Abstract
Trypanosoma cruzi is the aetiological agent of Chagas disease, which affects approximately eight million people in the Americas. This parasite exhibits genetic variability, with at least six discrete typing units broadly distributed in the American continent. T. cruzi I (TcI) shows remarkable genetic diversity; a genotype linked to human infections and a domestic cycle of transmission have recently been identified, hence, this strain was named TcIDom. The aim of this work was to describe the spatiotemporal distribution of TcI subpopulations across humans, insect vectors and mammalian reservoirs in Colombia by means of molecular typing targeting the spliced leader intergenic region of mini-exon gene. We analysed 101 TcI isolates and observed a distribution of sylvatic TcI in 70% and TcIDom in 30%. In humans, the ratio was sylvatic TcI in 60% and TcIDom in 40%. In mammal reservoirs, the distribution corresponded to sylvatic TcI in 96% and TcIDom in 4%. Among insect vectors, sylvatic TcI was observed in 48% and TcIDom in 52%. In conclusion, the circulation of TcIDom is emerging in Colombia and this genotype is still adapting to the domestic cycle of transmission. The epidemiological and clinical implications of these findings are discussed herein.
Keywords: Chagas disease, genotypes, domestic cycle, sylvatic cycle
Chagas disease is caused by the kinetoplastid parasite Trypanosoma cruzi. This pathogen is mainly transmitted by the faeces of infected triatomine insects from the Reduviidae family. The disease is considered undertreated and is a serious public health problem in Latin America (Teixeira et al. 2006). There are 16-18 million people worldwide infected with this parasite, 50,000 of whom die every year. In 2005, 1,200 new cases were detected in endemic countries of the Americas (OMS 2008). Colombia has an estimated prevalence between 700,000-1,200,000 and 8.000,000 are at risk of acquiring the infection according to the geographical distribution of the insect vector species (INS 2012).
T. cruzi exhibits broad intraspecific genetic diversity and is classified into six discrete typing units (DTUs) identified as TcI-TcVI (Zingales et al. 2009, 2012). TcI presents the broadest geographical distribution, which covers the southern United States of America to northern Argentina and Chile. This DTU can be found in the sylvatic and domestic transmission cycles (Añez et al. 2004, Guhl & Ramírez 2011, Zingales et al. 2012). This near-clade exhibits tremendous genetic diversity based on initial studies of the spliced leader intergenic region of mini-exon gene (SL-IR), which have subdivided TcI into genotypes (TcIa-TcIe) associated with different transmission cycles (Herrera et al. 2009, Cura et al. 2010). Furthermore, the use of microsatellite markers has demonstrated the emergence of a domestic genotype in Venezuela, which was previously called VenDom (Llewellyn et al. 2009). Similarly, the use of ribosomal and mitochondrial markers has suggested the existence of genotypes associated with the domestic and sylvatic cycles of transmission (Ramírez et al. 2012a, b). Recent phylogenetic studies based on the nuclear and mitochondrial genomes of TcI populations have identified an emerging clade henceforth called TcIDom, which has been observed from Central America to South America due to human migration and is associated with domestic cycles of transmission. Human infection is a reflection of the adaptation of this genotype to human populations (Ramírez et al. 2012c, Zumaya-Estrada et al. 2012, Segovia et al. 2013).
Several studies have described the occurrence of TcI in domestic and sylvatic cycles of transmission, including its presence in patients with Chagas disease in Colombia (Zafra et al. 2008, 2011, Mantilla et al. 2010, Ramírez et al. 2010). These studies have identified the presence of sylvatic TcI as the causative agent for oral outbreaks of Chagas disease, suggesting the possibility that this type of parasite invades and infects human pantries (Ramírez et al. 2013a). Likewise, TcI has been identified in populations infecting domestic cycle vectors, as in the case of Triatoma infestans in Argentina and Paraguay; in the peridomestic cycle, as observed for T. infestans in Paraguay and in the sylvatic cycle, among Rhodnius neglectus and Rhodnius nasutus in Brazil and Mepraia spinolai/gajardoi in Chile (Cura et al. 2010). In Colombia, we have found the presence of TcI in Rhodnius prolixus, Panstrongylus geniculatus, Triatoma dimidiata, Rhodnius pallescens, Rhodnius robustus, Rhodnius colombiensis, Triatoma maculata and Triatoma venosa, as well as in sylvatic reservoirs such as Didelphis marsupialis, Dasypus novemcintus, Rattus rattus, Rhynchonycteris naso, Tamandua tetradactyla and Canis familiaris (Guhl & Ramírez 2013, Ramírez et al. 2013b). However, most studies have not yet identified TcI subpopulations circulating within these hosts.
