Abstract
Salmonellosis cases in the in the United States show distinct geographical trends, with the southeast reporting among the highest rates of illness. In the state of Georgia, USA, non-outbreak associated salmonellosis is especially high in the southern low-lying coastal plain. Here we examined the distribution of Salmonella enterica in environmental waters and associated wildlife in two distinct watersheds, one in the Atlantic Coastal Plain (a high case rate rural area) physiographic province and one in the Piedmont (a lower case rate rural area). Salmonella were isolated from the two regions and compared for serovar and strain diversity, as well as distribution, between the two study areas, using both a retrospective and prospective design. Thirty-seven unique serovars and 204 unique strain types were identified by pulsed-field gel electrophoresis (PFGE). Salmonella serovars Braenderup, Give, Hartford, and Muenchen were dominant in both watersheds. Two serovars, specifically S. Muenchen and S. Rubislaw, were consistently isolated from both systems, including water and small mammals. Conversely, 24 serovars tended to be site-specific (64.8%, n = 37). Compared to the other Salmonella serovars isolated from these sites, S. Muenchen and S. Rubislaw exhibited significant genetic diversity. Among a subset of PFGE patterns, approximately half of the environmental strain types matched entries in the USA PulseNet database of human cases. Ninety percent of S. Muenchen strains from the Little River basin (the high case rate area) matched PFGE entries in PulseNet compared to 33.33% of S. Muenchen strains from the North Oconee River region (the lower case rate area). Underlying the diversity and turnover of Salmonella strains observed for these two watersheds is the persistence of specific Salmonella serovars and strain types that may be adapted to these watersheds and landscapes.
Introduction
Salmonella enterica are zoonotic bacteria associated with a wide range of animals, including humans, where they are a significant cause of enteric disease and often attributed to foodborne transmission [1]. The incidence of salmonellosis has decreased only slightly in the past 26 years (19 cases per 100,000 in 1987 versus 15.2 cases per 100,000 in 2013) [2, 3]. While the implementation of HACCP (Hazard Analysis and Critical Control Points) for the food industry in the U.S. has reduced contamination of meats, milk, and eggs with foodborne pathogens [4], there has been increased recognition of Salmonella outbreaks associated with fresh produce [5–15]. The cultivation and processing of fruits and vegetables is intimately linked to the environment, where ample opportunities exist to introduce pathogens, through direct contamination from animals in crops and fields and the use of irrigation water that may be contaminated [5, 6, 8, 9, 16]. Although not widely linked to outbreaks, water itself is an important vehicle, especially in sporadic (non-outbreak associated) cases of gastrointestinal illnesses, including salmonellosis [17–19]. Sporadic cases are increasingly associated with a high burden of illnesses and are often not attributable to a particular source, such as food [20]. As a zoonotic agent, animals—livestock as well as wildlife—can be important contributors to the abundance and distribution of Salmonella in the environment and possible transmission to humans [21–26]. Salmonella enterica is intimately associated with the landscape, and its components (living and non-living). Efforts to examine the environmental ecology of this agent are needed to better understand how it may be controlled, especially in regards to non-foodborne and non-outbreak cases.
The notion that environmental parameters affect both the incidence and distribution of salmonellosis cases is illustrated in part by large regional differences in the rates of reported human cases in the U.S. Variations among states in reported cases are not always explained by differences in surveillance, demographics, patterns in food preparation, or food-distribution networks, suggesting that environmental and ecological factors could affect its relative distribution (e.g., biogeographical patterns [27]). Georgia remains among the states (all in the southeast) with the highest annual prevalence, at 24 cases per 100,000 [28], compared to the national average of roughly 15 cases per 100,000 [2] in the Foodborne Diseases Active Surveillance Network (FoodNet). The prevalence in the southern portion of the state, primarily in the Coastal Plain physiographic province, is markedly higher than that in the northern part of the state’s Piedmont province. In 2011, there were 70.1 cases per 100,000 people in Georgia Public Health District 8–1 and 28.8 cases per 100,000 in Public Health District 10 in 2011, representing the south (Coastal Plain) and north (Piedmont), respectively (GA Dept. of Public Health; dph.georgia.gov). Regional patterns in the epidemiology of salmonellosis are reflected not only by significant differences in reported infection rates, but also in the distribution of specific Salmonella serovars between physiographic provinces and individual counties [28].
Salmonella is ubiquitous in fresh and marine environmental surface waters [2, 29, 30], but contamination may come from many different routes such as effluent from wastewater treatment plants, contaminated runoff from urban or agricultural areas, overburdened septic systems, or local and migratory fauna [30–34]. Contamination of environmental waters with Salmonella may be of a greater public health concern than previously thought due to the ability of it to persist and, in some cases, grow outside of a host organism [29]. This characteristic increases the probability of survival between hosts [35]. The environment, including surface waters, can be considered as a part of the lifecycle of Salmonella, and therefore influences the biogeographical patterns of these pathogens.
Previous studies in the Atlantic Coastal Plain (south Georgia and north Florida) indicate that Salmonella are commonly detected in the environment, i.e., streams and ponds [36–39]. Frequency of detection ranges from 29% to 96% of samples among these studies, with concentrations reaching 5,400 MPN L-1 in the southern reaches of the Upper Suwanee Watershed, which spans southern Georgia and north Florida [36, 39]. At the uppermost reaches of the Upper Suwanee Watershed in south Georgia, the Little River is typical of the heavily vegetated, slow-moving stream systems in this region. We have previously shown that 79% of sites along the Little River were positive for Salmonella [37] with levels ranging from 2.5 MPN L-1 to 36.3 MPN L-1. Salmonella densities are positively impacted by precipitation and temperature (e.g., summer season) when serovars associated with human cases are also more likely to be detected [38]. Here we expanded on this work and examined the distribution and diversity of Salmonella serovars and strains across geographic space, time, and between water and wildlife reservoirs, which may affect exposure routes and transmission to humans.
Material and Methods
Description of study sites
The sample areas were located in two distinct physiographic regions of Georgia, USA—the low lying Coastal Plain and the higher elevation Piedmont physiographic province (Fig 1). The regions were also distinguished by prevalence of salmonellosis. Otherwise the selected areas were similar in watershed size, land use, population, and median incomes.
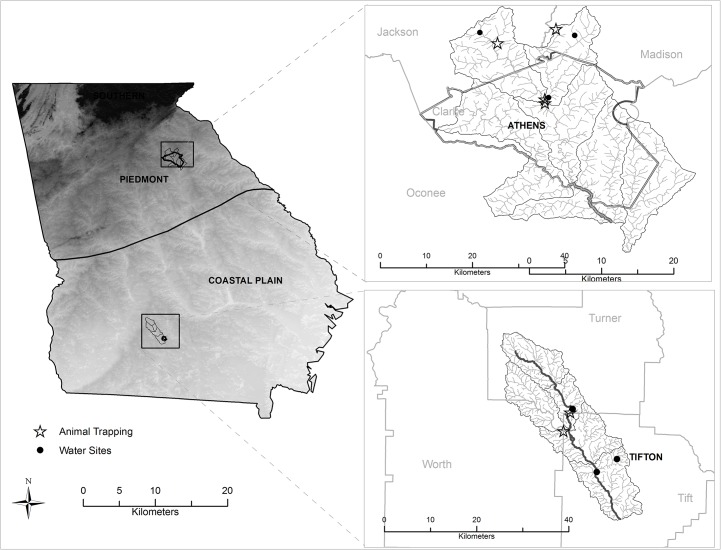
Fig 1. Map of sampling areas in the Oconee River watershed (near Athens in the Piedmont physiographic province) and Little River watershed (near Tifton in the Coastal Plain physiographic province).
Base map source: U.S. Geological Survey, Department of the Interior. (http://water.usgs.gov/lookup/getspatial?physio). Background: watershed produced using ESRI-ArcGIS (LM_LICENSE_FILE: 1700@wrrs.gly.uga.edu) based on U.S Geological Survey, National Elevation Dataset (NED), 2012. Site location: Department of Environmental Health Science-UGA. (Produced by Presotto A, 2015).
