Introduction
The study of human primary immunodeficiencies (PIDs) has identified factors critical for the development and function of the immune system [1]. While typical severe combined immunodeficiency (SCID) in humans is defined by a profound block in T lymphocyte production, with or without associated B and NK lymphocyte defects, more heterogeneous combined immunodeficiency (CID) disorders arise from gene defects that allow T lymphocytes to mature, but hinder their survival, release from the thymus into the periphery, or effector functions [2]. Individuals with CID may have both impairment of immune responses and defects in immune regulation; thus they may experience severe, recurrent and opportunistic infections as well as autoimmune disorders [3, 4].
Members of the coronin family of proteins are important regulators of the actin cytoskeleton, and mutations in CORO1A, encoding Coronin-1A, the predominant coronin expressed in lymphocytes, cause variable degrees of T cell lymphopenia, susceptibility to infection and immune dysregulation in mice and humans [5–7]. Monoclonal antibodies against Coronin-1A are being investigated as potential therapy for human autoimmune diseases and lymphoid malignancies [8, 9]. Here we report a new seventh case of human Coronin-1A deficiency and review our patient’s findings in the context of current knowledge.
History of Coronin-1A
Coronin was first identified by de Hostos and colleagues in 1991 through its association with F-actin in the crown-like structures of the slime mold Dictyostelium discoideum [10]. Several coronins with similar structure were subsequently discovered in mammals. Although originally associated with phosphoinositol-specific phospholipase (PI-PL) C [11], the similarity of Coronin-1A to the actin-binding coronin in Dictyostelium discoideum that is crucial for cell locomotion, phagocytosis, macropinocytosis and cytokinesis led to an initial focus on its cytoskeleton remodeling properties. Other roles have since become evident, among them Ca2+ mediated signaling via PLC-γ1 [12–14].
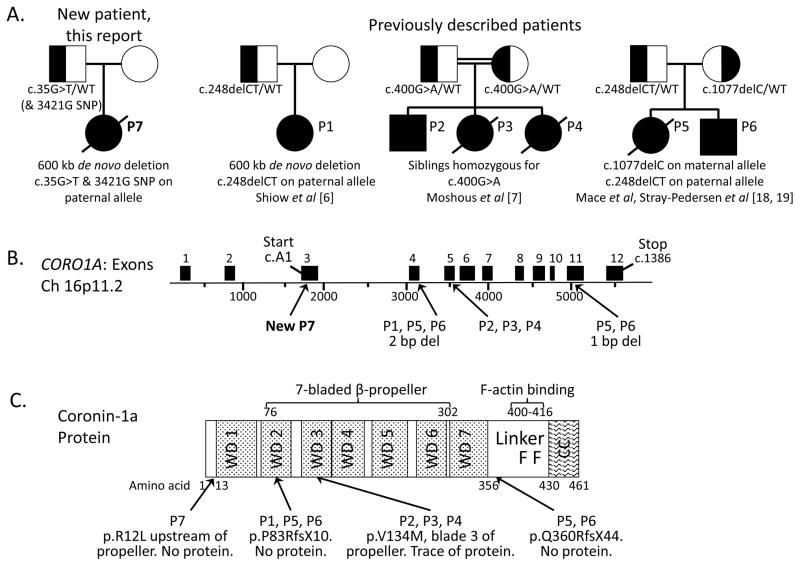
Studies of mammalian Coronin-1A began with the spontaneously occurring “peripheral T cell deficient” or Ptcd mouse [15]. Positional cloning revealed a Coro1a mutation underlying the failure of T cells of this mouse to exit the thymus, explaining their absence in the periphery despite intact thymic differentiation [5]. Several investigators then studied Coronin-1A knockout, hypomorphic and gain-of-function mice [5, 14, 16, 17]. Shiow et al. first identified a child with Coronin-1A deficiency whose phenotype echoed the mouse: few peripheral T cells despite a normal-sized thymus, with normal numbers of B and NK cells [6]. The T cell intrinsic nature of the human defect was demonstrated by immunologic cure by allogeneic hematopoietic cell transplantation (HCT). Additional Coronin-1A deficient patients have been reported [6, 7, 18, 19] (Fig. 1).
