl) at days 1, 3, 7 and 14. VEGF165 concentration of the collected sample buffer was measured by ELISA (900-TM10, PeproTech), and the concentration was converted to the amount of VEGF165 released from constructs. The release profile was presented as cumulative release, calculated as the percentage of the total amount released at the indicated time points divided by the amount of VEGF165 loaded initially.
The surface morphology of microtubes was probed using a Hitachi variable pressure scanning electron microscope on uncoated microtube samples. The high (1000X) magnification images were obtained and a representative image was presented in Figure 7.
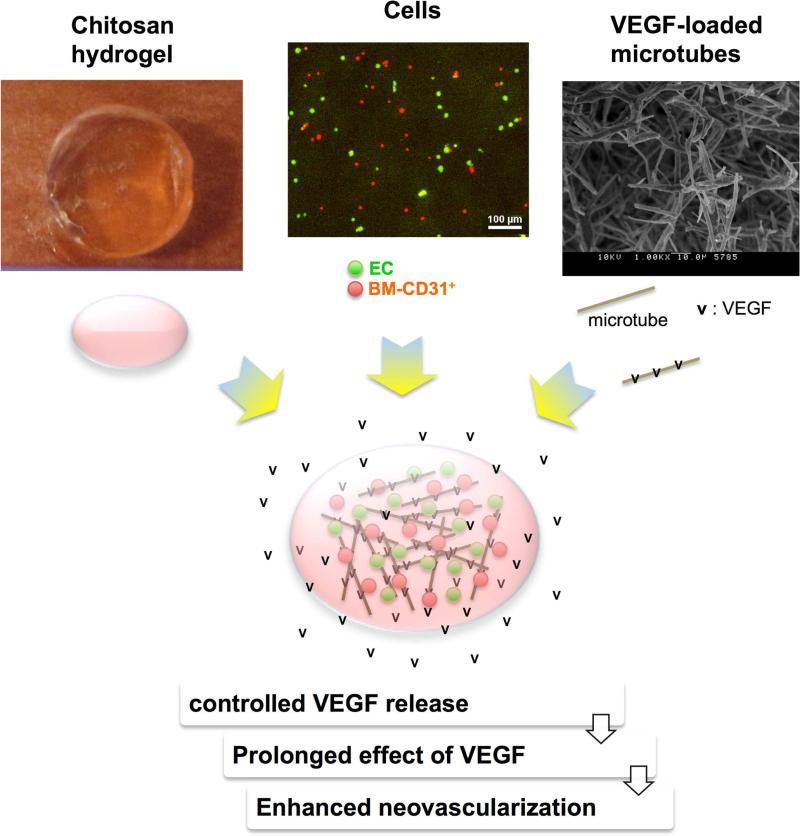
Figure 7. Advantages of the lipid microtubes loaded with VEGF.
The top portion shows photographs of a representative chitosan gel, ECs (green) and BM-CD31+ cells (red), and a high magnification (1000X) scanning electron micrograph of lipid microtubes. The bottom portion illustrates the combination of the three: cells (ECs as green dots and BM-CD31+ as red dots) and VEGF165 (v)-loaded microtubes (grey sticks) which are encapsulated by chitosan hydrogel (pink oval). The lipid microtubes provide controlled release of VEGF, prolonging the effects of VEGF and resulting in enhanced neovascularization.
2.4 TUNEL staining
To evaluate apoptosis, terminal deoxynucleotidyl transferase deoxyuride-5′-triphosphate biotin nick end labeling (TUNEL) reaction was conducted [11]. The hydrogels were collected, fixed with 4% paraformaldehyde solution for fixation and permeabilized. The fluorescein in situ cell death detection kit (Roche Applied Science, Indianapolis, IN, USA) was used for TUNEL staining.
2.5 Transplantation of hydrogel-encapsulated cell patches into ischemic hindlimbs
All experimental protocols were approved by the Emory University Institutional Animal Care and Use Committee. Hindlimb ischemia (HLI) was induced in male 129 SvJ mice (Charles River Laboratories, Wilmington, Massachusetts) 8 to 10 weeks old as previously described [12, 13]. Briefly, under anesthesia using tribromoethanol (Avertin), a ligation was made around the femoral artery and large branches were cauterized. Mice were transplanted with the cell-hydrogel patch, which was constructed as described in the previous section. These patches included chitosan hydrogels embedded with mBM-CD31+ cells (CD31), with mESC-ECs (EC), and both types of cells in the absence (CD31+EC) and presence of VEGF165 containing microtubes (CD31+EC+ VEGF). For control groups, mice injected intramuscularly with 100 μl PBS (PBS) or transplanted with chitosan hydrogels without any cells (No Cells) were included. Cells were labeled with chloromethylbenzamido (CellTracker™ CM-DiI, Invitrogen, Carlsbad, CA, USA) prior to mixing with chitosan hydrogel. This cell-hydrogel patch was incubated for a day in EGM2 20% FBS medium and transplanted into the ischemic site of a hind limb.
2.6 Blood flow measurements in ischemic hindlimbs
Serial blood flow of the hindlimb was measured for 2 weeks after the operation using a Laser Doppler perfusion imager (LDI, Moor Instruments, UK) as described previously [12, 13]. Mean values of perfusion were calculated from the stored digital color-coded images. The level of blood flow of the ischemic (right) limb was normalized to that of non-ischemic (left) limb to avoid data variations caused by ambient light and temperature.
2.7 Confocal microscopy and capillary density measurements
Before euthanasia, the mice were perfused with fluorescein-labeled Griffonia (Bandeiraea) Simplicifolia Lectin I (BSL1, Vector Laboratory Inc.) by direct cardiac injection to stain functional endothelial cells in blood vessels [12, 13]. After 15 minutes, the hindlimb tissue was harvested, fixed for 4 hours in 4% PFA, and incubated overnight in 30% sucrose solution. The tissues were then embedded in Optimal Cutting Temperature (OCT) compound (Sakura Finetek USA, Torrance, CA, USA), snap-frozen in liquid nitrogen, and sectioned at 50 μm in thickness. The tissue sections were counter-stained with DAPI (Invitrogen, Carlsbad, CA, USA) to visualize the nuclei. Incorporation of transplanted cells into the vasculature as ECs was determined using a Zeiss LSM 510 Meta confocal laser scanning microscope and LSM 510 Image software (Carl Zeiss, Jena, Germany). At two weeks after transplantation, the capillaries visualized by perfused BSL1 were measured and capillary density was calculated from at least 5 randomly selected fields (N = 5).
