Abstract
The mechanisms related to the spontaneous clearance of hepatitis C virus (HCV) have been primarily studied in regions where the infection is endemic. Results of prior studies have been extrapolated to populations with low endemicity, such as Mexico. Herein, we determined the cytokine profiles in serum samples from Mexican patients who spontaneously cleared HCV and patients chronically infected with HCV genotype 1a. Chronic HCV-infected patients displayed increased interleukin (IL)-8 and regulated upon activation, normal T-cell expressed and secreted (CCL-5) secretion, whereas patients who spontaneously cleared HCV showed augmented levels of IL-1 alpha, tumour necrosis factor-alpha, transforming growth factor-beta, monocyte chemoattractant protein-2 (CCL-8), IL-13 and IL-15. Our study suggeststhat cytokine profiles may predict disease outcome during HCV infection.
Keywords: hepatitis C virus, cytokines, viral clearance, genotype 1a
Hepatitis C virus (HCV) is an enveloped RNA virus belonging to the Flaviviridae family. Each year, HCV infects approximately three-four million individuals worldwide. It is estimated that 170 million people are chronically infected with HCV and at risk of developing chronic liver disease and 350,000 deaths occur each year due to HCV-related causes (Hanafiah et al. 2013). HCV is present worldwide, but its distribution pattern is not uniform. In Latin America, HCV genotype 1 is the most prevalent and the overall prevalence of HCV antibody is estimated to be 1.5% (Alvarado-Mora & Pinho 2013). In Mexico, an identified region with low HCV prevalence, the most prevalent viral genotypes are 1a and 1b followed by 2a and 3b (Panduro et al. 2011). In recent years, chronic diseases associated with liver malfunction have gained increasing importance in the world (Fierro et al. 2014); thus, the study of causal agents of liver damage is of great significance.
HCV is able to establish lifelong persistent infection in most individuals by successfully evading the immune system. However, approximately 30% of patients spontaneously clear the virus (Rehermann & Bertoletti 2015). The immunopathology associated with HCV infection has been predominantly studied in regions where infection is common and the results have been extrapolated to regions of low endemicity. The exact mechanisms responsible for viral clearance and recovery in humans are unknown. In this context, efforts to determine whether biomarkers, including serum cytokines, can accurately predict the outcome of HCV infection are valuable for establishing better HCV control strategies in Latin American regions.
In the present study, 33 serum samples from patients (> 18 years of age) admitted to the Molecular Biology Service of the Fray Antonio Alcalde Civil Hospital of Guadalajara (AHCFAA) from 2011-2013 were retrospectively analysed. Hepatitis was defined as described in a previously published study (Escobedo-Meléndez et al. 2012). Informed consent was obtained from all patients involved in the study. The local ethical committee of the AHCFAA approved the study protocol.
Samples from patients with liver disease and under treatment with a hepatotoxic drug, patients with chronic hepatitis associated with an aetiological agent distinct to viral hepatitis, patients with autoimmune hepatitis and patients identified as overweight or obese were excluded from the study. Clinical history and demographic data were collected for all participants through a structured questionnaire, as previously reported (Escobedo-Meléndez et al. 2012). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using an enzymatic method (Human, Germany) with an automatic analyser. The abnormal cut-off values for ALT and AST enzymes were 40 UI/mL and 50 UI/mL, respectively.
Clinical evaluation of HCV-infected patients - Chronic HCV infection was defined as a positive anti-HCV test (ELISA Third-Generation, AxSYM) result and the presence of serum HCV RNA for more than six months (COBAS® AmpliPrep and COBAS® TaqMan 48). Samples from patients who spontaneously cleared HCV tested positive for anti-HCV antibody in the absence of HCV RNA six months after the initial test, in accordance with the definition of spontaneous viral clearing (Hanafiah et al. 2013, EASL 2014). AST and ALT levels were determined by dry chemistry on a Vitros 250 analyser (Ortho Clinical Diagnostics, Johnson & Johnson, USA). Viral genotyping was performed at the Bayesian Markov model through a conventional line probe assay (VERSANT HCV Genotype 2.0 Assay LiPA, Germany) after HCV RNA extraction (QIAamp RNA mini kit) and RNA amplification (VERSANT HCV LiPA 2.0 Amplification Kit) following the manufacturer’s instructions.