The 334 km2 Little River watershed is near Tifton, Georgia in the South Atlantic Coastal Plain, and forms the headwaters for the larger Upper Suwannee Watershed. The Little River watershed is typified by broad floodplains with very poorly defined stream channels and gently sloping uplands. Approximately 45% of the watershed is woodland, 37% crops, 4% pastures, 7% idle, and 7% roads, urban, and water (as described in [37]). Swamp hardwoods occur along the stream edges and are often accompanied by thick undergrowth forming the riparian vegetation boundary along stream networks. Three sampling stations were selected representing first to fourth stream orders with varying levels of flow throughout the year. First order streams are headwaters and are small and narrow whereas fourth order streams are fed by multiple tributaries and are larger and broader. Stations were located in Tift County (upstream of the City of Tifton) in GA Public Health District 8–1. Tift County is rural but is considered to be urbanizing. The 2010 population was 40,118 (59.8 people km-2) with 6.9% of the population under the age of 5 [40]. The per capita income was $18,928 [40]. This district has the highest case rates for salmonellosis in the state (70.1 cases per 100,000 in 2011).
The Oconee River Basin consists of two headwater tributaries, the North Oconee River and the Middle Oconee River, which originate at the northern end of the basin in the Piedmont Upland physiographic province, at an elevation of about 305 m above mean sea level. These headwater streams are generally well entrenched, flow through narrow floodplains, and have steep gradients ranging from 0.15 to 1.4 m km-1. These rivers flow for approximately 100 km to a point just south of Athens, Georgia, where they join to form the Oconee River. Land use in the upper portion of the basin is primarily rural, with poultry farming, dairy farms and grazing for beef production as primary uses. Three sampling stations were selected along the North Oconee River and its tributaries in Jackson County, upstream of the city of Athens, and included first to fourth ordered streams. Jackson County recorded a population of 60,485 in 2010 (68.8 people km-2) with 6.8% of the population under the age of 5 [40]. The per capita income was $22,830 [40]. Jackson County is located in GA Public Health District 10, which is a lower case rate area for salmonellosis in Georgia (28.9 cases per 100,000 in 2011).
Sample collection
Water samples were collected monthly from December 2010 to November 2011 at each of the six stations (Fig 1), which were accessible on foot with public access. Sterile 1-L polypropylene bottles were filled by hand at the deepest part of the stream. For a limited number of collection events (n = 3 in each watershed), samples were obtained from well water, after flushing the spigot for 5 min, at farms near the sampling sites (in coordination with owners). All samples were maintained on ice, transported to the laboratory, and processed within 6 h of collection. Wildlife near each of the six sites was also sampled. Songbirds, raccoons, and opossums were the focus of the active surveillance. Mist nets were used as previously described [41] to capture birds. Birds were held in individual disposable paper bags until they defecated. After 1 h, even if the bird had not defecated, it was released to avoid capture-related mortality. Opossums and raccoons were captured with baited live box traps (Havahart, Woodstream Corp, Lititz, PA). Briefly, traps baited with sardines were deployed at sunset and checked at dawn. If an animal was captured, the trap was turned so that it would stand vertically and the animal was gently forced to the bottom and hand injected with an anesthetic. Once the animal had reached a light plane of anesthesia, it was removed from the trap, and approximately 1 g of feces was removed directly from the rectum by digital extraction. Fresh fecal material was immediately immersed in 10 ml of dulcitol selenite broth and maintained at room temperature until submitted to the Athens Diagnostic Laboratory for isolation. Samples were submitted to the laboratory within 48 h. The University of Georgia Animal Care and Use and Procedures Committee approved protocols involving capture and handling of animals associated with this project (AUP # A2010 08-159-Y3-A0).
Salmonella isolation
Each water sample (≤100 ml) was filtered in duplicate onto 0.45 μm 47-mm mixed cellulose ester membranes, inserted into a sterile 50-ml centrifuge tube containing 20 ml of 1% sterile peptone broth and incubated overnight at 37°C. A 100-μl aliquot of turbid broth culture was used to inoculate a15-ml tube 10 ml Rappaport-Vassiliadis (RV) broth, which was then incubated at 37°C for ~24 h. One loopful of overnight growth from RV broth was spread onto XLD agar plates and incubated for 24 h. Presumptive Salmonella colonies (H2S positive) were picked and transferred to LB agar stabs. Cultures were streaked for isolation three times before final Salmonella confirmation and serovar determination (see below). Each sample was scored as positive or negative for Salmonella presence following confirmation steps.
For animal feces, Dulcitol Selenite (Difco; Detroit, MI) was inoculated with fecal samples and incubated at 42 ± 0.5°C. A 10 μl loopful of overnight growth from enrichment broth was streaked onto a XLT4/BGN bi-plate (Remel Inc.; Lenexa, KS) followed by 37°C overnight incubation [42, 43].
All H2S positive colonies were further characterized biochemically to identify Salmonella. Real time PCR was used to screen Selenite broths that were culture negative for Salmonella [44]. PCR template was prepared from a pool of three Selenite broth cultures. DNA was isolated from 1 ml of the pooled enrichment using Ultra Clean Fecal DNA Kit (MO Bio Inc., Carlsbad, CA). Positive pools were then tested individually by PCR and subcultured simultaneously onto Salmonella selective media. A delayed secondary enrichment was done for culture negative, PCR positive Selenite enrichment broths [43]. Any suspect, biochemically atypical Salmonella were confirmed by PCR [44]. Salmonella isolates were forwarded to the National Veterinary Service Laboratory (Ames, IA) for serotyping.
Molecular typing of Salmonella isolates by pulsed-field gel electrophoresis
Pulsed-field gel electrophoresis (PFGE) was used to determine the genetic relatedness [45–47] among Salmonella isolates obtained during this study, archived isolates from environmental and animal sources, and human isolates represented in the PulseNet USA national database, which included reports from states in the southeast US (GA, FL, SC, and AL). A master database of Salmonella PFGE patterns was generated in BioNumerics (Applied Maths; Austin, TX). Comparisons were made between PFGE patterns using Dice coefficient [48] and unweighted pair group method of arithmetic averages (UPGMA) clustering. Clusters were identified based on a 75% similarity cut-off.
Results
Serovar distribution in Salmonella isolated from the Little River and Oconee River Basins
In total, 1,029 isolates from the two watersheds (water and animals) and archived environmental and animal samples were processed for serovar and PFGE pattern. These included 355 isolates from water and animals collected in the Little River (Upper Suwannee) and Oconee River watersheds (2005–2011) and an additional 674 archived isolates from animal sources (various species) obtained from the Salmonella Reference Collection (SARA 1–72) [49] and past studies [21, 37, 48, 50–53]. Thirty-seven unique serovars were identified from salmonellae isolated from water and animals in the Little River and Oconee River watersheds. These included 15 serovars that were ranked among the top 20 for human cases in the US and in Georgia [31]. Eighteen serovars were recovered only from water (represented by 53 isolates) and included six of the top 20 ranked serovars in human cases (U.S. and Georgia). Only two serovars, Braenderup and Paratyphi B var L (+) tartate +, were found in both watersheds and none of the PFGE types were shared between the two regions (Table 1). Only five serovars were recovered solely from animals (cattle, hogs, opossum, raccoons or song birds) and none included serovars commonly associated with human cases. Two serovars were found in animals from both watersheds (Dublin and Muenster) (Table 1). Most isolates (82%; 291/355) were associated with serovars found in both water and animals, including 4 isolates from shallow wells in the Little River watershed. In all, 14 serovars were recovered from both sources and nine of these were among the top 20 for human cases (US and Georgia) (Table 1). Unlike water-only and animal-only serovars, most of the serovars from both sources were also found in both watersheds (11 were shared).