2.8 Real-time RT-PCR
Total RNA was extracted from cell-injected ischemic hindlimb tissue using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. First-strand cDNA was generated using the Multiscribe Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Gene expression was determined by real-time quantitative PCR (7500 Fast Real-Time PCR System, Applied Biosystems) using TaqMan PCR Master Mix (Applied Biosystems). Relative mRNA expression of target gene normalized to that of glyceraldehye-3-phosphate dehydrogenase (GAPDH) was calculated using the formula: Relative Expression Level = 2−ΔCT, where ΔCT = CT gene of interest – CT GAPDH as previously described [7]. The primers and probes were designed using Primer Express 3.0 (Applied Biosystems) and are described in Table 1.
Table 1.
Sequences of primers and probes for qRT-PCR
| Genes | Forward (5’ to 3’) | Reverse (5’ to 3’) | Probe (5’ to 3’) |
|---|---|---|---|
| Gapdh | CGTGTTCCTACCCCCAATGT | TGTCATCATACTTGGCAGGTTTCT | TCGTGGATCTGACGTGCCGCC |
| Vegfa | GCAGGCTGCTGTAACGATGA | GCATGATCTGCATGGTGATGTT | CCCTGGAGTGCGTGCCCACG |
| Fgf2 | GTCACGGAAATACTCCAGTTGGT | CCGTTTTGGATCCGAGTTTATACT | TGTGGCACTGAAACGAACTGGG |
| Igf1 | TGCTTCCGGAGCTGTGATCT | CGGGCTGCTTTTGTAGGCT | AGGAGACTGGAGATGTACTGTGCCCCAC |
| Ang1 | GGGACAGCAGGCAAACAGA | TGTCGTTATCAGCATCCTTCGT | TTGATCTTACACGGTGCCGATT |
| Tgfβ | AAACGGAAGCGCATCGAA | GGGACTGGCGAGCCTTAGTT | CCATCCGTGGCCAGATCCTGTCC |
2.9 Statistical Analysis
All data were expressed as means ± S.E.M and statistical analysis was conducted using GraphPad Prism. Student's t test was used for the statistical analysis for continuous variables between two groups and repeated ANOVA followed by multiple comparison with Bonferroni's method for variables among more than 2 groups. A P value < 0.05 was considered statistically significant.
3. Results
3.1 High cell viability in chitosan hydrogel
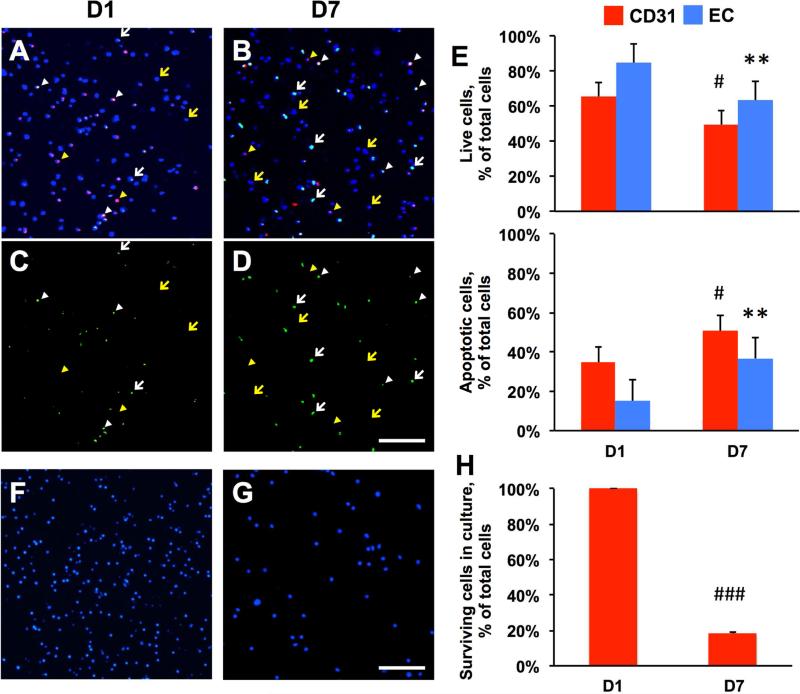
We conducted experiments to examine the cell viability of mouse mBM-CD31+ and mESC-ECs in chitosan hydrogel. First, we embedded CM-DiI-labeled mBM-CD31+ cells (1 × 105 cells) and non-labeled mESC-ECs (1 × 105 cells) into a hydrogel patch which consisted of 0.5% chitosan hydrogel without lipid microtubes bearing VEGF165. The cells were cultured in the hydrogel patches in EGM2 20% FBS medium for 7 days. The cell viability was evaluated at days 1 and 7 via confocal microscopic examination after TUNEL and DAPI staining (Fig. 1 A-D). At days 1 and 7, TUNEL-positive mBMCD31+ cells were 34.6 ± 7.3% and 50.6 ± 5.0%, and TUNEL-positive mESC-ECs were 15.2 ± 7.5% and 36.7 ± 8.1%, respectively, suggesting that approximately 16 to 25% of cells underwent apoptosis over the 6 day culture period (Fig. 1E). For comparison, we evaluated the cell viability in culture without chitosan hydrogel. mBM-CD31+ cells (1 × 106 cells) were directly plated on a 24-well culture dish, and the number of surviving cells was evaluated by microscopic examination after staining with DAPI at days 1 and 7 (Fig. 1F and G). Compared to day 1 (100%), 18.2 ± 0.6% of cells survived at day 7, showing that more than 80% of cells died (Fig. 1H). Together, these results suggested that chitosan hydrogel substantially enhanced cell survival and is appropriate for encapsulating mixed populations of mBM-CD31+ cells and mESC-ECs.
Figure 1. Better survival of co-cultured mBM-CD31+ cells and mESC-ECs in chitosan hydrogel.