Patients with positive viral RNA were stratified into four groups according to their fibrosis stage, as determined by transitional elastography using a FibroScan® instrument (Echosens, France). As validated by the manufacturer, the stage of fibrosis in patients with chronic HCV infection was defined as follows: F1, initial fibrosis, F2, moderate fibrosis, F3, advanced fibrosis, and F4, cirrhosis stage.
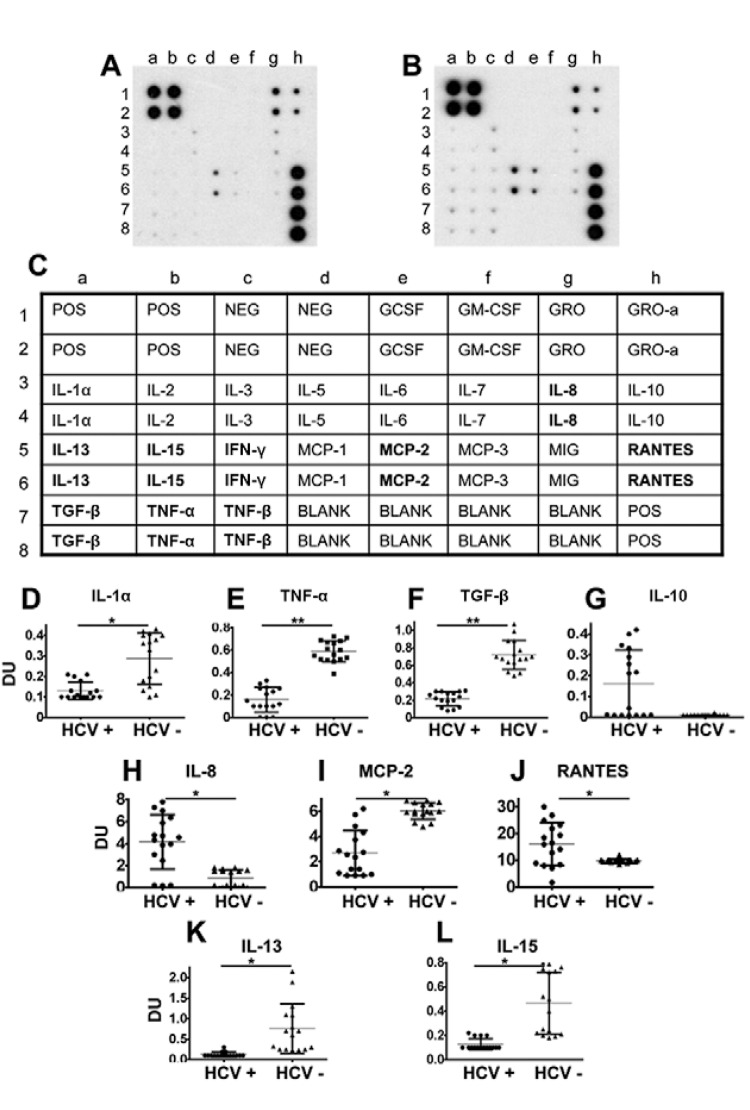
Analysis of serum cytokines - As reported previously, a standardised (Fierro et al. 2011) dot blot-based assay was used according to the manufacturer’s instructions (Ray-Biotech, USA) to detect the relative expression levels of 23 cytokines in serum samples collected from patients. The following cytokines were analysed: pro-inflammatory cytokines [interleukin (IL)-8, tumour necrosis factor-alpha (TNF-α), IL-2, IL-1 and IL-6], anti-inflammatory cytokines [tumour growth factor-beta (TGF-β) and IL-10], fibrogenic (IL-13), immunoregulatory cytokines [IL-5, IL-15, interferon-gamma (IFN-γ), IL-7 and TNF-β], chemokines [monocyte chemoattractant protein (MCP)-1 (CCL-2), MCP-2 (CCL-8), MCP-3 (CCL-7), monokine-induced by IFN-γ (MIG) (CXCL-9) and regulated upon activation, normal T-cell expressed and secreted (RANTES) (CCL-5)] and growth factors [growth-regulated oncogene-alpha (GRO-α), GRO, IL-3, granulocyte-macrophage colony-stimulating factor (GMCSF) and granulocyte colony-stimulating factor (GCSF)] (Fierro et al. 2011). Briefly, the membranes were blocked with a blocking buffer and 1 mL of a 1:500 dilution of the patient serum was added. Next, the membranes were incubated at room temperature (RT) for 2 h. After the membranes were washed, 1 mL of primary biotin-conjugated antibodies was added to detect the 23 cytokines previously described and the membranes were incubated at RT for 2 h. The membranes were incubated with 2 mL of horseradish peroxidase-conjugated streptavidin at RT for 1 h, which was developed using an enhanced chemiluminescence-type solution subsequently exposed to film and processed by autoradiography. The densitometry analysis was performed with an Alpha-Innotech FluorChem Imaging System. For each membrane, the individual background value obtained from the densitometry values for the blank controls included in each assay (A in Figure) were subtracted from the densitometry values corresponding to each cytokine. The densitometry units corresponding to the relative expression levels of each cytokine are shown.