Table 1. Salmonella serovars isolated from the Little River and Oconee River watersheds (2005–2011).
| Source | Serovar | Little River | Oconee River | Both watersheds | ||
|---|---|---|---|---|---|---|
| # of isolates | # PFGE types | # of isolates | # PFGE types | # isolates with shared PFGE type a | ||
| Water | 16:z:10 | 1 | 1 | 0 | 0 | 0 |
| Only | 30:-:lw | 1 | 1 | 0 | 0 | 0 |
| 47:z4z23:- | 3 | 1 | 0 | 0 | 0 | |
| Braenderup | 13 | 3 | 2 | 2 | 0 | |
| Enteritidis | 2 | 2 | 0 | 0 | 0 | |
| Heidelberg | 0 | 0 | 1 | 1 | 0 | |
| Infantis | 0 | 0 | 1 | 1 | 0 | |
| Kentucky | 4 | 0 | 0 | 0 | 0 | |
| Kiambu | 0 | 0 | 1 | 1 | 0 | |
| Liverpool | 2 | 2 | 0 | 0 | 0 | |
| Livingstone | 0 | 0 | 1 | 1 | 0 | |
| Oranienburg | 0 | 0 | 3 | 2 | 0 | |
| Ouakam | 0 | 0 | 1 | 1 | 0 | |
| Paratyphi B b | 3 | 1 | 5 | 2 | 0 | |
| Senftenberg | 0 | 0 | 3 | 2 | 0 | |
| Tamberma | 1 | 1 | 0 | 0 | 0 | |
| Thompson | 0 | 0 | 4 | 1 | 0 | |
| Typhimurium | 0 | 0 | 1 | 1 | 0 | |
| Animal | III 44:z4, z32:- | 1 | 1 | 0 | 0 | 0 |
| Only | O-:lz4, z23:- | 1 | 1 | 0 | 0 | 0 |
| Arizona | 1 | 1 | 0 | 0 | 0 | |
| Dublin | 3 | 1 | 1 | 1 | 4 | |
| Muenster | 3 | 1 | 1 | 1 | 4 | |
| Water + | IV 40: z4: 32 | 1 | 1 | 3 | 1 | 0 |
| Animal | Anatum | 6 | 4 | 1 | 1 | 0 |
| Bareilly | 12 | 5 | 7 | 3 | 0 | |
| Gaminara | 8 | 7 | 2 | 1 | 0 | |
| Give | 27 | 7 | 15 | 13 | 15 | |
| Hartford | 16 | 8 | 21 | 2 | 0 | |
| Inverness | 2 | 2 | 0 | 0 | 0 | |
| Mbandaka | 9 | 2 | 3 | 2 | 0 | |
| Meleagridis | 15 | 4 | 0 | 0 | 0 | |
| Montevideo | 5 | 3 | 4 | 3 | 0 | |
| Muenchen | 11 | 10 | 36 | 25 | 5 | |
| Newport | 2 | 1 | 6 | 5 | 0 | |
| Rubislaw c | 41 | 36 | 29 | 14 | 4 | |
| Saintpaul | 9 | 4 | 0 | 0 | 0 | |
Serovars noted in bold are those ranked among the top 20 in human cases for the US (2009–2011); serovars in italics are those ranked in the top 20 in human cases in Georgia (31).
a Isolates shared one PFGE type (per serovar)
b Paratyphi B var L (+) tartate +
c Included one isolate collected from shallow well water in the Little River watershed
Regardless of source, Salmonella enterica Rubislaw, Give, Hartford, Braenderup, and Muenchen were the serovars most frequently isolated from either region, accounting for 53% and 62% of total isolates from the Little River and Oconee River watersheds, respectively. Salmonella Muenchen and Rubislaw were the most frequently encountered serovars in both river basins. With the exception of S. Braenderup, most other Salmonella serovars encountered in both watersheds were transient, being isolated only once for a given year.
Strain distribution in Salmonella isolated from the Little River and Oconee River Basins
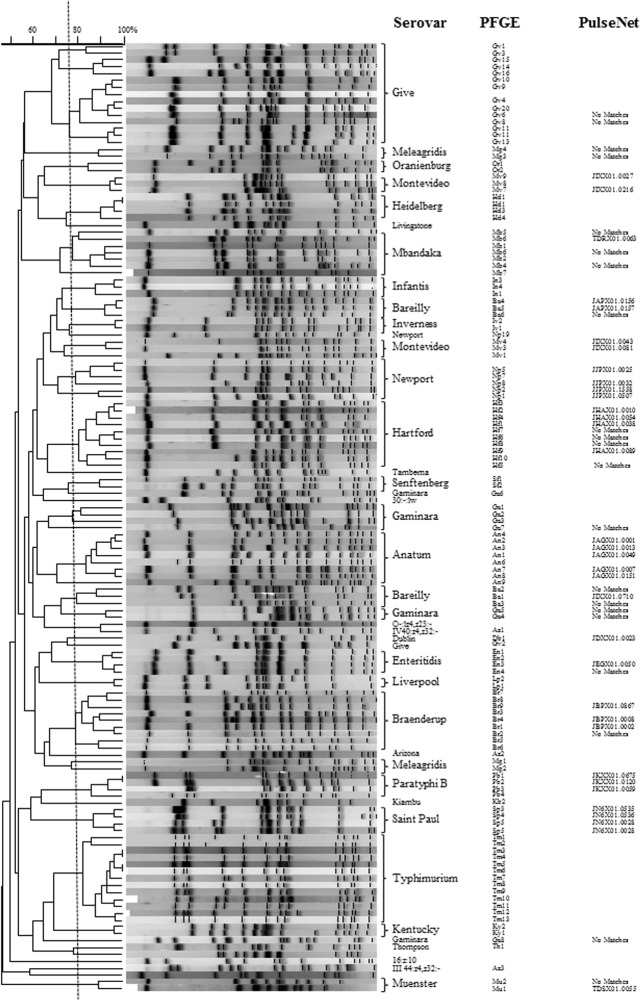
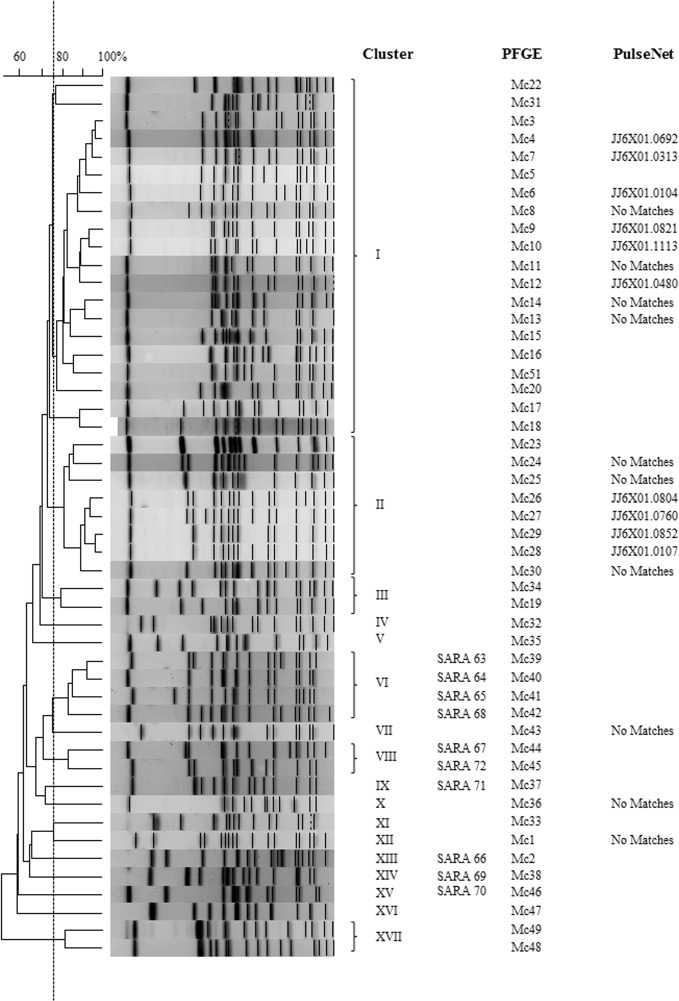
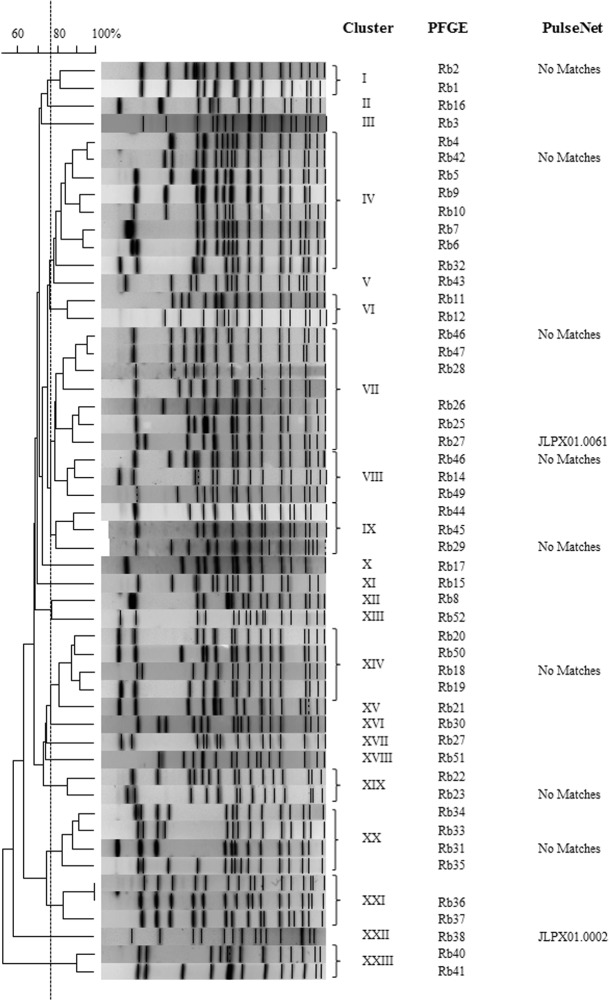
Salmonella PFGE patterns for environmental and archived isolates were compared to each other to evaluate trends in strain persistence and relatedness from the two sample sites. In addition to patterns in serovar distribution (Table 1), there was also considerable strain diversity in Salmonella isolated from the two study sites. Among 37 Salmonella serovars isolated from either region, there were 204 unique PFGE patterns for the water and animal isolates analyzed. Frequently PFGE patterns clustered together by serovar (≥75% similarity) (Fig 2); however, there were several Salmonella serovars where PFGE patterns with <75% similarity generated two or more clusters (S. Bareilly- 2 clusters; S. Gaminara- 6 clusters; S. Give- 4 clusters; S. Meleagridis- 2 clusters). Two Salmonella serovars, S. Muenchen and S. Rubislaw, exhibited the greatest diversity in PFGE patterns, necessitating separate cluster analyses (Figs 3 and 4). Salmonella Muenchen PFGE patterns fell into one of 16 clusters; clusters I & II represented 41% and 16% of PFGE profiles, respectively. Salmonella Rubislaw demonstrated even greater diversity in PFGE profiles, with patterns falling into one of twenty-three clusters. No single S. Rubislaw PFGE cluster accounted for more than 15% of the total patterns. While there was a high level of diversity in PFGE profiles for both serovars, more patterns matched human clinical cases entered in PulseNet for S. Muenchen (44%; n = 25) than for S. Rubislaw (18%; n = 22).