A-D. TUNEL staining and confocal examination. mBM-CD31+ cells (CM-DiI labeled, red fluorescence) and mESC-ECs (non-labeled) were embedded in chitosan hydrogel and cultured. The hydrogel patches were collected at days 1 (D1, A and C) and 7 (D7, B and D) and were stained for DAPI (blue) and TUNEL (green). A and B are merged images of all three channels (DAPI, CM-DiI, TUNEL), whereas C and D show only TUNEL positive signals. Confocal microscopy examination showed live mBM-CD31+ cells (yellow arrowheads, red cells), apoptotic mBM-CD31+ cells (white arrowheads, green/red cells), live mESC-ECs (yellow arrows, blue nuclei), and apoptotic mESC-ECs (white arrows, green cells). E. Quantitative evaluation of the percent of live cells and apoptotic cells per total cells (live cells plus apoptotic cells). F-G. Cell survival in culture without chitosan hydrogel. F and G are representative images of DAPI-stained mBM-CD31+ cells in culture dishes at days 1 (F) and 7 (G). H. Quantitative evaluation of the percent of surviving cells at day 7 per cells at day 1. Scale bar: 100 μm. N = 3, ###P < 0.001, #P < 0.05 vs. D1 CD31, **P < 0.01 vs. D1 EC.
3.2 Effect of VEGF-loaded microtubes on cells encapsulated in hydrogel
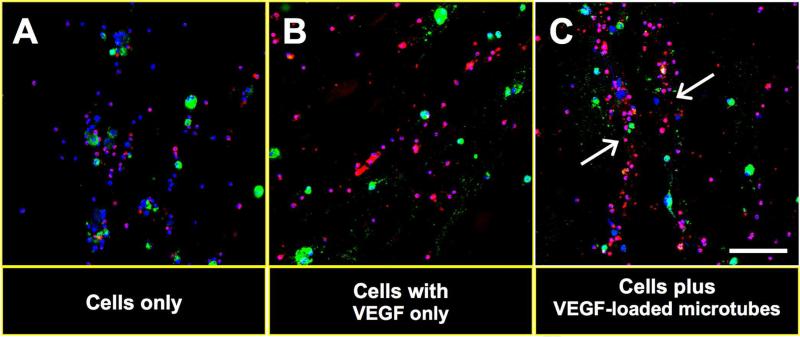
Next we determined the effects of VEGF165-loaded microtubes on cell biological and morphological changes in this cell-chitosan hydrogel mixture. We embedded 1 × 105 CM-DiI (red fluorescent dye)-labeled mBM-CD31+ cells and 1 × 105 SP-DiO (green fluorescent dye)-labeled mESC-ECs into 0.5% chitosan hydrogel with or without VEGF165-loaded microtubes. Four days later, the cell-hydrogel mixture was collected, fixed with 4% paraformaldehyde, and subjected to confocal microscopy. Confocal microscopic examination demonstrated that while the two incorporated cell types showed no specific patterns in the absence of VEGF165 or microtubes (Fig. 2A and B), they were linearly aligned and formed tube-like structures in the presence of VEGF165- loaded microtubes (Fig. 2C). These data suggest that VEGF-loaded microtubes induced primitive vascular tube-like alignment of the cells in chitosan hydrogel.
Figure 2. Tubular alignment of cell mixture in chitosan hydrogel after application of VEGF-loaded microtubes.
mBM-CD31+ cells (CM-DiI labeled, red) and mESC-ECs (SP-DiO labeled, green) were embedded in chitosan hydrogel in the absence of VEGF165-loaded microtubes (A), presence of VEGF165 only (B), and presence of VEGF165-loaded microtubes (C). The hydrogel-cell mixture was collected at day 4 and stained with DAPI (blue). Confocal microscopic examination showed tube-like cellular arrangement (white arrows) only in the presence of VEGF165-loaded microtubes (C). Scale bar: 100 μm.
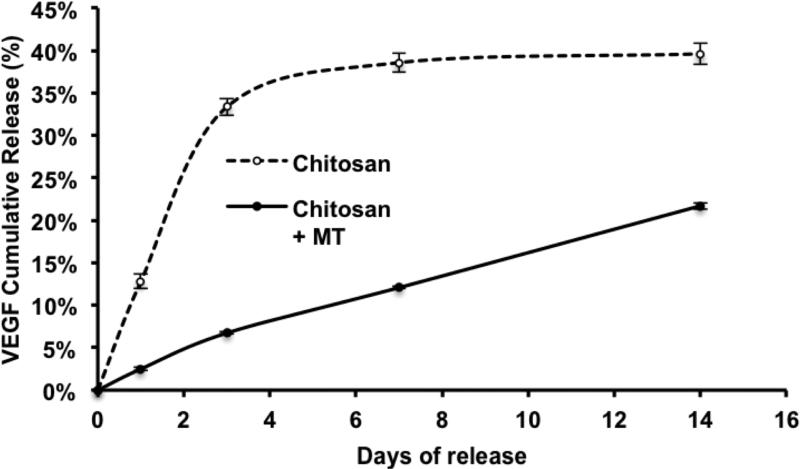
To examine the effect of microtubes on the release of VEGF165, the release kinetics of VEGF165 from hydrogel constructs were examined with and without microtubes. VEGF165 (20 ng) or lipid microtubes (0.5 mg) loaded with VEGF165 (20 ng) were embedded in chitosan hydrogel constructs and incubated in 2% BSA in PBS. At days 1, 3, 7 and 14, the supernatants were collected and assayed for VEGF165 by ELISA (Fig. 3). Media was replaced with fresh 2% BSA in PBS after collection of the supernatant. The cumulative release curve showed that for the VEGF165 loaded in microtubes group, 25% of the total VEGF165 was released over 14 days in a linear manner. Without microtubes, about 40% of VEGF165 was released over 14 days. However, about 90% of this release occurred within the first 3 days, which is a characteristic burst release, reducing the bioavailability of VEGF165 over a sustained period of time. These results suggest that microtubes provided sustained release of VEGF165 and prolonged the window for the effects of VEGF.
Figure 3. Release profiles of VEGF from lipid microtubes.
The release kinetics of VEGF165 from chitosan hydrogel (Chitosan) containing bare VEGF165 or chitosan hydrogel containing VEGF165-loaded microtubes (Chitosan + MT) were examined. The cumulative release was calculated as the percentage of total amount released at the indicated time points divided by the initially loaded amount of VEGF165. Data were shown as means ± S.E.M.