Understanding the genetic variability of T. cruzi is a tool to understand the dynamics of transmission and the severity of some symptoms in the acute and chronic phases of disease (Falla et al. 2009). Recently, we have determined the biological properties of TcIDom compared to sylvatic TcI strains; this study showed that TcIDom strains cause less parasitaemia than sylvatic TcI strains and concluded that TcIDom strains have a low rate of tissue invasion, while sylvatic TcI strains have a high rate of tissue invasion, suggesting the importance of these sympatric genotypes (Cruz et al. 2015). Therefore, the objective of this study was to retrospectively detect TcIDom and sylvatic TcI strains using specific primers that amplify SL-IR from vectors, reservoirs and humans isolates from different regions of Colombia between 1984-2012.
SUBJECTS, MATERIALS AND METHODS
Study areas and ethics statement - T. cruzi isolates from humans, triatomine bugs and mammalian reservoirs from 19 departments (Amazonas, Arauca, Boyacá, Bolívar, Caquetá, Caldas, Casanare, Cesar, Cundinamarca, Guainía, Guajira, Huila, Magdalena, Meta, Norte de Santander, Putumayo, Santander, Tolima and Vaupes) in Colombia reported as having high, medium and low endemicity (Guhl & Vallejo 1999), were obtained from a cryobank as part of the epidemiological surveillance of Chagas disease in the country by the National Institute of Health in Colombia from 1984-2012. The sampling areas were at altitudes ranging from 0-2,100 m above sea level, including a wide range of different ecotopes from savannah to mountains. Triatomines (R. robustus, R. colombiensis, Rhodnius pictipes, R. prolixus, T. venosa, T. dimidiata, T. maculata and P. geniculatus) and mammals (D. marsupialis, C. familiaris, Caluromys lanatus, Oryzomys and R. rattus) were captured and released after blood collection at domestic (within dwellings), peridomestic (near dwellings) and sylvatic (more than 250 m from dwellings) locations. The blood was taken by technicians who were previously trained by veterinarians. In the collections locations, no specific permission to conduct field studies was required; the environmental ministry in Colombia allows for the collection of blood samples if the animals are not killed or considered endangered or protected. The species sampled are not endangered or protected. Regarding the domestic animals, oral informed consent was provided by the owners. The animals were anaesthetised and a blood sample of 1-2 mL was collected. After blood collection, the animals were released and manipulated following the international guiding principles for biomedical research involving animals, as issued by the Council for International Organizations of Medical Sciences. Trypanosomes were isolated from human patients following ethical clearance using a written informed consent approved by the National Institute of Health in Colombia.
Parasite isolation and DNA extraction - We obtained 101 isolates (45 from humans, 32 from mammalian reservoirs and 23 from insect vectors) from 19 departments in Colombia. DNA was extracted from 200-µL aliquots of the exponential phase cultures using a QIAamp DNA Isolation Kit. The DNA quality and concentration were measured at 260 nm and stored at -20ºC.
Genotyping methods - The T. cruzi isolates were initially genotyped using the SL-IR, 24Sα and 18S regions to detect TcI. We used the SL-IR region to discriminate TcIDom genotype and TcI sylvatic isolates. The polymerase chain reaction (PCR) reaction was performed in a final volume of 20 µL, which contained 2 µL of 10X reaction buffer (Invitrogen), 0.16 µL of a deoxynucleotide triphosphate mix, 0.6 µL of MgCl2, 1 µL of each primer (1Am: 5’-TGTGTGTGTATGTATGTG-3’; 1B: 5’-CGGAGCGGTGTGTGCAG-3’), 0.1 µL of Taq DNA polymerase (Invitrogen) and 5 µL of DNA (Villa et al. 2013). The thermal profile consisted of an initial denaturation at 94ºC for 4 min followed by 35 cycles at 94ºC for 30 s, 20 s at 55ºC and 30 s at 72ºC with a final extension at 72ºC for 10 min. To determine the size of the band, the amplification products were submitted to gel electrophoresis in a 2% agarose gel using as controls reference strains MHOM/CO/01/ DA (TcIDom) and MHOM/CO/10/ GC (sylvatic TcI) and the images were analysed on a transilluminator. To determine the congruence for the correct assignment of TcIDom between SL-IR and mitochondrial alleles (as previously determined), 40 strains were analysed by 10 multilocus sequence typing (MLST) mitochondrial markers, as reported elsewhere (Messenger et al. 2012).