Fig 2. Dendrogram of representative Salmonella PFGE patterns for 37 Salmonella serovars (excluding S. enterica serovars Muenchen and Rubislaw) collected from Oconee and Little River watersheds and archived isolates with similar PFGE profiles.
Salmonella PFGE patterns generated in this study were compared to a BioNumerics database of PFGE entries of Salmonella isolates from various animal species and to the CDC PulseNet data base of isolates from human cases. Vertical line indicates 75% similarity.
Fig 3. Dendrograms of representative Salmonella PFGE patterns for Salmonella serovars Muenchen collected from Oconee and Little River watersheds and archived isolates with similar PFGE profiles.
Vertical line indicates 75% similarity.
Fig 4. Dendrograms of representative Salmonella PFGE patterns for Salmonella serovars Rubislaw collected from Oconee and Little River Basins and archived isolates with similar PFGE profiles.
Vertical line indicates 75% similarity.
While there were several Salmonella serovars common to both watersheds, only 5 of 204 PFGE types observed were shared among isolates from the two regions (Table 1). Most Salmonella PFGE types (82%) were rare, only appearing once. There were 11 unique PFGE types that were identified in isolates from both water and animals within the same watershed sampling area (Table 2). For eight of these, water and animal isolates were collected within the same season or year and included Salmonella serovars S. Anatum, S. Bareilly, S. Give, S. Hartford, S. Mbandaka, S. Montevideo, and S. Newport. Most PFGE types were specific to water or to animals.
Table 2. Salmonella strains (PFGE types) present in both animals and water of the Little River or North Oconee River watersheds (Georgia, USA).
| Serovar | Watershed | # of Strains | Sources | Season/Year Collected |
|---|---|---|---|---|
| Anatum | Little River | 1* | Water | Winter 2011 |
| Opossum | Spring 2011 | |||
| Barielly | Little River | 1* | Water | Spring 2005, Summer 2007, Fall 2011 |
| Raccoon | Summer 2011 | |||
| Oconee River | 1 | Opossum | Fall 2010, Summer 2011 | |
| Water | Summer 2011 | |||
| Give | Little River | 1 | Opossum | Fall 2010, Winter, Spring, Summer, Fall 2011 |
| Water | Fall 2011 | |||
| Hartford | Oconee River | 1* | Raccoon | Winter and Spring 2011 |
| Water | Summer and Fall 2011 | |||
| Mbandaka | Little River | 1 | Songbird | Spring 2011 |
| Opossum | Summer 2011 | |||
| Water | Fall 2011 | |||
| Montevideo | Little River | 1* | Water | Winter and Fall 2011 |
| Raccoon | Winter 2011 | |||
| Muenchen | Oconee River | 1* | Water | Spring 2005 |
| Opossum | Summer 2011 | |||
| Newport | Little River | 1* | Raccoon | Winter 2011 |
| Water | Summer 2011 | |||
| Rubislaw | Oconee River | 2 | Water | Spring 2005 |
| Opossum | Winter, Summer, Fall 2011 |
* indicates PFGE pattern matching isolate in CDC PulseNet database. [Winter (Jan, Feb, Mar), Spring (Apr, May, Jun), Summer (Jul, Aug, Sep), and Fall (Oct, Nov, Dec).]
Despite turnover of Salmonella strains, there were several Salmonella strains isolated at least twice from either watershed, representing 19 serovars and 34 PFGE types. Most of the PFGE types were encountered sporadically, often isolated once in a given year. Just over half (18/34) were encountered multiple years; 14 that were originally isolated in 2005 were still present in 2010 and 2011 (4 PFGE types in the Little River and 10 in the Oconee River) (Table 3). Only two PFGE types were consistently present between 2005 and 2011; S. Braenderup type Br1 and S. Saintpaul type Sp5 were isolated in the Little River in 4 and 3 separate years, respectively.
Table 3. Salmonella serovars and PFGE types isolated multiple times in the Little River or Oconee River watersheds (both water and animal sources) between 2005 and 2011.