3.3 Improvement of blood flow recovery and increased capillary density with engineered cells
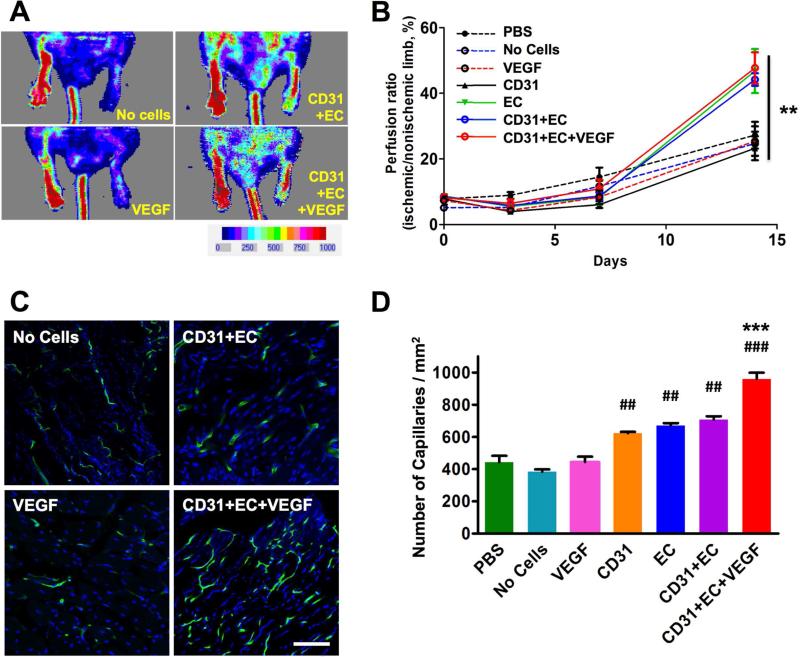
To examine the ischemic reparative and vascularizing capacity of mBM-CD31+ cells and mESC-ECs encapsulated in chitosan hydrogel, we implanted a cell-hydrogel patch into mice in a mouse HLI model. mBM-CD31+ cells and mESC-ECs, 1 × 105 each, both labeled with CM-DiI, were mixed with chitosan hydrogel (0.5 %) and microtubes (5 mg/ml) containing VEGF165 (200 ng/ml) and were exposed to UV light to crosslink the chitosan hydrogel. The cell-hydrogel patches were cultured for one day in EGM2 20% FBS medium and transplanted onto the muscle immediately following induction of HLI. As controls, we used PBS (PBS), chitosan hydrogel only (No Cells), and chitosan hydrogel with VEGF165-containing microtubes without cells (VEGF). The blood flow of chitosan hydrogels embedded with mBM-CD31+ cells (CD31), chitosan hydrogels embedded with mESC-ECs (EC), and chitosan hydrogels with mBM-CD31+ cells and mESC-ECs with VEGF165-containing microtubes (CD31+EC+VEGF) groups were measured by Laser Doppler Perfusion Imaging (LDPI). LDPI revealed significantly enhanced blood perfusion in the groups having mESC-ECs (EC, CD31+EC, and CD31+EC+VEGF) compared to PBS, No Cells or VEGF groups at two weeks after induction of HLI (Fig. 4A and B).
Figure 4. Increase in blood flow and capillary density in ischemic hindlimbs treated with various cells and cell-chitosan patch.
A. The blood perfusion was measured by LDPI at days 3, 7, and 14. The representative LDPI images at day 14 are shown. B. The quantitation of blood perfusion was plotted. N = 4 to 6, **P < 0.01. C. Representative images of capillaries in hindlimb. D. Quantitative analyses of capillary density at day 14. Scale bars: 100 μm. N = 5, ###P < 0.001 and ##P < 0.01 vs. PBS; ***P < 0.001 vs. CD31+EC. CD31: mBM-CD31+ cells, EC: mESC-EC, VEGF: VEGF165-loaded microtubes.
We next measured capillary density to evaluate neovascularization. The mice were perfused with FITC-conjugated BSL-1 to stain the blood vessels at 2 weeks after HLI and cell transplantation, and the hindlimb tissues were processed for confocal microscopic examination. The capillary density in the hindlimb muscle was significantly higher in the cell-hydrogel patch-treated groups (CD31, EC, CD31+EC, and CD31+EC+ VEGF) compared to PBS, No Cells or VEGF groups (Fig. 4C and D). Among the cell-hydrogel patch transplanted groups, the capillary density tended to increase in the combined cell group (CD31 and EC), compared to the single cell groups (CD31 or EC), although there was no significant difference. The capillary density was the highest when VEGF165-containing microtubes were included with the two cell types (CD31+EC+VEGF). Taken together, these data suggest that mESC-ECs encapsulated in chitosan hydrogel can efficiently repair hindlimb ischemia and promote postnatal neovascularization, and these effects were augmented when combined with mBM-CD31+ cells and VEGF165-containing microtubes in the hydrogel.
3.4 Cell retention and incorporation into vasculature in vivo
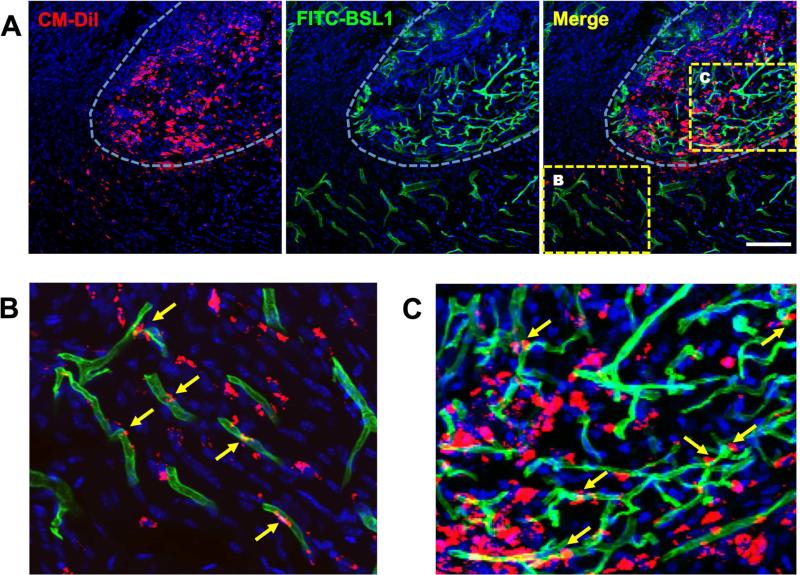
Next, we examined the in vivo behavior of the transplanted cells. Since we pre-labeled both mBM-CD31+ cells and mESC-ECs with CM-DiI, we were able to track their fate in the tissue sections. Confocal microscopic examination of muscle tissues harvested at 2 weeks after HLI demonstrated that many of the transplanted cells (red) survived within the hydrogel-degrading area and fewer cells migrated into the surrounding tissue (Fig. 5A). A portion of the transplanted cells was incorporated into vasculature (green fluorescence) within and outside of the hydrogel area, suggesting vasculogenesis (Fig. 5B and C). Notably, many more blood vessels were observed within the hydrogel area (Fig. 5A and C), indicating ingrowth of blood vessels from host and angiogenesis. These data suggest that mBM-CD31+ cells and mESC-ECs contribute to new vessel formation within and outside of the hydrogel patches.