RESULTS
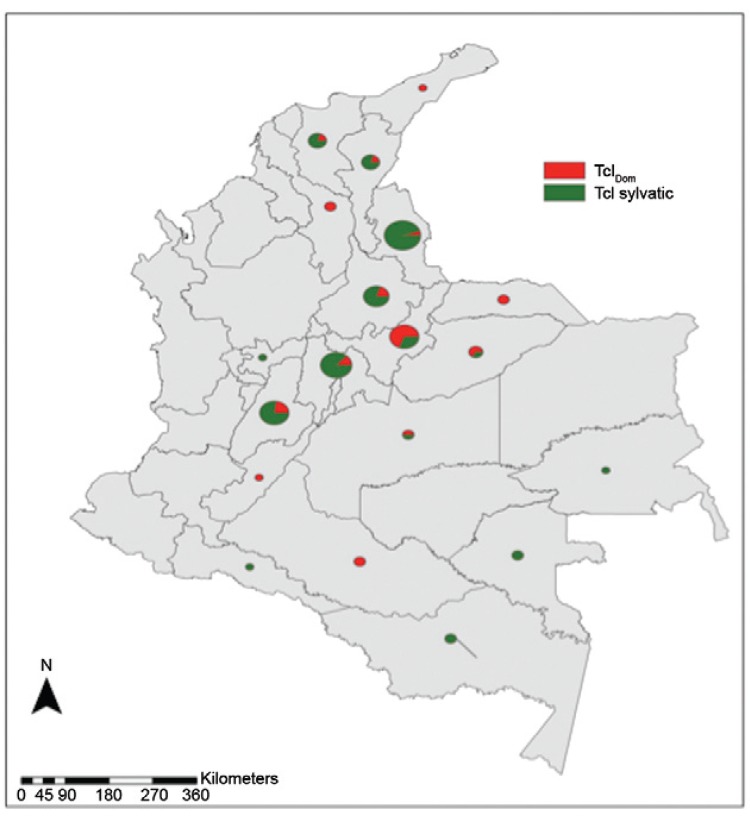
We conducted retrospective discrimination for 45 isolates from humans, 32 isolates from reservoirs and 24 isolates from triatomines originating in 19 departments of Colombia from 1984-2012. The DNA bands obtained from the amplification products were 231 and 450 bp for TcIDom isolates and sylvatic TcI, respectively (Fig. 1). In a blinded manner, we compared the congruence of SL-IR and mitochondrial MLST (mtMLST) for detecting TcIDom and were able to detect complete congruence between domestic TcI (SL-IR) and mitochondrial alleles by means of MLST. A higher frequency of TcI sylvatic isolates (70%) was observed, followed by TcIDom (30%) across the 101 isolates. Regarding the geographical distribution, the departments with a greater number of sylvatic TcI isolates were Norte de Santander and Cundinamarca; for the case of TcIDom, Boyacá showed the highest prevalence for this genotype (Fig. 2, Table).
Fig. 1: TcIDom strains were identified by amplying a 231 bp fragment and sylvatic TcI strains were identified by amplifying a 450-550 bp fragment. M: 100 bp ladder; 1: MHOM/CO/10/GC strain; 2: MHOM/CO/01/DA; 3: MHOM/CO/94/EA; 4: MHOM/CO/87/R12; 5: MHOM/CO/00/Coyaima; 6: MHOM/CO/11/HV; 7: negative control.
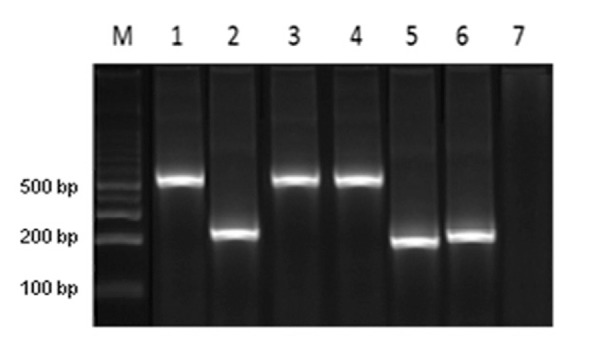
Fig. 2: geographical distribution of TcIDom genotype and sylvatic TcI isolates detected in the 101 isolates analysed.
TABLE. Biological distribution of Trypanosoma cruzi I (TcI) genotypes circulating in Colombian hosts (humans, mammals and triatomine bugs).