The cytokine profiles during hepatitis C virus (HCV) infection distinguish viral clearance from persistent infection. Representative dot blots of the study groups are shown (A: chronically HCV-infected patients with detectable viral RNA titres; B: patients who spontaneously cleared HCV; C: an array map). The dot blot assay was used according to the manufacturers’ instructions to detect the cytokine levels in serum samples collected from patients (Ray-Biotech). For each membrane, the individual background levels were subtracted. Individual densitometry analyses and averages for each group are shown [D: interleukin-1 alpha (IL-1α); E: tumour necrosis factor-alpha (TNF-α); F: tumour growth factor-beta (TGF-β); G: IL-10; H: IL-8; I: monocyte chemoattractant protein (MCP)-2; J: regulated upon activation, normal T-cell expressed and secreted (RANTES); K: IL-13; L: IL-15]. Chronically HCV-infected patients were defined as patients with positive anti-HCV test results and detectable HCV genotype 1a viral RNA titres (HCV+) and patients who spontaneously cleared HCV presented positive anti-HCV test results and undetectable HCV RNA titres (HCV-). Differences with p < 0.05 were considered statistically significant (*: p < 0.05; **: p < 0.001). DU: densitometry units (refer to the relative expression levels of detected cytokines); IFN-γ: interferon-gamma; MIG: monokine-induced by IFN-γ; TNF-β: tumour necrosis factor-beta.
Ethics - Blood from patients and controls was obtained by venipuncture with approval from the local ethical committee of the HCFAA (IRB: HCG/CI-883/09). The protocol was conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Statistical analysis - The data are reported as individual densitometry analyses for each patient for each cytokine. The mean ± standard deviation (SD) for study groups is indicated. The data are presented as the mean ± SD. Statistical comparisons were performed using GraphPad Prism v.5.01. A nonparametric Mann-Whitney U test was used to calculate the statistical significance of the assay results. A p value ≤ 0.05 was considered statistically significant. Significant p values were corrected using the Bonferroni method to ensure that there were differences between the compared groups.