| Serovar | PFGE type | PulseNet ID | Watershed | Source | Date of Collection |
|---|---|---|---|---|---|
| 47:z4z23:- | Tb1 | Not determined | Little River | Water | Feb, Mar, Dec 2007 |
| Anatum | An2 | JAGX01.0001 | Little River | Water | Mar 2011 |
| Opossum | Apr 2011 | ||||
| An7 | JAGX01.0007 | Little River | Water | Jun, Aug 2007 | |
| Bareilly | Ba1 | JIXX01.0710 | Oconee River | Water | Sep, Oct 2011 |
| Ba2* | No matches | Oconee River | Water | Aug 2011 | |
| Opossum | Dec 2010, Sep 2011 | ||||
| Ba4* | JAPX01.0156 | Little River | Water | May, Sep 2005, Oct 2011 | |
| Raccoon | Aug 2011 | ||||
| Ba5 | JAPX01.0157 | Little River | Water | Feb, Jun 2011 | |
| Ba6 | No matches | Little River | Water | Aug, Sep 2005 | |
| Braenderup | Br1* | JBPX01.0002 | Little River | Water | May, Jul, Aug 2005; Jan 2006; Jun, Jul, Aug, Sep 2007; Feb 2011 |
| Dublin | Db1* | JDXX01.0023 | Little River | Songbird | Dec 2010, 2011 |
| Gaminara | Ga4* | No matches | Little River | Water | Aug 2005, Feb 2007 |
| Give | Gv4* | No matches | Little River | Opossum | Dec 2010; Feb, May Aug, Oct, Nov 2011 |
| Gv11* | No matches | Oconee River | Water | Apr 2005, Feb 2011 | |
| Hartford | Hf1* | JHAX01.0038 | Oconee River | Water | Apr 2005, Feb 2011 |
| Hf2 | JHAX01.0010 | Oconee River | Water | Jun, Nov 2011 | |
| Raccoon | Feb, Apr, Jul 2011 | ||||
| Hf9 | JHAX01.0089 | Little River | Water | Jun, Aug, Nov 2005 | |
| Mbandaka | Mb3 | No Matches | Little River | Water | Oct 2011 |
| Songbird | Apr 2011 | ||||
| Opossum | Aug 2011 | ||||
| Meleagridis | Mg3 | No matches | Little River | Water | Jan, Feb, Mar, Oct 2011 |
| Mg4 | No matches | Little River | Water | Feb, Sep 2007 | |
| Muenchen | Mc4* | JJ6X01.0692 | Oconee River | Water | Apr 2005 |
| Opossum | Jul 2011 | ||||
| Mc24* | No matches | Oconee River | Water | Apr 2005, Jun 2011 | |
| Mc28 | JJ6X01.0107 | Little River | Water | Aug, Nov 2005 | |
| Montevideo | Mv3 | JIXX01.0081 | Little River | Water | Mar, Oct 2011 |
| Raccoon | Feb 2011 | ||||
| Newport | Np5* | JJPX01.0025 | Oconee River | Water | Apr 2005, Mar 2011 |
| Np8 | JJPX01.0032 | Little River | Water | Sep 2011 | |
| Raccoon | Feb 2011 | ||||
| Paratyphi B | Pb3* | JKXX01.0059 | Little River | Water | Nov 2005, Jun 2007 |
| Oconee River | Water | Apr 2005, Jan 2011 | |||
| Rubislaw | Rb20* | JLPX01.0108 | Little River | Water | Jul, Dec 2005; Jan 2011 |
| Rb27* | JLPX01.0061 | Oconee River | Water | Apr 2005, May 2011 | |
| Rb34* | No matches | Oconee River | Water | Apr 2005 | |
| Opossum | Feb, Aug, Nov 2011 | ||||
| Rb36* | No matches | Oconee River | Water | Apr 2005 | |
| Opossum | Feb, Mar 2011 | ||||
| Saintpaul | Sp5* | JN6X01.0028 | Little River | Water | Jul 2005; Sep, Nov 2007; Feb 2011 |
| Senftenberg | Sf2* | Not determined | Oconee River | Water | Dec 2010, Oct 2011 |
| Thompson | Th1 | Not determined | Oconee River | Water | Apr 2005; Sep, Oct 2011 |
*isolated in multiple years
Distribution of Salmonella strains associated with human illness in the Little River and Oconee River watersheds
The incidence of salmonellosis in Georgia is skewed within the state, with the highest incidence occurring in the southern region. To address whether the distribution of specific serovars or strains in the environment might be associated with these trends in human cases, select Salmonella PFGE types identified between Little River and Oconee River isolates were compared to CDC PulseNet database for matching PFGE patterns among human isolates. Due to the high number of PFGE patterns identified, only a subset were compared, representing 1) the most common Salmonella serovars in human cases, 2) a Salmonella strain that was persistent in either watershed, or 3) present in both water and wildlife from that locale (n = 113). Approximately, half of the Salmonella isolated from water and wildlife sources had matching PFGE patterns with PulseNet database of human isolates (Table 4). Human cases from the state of Georgia were reported for 7 and 4 of the Salmonella PFGE types from Little River and Oconee River watersheds, respectively (represented by serovars: S. Anatum, S. Braenderup, S. Hartford, S. Montevideo, S. Muenchen, S. Newport, S. Paratyphi B, and S. SaintPaul). There was no significant difference in preponderance of Salmonella strains that had matching PFGE patterns with human isolates in PulseNet between the two river basins (Table 4); however, there was a difference in the distribution of S. Muenchen strains associated with human cases. Ninety percent of S. Muenchen strains (n = 10) from the Little River had matching PFGE profiles with PulseNet entries, while only one third of S. Muenchen PFGE patterns for Oconee River isolates (n = 24) had matches with PulseNet database.
Table 4. Salmonella PFGE types isolated from the Little River and Oconee River watersheds associated with human illnesses.
| Little River | Oconee River | |
|---|---|---|
| Total PFGE types submitted to CDC PulseNet | 75 | 40 |
| PFGE types with matches to patterns in PulseNet | 33 | 19 |
| #Total isolates with PFGE pattern matching PulseNet database (all serovars) | 74 (46% b ; n = 161) | 49 (50% b ; n = 98) |
| • Muenchen isolates | 9 (90% c ; n = 10) | 8 (33% c ; n = 24) |
| • Rubislaw isolates | 5 (21% c ; n = 24) | 2 (7% c ; n = 29) |
| PFGE types associated with illnesses in Georgia a | 7 | 4 |
| #Cases in Georgia associated with matching PFGE types a | 28 | 32 |
a Of the PulseNet matches, the search of database was restricted to year of isolation for environmental strain
b Of PFGE patterns submitted to PulseNet, proportion of isolates with PulseNet matches
c For S. Muenchen or S. Rubislaw PFGE patterns submitted to PulseNet, the proportion of isolates with PulseNet matches
Discussion
The overall Salmonella serovar composition noted in this study and in prior work was similar between these two rural watersheds [36, 37, 53]. Salmonella serovars generally associated with food animals were rare in both watersheds (e.g., S. Newport, S. Enteritidis, S. Typimurium) [54–56], whereas elsewhere in the United States and Canada, such Salmonella serovars have been frequently isolated from watersheds [57, 58]. The serovar composition found in the present study also differed from the findings in agricultural ponds within the coastal plain of Georgia, where serovars associated with food production were common and displayed a relatively low diversity of serovar type [39]. In the natural and flowing river systems that were the focus of this study, serovar diversity was high and only two serovars common in food were observed, and were found only rarely (S. Newport and S. Saintpaul) [59, 60]. Here, S. Muenchen and S. Rubislaw were the most commonly detected isolates in this study, with 47 and 70 isolates, respectively. These serovars have also been found in other natural waters [53, 61], including other areas of the Atlantic Coastal Plain [36, 53, 61]. These serovars are also often associated with human cases in southern Georgia, where they ranked 4th and 11th in reported cases between 2000 and 2006 (Georgia Dept. of Health; District 8–1). Interestingly, common poultry Salmonella serovars such as S. Enteritidis and S. Heidelberg [62] were very rarely isolated even though north Georgia has the greatest concentration of poultry production in the state [63] and application of poultry manures to fertilize pasture land is a common practice in many areas of the state [38, 64].
Fourteen of the 37 serovars identified in this study, representing 82% of the isolates, were recovered both from surface waters and wildlife captured nearby (one serovar, Rubislaw, was also identified from well water). Eleven PFGE types were identical between water and animals. In contrast, only five serovars were recovered from animals alone. While 18 serovars were found solely in water, these represented only 15% of the isolates. Salmonella isolation was especially common in opossums and raccoons caught in proximity to the water collection sites in both watersheds. It is unknown how much these animal species and others [65, 66] contribute to Salmonella loading in either river basin, but the results indicate that a significant population of Salmonella strains may be moving between wildlife hosts and the environment, including water.
Interestingly, many of the Salmonella serovars identified in the Little River (southern Georgia) have been associated with outbreaks epidemiologically linked to fresh produce [15]. There is a significant delineation in agricultural output within the state of Georgia, where the southern region is noted more for produce production. As water is especially important in cultivation and production of produce, the watersheds in southern Georgia are potentially important conduits for introducing Salmonella contamination to food products. Irrigation ponds in the region have been shown to harbor Salmonella, but at a lower diversity than noted in the watershed studies presented here [39]. Differences between a pond environment and a flowing river system may reflect differences in loading and contamination and may also reflect ecological differences in the system.