| Species | TcI genotypes |
||
|---|---|---|---|
| Sylvatic TcI n (%) | TcIDom n (%) | ||
| Humans | Homo sapiens | 27 (60) | 18 (40) |
| Mammals | Caluromys lanatus | 1 (100) | 0 (0) |
| Canis familiaris | 0 (0) | 1 (100) | |
| Didelphis marsupialis | 27 (100) | 0 (0) | |
| Oryzomys | 1 (100) | 0 (0) | |
| Rattus rattus | 2 (100) | 0 (0) | |
| Insects | Pastrongylus geniculatus | 0 (0) | 1 (100) |
| Rhodnius colombiensis | 5 (83.4) | 1 (16.6) | |
| Rhodnius pictipes | 1 (100) | 0 (0) | |
| Rhodnius prolixus | 3 (60) | 2 (40) | |
| Rhodnius robustus | 0 (0) | 1 (100) | |
| Triatoma dimidiata | 1 (33.3) | 2 (66.6) | |
| Triatoma maculata | 1 (100) | 0 (0) | |
| Triatoma venosa | 0 (0) | 1 (100) | |
| Not classified | 2 (40) | 3 (60) | |
|
| |||
| Total | 71 (70) | 30 (30) | |
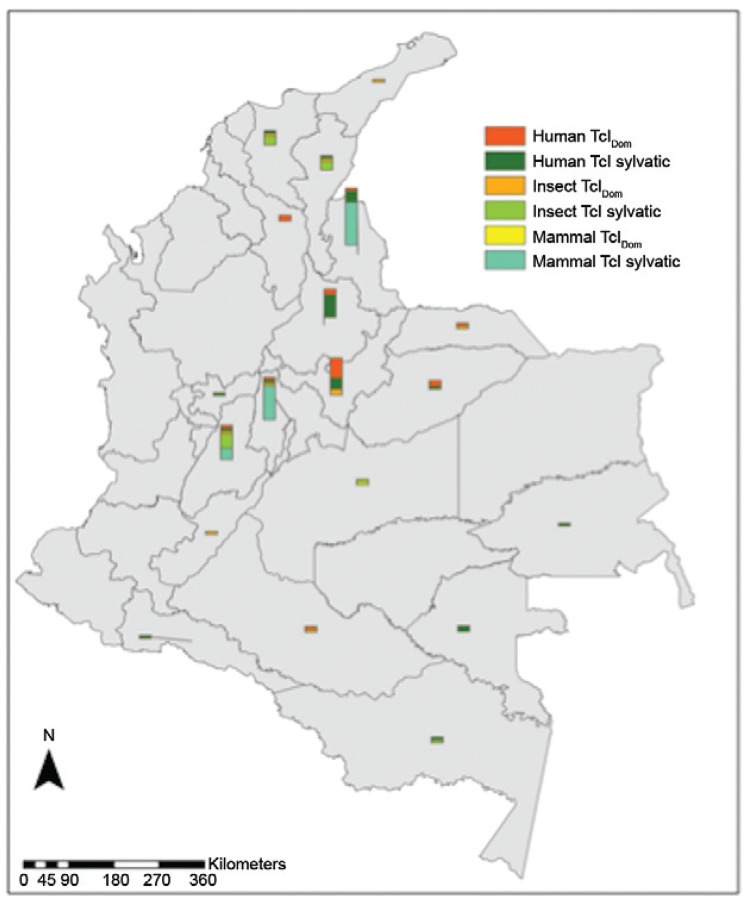
Regarding the discrimination of sylvatic TcI and TcIDom in human isolates, we observed a predominance of sylvatic TcI (60%). Moreover, those isolates obtained from congenital transmission cases were typed as TcIDom (40%). We also analysed 32 isolates from mammal reservoirs, including C. lanatus, C. familiaris, D. marsupialis, Oryzomys and R. rattus, observing the predominance of sylvatic TcI with the exception of C. familiaris, which was typed as TcIDom. To discriminate TcI genotypes among the insects, we analysed 24 isolates from P. geniculatus, R. colombiensis, R. pictipes, R. prolixus, R. robustus, T. dimidiata, T. maculata and T. venosa, where we detected a similar frequency: sylvatic TcI (48%) and TcIDOM (52%) (Fig. 3, Table). The domestic insect vectors, such as R. prolixus and T. dimidiata, were infected with TcIDom; furthermore, three isolates from P. geniculatus, R. robustus and T. venosa (sylvatic insect vectors) were found to be infected with the domestic genotype. Fig. 3 shows the geographical and biological distributions of the TcI genotypes across the 101 isolates. Humans had a higher frequency of TcIDom in the Boyacá department; in contrast, sylvatic TcI was mainly prevalent in the Santander department. In mammal reservoirs, we observed a higher frequency of sylvatic TcI in the Norte de Santander department. Regarding the insect vectors, a greater number of isolates were typed as TcIDom in the Tolima department (Fig. 3).
Fig. 3: biological distribution of TcIDom genotype and sylvatic TcI isolates detected in the 101 isolates analysed.
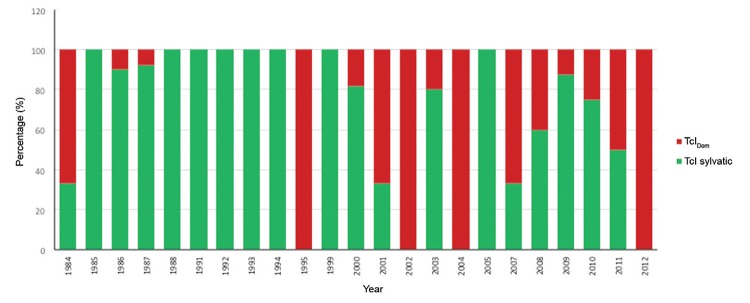
Finally, due to the recent description of the TcIDom genotype, this study retrospectively determined the distribution of this genotype in TcI samples collected since 1984. A predominance of sylvatic TcI isolates was observed across the timeline, but an increase in the number of TcIDom cases was clearly identified (Fig. 4).