RESULTS
Based on HCV antibody and RNA viral profiles, patients were stratified into two groups: patients who spontaneously cleared HCV and patients with chronic HCV infection who continued to have detectable RNA titres. There were no significant gender or age differences found between the analysed groups. However, the patients with chronic HCV infection and detectable viral RNA presented significantly increased ALT and AST levels compared with the patients who had spontaneously cleared HCV. Genotype 1a was found in all of the chronically infected patients with detectable RNA. Fibrosis staging determined by elastography was conducted in a small number of participants (6) from the chronic HCV group. Patients with distinct stages of liver disease were included in the study and cirrhosis (F4) was found exclusively in one of these patients (Table).
TABLE. Clinical and demographic characteristics of the patients.
| Characteristics | HCV+ (n = 16) | HCV- (n = 17) | p |
|---|---|---|---|
| Gender [female (%)] | 68.75 | 76.4 | - |
| Mean age (years ± SD) | 53.13 ± 10.3 | 49.12 ± 12.83 | 0.332 |
| Mean ALT (UI/L ± SD) | 64.43 ± 41.14 | 29.8 ± 15.07 | 0.005 |
| Mean AST (UI/L ± SD) | 61.86 ± 42.45 | 29.8 ± 16.74 | 0.012 |
| Positive anti-HCV | 16 | 17 | - |
| Viral genotype | 1a | Undetectable | - |
| Mean viral titre (UI/L ± SD) | 1.064 x 10^7 ± 1.56 x 10^7 | Undetectable | - |
|
| |||
| Lipid profile (mg/dL) | |||
| COL | 170.2 ± 52.67 | 177.3 ± 59.42 | 0.724 |
| TG | 157.3 ± 76.96 | 148.6 ± 63.4 | 0.728 |
| HDL | 38.27 ± 9.74 | 40.29 ± 7.6 | 0.514 |
| LDL | 100.5 ± 50.78 | 119.9 ± 34 | 0.210 |
| VLDL | 31.4 ± 15.28 | 30.29 ± 13 | 0.826 |
| Body mass index (kg/m2) | 22.94 ± 1.38 | 22.9 ± 1.26 | 0.925 |
| Grade of fibrosis (n) | |||
| F1 | 1 | - | - |
| F1-F2 | 1 | - | - |
| F2 | 1 | - | - |
| F2-F3 | 2 | - | - |
| F4 | 1 | - | - |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; COL: cholesterol; HCV-: patients who exhibited positive anti-hepatitis C virus test results and undetectable HCV RNA titres for more than six months. Averages of quantitative variables are expressed as mean ± standard deviation (SD); HCV+: chronically HCV-infected patients who exhibited positive anti-HCV test results and detectable HCV genotype 1a viral RNA titres for more than six months; HDL: high density lipoprotein; LDL: low density lipoprotein; TG: triglyceride; VLDL: very low density lipoprotein.
In an attempt to rule out the influence of overweight and obesity on the clearance or evolution of chronic HCV infection, this study only included samples from patients with a normal body mass index. No differences in the lipid profiles of the analysed groups were found (Table). Representative dot blots showing the relative expression of cytokines in the study groups are shown in A, B in Figure. A low detection rate of certain cytokines, including IL-2, IL-6, IL-5, IFN-γ, IL-7, TNF-β, MCP-1 (CCL-2), MCP-3 (CCL-7), MIG (CCL-9), GRO-α, GRO, IL-3, GMCSF and GCSF, was observed in our study groups (A-C in Figure). Interestingly, compared with chronic HCV-infected patients with detectable RNA, the patients who spontaneously cleared HCV displayed increased levels of IL-1α, TNF-α, TGF-β, MCP-2 (CCL-8), IL-13 and IL-15 (D-F, I, K, L in Figure, respectively). In contrast, the chronic HCV-infected patients with detectable RNA showed increased levels of IL-8 and RANTES (CCL-5) compared with patients who spontaneously cleared HCV (H, J in Figure, respectively). No significant differences were found in IL-10 levels. However, there was a trend towards increased IL-10 levels in the chronic HCV-infected patients with detectable RNA (G in Figure).