Figure 5. Incorporation of cells into vasculature.
Confocal microscopic images of ischemic limb tissues transplanted with a chitosan patch encapsulating mBM-CD31+ cells and mESC-ECs with VEGF165-loaded microtubes were analyzed for the incorporation of cells into vasculature. A. All cells were labeled with CM-DiI (red). The mouse was perfused with FITC-BSL1 (green) to visualize the functional vessels at day 14. DAPI (blue). White dotted line marks transplanted chitosan hydrogel area which shows encapsulated cells (red) and invested blood vessels (green). B and C are magnified images of the boxed areas in A. B. Transplanted cells were migrated from hydrogel to the neighboring areas and were incorporated into the blood vessels as endothelial cells, showing vasculogenesis (yellow arrows). C. Transplanted cells in chitosan hydrogel area showed extensive vasculogenesis and angiogenesis by incorporation of transplanted cells (yellow arrows) into vessels and ingrowth of host vessels from surrounding vessels. Scale bar: 100 μm.
3.5 Elevated expression of angiogenic factors with engineered cells
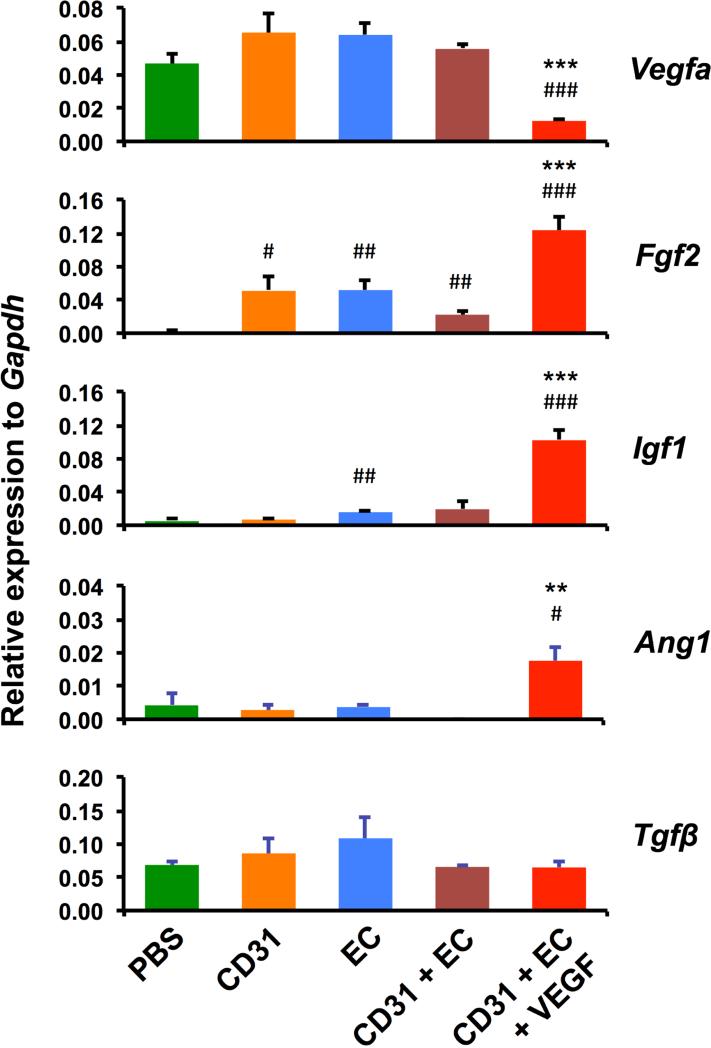
We next investigated the angiogenic effects of cell-hydrogel patches on ischemic hindlimb by measuring expression of angiogenic factors (vegfa, fgf2, Igf1, Ang1, Tgfβ) via real-time RT-PCR with muscle tissues harvested at 4 weeks. Except for vegfa, classical angiogenic factors, Fgf2, Igf1, and Ang1 were more highly expressed in the VEGF-containing cell-hydrogel patch group compared to all the other groups (Fig. 6). In general, any cell transplantation group showed increased expression of at least one of these three angiogenic factors. Surprisingly, Vegfa expression was not increased in any cell transplantation group but rather was suppressed in the group containing VEGF containing microtubes, suggesting potential feedback suppression at this 4 week time point. An atypical angiogenic factor, Tgfβ, was not significantly different between the groups. Together, these data indicate that transplantation of combined mBM-CD31+ cells and mESC-ECs encapsulated within chitosan containing VEGF increased expression of the representative angiogenic factors.
Figure 6. Increased mRNA expression of angiogenic factors in ischemic hindlimbs treated with chitosan-cell patch.
The expression of Vegfa, Fgf2, Igf1, Ang1 and Tgfβ genes were examined by real-time RT-PCR of hindlimb tissues harvested at 4 weeks following HLI surgery. The expression level of each gene is plotted as a relative value to the expression level of Gapdh. N = 3. ###P < 0.001, ##P < 0.01, and #P < 0.05 vs. PBS; ***P < 0.001 and **P < 0.01 vs. CD31+EC.