The overall level of Salmonella and their diversity in natural watersheds supports the idea that landscapes may be an important feature in sporadic transmission to humans. Similar disparities in the geographic distribution of human illnesses associated with other zoonotic bacteria such as Escherichia coli O157:H7 and Campylobacter sp. have been observed elsewhere, where higher illness rates are found in regions with high livestock densities, likely due to increased animal contact and environmental exposure [18, 19, 67, 68]. Evidence from recent work suggests that direct environmental exposures may be important in non-outbreak scenarios. For example, the prevalence of private wells for drinking water and use of septic systems are noted risk factors for non-outbreak associated salmonellosis, especially among children [17, 20]. Similar risk factors were also noted for specific serovars common in the southeast U.S. [20] While both of the rural areas investigated in this study rely on septic systems for waste disposal, there is a higher rate of private (untreated) wells as the source of drinking water in the southern part of the state (28.7% of the total population and 95% of the rural population [69].
Salmonella enterica is a commonly detected pathogen in the waters of Georgia, and here showed a very high level of diversity both at the serovar and PFGE-type strain level, especially in the Little River watershed in the Atlantic Coastal Plain. Similar observations have been made regarding the genetic diversity of Salmonella isolated from other river systems [58], including the Suwanee watershed of South Georgia/North Florida [36, 58]. This temporal turnover of Salmonella strain types in the two river basins may follow point-source contamination and possible impact of land use on prevalence and diversity within these environs. While there was significant genetic diversity in each of these two river basins, we did identify matching PFGE patterns between Salmonella recently isolated from the Oconee River and Little River and those from earlier studies of the two watersheds [37, 53]. This suggests certain Salmonella strains persist in this environment due to either continued contribution by an animal population or long-term persistence within this aquatic environment, suggesting a specific niche within the river or watershed. For example, evidence indicates that sediment may support long-term survival and persistence for Escherichia coli O157:H7 [70], Salmonella [70, 71], and Campylobacter [72]. Storm events can churn up this sediment and reintroduce these dormant, persistent Salmonella strains into the water column. Salmonella levels within the water column significantly increase in the river following storm events and there is a positive correlation in Salmonella prevalence, concentration, and rainfall [37].
Over all serovars tested, there were no differences between the Oconee River and the Little River basin in the percentage of environmental Salmonella strains that matched human clinical strains (~50% each, determined by identical PFGE patterns with human isolates reported to the PulseNet database), despite the fact that prevalence of salmonellosis is higher in the south (including the Little River area). When focusing on specific Salmonella serovars, we identified distinct differences between the watersheds in the proportions of strains matching those of human isolates in PulseNet. For example, 90% of the S. Muenchen isolates from the Little River watershed matched entries in PulseNet versus only 33% from the Oconee River watershed. The greater preponderance of matches between environmental and human isolates for the Little River and the Oconee River is especially significant due to the high level of genetic diversity inherent in these populations and may suggest differences in climate, landscape, and human activities between the two watersheds. In addition to epidemiological studies that may help to determine where and how humans may be exposed through the environment (including water), in the future whole genome sequencing of these environmental, animal, and human isolates, with matching PFGE patterns, will allow us to discern genetics that underlie the pathogenic potential of environmental Salmonella and genetic markers that identify point source for contamination.
Conclusion
Studies examining risk factors for salmonellosis can no longer focus only on impacts associated with food production. Salmonella is a broad zoonotic agent that is likely part of the ecology of the landscape, with high rates of exchange probable between humans, water, and wildlife. There are several inherent differences between North and South Georgia in its geography, geology, land use, and ecology that may be driving the rates of salmonellosis within the state. The recent publication of the Salmonella Atlas for 32 major Salmonella serovars by the CDC further supports geographical differences in the incidence of disease in the United States [73]. Understanding ecological interactions between pathogens, the environment, and humans is essential for reducing the burden of human illnesses due to Salmonella.
Acknowledgments
We wish to thank Richard Meinersmann and Paula Fedorka-Cray for providing archived Salmonella isolates from their 2005 study of the Oconee River basin [53]. We sincerely appreciate the various undergraduate students who participated in PFGE studies including Mariama Barry, Amanda Booker, Ricky Doshi, Karisma Govan, Michelle Lee, Arthur Liang, Mohamed Mustafa, Samareh Namdar, Adane Nune, Nuratulahi Oke, Mimi Opanuga, Natasha Patel, Maha Sayed, Natasha Shyan, Leah Stuart, Thanh Loan Ngoc Vu, Nancy Wang, and William Webster.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grant 1R15AI089565-01 from the National Institutes of Health (National Institute of Allergy and Infectious Disease) to EKL, JJM, SH, SS, MM and SV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7–15. Epub 2011/01/05. 10.3201/eid1701.091101p1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, et al. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. MMWR Morb Mortal Wkly Rep. 2014;63(15):328–32. Epub 2014/04/18. . [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen SJ, Bishop R, Brenner FW, Roels TH, Bean N, Tauxe RV, et al. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987–1997. J Infect Dis. 2001;183(5):753–61. Epub 2001/02/22. 10.1086/318832 . [DOI] [PubMed] [Google Scholar]
- 4. Anonymous. Pathogen reduction; hazard analysis and critical control point (HACCP) systems; final rule. Federal Reg. 1996;61:38805–989. [Google Scholar]
- 5. Greene SK, Daly ER, Talbot EA, Demma LJ, Holzbauer S, Patel NJ, et al. Recurrent multistate outbreak of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol Infect. 2008;136(2):157–65. Epub 2007/05/04. 10.1017/S095026880700859X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hedberg CW, Angulo FJ, White KE, Langkop CW, Schell WL, Stobierski MG, et al. Outbreaks of salmonellosis associated with eating uncooked tomatoes: implications for public health. The Investigation Team. Epidemiol Infect. 1999;122(3):385–93. Epub 1999/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lynch M, Painter J, Woodruff R, Braden C. Surveillance for foodborne-disease outbreaks—United States, 1998–2002. MMWR Surveill Summ. 2006;55(10):1–42. Epub 2006/11/10. . [PubMed] [Google Scholar]
- 8. Mohle-Boetani JC, Farrar J, Bradley P, Barak JD, Miller M, Mandrell R, et al. Salmonella infections associated with mung bean sprouts: epidemiological and environmental investigations. Epidemiol Infect. 2009;137(3):357–66. Epub 2008/02/26. 10.1017/S0950268808000411 . [DOI] [PubMed] [Google Scholar]