Viral and host factors are related to the progression of HCV infection. In 2009, it was demonstrated that HCV viral clearance is associated with genetic variation in the IL-28B gene (Ge et al. 2009, Thomas et al. 2009, Rauch et al. 2010). Numerous studies have been conducted on this family of cytokines and have led to several inconsistencies and controversies, including the possible correlation between serum protein levels and disease outcomes in chronic viral hepatitis patients (Torres et al. 2014). Thus, it is accepted that variations in the profile of cytokines involved in the immune response may contribute to the ability to clear HCV. These studies have mainly been conducted in regions where the virus is endemic (Shi et al. 2012), thus information on populations in regions of low endemicity is scarce. Herein, we characterised serum cytokine expression profiles in Mexican HCV-infected patients and found subtle differences in the cytokine profiles between distinct clinical courses of HCV infection. In our study, persistent infection with detectable genotype 1a viral content resulted in a proinflammatory profile characterised by increased levels of IL-8 and RANTES (CCL-5) and a trend towards increased IL-10 levels. These findings correlate with in vitro studies that have shown that natural killer cells from HCV-infected patients are impaired in their capacity to activate dendritic cells due to the overproduction of IL-10 (Jinushi et al. 2004). Likewise, the increased levels of IL-8 found in the chronic HCV group in the present study may be related to the association between IL-8 production and IFN-α inhibition described in both in vivo and in vitro studies (Khabar et al. 1997, Jia et al. 2007). Moreover, the analysis of cytokines in each patient revealed a trend towards higher serum concentrations of IL-10 and IL-8 in patients with more severe liver damage (F2-F3 and F4) (data not shown). Altogether, our findings suggest that IL-10 and IL-8 overproduction may contribute to a lower probability of viral clearance, whereas homeostasis between pro-inflammatory [IL-1 α, TNF-α, MCP-2 (CCL-8)], anti-inflammatory (TGF-β), fibrogenic (IL-13) and immunoregulatory (IL-15) profiles allows for viral clearance, as observed in patients with undetectable viral RNA levels. However, large-scale studies with adequate statistical power to support this hypothesis are needed. Furthermore, given that our data do not rule out the possibility that cytokine levels may be a consequence and not the cause of HCV clearance, future studies aimed at analysing the possibility of a reverse causation are necessary.
The progression of HCV infection is associated with the characteristics of lipid profiles in chronic HCV patients. In fact, it is accepted that lipid components associated with HCV infection are finely modulated in the Mexican population (Fierro et al. 2014). In the present study, no association between lipid profiles and viral clearance was found. Future studies that include overweight and obese individuals will allow the determination of the exact role of lipids in viral clearance.
We previously reported the influence of cytokines on the development of distinct clinical courses in children infected with hepatitis A (Fierro et al. 2012, Castro-Garcia et al. 2014) and on the development of occult hepatitis B virus infection in native Mexican groups (Fierro et al. 2011). Herein, our findings suggest that cytokine expression can influence the extent of HCV development and provide important insights into cytokine-mediated mechanisms underlying the long-term persistence of HCV. In this context, efforts to determine whether biomarkers, including serum cytokines, can accurately predict the outcome of HCV infection are valuable for establishing better HCV control strategies in Latin American regions.
ACKNOWLEDGEMENTS
To Flor P Castro and Jesus Meza, for the technical assistance.
Funding Statement
Financial support: CONACYT (127229, 188240)
Footnotes
Financial support: CONACYT (127229, 188240)
RT-V, MET-T and JLT-O were supported by PhD scholarships from the CONACYT, NAF, SR and AP are sponsorship recipients of the Red Temática de Colaboración Académica en Fisiopatología de las Enfermedades Hepáticas (SEP-Promep).