Fig. 4: temporal distribution of TcIDom genotype and sylvatic TcI isolates detected in the 101 isolates analysed from 1984-2012.
DISCUSSION
T. cruzi exhibits remarkable genetic diversity, comprising at least six DTUs with the recent emergence of TcBat (Marcili et al. 2009, Zingales et al. 2012, Ramírez et al. 2014). TcI is a peculiar DTU that shows extant genetic diversity, elucidated by means of distinct genetic markers such as microsatellites, nuclear and mitochondrial MLST and SL-IR (Llewellyn et al. 2009, Guhl & Ramírez 2011, Ramírez et al. 2012b). Prior studies have suggested the cryptic subdivision of TcI into TcIa-TcIe based on SL-IR, but this nomenclature presents serious drawbacks due to the incorrect use of the term(s) “haplotypes” and “genotypes” (Herrera et al. 2007, 20, 2009, Cura et al. 2010). However, the scientific community insists on using the SL-IR nomenclature. Herein, we conducted the first molecular epidemiology approach to report the TcI subpopulations circulating in Colombia, using a previously reported PCR assay to discriminate domestic (TcIDom) and sylvatic TcI populations (Villa et al. 2013).
We compared the SL-IR PCR assay for discriminating domestic and sylvatic TcI populations with the markers employed for describing this genotype (mtMLST). Our results showed complete concordance between the allelic types reported by mtMLST and designation of a domestic genotype by SL-IR. These results support the previous findings of Villa et al. (2013), who applied this PCR assay to previously characterised biological clones, confirming the potential of this assay in discriminating TcIDom across the Americas.
Guhl and Ramírez (2013) reported a broad study about the molecular epidemiology of T. cruzi in Colombia, but did not depict the circulating TcI genotypes across the country. Here, we conducted spatiotemporal sampling to describe the circulating populations of TcI in Colombia. Our results demonstrate the predominance of sylvatic TcI (70%) populations in all the key players of the life-cycle of T. cruzi (Table). This finding is not novel because previous authors have reported the absolute occurrence of TcI sylvatic populations in the country; indeed, the study by Guhl and Ramírez (2013) shows that TcI, TcIII and TcIV (sylvatic DTUs) are commonly detected across humans, triatomines and mammal reservoirs (Guhl & Ramírez 2013, Ramírez et al. 2013a). Regarding the TcI genotypes detected in humans, we report sylvatic TcI in 60% and TcIDom in 40%. This finding is interesting because of the epidemiological and clinical relevance of the higher frequency of sylvatic TcI populations in Colombia. Human Chagas disease in Colombia is mainly associated with TcI infection and some authors have tried to elucidate the effect on genetic diversity and clinical outcome. Some authors have implicated the sylvatic populations of TcI with the more severe cardiac forms of chronic Chagas disease (Mantilla et al. 2010, Ramírez et al. 2010, 2013a, Zafra et al. 2011). Additionally, a recent survey that sought to detect the TcI populations associated with oral transmission of T. cruzi implicated the sylvatic mitochondrial haplotypes (Ramírez et al. 2013a). Additionally, a recent report in a Colombian human immunodeficiency virus patient co-infected with T. cruzi showed a tailored histiotropism of TcI populations, reporting sylvatic TcI in brain tissue and domestic TcI in cardiac tissue (Hernández et al. 2014). Finally, the description of the biological features of TcIDom and sylvatic TcI in murine models suggests that sylvatic TcI causes higher histopathological damage than TcIDom (Cruz et al. 2015). All of these advances demonstrate the important features and sympatric differences between sylvatic and domestic TcI populations. Our results show a marked prevalence of sylvatic TcI, which might explain the severity of cardiac forms of Chagas disease in Colombia.
We also evaluated the TcI genotypes circulating in mammal reservoirs. In these hosts, a major distribution of sylvatic TcI was observed, with the exception of one isolate from C. familiaris that was typed as TcIDom (Table, Fig. 3). The mammal reservoirs surveyed were mainly sylvatic and in some cases synanthropic as D. marsupialis. This pattern also implies the zoonotic behaviour of T. cruzi transmission and highlights the reservoirs as the autochthonous vehicles of the intrusion of TcI sylvatic populations into human dwellings. Our findings are supported by those reported in Ecuador and the Argentinean Chaco, where the sylvatic reservoirs harbour mostly sylvatic TcI populations, which have been shown to arise from inbreeding and recombination with domestic populations (Ocaña-Mayorga et al. 2010, Alvarado-Otegui et al. 2012). This finding intrinsically demonstrates the importance of mammal reservoirs in the transmission dynamics of TcI, serving as a link between both ecotopes. In the case of unique TcIDom infections in mammal reservoirs (C. familiaris), we found this infection in the Boyacá department in a domestic dwelling. A recent report of DTUs surveyed in dogs from Colombia demonstrates that dogs are important synanthropic reservoirs of T. cruzi, which also demonstrates that these animals may play a relevant role in the diversification of the T. cruzi taxon, where the domestic population can be moved back to the sylvatic ecotope (Ramírez et al. 2013b).