- 9. Proctor ME, Hamacher M, Tortorello ML, Archer JR, Davis JP. Multistate outbreak of Salmonella serovar Muenchen infections associated with alfalfa sprouts grown from seeds pretreated with calcium hypochlorite. J Clin Microbiol. 2001;39(10):3461–5. Epub 2001/09/28. 10.1128/JCM.39.10.3461-3465.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anonymous. Multistate outbreaks of Salmonella serotype Poona infections associated with eating cantaloupe from Mexico—United States and Canada, 2000–2002. MMWR Morb Mortal Wkly Rep. 2002;51(46):1044–7. Epub 2002/12/19. . [PubMed] [Google Scholar]
- 11. Anonymous. Outbreak of Salmonella serotype Enteritidis infections associated with raw almonds—United States and Canada, 2003–2004. MMWR Morb Mortal Wkly Rep. 2004;53(22):484–7. Epub 2004/06/11. . [PubMed] [Google Scholar]
- 12. Anonymous. Multistate outbreaks of Salmonella infections associated with raw tomatoes eaten in restaurants—United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2007;56(35):909–11. Epub 2007/09/07. . [PubMed] [Google Scholar]
- 13. Anonymous. Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items—United States, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(34):929–34. Epub 2008/08/30. . [PubMed] [Google Scholar]
- 14. Anonymous. Outbreak of Salmonella serotype Saintpaul infections associated with eating alfalfa sprouts—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(18):500–3. Epub 2009/05/16. . [PubMed] [Google Scholar]
- 15. Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis. 2013;19(8):1239–44. Epub 2013/07/24. 10.3201/eid1908.121511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uesugi AR, Danyluk MD, Mandrell RE, Harris LJ. Isolation of Salmonella Enteritidis phage type 30 from a single almond orchard over a 5-year period. J Food Prot. 2007;70(8):1784–9. Epub 2007/09/07. . [DOI] [PubMed] [Google Scholar]
- 17. Denno DM, Keene WE, Hutter CM, Koepsell JK, Patnode M, Flodin-Hursh D, et al. Tri-county comprehensive assessment of risk factors for sporadic reportable bacterial enteric infection in children. J Infect Dis. 2009;199(4):467–76. Epub 2009/03/14. 10.1086/596555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michel P, Wilson JB, Martin SW, Clarke RC, McEwen SA, Gyles CL. Temporal and geographical distributions of reported cases of Escherichia coli O157:H7 infection in Ontario. Epidemiol Infect. 1999;122(2):193–200. Epub 1999/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nygard K, Andersson Y, Rottingen JA, Svensson A, Lindback J, Kistemann T, et al. Association between environmental risk factors and Campylobacter infections in Sweden. Epidemiol Infect. 2004;132(2):317–25. Epub 2004/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarkson LS, Tobin-D'Angelo M, Shuler C, Hanna S, Benson J, Voetsch AC. Sporadic Salmonella enterica serotype Javiana infections in Georgia and Tennessee: a hypothesis-generating study. Epidemiol Infect. 2010;138(3):340–6. Epub 2009/09/03. 10.1017/S0950268809990586 . [DOI] [PubMed] [Google Scholar]
- 21. Hernandez SM, Keel K, Sanchez S, Trees E, Gerner-Smidt P, Adams JK, et al. Epidemiology of a Salmonella enterica subsp. enterica serovar Typhimurium strain associated with a songbird outbreak. Applied and Environmental Microbiology. 2012;78(20):7290–8. 10.1128/AEM.01408-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Refsum T, Handeland K, Baggesen DL, Holstad G, Kapperud G. Salmonellae in avian wildlife in Norway from 1969 to 2000. Applied and Environmental Microbiology. 2002;68(11):5595–9. Epub 2002/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Handeland K, Nesse LL, Lillehaug A, Vikoren T, Djonne B, Bergsjo B. Natural and experimental Salmonella Typhimurium infections in foxes (Vulpes vulpes). Vet Microbiol. 2008;132(1–2):129–34. Epub 2008/06/13. 10.1016/j.vetmic.2008.05.002 . [DOI] [PubMed] [Google Scholar]
- 24. Compton JA, Baney JA, Donaldson SC, Houser BA, San Julian GJ, Yahner RH, et al. Salmonella infections in the common raccoon (Procyon lotor) in western Pennsylvania. J Clin Microbiol. 2008;46(9):3084–6. Epub 2008/07/04. 10.1128/JCM.00685-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashbolt R, Kirk MD. Salmonella Mississippi infections in Tasmania: the role of native Australian animals and untreated drinking water. Epidemiol Infect. 2006;134(6):1257–65. Epub 2006/05/05. 10.1017/S0950268806006224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith KE, Anderson F, Medus C, Leano F, Adams J. Outbreaks of salmonellosis at elementary schools associated with dissection of owl pellets. Vector Borne Zoonotic Dis. 2005;5(2):133–6. Epub 2005/07/14. 10.1089/vbz.2005.5.133 . [DOI] [PubMed] [Google Scholar]
- 27. Martiny JB, Bohannan BJ, Brown JH, Colwell RK, Fuhrman JA, Green JL, et al. Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol. 2006;4(2):102–12. Epub 2006/01/18. 10.1038/nrmicro1341 . [DOI] [PubMed] [Google Scholar]
- 28. CDC. 2013. National Salmonella surveillance annual report A, GA, US Dept of Health and Human Services, CDC; http://wwwcdcgov/ncezid/dfwed/pdfs/salmonella-annual-report-2011-508cpdf. [Google Scholar]
- 29. Cherry WB, Biddle JW, Hanks JB, Thomason BM, Murlin AM, Croom JM. Salmonellae as an index of pollution of surface waters. Appl Microbiol. 1972;24(3):334–&. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heinitz ML, Ruble RD, Wagner DE, Tatini SR. Incidence of Salmonella in fish and seafood. J Food Prot. 2000;63(5):579–92. . [DOI] [PubMed] [Google Scholar]
- 31. Bairdparker AC. Foodborne salmonellosis. Lancet. 1990;336(8725):1231–5. . [DOI] [PubMed] [Google Scholar]
- 32. Borchardt MA, Chyou PH, DeVries EO, Belongia EA. Septic system density and infectious diarrhea in a defined population of children. Environ Health Perspect. 2003;111(5):742–8. Epub 2003/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brands DA, Inman AE, Gerba CP, Mare CJ, Billington SJ, Saif LA, et al. Prevalence of Salmonella spp. in oysters in the United States. Applied and Environmental Microbiology. 2005;71(2):893–7. Epub 2005/02/05. 10.1128/AEM.71.2.893-897.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Callaway TR, Keen JE, Edrington TS, Baumgard LH, Spicer L, Fonda ES, et al. Fecal prevalence and diversity of Salmonella species in lactating dairy cattle in four states. J Dairy Sci. 2005;88(10):3603–8. Epub 2005/09/16. 10.3168/jds.S0022-0302(05)73045-9 . [DOI] [PubMed] [Google Scholar]
- 35. Winfield MD, Groisman EA. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli . Applied and Environmental Microbiology. 2003;69(7):3687–94. Epub 2003/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rajabi M, Jones M, Hubbard M, Rodrick G, Wright AC. Distribution and genetic diversity of Salmonella enterica in the Upper Suwannee River. Int J Microbiol. 2011;2011:461321 Epub 2012/02/22. 10.1155/2011/461321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haley BJ, Cole DJ, Lipp EK. Distribution, diversity, and seasonality of waterborne salmonellae in a rural watershed. Applied and Environmental Microbiology. 2009;75(5):1248–55. Epub 2009/01/07. 10.1128/AEM.01648-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vereen E Jr., Lowrance RR, Jenkins MB, Adams P, Rajeev S, Lipp EK. Landscape and seasonal factors influence Salmonella and Campylobacter prevalence in a rural mixed use watershed. Water Res. 2013;47(16):6075–85. Epub 2013/08/24. 10.1016/j.watres.2013.07.028 . [DOI] [PubMed] [Google Scholar]
- 39. Li B, Vellidis G, Liu H, Jay-Russell M, Zhao S, Hu Z, et al. Diversity and antimicrobial resistance of Salmonella enterica isolates from surface water in southeastern United States. Applied and Environmental Microbiology. 2014;80(20):6355–65. Epub 2014/08/12. 10.1128/AEM.02063-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Census Bureau: State and County QuickFacts. Data derived from Population Estimates ACS, Census of Population and Housing, State and County Housing Unit Estimates, County Business Patterns, Nonemployer Statistics, Economic Census, Survey of Business Owners, Building Permits, Last Revised: Tuesday-J-E.