4. Discussion
In this study, we have demonstrated for the first time the utility and effects of combining adult and embryonic stem cells for vascular regeneration. Moreover, this is the first study demonstrating the effects of the chitosan hydrogel to enhance vascular cell viability in ischemic tissues. This new approach of encapsulating two complementary cells in chitosan hydrogel enhanced cell retention in ischemic tissues, induced neovascularization, and efficiently repaired tissue ischemia. Addition of VEGF-containing microtubes to the cell-chitosan patch further augmented neovascularization in vivo compared to the patch without VEGF.
The current study clearly provides support for the beneficial application of cells encapsulated in chitosan hydrogel because of enhanced cell retention and induction of vessel formation. Although cell therapy is a promising option, it has been limited by low cell retention in the ischemic tissue [43, 44]. To resolve this problem, biomaterials have been widely attempted, particularly in animal models of myocardial infarction (reviewed in [45]). However, only a few studies have assessed the performance of cell therapy accompanied by synthetic or natural biomaterial carriers such as alginate hydrogel, hyaluronic acid hydrogel, and poly lactic acid in an ischemic hindlimb [46-51]. We recently reported the fabrication of a novel water-soluble photocrosslinkable chitosan based hydrogel with negligible cytotoxicity [35]. Chitosan, a derivative of chitin, is a natural polymer and has outstanding biocompatibility, enzyme-mediated degradation profiles, and non-toxic byproducts [52]. Additionally, it has a positive surface charge which promotes cell attachment and differentiation, and active end residues that enable coupling of bioactive proteins [53-56]. Thus, for this study, BM-CD31+ cells and ESC- ECs were engineered in this novel chitosan based hydrogel, and more than 80% of cells survived after 6 days in culture (comparing D7 vs. D1) while without hydrogel, more than 80% cells died. In the in vivo studies, two weeks after transplantation, a large number of cells were present and they contributed to vessel formation in the hydrogel-degrading area. Furthermore, some of the cells migrated into the surrounding tissue, incorporating themselves into the vasculature. These data suggest that chitosan hydrogel plays a crucial role as a cell reservoir by keeping cells viable and available for long-term engraftment, ultimately maximizing the neovascularization and ischemia reparative effects of the encapsulated cells. Additionally, the pore sizes and the degradation rate of the hydrogel supported cell survival and migration within or outside of the hydrogel system.
Adult stem/progenitor cells and ESC-derived ECs have their respective advantages and disadvantages for cell therapy. The collective data to date has shown that the dominant mechanisms underlying vascular regenerative effects of adult stem or progenitor cells are their paracrine activities (reviewed in [57]). Based on this, we sought to identify higher angiogenic cells regardless of their stem cell status and discovered that a subpopulation of BM or peripheral blood MNCs expressing CD31 have these high angiogenic activities and are effective for vascular repair [12, 13]. On the other hand, ESC- or iPSC-derived endothelial cells have emerged as a promising cell source for vascular regeneration due to their high vasculogenic potential, and have demonstrated efficacy for vascular regeneration [14-17, 25]. However, one major drawback of using ESC-ECs is the difficulty in obtaining enough cells, due to their poor proliferation and maintenance properties once they are endothelially differentiated [15, 24, 58-62]. Therefore, we used a combination of cells: CD31+ cells, which are abundant and have robust angiogenic activities but with limited vasculogenic activities, and ESC-ECs, which are harder to obtain but have higher vasculogenic properties.
We anticipated synergistic effects from ESC-ECs (vasculogenic) and BM-CD31+ cells (angiogenic) and hoped to reduce the required number of cells. The LDPI data showed that the cell hydrogel patch including ESC-ECs enhanced blood flow recovery from hindlimb ischemia. Histologically, numerous perfusable vessels were observed within the hydrogel area and overall capillary density was increased in these groups in ischemic hindlimb muscle. These data suggest that ESC-EC-including chitosan patch groups have robust vascularizing effects. However, synergistic effects were not observed by combining BM-CD31+ cells and ESC-ECs. We speculate that the reason could be the capability of chitosan hydrogel that allows robust retention of cells so that even a single cell type is sufficient to induce ischemia recovery. Since we expected higher cell survival, in this study, we used one fifth of the cell dose (total 2 × 105 per animal) that we normally used for other cell injection studies in hindlimb ischemia. It thus remains to be determined whether further reduction of the cell number can lead to synergistic effects.
After addition of VEGF165-releasing microtubes to the cell combination, the neovascularization and proangiogenic factors in tissues were substantially increased, although the ischemia recovery rate was similar. The role of VEGF appears versatile. VEGF might have exerted direct angiogenic effects independent of injected cells. However, the qRT-PCR data demonstrated an increase in multiple angiogenic factors after addition of VEGF, suggesting that VEGF might have activated an angiogenic cascade. By providing an angiogenic milieu for a prolonged time within and near the hydrogel area, VEGF appeared to stimulate vessel-formation activities of BM-CD31+ and ESC-ECs and to induce angiogenesis from host vessels, culminating in more robust vessel formation. This assumption is supported by the in vitro data showing that addition of VEGF-releasing microtubes to BM-CD31+ and ESC-ECs induced tube-like structures in hydrogel, and the in vivo data showed robust infiltration of host vessels into the hydrogel area. Inclusion of growth-factor releasing microtubes in the hydrogel is another novel aspect of our study and was found to be effective. In earlier studies of vascular regeneration, angiogenic growth factors such as VEGF, FGF2, and GM-CSF were used to promote angiogenesis [63-68]. However, due to the low and transient levels of these factors at sites of ischemia, several approaches have been attempted to provide a protective environment and controlled release of growth factors by incorporation into biomaterials [69-78]. In the current study, in order to deliver VEGF efficiently, we used lipid-based microtubes, which were demonstrated to be efficient depots for controlled release of protein factors within hydrogel matrices for longer durations of time (about 3-4 weeks) without provoking cytotoxicity or inflammatory responses [39-41]. In fact, the microtubes capture and slowly release VEGF, prolonging the effects of VEGF (Fig. 7). In our platform this lipid microtube technique together with hydrogel encapsulation contributed to steady release of VEGF from the construct.
This present study describes the application of both adult and embryonic cells in a synthetic hydrogel matrix. For clinical application, further studies are required to engineer human PSC-ECs with human CD31+ MNCs isolated from human peripheral blood in the chitosan hydrogel system along with VEGF-releasing microtubes. However, this study provides clear evidence that this cell therapy platform would be a valuable therapeutic option for the treatment of many human cardiovascular diseases.