Regarding the insect vectors, sylvatic TcI (48%) and TcIDOM (52%) were detected (Table, Fig. 3). This distribution was interesting in light of the high frequency of domesticated vectors in some departments, such as Boyacá, where R. prolixus and T. dimidiata are mainly adapted to the domestic foci where they harbour TcIDom infection. Previously, based on SL-IR genotyping, Herrera et al. (2009) reported T. dimidiata infected with genotypes TcIa and TcIb, which we now report as TcIDom (Falla et al. 2009, Herrera et al. 2009). This finding implies that the domiciliation process of triatomines has enhanced the ability of TcIDom to adapt to this domestic focus and be transmitted to humans. This conclusion is supported by our recent findings where TcIDom isolates cause greater parasitaemia compared with sylvatic TcI isolates, implying that more parasites in the blood of infected individuals will increase the likelihood of infection and maintenance of this genotype in human dwellings (Cruz et al. 2015). Another notable result is that three isolates from sylvatic insect vectors (P. geniculatus, R. robustus and T. venosa) were found to be infected with TcIDom, implying that these sylvatic vectors are able to invade human dwellings and become infected with domestic populations. In contrast, T. venosa was captured in the Boyacá department, where there is not a clear delineation of a sylvatic focus, suggesting a peculiar domiciliation of this triatomine species. In the case of P. geniculatus, this insect was captured in a human dwelling, but was not domiciliated, suggesting that a clear classification of domestic/sylvatic status must be readdressed for this species in light of the recent implication of P. geniculatus in oral outbreaks in Colombia and Venezuela and reports of domiciliation (Wolff & Castillo 2000, Carrasco et al. 2005, 20, 2012, Alarcón de Noya et al. 2010, Ramírez et al. 2013a, Segovia et al. 2013).
Finally, we were also interested in determining the temporal variation of TcI genotypes from 1982-2012 (Fig. 4). Our results corroborate the ancient emergence of TcIDom as previously reported, showing its emergence close to the late Pleistocene (Ramírez et al. 2012c). This finding suggests the paramount importance of tracking the presence of this genotype across the players in the life-cycle of T. cruzi and also in different countries of the Americas. Recently, Zumaya-Estrada et al. (2012) tracked the dispersion and genetic diversity of this genotype, showing its low genetic diversity (proof of host adaptation) and its dispersion from the north to the south in accordance with human migration. An intensive sampling and case-control study is needed to unravel the strict associations between TcIDom and human pathogenesis; the advent of genomics will provide more information about the biological features of this enigmatic genotype.
Here, we conducted the first retrospective molecular epidemiology study to determine the biological and geographical distribution of TcIDom and sylvatic TcI across Colombia. We validated an easy PCR assay for discriminating these intra-DTU genotypes with a high congruence with mitochondrial alleles retrieved from MLST, as reported elsewhere (Messenger et al. 2012). We believe that depicting the real distribution of TcIDom is of paramount importance to unravel the history of Chagas disease and the association of this genotype with the clinical manifestations of this tropical pathology and to depict ancestral events of domiciliation. Similarly, we encourage the scientific community to avoid the use of SL-IR genotypes (TcIa-TcIe) and begin employing the TcIDom description to prevent serious problems in genotype designation, as shown elsewhere (Muñoz-Calderón et al. 2013). Finally, we suggest to those scientists working on the molecular epidemiology of Chagas disease to determine its prevalence in other endemic countries of the Americas thanks to the rise of a single PCR assay validated here.
ACKNOWLEDGEMENTS
To Camila González, from Universidad de Los Andes, for aid in the construction of the maps. Juan David Ramírez is principal professor at Universidad del Rosario.