REFERENCES
- Alvarado-Mora MV, Pinho JR. Epidemiological update of hepatitis B, C and delta in Latin America. Antivir Ther. 2013;18:429–433. doi: 10.3851/IMP2595. [DOI] [PubMed] [Google Scholar]
- Castro-Garcia FP, Corral-Jara KF, Escobedo-Meléndez G, Sandoval-Hernandez MA, Rosenstein Y, Roman S, Panduro A, Fierro NA. Conjugated bilirubin adjusts cytokine profiles in hepatitis A virus infection by modulating function of signal transducer and activator of transcription factors. Immunology. 2014;143:578–587. doi: 10.1111/imm.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASL. European Association for Study of Liver EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Escobedo-Meléndez G, Fierro NA, Roman S, Maldonado-González M, Zepeda-Carrillo E, Panduro A. Prevalence of hepatitis A, B and C serological markers in children from western Mexico. Ann Hepatol. 2012;2:194–201. [PubMed] [Google Scholar]
- Fierro NA, Escobedo-Meléndez G, Paz L, Realpe M, Roman S, Panduro A. Cytokine expression profiles associated with distinct clinical courses in hepatitis A virus-infected children. Pediatr Infect Dis J. 2012;31:870–871. doi: 10.1097/INF.0b013e318258e808. [DOI] [PubMed] [Google Scholar]
- Fierro NA, Gonzalez-Aldaco K, Torres-Valadez R, Martinez-Lopez E, Roman S, Panduro A. Immunologic, metabolic and genetic factors in hepatitis C virus infection. World J Gastroenterol. 2014;20:3443–3456. doi: 10.3748/wjg.v20.i13.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro NA, Roman S, Realpe M, Hernandez-Nazara Z, Zepeda-Carrillo EA, Panduro A. Multiple cytokine expression profiles reveal immune-based differences in occult hepatitis B genotype H-infected Mexican Nahua patients. Mem Inst Oswaldo Cruz. 2011;106:1007–1013. doi: 10.1590/s0074-02762011000800018. [DOI] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL-28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Hanafiah KM, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;4:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wei L, Jiang D, Wang J, Cong X, Fei R. Antiviral action of interferon-alpha against hepatitis C virus replicon and its modulation by interferon-gamma and interleukin-8. J Gastroenterol Hepatol. 2007;22:1278–1285. doi: 10.1111/j.1440-1746.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- Khabar KS, Al-Zoghaibi F, Al-Ahdal MN, Murayama T, Dhalla M, Mukaida N, Taha M, Al-Sedairy ST, Siddiqui Y, Kessie G, Matsushima K. The alpha chemokine, interleukin 8, inhibits the antiviral action of interferon alpha. J Exp Med. 1997;1867:1077–1085. doi: 10.1084/jem.186.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panduro A, Escobedo-Meléndez G, Fierro NA, Ruiz-Madrigal B, Zepeda-Carrillo EA, Román S. Epidemiology of viral hepatitis in Mexico. Salud Publica Mexico. 2011;53:37–45. [PubMed] [Google Scholar]
- Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J, Colombo S, Cerny A, Dufour JF, Furrer H, Günthard HF, Heim M, Hirschel B, Malinverni R, Moradpour D, Müllhaupt B, Witteck A, Beckmann JS, Berg T, Bergmann S, Negro F, Telenti A, Bochud PY, Swiss Hepatitis C Cohort Study. Swiss HIV Cohort Study Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–1345. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712–721. doi: 10.1002/hep.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Pan Y, Wang M, Wang D, Li W, Jiang T, Zhang P, Chi X, Jiang Y, Gao Y, Zhong J, Sun B, Xu D, Jiang J, Niu J. IL28B genetic variation is associated with spontaneous clearance of hepatitis C virus, treatment response, serum IL-28B levels in Chinese population. PLoS ONE. 2012;7: doi: 10.1371/journal.pone.0037054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres C, Brahm J, Venegas M. Lambda interferon serum levels in patients with chronic hepatitis C virus infection according to their response to therapy with pegylated interferon and ribavirin. J Interferon Cytokine Res. 2014;34:106–110. doi: 10.1089/jir.2013.0005. [DOI] [PubMed] [Google Scholar]