5. Conclusions
Engineering of BM-CD31 cells together with ESC-derived ECs in chitosan hydrogel which contains VEGF165-releasing microtubes augmented neovascularization and efficiently induced ischemic vascular repair. This study clearly demonstrates the utility of biomaterial-encapsulated adult and pluripotent stem cells for vascular regeneration. These engineered cellular constructs could form a robust platform for treating various cardiovascular and neurovascular diseases.
Acknowledgements
This work was supported in part by a Wallace H. Coulter Translational Research Grant, NIH grant DP3DK094346, NSF-EBICS grant CBET-0939511, Faculty Research Assistance Program of Yonsei University College of Medicine 2015, and Bio and Medical Technology Development Grant by Ministry of Science, ICT and Future Planning, Korea. We would also like to acknowledge the Robert P. Apkarian Integrated Electron Microscopy Core of Emory University for their help with the electron microscopy of the lipid microtubes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Kinoshita M, Fujita Y, Katayama M, Baba R, Shibakawa M, Yoshikawa K, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012;224(2):440–5. doi: 10.1016/j.atherosclerosis.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. Journal of vascular surgery. 2012;56(1):264–6. doi: 10.1016/j.jvs.2012.03.255. [DOI] [PubMed] [Google Scholar]
- 3.Raval Z, Losordo DW. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res. 2013;112(9):1288–302. doi: 10.1161/CIRCRESAHA.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104(9):1095–102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. The Journal of experimental medicine. 2007;204(13):3257–69. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 9.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122(5):517–26. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111(16):2073–85. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107(5):602–14. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56(7):593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nature biotechnology. 2007;25(3):317–8. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 15.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nature biotechnology. 2010;28(2):161–6. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, et al. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116(21):2409–19. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 17.Park SW, Jun Koh Y, Jeon J, Cho YH, Jang MJ, Kang Y, et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116(25):5762–72. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- 18.Descamps B, Emanueli C. Vascular differentiation from embryonic stem cells: novel technologies and therapeutic promises. Vascular pharmacology. 2012;56(5-6):267–79. doi: 10.1016/j.vph.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Reed DM, Foldes G, Harding SE, Mitchell JA. Stem cell-derived endothelial cells for cardiovascular disease: a therapeutic perspective. British journal of clinical pharmacology. 2013;75(4):897–906. doi: 10.1111/j.1365-2125.2012.04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N, Nishikawa S. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93(4):1253–63. [PubMed] [Google Scholar]
- 23.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(7):4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408(6808):92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 25.Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, Milligan G, et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(7):1389–97. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 26.Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(11):e72–9. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann J, Glassford AJ, Doyle TC, Robbins RC, Schrepfer S, Pelletier MP. Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. The Thoracic and cardiovascular surgeon. 2010;58(3):136–42. doi: 10.1055/s-0029-1240758. [DOI] [PubMed] [Google Scholar]
- 28.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(5):984–91. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurila JP, Laatikainen L, Castellone MD, Trivedi P, Heikkila J, Hinkkanen A, et al. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy. 2009;11(6):726–37. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang NF, Okogbaa J, Babakhanyan A, Cooke JP. Bioluminescence imaging of stem cell-based therapeutics for vascular regeneration. Theranostics. 2012;2(4):346–54. doi: 10.7150/thno.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96(2):151–63. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 32.Menasche P. Skeletal myoblasts as a therapeutic agent. Progress in cardiovascular diseases. 2007;50(1):7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 34.Qian H, Yang Y, Huang J, Gao R, Dou K, Yang G, et al. Intracoronary delivery of autologous bone marrow mononuclear cells radiolabeled by 18F-fluoro-deoxy glucose: tissue distribution and impact on post-infarct swine hearts. Journal of cellular biochemistry. 2007;102(1):64–74. doi: 10.1002/jcb.21277. [DOI] [PubMed] [Google Scholar]
- 35.Valmikinathan CM, Mukhatyar VJ, Jain A, Karumbaiah L, Dasari M, Bellamkonda RV. Photocrosslinkable chitosan based hydrogels for neural tissue engineering. Soft Matter. 2012;8(6):1964–76. doi: 10.1039/c1sm06629c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, et al. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation. 1994;90(5 Pt 2):II228–34. [PubMed] [Google Scholar]
- 37.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. The Journal of clinical investigation. 1994;93(2):662–70. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kofidis T, Nolte D, Simon AR, Metzakis A, Balsam L, Robbins R, et al. Restoration of blood flow and evaluation of corresponding angiogenic events by scanning electron microscopy after a single dose of VEGF in a model of peripheral vascular disease. Angiogenesis. 2002;5(1-2):87–92. doi: 10.1023/a:1021561507227. [DOI] [PubMed] [Google Scholar]
- 39.Jain A, McKeon RJ, Brady-Kalnay SM, Bellamkonda RV. Sustained delivery of activated Rho GTPases and BDNF promotes axon growth in CSPG-rich regions following spinal cord injury. PloS one. 2011;6(1):e16135. doi: 10.1371/journal.pone.0016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson MR, Lee HJ, Bellamkonda RV, Guldberg RE. Sustained release of BMP-2 in a lipid-based microtube vehicle. Acta biomaterialia. 2009;5(1):23–8. doi: 10.1016/j.actbio.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meilander NJ, Yu X, Ziats NP, Bellamkonda RV. Lipid-based microtubular drug delivery vehicles. Journal of controlled release : official journal of the Controlled Release Society. 2001;71(1):141–52. doi: 10.1016/s0168-3659(01)00214-0. [DOI] [PubMed] [Google Scholar]
- 42.Meilander NJ, Pasumarthy MK, Kowalczyk TH, Cooper MJ, Bellamkonda RV. Sustained release of plasmid DNA using lipid microtubules and agarose hydrogel. Journal of controlled release : official journal of the Controlled Release Society. 2003;88(2):321–31. doi: 10.1016/s0168-3659(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116(11 Suppl):I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 45.Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109(8):910–22. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 46.Katare R, Riu F, Rowlinson J, Lewis A, Holden R, Meloni M, et al. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(8):1872–80. doi: 10.1161/ATVBAHA.113.301217. [DOI] [PubMed] [Google Scholar]