Funding Statement
Financial support: COLCIENCIAS, Red Chagas (380-2011/5014-537-30398)
Footnotes
Financial support: COLCIENCIAS, Red Chagas (380-2011/5014-537-30398)
REFERENCES
- Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz R, Mauriello L, Zavala R, Suarez JA, Abate T, Naranjo L, Paiva M, Mendoza I, Acquatella H, Torres J, Noya O. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas Venezuela. J Infect Dis. 2010;201:1309–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- Alvarado-Otegui JA, Ceballos LA, Orozco MM, Enriquez GF, Cardinal MV, Cura C, Schijman AG, Kitron U, Gurtler RE. The sylvatic transmission cycle of Trypanosoma cruzi in a rural area in the humid Chaco of Argentina. Acta Trop. 2012;124:79–86. doi: 10.1016/j.actatropica.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Añez N, Crisante G, Silva FM, Rojas A, Carrasco H, Umezawa ES, Stolf AM, Ramírez JL, Teixeira MM. Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas disease. Trop Med Int Health. 2004;9:1319–1326. doi: 10.1111/j.1365-3156.2004.01333.x. [DOI] [PubMed] [Google Scholar]
- Carrasco HJ, Segovia M, Llewellyn MS, Morocoima A, Urdaneta-Morales S, Martínez C, Martínez CE, García C, Rodríguez M, Espinosa R, Alarcón de Noya B, Díaz-Bello Z, Herrera L, Fitzpatrick S, Yeo M, Miles MA, Feliciangeli MD. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Negl Trop Dis. 2012;6: doi: 10.1371/journal.pntd.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco HJ, Torrealba A, García C, Segovia M, Feliciangeli MD. Risk of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) transmission by Pastrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan Distric) and neighboring states, Venezuela. Int J Parasitol. 2005;35:1379–1384. doi: 10.1016/j.ijpara.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Cruz L, Vivas A, Hernández C, Montilla M, Florez C, Parra E, Ramírez JD. Comparative study of the biological properties of Trypanosoma cruzi I genotypes in a murine experimental model. Infect Genet Evol. 2015;29:110–117. doi: 10.1016/j.meegid.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, Cardinal MV, Kjos S, Gurgel-Gonçalves R, Blanchet D, Pablos LM, Tomasini N, Silva A, Russomando G, Cuba CA, Aznar C, Abate T, Levin MJ, Osuna A, Gurtler RE, Diosque P, Solari A, Triana-Chávez O, Schijman AG. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol. 2010;40:1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falla A, Herrera C, Fajardo A, Montilla M, Vallejo GA, Guhl F. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop. 2009;110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Guhl F, Ramírez JD. Trypanosoma cruzi I diversity: towards the need of genetic subdivision? Acta Trop. 2011;119:1–4. doi: 10.1016/j.actatropica.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Guhl F, Ramírez JD. Retrospective molecular integrated epidemiology of Chagas disease in Colombia infection. Infect Genet Evol. 2013;20:148–154. doi: 10.1016/j.meegid.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Guhl F, Vallejo GA. Interruption of Chagas disease transmission in the Andean countries: Colombia. Mem Inst Oswaldo Cruz. 1999;94(Suppl. I):413–415. doi: 10.1590/s0074-02761999000700081. [DOI] [PubMed] [Google Scholar]
- Hernández DC, Cucunubá ZM, Parra E, Toro G, Zambrano P, Ramírez JD. Chagas disease (Trypanosoma cruzi) and HIV co-infection in Colombia. Int J Infect Dis. 2014;26:146–148. doi: 10.1016/j.ijid.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Herrera C, Bargues M, Falla A, Fajardo A, Montilla M, Vallejo GA, Guhl F. Genetic variability and phylogenetic relationships within Trypanosoma cruzi I isolates in Colombia based on mini-exon gene sequences. 897364J Parasitol Res. 2009;2009 doi: 10.1155/2009/897364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C, Bargues MD, Fajardo A, Montilla M, Triana O, Vallejo GA, Guhl F. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect Genet Evol. 2007;7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- INS - Instituto Nacional de Salud Colombia Informe del evento enfermedad de Chagas 2012. 2012 ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/sivigila/Paginas/vigilancia-rutinaria.aspx.
- Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J, Torrico F, Diosque P, Valente V, Valente SA, Gaunt MW. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog. 2009;5: doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla JC, Zafra GA, Macedo AM, González CI. Mixed infection of Trypanosoma cruzi I and II in a Colombian cardiomyopathic patient. Hum Pathol. 2010;41:610–613. doi: 10.1016/j.humpath.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Marcili A, Lima L, Cavazzana MJ, Junqueira AC, Veludo HH, Silva FM, Campaner M, Paiva F, Nunes VL, Teixeira MM. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009;136:641–655. doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- Messenger LA, Llewellyn MS, Bhattacharyya T, Franzén O, Lewis MD, Ramírez JD, Carrasco HJ, Andersson B, Miles MA. Multiple mitochondrial introgression events and heteroplasmy in Trypanosoma cruzi revealed by maxicircle MLST and next generation sequencing. PLoS Negl Trop Dis. 2012;6: doi: 10.1371/journal.pntd.0001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Calderón A, Díaz-Bello Z, Valladares B, Noya O, López MC, Alarcón de Noya B, Thomas MC. Oral transmission of Chagas disease: typing of Trypanosoma cruzi from five outbreaks occurred in Venezuela shows multiclonal and common infections in patients, vectors and reservoirs. Infect Genet Evol. 2013;17:113–122. doi: 10.1016/j.meegid.2013.03.036. [DOI] [PubMed] [Google Scholar]
- Ocaña-Mayorga S, Llewellyn MS, Costales JA, Miles MA, Grijalva MJ. Sex, subdivision and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLoS Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMS - Organización Mundial de la Salud Informe enfermedad de Chagas: control y eliminación. 2008 apps.who.int/gb/ebwha/pdf_files/EB124/B124_17-sp.pdf.