Figure 1.
A, Pedigrees of 4 families reported to date with CORO1A deficiency. Left: new patient P7; previously described patients are shown in order of publication. Note while Moshous et al [7] used mutation numbering 717G>A for P2, P3 and P4 corresponding to CORO1A transcript variant 2, NM_007074.3, we list all mutations here using the initial A of the first translated codon ATG as cDNA1 (variant 1, NM_001193333), per Shiow et al [6].
B, CORO1A gene locus indicating transcript NM_001193333 and positions of known disease-causing mutations.
C, Major structural domains of Coronin-1A protein, indicating mutation sites. WD, tryptophan-asparagine repeat region; linker domain, aa 356–429 containing positively charged residues 400–416 forming 2 F-actin binding sites [24]; CC, coiled-coil domain, aa 430–461, required for homo-trimerization.
Coronin-1A: structure, binding partners and mechanisms of action
Coronins contain multiple repeated motifs of about 40 amino acids that have “WD repeats” (single letter amino acid codes for tryptophan, W, and asparagine, D), similar to the β subunits of G proteins [20]. Previously known as p57, clabp (coronin-like actin binding protein) or TACO (tryptophan aspartate-containing coat protein), Coronin-1A is more highly expressed than other coronins in leukocytes [11, 21, 22]. It is a short, conventional coronin, with an N-terminal region with 7 WD repeats, a central linker, and a C-terminal coiled coil (CC) (Fig. 1C) [11, 20]. The WD regions form a 7-bladed β propeller [23] that mediates plasma membrane binding. Positively charged residues in the linker region form 2 potential F-actin-binding sites. The C-terminal extension contains a leucine zipper coiled-coil domain that mediates homo-trimerization and association with the cytoskeleton [24]. Thus Coronin-1A can link the plasma membrane to the actin cytoskeleton, directly or indirectly, inducing cytoskeletal remodeling in response to extracellular signals. This activity is important for signal transduction, migration, phagocytosis, and vesicle trafficking [25, 26].
In addition to binding F-actin, Coronin-1A also binds to the actin related protein (Arp) 2/3 complex [27]. While the Arp2/3 binding site of coronin in S. cerevisiae resides in the C-terminal linker and coiled coil domain [28, 29], its precise location in mammals is still undetermined. Coronin-1A freezes the Arp2/3 complex in its inactive conformation, preventing actin polymerization and further modulating cytoskeleton dynamics.
Association of Coronin-1A with the F-actin cytoskeleton was originally suggested as its mechanism to promote lymphocyte survival, activation and chemotaxis [17, 24]. However, further analysis revealed a perhaps more crucial role in mediating the release of intracellular Ca2+ ions through interaction with PLC-γ1 [12–14]. Defects in both survival and migration occur due to defective signaling and calcineurin activation in Coronin-1A-deficient naïve T cells [14, 30].
Coronin-1A in association with cofilin and actin-interacting protein 1 (Aip1), is also involved in the disassembly of actin filaments [31], perhaps because its coiled-coil domain blocks the binding of cofilin to freshly polymerized actin while allowing older filaments to be degraded [32]. One model suggests that Coronin-1A and -1B together coordinate Arp2/3 assembly and actin depolymerizing factor (ADF)/cofilin disassembly, enhancing the flux of actin through the filament assembly/disassembly cycle [33, 34]. Finally, translocation of Rac1 to the plasma membrane also involves Coronin-1A, which in concert with other proteins, including ArhGEF7, Pak1 and RhoGD1α, sets up a relay mechanism to amplify Rac1 signals in response to F-actin polymerization or other changes induced by engagement of cell surface receptors and co-stimulatory molecules [35].
Thus, while Coronin-1A is clearly important for cytoskeleton remodeling and calcium signaling, the precise sequence of intracellular events is yet undetermined; it is unknown whether Coronin-1A-mediated Ca2+ release causes reorganization of the cytoskeleton or vice versa [36].