- 41. Bibby CJ, Burgess N. D., Hill D. A., and Mustoe S.. Bird Census Techniques, 2nd Edition Academic Press, London: 2000. [Google Scholar]
- 42. Hajna AA, Damon SR. New enrichment and plating media for the isolation of Salmonella and Shigella organisms. Appl Microbiol. 1956;4(6):341–5. Epub 1956/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waltman WD, Horne AM, Pirkle C, Dickson TG. Use of delayed secondary enrichment for the isolation of Salmonella in poultry and poultry environments. Avian Diseases. 1991;35(2029264):88–92. [PubMed] [Google Scholar]
- 44. Stone GG, Oberst RD, Hays MP, McVey S, Chengappa MM. Detection of Salmonella serovars from clinical samples by enrichment broth cultivation-PCR procedure. J Clin Microbiol. 1994;32(7):1742–9. Epub 1994/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hunter SB, Vauterin P, Lambert-Fair MA, Van Duyne MS, Kubota K, Graves L, et al. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol. 2005;43(3):1045–50. Epub 2005/03/08. 10.1128/JCM.43.3.1045-1050.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koort JM, Lukinmaa S, Rantala M, Unkila E, Siitonen A. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J Clin Microbiol. 2002;40(9):3497–8. Epub 2002/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3(1):59–67. Epub 2006/04/11. 10.1089/fpd.2006.3.59 . [DOI] [PubMed] [Google Scholar]
- 48. Zamperini K, Soni V, Waltman D, Sanchez S, Theriault EC, Bray J, et al. Molecular characterization reveals Salmonella enterica serovar 4,[5],12:i:- from poultry is a variant Typhimurium serovar. Avian Diseases. 2007;51(4):958–64. Epub 2008/02/07. . [DOI] [PubMed] [Google Scholar]
- 49. Beltran P, Plock SA, Smith NH, Whittam TS, Old DC, Selander RK. Reference collection of strains of the Salmonella typhimurium complex from natural populations. J Gen Microbiol. 1991;137(3):601–6. Epub 1991/03/01. . [DOI] [PubMed] [Google Scholar]
- 50. Dorea FC, Cole DJ, Hofacre C, Zamperini K, Mathis D, Doyle MP, et al. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Applied and Environmental Microbiology. 2010;76(23):7820–5. Epub 2010/10/05. 10.1128/AEM.01320-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liljebjelke KA, Hofacre CL, Liu T, White DG, Ayers S, Young S, et al. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodborne Pathog Dis. 2005;2(1):90–102. Epub 2005/07/05. 10.1089/fpd.2005.2.90 . [DOI] [PubMed] [Google Scholar]
- 52. Hudson CR, Quist C, Lee MD, Keyes K, Dodson SV, Morales C, et al. Genetic relatedness of Salmonella isolates from nondomestic birds in Southeastern United States. J Clin Microbiol. 2000;38(5):1860–5. Epub 2000/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meinersmann RJ, Berrang ME, Jackson CR, Fedorka-Cray P, Ladely S, Little E, et al. Salmonella, Campylobacter and Enterococcus spp.: their antimicrobial resistance profiles and their spatial relationships in a synoptic study of the Upper Oconee River basin. Microb Ecol. 2008;55(3):444–52. Epub 2007/08/10. 10.1007/s00248-007-9290-6 . [DOI] [PubMed] [Google Scholar]
- 54.Anonymous. Salmonella Annual Report 2010. wwwcdcgov/ncezid/pdfs/salmonella-annual-report-2010-508cpdf. 2013;Accessed 6/18/2014.
- 55.Anonymous. National Salmonella Surveillance Annual Report, 2011. wwwcdcgov/ncezid/dfwed/pdfs/salmonella-annual-report-2011-508cpdf. 2013;Accessed 6/18/2014.
- 56.Anonymous. Salmonella annual summary 2009. Centers for Disease Control and Prevention; National Salmonella Surveillance Data. 2009;http://www.cdc.gov/ncezid/PDFs/SalmonellaAnnualSummaryTables2009.pdf; Accessed 03/25/2013.
- 57. Thomas JL, Slawson RM, Taylor WD. Salmonella serotype diversity and seasonality in urban and rural streams. J Appl Microbiol. 2013;114(3):907–22. Epub 2012/11/22. 10.1111/jam.12079 . [DOI] [PubMed] [Google Scholar]
- 58. Walters SP, Gonzalez-Escalona N, Son I, Melka DC, Sassoubre LM, Boehm AB. Salmonella enterica diversity in central Californian coastal waterways. Applied and Environmental Microbiology. 2013;79(14):4199–209. Epub 2013/04/30. 10.1128/AEM.00930-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edrington TS, Schultz CL, Bischoff KM, Callaway TR, Looper ML, Genovese KJ, et al. Antimicrobial resistance and serotype prevalence of Salmonella isolated from dairy cattle in the southwestern United States. Microb Drug Resist. 2004;10(1):51–6. Epub 2004/05/14. 10.1089/107662904323047808 . [DOI] [PubMed] [Google Scholar]
- 60. Zhao S, McDermott PF, Friedman S, Abbott J, Ayers S, Glenn A, et al. Antimicrobial resistance and genetic relatedness among Salmonella from retail foods of animal origin: NARMS retail meat surveillance. Foodborne Pathog Dis. 2006;3(1):106–17. Epub 2006/04/11. 10.1089/fpd.2006.3.106 . [DOI] [PubMed] [Google Scholar]
- 61. Jokinen C, Edge TA, Ho S, Koning W, Laing C, Mauro W, et al. Molecular subtypes of Campylobacter spp., Salmonella enterica, and Escherichia coli O157:H7 isolated from faecal and surface water samples in the Oldman River watershed, Alberta, Canada. Water Res. 2011;45(3):1247–57. Epub 2010/10/26. 10.1016/j.watres.2010.10.001 . [DOI] [PubMed] [Google Scholar]
- 62. Liljebjelke KA, Hofacre CL, Liu TR, White DG, Ayers S, Young S, et al. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodborne Pathog Dis. 2005;2(1):90–102. . [DOI] [PubMed] [Google Scholar]
- 63. Vieira AR, Hofacre CL, Smith JA, Cole D. Human contacts and potential pathways of disease introduction on Georgia poultry farms. Avian Diseases. 2009;53(1):55–62. Epub 2009/05/13. . [DOI] [PubMed] [Google Scholar]
- 64. Pedroso AA, Hurley-Bacon AL, Zedek AS, Kwan TW, Jordan AP, Avellaneda G, et al. Can probiotics improve the environmental microbiome and resistome of commercial poultry production? Int J Environ Res Public Health. 2013;10(10):4534–59. Epub 2013/09/28. 10.3390/ijerph10104534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lockhart JM, Lee G, Turco J, Chamberlin L. Salmonella from gopher tortoises (Gopherus polyphemus) in south Georgia. J Wildl Dis. 2008;44(4):988–91. Epub 2008/10/30. 10.7589/0090-3558-44.4.988 . [DOI] [PubMed] [Google Scholar]
- 66. Hernandez E, Rodriguez JL, Herrera-Leon S, Garcia I, de Castro V, Muniozguren N. Salmonella Paratyphi B var Java infections associated with exposure to turtles in Bizkaia, Spain, September 2010 to October 2011. Euro Surveill. 2012;17(25). Epub 2012/07/04. . [PubMed] [Google Scholar]
- 67. Innocent GT, Mellor DJ, McEwen SA, Reilly WJ, Smallwood J, Locking ME, et al. Spatial and temporal epidemiology of sporadic human cases of Escherichia coli O157 in Scotland, 1996–1999. Epidemiol Infect. 2005;133(6):1033–41. Epub 2005/11/09. 10.1017/S0950268805003687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kistemann T, Zimmer S, Vagsholm I, Andersson Y. GIS-supported investigation of human EHEC and cattle VTEC O157 infections in Sweden: geographical distribution, spatial variation and possible risk factors. Epidemiol Infect. 2004;132(3):495–505. Epub 2004/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tyson A, Kelley L.A. Improving drinking water for the rural resident, Georgia Farm A-Syst/Home A-Syst. University of Georgia Coperative Extension Bulletin; 2012;1152–21. [Google Scholar]
- 70. Benjamin L, Atwill ER, Jay-Russell M, Cooley M, Carychao D, Gorski L, et al. Occurrence of generic Escherichia coli, E. coli O157 and Salmonella spp. in water and sediment from leafy green produce farms and streams on the Central California coast. Int J Food Microbiol. 2013;165(1):65–76. Epub 2013/05/24. 10.1016/j.ijfoodmicro.2013.04.003 . [DOI] [PubMed] [Google Scholar]
- 71. Gorski L, Parker CT, Liang A, Cooley MB, Jay-Russell MT, Gordus AG, et al. Prevalence, distribution, and diversity of Salmonella enterica in a major produce region of California. Applied and Environmental Microbiology. 2011;77(8):2734–48. 10.1128/AEM.02321-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abulreesh HH, Paget TA, Goulder R. Recovery of thermophilic campylobacters from pond water and sediment and the problem of interference by background bacteria in enrichment culture. Water Res. 2005;39(13):2877–82. Epub 2005/06/28. 10.1016/j.watres.2005.05.004 . [DOI] [PubMed] [Google Scholar]
- 73.Anonymous. An Atlas of Salmonella in the United States, 1968–2011. http://wwwcdcgov/salmonella/pdf/salmonella-atlas-508cpdf [Accessed: 05/02/2014]. 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.