- 47.Mima Y, Fukumoto S, Koyama H, Okada M, Tanaka S, Shoji T, et al. Enhancement of cell-based therapeutic angiogenesis using a novel type of injectable scaffolds of hydroxyapatite-polymer nanocomposite microspheres. PloS one. 2012;7(4):e35199. doi: 10.1371/journal.pone.0035199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratliff BB, Ghaly T, Brudnicki P, Yasuda K, Rajdev M, Bank M, et al. Endothelial progenitors encapsulated in bioartificial niches are insulated from systemic cytotoxicity and are angiogenesis competent. American journal of physiology Renal physiology. 2010;299(1):F178–86. doi: 10.1152/ajprenal.00102.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci U S A. 2008;105(38):14347–52. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang ZC, Liao WY, Tang AC, Tsai SJ, Hsieh PC. The enhancement of endothelial cell therapy for angiogenesis in hindlimb ischemia using hyaluronan. Biomaterials. 2011;32(1):75–86. doi: 10.1016/j.biomaterials.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 51.Moon SH, Kim JS, Park SJ, Lee HJ, Do JT, Chung HM. A system for treating ischemic disease using human embryonic stem cell-derived endothelial cells without direct incorporation. Biomaterials. 2011;32(27):6445–55. doi: 10.1016/j.biomaterials.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 52.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133–42. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 53.Cao Z, Gilbert RJ, He W. Simple agarose-chitosan gel composite system for enhanced neuronal growth in three dimensions. Biomacromolecules. 2009;10(10):2954–9. doi: 10.1021/bm900670n. [DOI] [PubMed] [Google Scholar]
- 54.Dillon GP, Yu X, Bellamkonda RV. The polarity and magnitude of ambient charge influences three-dimensional neurite extension from DRGs. Journal of biomedical materials research. 2000;51(3):510–9. doi: 10.1002/1097-4636(20000905)51:3<510::aid-jbm28>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 55.Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. Journal of biomaterials science Polymer edition. 1998;9(10):1049–69. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 56.Jiang T, Kumbar SG, Nair LS, Laurencin CT. Biologically active chitosan systems for tissue engineering and regenerative medicine. Current topics in medicinal chemistry. 2008;8(4):354–64. doi: 10.2174/156802608783790974. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Yoon YS. Revisiting cardiovascular regeneration with bone marrow-derived angiogenic and vasculogenic cells. British journal of pharmacology. 2013;169(2):290–303. doi: 10.1111/j.1476-5381.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman O, Feraud O, Boyer-Di Ponio J, Driancourt C, Clay D, Le Bousse-Kerdiles MC, et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells. 2009;27(8):1750–9. doi: 10.1002/stem.100. [DOI] [PubMed] [Google Scholar]
- 59.Lu SJ, Feng Q, Caballero S, Chen Y, Moore MA, Grant MB, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nature methods. 2007;4(6):501–9. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(1):80–9. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, et al. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(10):2127–34. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- 62.Yamahara K, Sone M, Itoh H, Yamashita JK, Yurugi-Kobayashi T, Homma K, et al. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PloS one. 2008;3(2):e1666. doi: 10.1371/journal.pone.0001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nature medicine. 2003;9(5):604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 64.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. The Journal of experimental medicine. 2001;193(9):1005–14. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawamoto A, Murayama T, Kusano K, Ii M, Tkebuchava T, Shintani S, et al. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation. 2004;110(11):1398–405. doi: 10.1161/01.CIR.0000141563.71410.64. [DOI] [PubMed] [Google Scholar]
- 66.Rafii S, Heissig B, Hattori K. Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene therapy. 2002;9(10):631–41. doi: 10.1038/sj.gt.3301723. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature medicine. 1999;5(4):434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 68.Yau TM, Kim C, Li G, Zhang Y, Weisel RD, Li RK. Maximizing ventricular function with multimodal cell-based gene therapy. Circulation. 2005;112(9 Suppl):I123–8. doi: 10.1161/CIRCULATIONAHA.104.525147. [DOI] [PubMed] [Google Scholar]
- 69.Hosaka A, Koyama H, Kushibiki T, Tabata Y, Nishiyama N, Miyata T, et al. Gelatin hydrogel microspheres enable pinpoint delivery of basic fibroblast growth factor for the development of functional collateral vessels. Circulation. 2004;110(21):3322–8. doi: 10.1161/01.CIR.0000147779.17602.18. [DOI] [PubMed] [Google Scholar]
- 70.Layman H, Sacasa M, Murphy AE, Murphy AM, Pham SM, Andreopoulos FM. Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta biomaterialia. 2009;5(1):230–9. doi: 10.1016/j.actbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 71.Matsui M, Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta biomaterialia. 2012;8(5):1792–801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 72.Kawamura I, Takemura G, Tsujimoto A, Watanabe T, Kanamori H, Esaki M, et al. Treatment of leg ischemia with biodegradable gelatin hydrogel microspheres incorporating granulocyte colony-stimulating factor. Journal of cardiovascular pharmacology. 2011;57(4):416–23. doi: 10.1097/FJC.0b013e31820c9776. [DOI] [PubMed] [Google Scholar]
- 73.Li L, Okada H, Takemura G, Esaki M, Kobayashi H, Kanamori H, et al. Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol. 2009;53(25):2378–88. doi: 10.1016/j.jacc.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 74.Ruvinov E, Leor J, Cohen S. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials. 2010;31(16):4573–82. doi: 10.1016/j.biomaterials.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 75.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. Journal of thrombosis and haemostasis : JTH. 2007;5(3):590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 76.Cao L, Arany PR, Wang YS, Mooney DJ. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials. 2009;30(25):4085–93. doi: 10.1016/j.biomaterials.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, et al. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. American journal of physiology Heart and circulatory physiology. 2010;298(6):H1959–65. doi: 10.1152/ajpheart.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shin SH, Lee J, Lim KS, Rhim T, Lee SK, Kim YH, et al. Sequential delivery of TAT-HSP27 and VEGF using microsphere/hydrogel hybrid systems for therapeutic angiogenesis. Journal of controlled release : official journal of the Controlled Release Society. 2013;166(1):38–45. doi: 10.1016/j.jconrel.2012.12.020. [DOI] [PubMed] [Google Scholar]