Mouse models: knockouts and gain-of-function mutants
Analysis of mice with both knockout and gain-of-function point mutations in the Coro1a gene has delineated the role of Coronin-1A in various stages of T cell development, [5, 14, 16, 17]. In all knockout mice the mature single positive T cells in the thymus and periphery were maximally affected, while immature double negative (DN) and double positive (DP) thymocytes remained unaffected, correlating with expression levels of the Coronin-1A protein. Notwithstanding the continuing disagreement on the mode of action of the protein, all studies have shown that Coronin-1A was essential for TCR-mediated proliferation, IL-2 production, constitutive activation of the JNK signaling pathway, decreased phosphorylation of IκB upon stimulation, progression of T cells through the cell cycle, and resistance to apoptosis [14, 16, 17]. Furthermore, down-regulation of Coronin-1A and the Arp2/3 complex by GRAIL resulted in the induction of T cell anergy, suggesting a role for Coronin-1A in modulation of T cell signaling [37].
While Coronin-1 deficient mice showed no increased susceptibility to effects of LCMV infection, they were highly susceptible to VSV infection, attributed to a delay in CD4+ helper T cell function and helper-dependent IgG responses [38].
Despite their immune defects, Coronin-1 deficient mice have been fertile and healthy [13, 17]. However, Coronin-1 is expressed at excitatory neuronal synapses, where it modulates the cAMP/PKA pathway [39], and mice lacking Coronin-1 displayed behavioral abnormalities including increased aggression, as well as defective learning and memory [39]. Further roles for Coronin-1 may emerge.
Coronin-1A and human immunodeficiency
Previously, 6 patients from 3 families have been reported to have CID associated with mutations in CORO1A (Fig. 1, Table 1) [6, 7, 18, 19]. Here, we describe a seventh patient with a novel CORO1A mutation as well as the second instance with a chromosome 16p11.2 de novo deletion. This female was born at term after an uneventful pregnancy to healthy non-consanguineous parents with no family history of immunodeficiency, early death or miscarriage, though a half brother had a seizure disorder. Newborn screening for SCID with a TREC test was not performed. During her first year she experienced oral thrush, severe diaper dermatitis, pneumonia and 10 episodes of otitis. She also exhibited seizures and delayed development of gross motor skills and language. She received routine killed vaccinations through 9 months of age (not live rotavirus vaccine), and at 12 months live viral vaccines were omitted due to illness. At 14 months she presented with a 7-week history of fever, lymphadenopathy and night sweats. Her chest radiograph and chest computerized tomography scan revealed no infiltrates, and thymic tissue was present. She had CD4+ and CD8+ T cell lymphopenia, nearly absent naïve T cells, nearly undetectable T-cell receptor excision circles (TRECs), and no evidence of maternal T cell engraftment (Table 1). CD19+ B cells and CD16/56+ NK cells were present; she had low serum IgG, but normal IgM and IgA. Her initial serum Epstein-Barr virus (EBV) PCR revealed a viral load of 28,870 copies/mL (low positive: 10,000–100,000). Cervical lymph node biopsy showed EBV-associated diffuse large B-cell lymphoma (CD20[bright], PAX-5+ and BCL2+; negative for CD5, CD10 and surface immunoglobulin light chains). The lymphoma cells had a Ki-67 proliferation index of 70% and an EBV type III latency pattern (positive for EBER, LMP1 and EBNA-2). Cytogenetic analysis demonstrated a normal female karyotype, but molecular testing showed a clonal IGH gene rearrangement with no clonal TCR rearrangement. Despite antibiotics, acyclovir, intravenous immunoglobulin, rituximab, and chemotherapy that included low-intensity preconditioning for a planned HCT, she developed severe gastrointestinal bleeding and fatal respiratory failure. Autopsy revealed lymphoma involving the central nervous system, gastrointestinal tract, liver, lungs, and thymus.
Table 1.