- Ramírez JD, Duque MC, Montilla M, Cucunubá ZM, Guhl F. Multilocus PCR-RFLP profiling in Trypanosoma cruzi I highlights an intraspecific genetic variation pattern. Infect Genet Evol. 2012a;12:1743–1750. doi: 10.1016/j.meegid.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Ramírez JD, Duque MC, Montilla M, Cucunubá ZM, Guhl F. Natural and emergent Trypanosoma cruzi I genotypes revealed by mitochondrial (Cytb) and nuclear (SSU rDNA) genetic markers. Exp Parasitol. 2012b;132:487–494. doi: 10.1016/j.exppara.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Ramírez JD, Guhl F, Messenger L, Lewis M, Montilla M, Cucunubá Z, Miles M, Llewellyn M. Contemporary cryptic sexuality in Trypanosoma cruzi. Mol Ecol. 2012c;21:4216–4226. doi: 10.1111/j.1365-294X.2012.05699.x. [DOI] [PubMed] [Google Scholar]
- Ramírez JD, Guhl F, Rendón LM, Rosas F, Marin JA, Neto, Morillo CA. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic chagasic patients. PLoS Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez JD, Montilla M, Cucunubá ZM, Flórez AC, Zambrano P, Guhl F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis. 2013a;7: doi: 10.1371/journal.pntd.0002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez JD, Tapia G, Muñoz G, Poveda C, Rendón LM, Hincapié E, Guhl F. Trypanosome species in Neotropical bats: biological, evolutionary and epidemiological implications. Infect Genet Evol. 2014;22:250–256. doi: 10.1016/j.meegid.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez JD, Turriago B, Tapia G, Guhl F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet Parasitol. 2013b;196:216–219. doi: 10.1016/j.vetpar.2012.12.054. [DOI] [PubMed] [Google Scholar]
- Segovia M, Carrasco HJ, Martínez CE, Messenger AN, Londoño JC, Espinosa R, Martínez C, Alfredo M, Bonfante-Cabarcas. Lewis M, Miles M, Llewellyn MS. Molecular epidemiologic source tracking of orally transmitted Chagas disease, Venezuela. Emerg Infect Dis. 2013;19:1098–1101. doi: 10.3201/eid1907.121576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira ARL, Nitz N, Guimaro M, Gomes C, Santos-Buch CA. Chagas disease. Postgrad Med J. 2006;82:788–798. doi: 10.1136/pgmj.2006.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LM, Guhl F, Zabala D, Ramírez JD, Urrea DA, Hernández DC, Cucunubá Z, Montilla M, Carranza JC, Rueda K, Trujillo JE, Vallejo GA. The identification of two Trypanosoma cruzi I genotypes from domestic and sylvatic transmission cycles in Colombia based on a single polymerase chain reaction amplification of the spliced-leader intergenic region. Mem Inst Oswaldo Cruz. 2013;108:932–935. doi: 10.1590/0074-0276130201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Castillo D. Evidencias de domesticación y aspectos biológicos de Panstrongylus geniculatus (Latreille, 1811) (Hemiptera: Reduviidae) Acta Entomol Chil. 2000;24:77–83. [Google Scholar]
- Zafra G, Mantilla J, Jácome J, Macedo AM, González CI. Direct analysis of genetic variability in Trypanosoma cruzi populations from tissues of Colombian chagasic patients. Hum Pathol. 2011;42:1159–1168. doi: 10.1016/j.humpath.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Zafra G, Mantilla JC, Valadares HM, Macedo AM, González CI. Evidence of Trypanosoma cruzi II infection in Colombian chagasic patients. Parasitol Res. 2008;103:731–734. doi: 10.1007/s00436-008-1034-0. [DOI] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira M, Schijman AG, Llewellyn M, Lages-Silva E, Machado CR, Andrade SG, Sturm N. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Zumaya-Estrada F, Messenger LA, Lopez-Ordoñez T, Lewis M, Flores-Lopez CA, Martínez-Ibarra AJ, Pennington PM, Cordon-Rosales C, Carrasco HV, Segovia M, Miles MA, Llewellyn S. North American import? Charting the origins of an enigmatic Trypanosoma cruzi domestic genotype. 226Parasit Vectors. 2012;5 doi: 10.1186/1756-3305-5-226. [DOI] [PMC free article] [PubMed] [Google Scholar]