Characteristics of individual Coronin-1A deficient patients, listed within sibships, and findings common to all 7 cases.
| Feature1 | New Patient P7 (this report) | Previous published reports | Common findings | ||
|---|---|---|---|---|---|
| P1 [5,6] | P2, P3, P4 [7] | P5, P6 [18,19] | |||
| Age at immune evaluation, sex | 14 mo F | 15 mo F | P2, 12 mo M; P3, 7.5 mo F; P4, 14 mo F | P5, 7 yr F; P6, 7 yr M | CID, onset of infections in infancy |
| Genotype | R12L/del16p11 | c248delCT/del16p11 | V134M/V134M | c248delCT/Q360RfsX44 | Interstitial del 16p11; recessive variants |
| Outcome | Died of lymphoma prior to HCT2 | Alive and well after HCT | P2 alive, no HCT; P3 died prior to HCT; P4 died with HCT | P5 died with HCT; P6 alive, HCT planned | HCT can cure otherwise lethal infections and organ damage |
| Immunoglobulins (mg/dL) | |||||
| IgG (762±209) | 50 | NA3 (on IVIG) | Normal | Normal | Low - normal |
| IgM (58±23) | 151 | 46 | Normal | P5 low; P6 normal | Low - normal |
| IgA (50±24) | 60 | 42 | Normal | P5 low; P6 normal | Low - normal |
| Lymphocyte subsets (cells/uL) | |||||
| CD3 T (2100–6200) | 98 | 110 | Low | Low | Low |
| CD4 T (1300–3400) | 39 | 50 | Low | Low | Low |
| CD8 T (620–2000) | 49 | 30 | Low | Low | Low |
| CD45RA T | Nearly absent | Low | Low | Very low | Low |
| CD45RO T (<65%) | 90% | NA | 98% | NA | High |
| TRECs at diagnosis (>33/μl) | 2 (normal PCR control) | NA | NA | NA | Low |
| CD19 B (300–2600) | 59 | 410 | Low | Low | Low - normal |
| CD16/56 NK (>200) | 121 | 340 | Normal | Low | Low - normal |
| NK cell function | Normal | NA | NA | Low | Low - normal |
| Mitogen response (% of normal) | |||||
| PHA | <10% | 10–30% | Low-normal | Low | Low - normal |
| PWM | <10% | <10% | NA | Low - normal | Low - normal |
| ConA | <10% | <10% | NA | Low | Low |
| Antibody production after vaccination | |||||
| Tetanus (>0.15 IU/ml) | 1.65 | 0.02 | Normal | Normal | Poor specific antibody responses |
| H. influenzae B (>1.0 IU/ml) | NA | 0.7 | <1.0 | NA | |
| S pneumoniae (serotypes protective) | 0 post 13-valent conjugate vaccine | NA | P2, P3 NA; P4 normal | Low - normal | |
| Other studies | |||||
| Epstein-Barr virus | >25,000 copies; EBV+B cell lymphoma at 14 mo | NA | P4, EBV+ B cell lymphoproliferation, lymphoma at 12 mo | P5, stage IV Hodgkin lymphoma and diffuse large B cell lymphoma, both EBV+ | Severe EBV infections, early onset EBV+ lymphoma |
| TCR Vβ | High CD8 Vβ5.1 | NA | P2, high Vβ4; P2 and P4, low TCR diversity | Abnormal TCR | Limited TCR diversity |
Normal ranges for ages 12–24 months in parentheses.
HCT, hematopoietic cell transplant.
NA, not available
A genomic DNA copy number array study revealed a heterozygous interstitial deletion of chromosome 16p11.2, the region containing CORO1A. Further testing demonstrated a hemizygous CORO1A missense mutation c.35G>T, (Fig. 1), resulting in an arginine to leucine transversion, p.R12L, in a highly species-conserved region immediately preceding the first WD domain (amino acids 13–63). The mutation was predicted to be damaging [40], and indeed, no Coronin-1A protein was detected in an EBV transformed cell line from the patient, as in the first Coronin-1A deficient patient described by Shiow et al [6] (data not shown). Our hemizygous patient’s mutant allele harbored a coding SNP that was absent in the mother, whose CORO1A copy number was normal diploid and whose sequence had no mutations. Therefore the patient’s CORO1A SNP and missense mutation were likely to be paternally derived, with her deletion arising de novo on her maternal allele.
All Coronin-1A deficient patients to date have had a T(−/low), B(low/+), NK(low/+) phenotype (Table 1). Lymphocyte proliferative responses to mitogen stimulation were impaired, but not absent. Serum immunoglobulins were detectable, but specific antibody titers following vaccines were low to absent, except against tetanus toxoid. The patients showed markedly reduced naïve peripheral blood T cells, and where measured, TRECs were nearly undetectable. However, in contrast to typical SCID, all Coronin-1A deficient patients had thymic tissue detected by imaging, despite profound peripheral T lymphopenia. All suffered from upper respiratory tract infections; and inability to control EBV was a prominent feature, associated with fatal lymphoproliferative syndrome and lymphoma at a particularly young age.
Our newly reported patient P7, like P1, the original patient of Shiow et al [6], had a complete loss of Coronin-1A protein expression (data not shown). Each had a de novo hemizygous 600 kb deletion of over 30 genes on chromosome 16p along with a missense mutation or 2 nucleotide frame-shifting deletion of the retained CORO1A allele (Fig. 1). In both of these patients, as well as P5 and P6 [19], whose paternally derived deletion c.248delCT was identical to that in P1, early frameshifts leading to truncation codons were predicted to cause nonsense mediated decay of mRNA and therefore no detectable protein. P5 and P6 also had a novel CORO1A microdeletion, c.1077delC, leading to replacement of glutamine 360 in the C-terminal extension with an arginine and premature truncation, p.Q360RfsX44 (Fig. 1B, 1C). Loss of the coiled coil domain, required for stability [23], explains the lack of protein in P6. The 3 siblings P2, P3 and P4 (Fig. 1), described by Moshous et al [7, 41], were homozygous for a missense mutation, c.400G>A, causing transversion to methionine of an evolutionarily conserved valine (p.V134M) in the WD2 β-propeller region. While the mutation did not affect transcription of CORO1A mRNA, very low amounts of protein were detectable in the cells of these patients [7].
All affected individuals had multiple ear, sinus and respiratory infections. Our patient P7, siblings P2, P3 and P4 and patient P5 developed EBV-induced B-cell lymphoproliferative syndrome, and 2 patients developed B-cell lymphomas before reaching the age of 2 years. While patients with other PIDs such as X-linked lymphoproliferative syndrome, Wiskott-Aldrich syndrome, autoimmune lymphoproliferative syndrome, CD27 deficiency and other PIDs also are at risk for developing uncontrolled lymphoproliferation and lymphoma following EBV infection [42], the Coronin-1A deficient patients are the youngest to be described to our knowledge. On the other hand, unlike P1, the siblings with missense mutation and possible residual Coronin-1 activity accounting for their later onset and milder phenotype (P2, P3 and P4) did not develop chickenpox vesicles following live attenuated varicella vaccination [41].
P5 and P6, like all the others, had profound T cell lymphopenia, suffered from opportunistic viral infections, and had low NK and absent memory B cells [18, 19]. They also had shortened telomeres and low mucosal invariant T cells, findings not previously associated with Coronin-1A deficiency. Haploinsufficiency for Coronin-1A does not result in detectable immune deficiency. However the father of patients P5 and P6 (carrying c.248delCT/WT) had reduced T cell numbers, while the mother (c.1077delC/WT), had reduced NK cells [19].
Human NK cell lines in which Coronin-1A was knocked-down in vitro had decreased cytotoxic function. Immune synapses and granule polarization occurred, but increased synaptic density of F-actin caused the granules to be inappropriately positioned at the synapse, accounting for decreased efficiency of NK ‘initial kill’ [18]. Analysis of NK cell function in P6 (c.248delCT/1077delC) also showed insufficient F-actin deconstruction at the synapse, resulting in fewer ‘permissive clearances’ for lytic granules and therefore reduced NK cell degranulation [18].
While the mode of action of human Coronin-1A is not completely known, the T cell lymphopenia observed in the Coronin-1A-deficient patients, despite the presence of a thymus [6, 7, 18, 19], indicates that Coronin-1A deficiency might result in both impaired central T cell development due to compromised thymic maturation of SP cells and increased apoptosis [44] as well as peripheral defects, including unresponsiveness to TCR ligation, reduced migration in response to chemokines and increased apoptosis [4].
HCT was curative in 2 patients, who survived and became infection-free (HCT was planned for P6 at the time of publication [19]). The post-HCT patients, however, had mild to substantial neurological abnormalities [6, 7]. In P1 this may be explained by the 16p11.2 chromosomal deletion, known to be associated with autism [45, 46]. Patient P2 is in a highly consanguineous family in which other recessive neurodevelopmental genetic impairments may be segregating [7]. However, Coronin-1A may play a role in human neurodevelopment, as it does in mice, as described above. Data from additional Coronin-1A deficient patients will be needed to clarify this.
Coronin-1A as a therapeutic target in autoimmune and autoactivation disorders and leukemia
Although not yet observed in humans, mutations in Coro1a in mice impart protection from the development of autoimmune diseases. The role of Coronin-1A in modulating autoimmunity was first uncovered when a search for genetic loci associated with systemic lupus erythematosus (SLE) revealed a nonsense mutation in the Coro1a gene to be lupus suppressing [16]. Further studies with Coronin-1A deficient mice showed that immunization with a myelin oligodendrocyte glycoprotein (MOG) peptide induced only mild symptoms of experimental autoimmune encephalomyelitis (EAE) in the Coro1a−/− mice, compared to wild type controls [47, 48]. This could be due to either a reduced pool of disease-inducing naïve T cells in the Coro1a−/− mice, or the migration defect of Coronin-1A deficient T cells [47, 48]. However, following repeated immunizations with the MOG peptide, Coro1a−/− mice developed even more pronounced disease than did wild type mice. Coronin-1A was implicated in regulation of Th17 CD4+ T cells by the finding that Coro1a−/− Th17 cells produced increased amounts of IL-17 cytokine, which is crucial for the development of EAE. The authors hypothesized that Coronin-1A acts as a negative regulator of IL-17 production through the SMAD3-mediated TGF-β signaling pathway [47].
Immunohistochemistry of brain samples from patients suffering from multiple sclerosis (MS) showed the presence of Coronin-1A expressing T cells and macrophages in active MS lesions, along with increased MHC class-II and reduced proteolipid protein [8]. Coronin-1A was recognized by MS patient cerebrospinal fluid (CSF) antibodies reactive to the peptide UH-CIS6 [49], suggesting Coronin-1A as a novel antibody target in the treatment of MS [8].
In vitro experiments with an anti-Coronin-1A monoclonal antibody ZCH-2B8a demonstrated complement mediated cytotoxicity against human acute lymphoblastic leukemia and other B-lineage leukemia cell lines, showing potential as a therapy for B cell malignancies [9]. Further experiments with the same antibody showed that expression of Coronin-1A was increased in activated T cells, which might therefore also be preferentially targeted with ZCH-2B8a. Coronin-1A was also overexpressed in T cells from patients with aplastic anemia and hemophagocytic lymphohistiocytosis compared to controls, suggesting that, beyond treating B cell malignancies, an antibody targeting Coronin-1A could be used to treat immune disorders mediated by activated T cells [50].
Summary
Since the discovery of coronins in 1991 significant research has been carried out to understand their molecular structure and cellular mechanisms of the action. While a number of binding partners have been discovered, the precise mechanisms of action of Coronin-1A are still being elucidated, both in vitro and in vivo. The role of Coronin-1A in the development and function of the immune system is irrefutable, in both humans and mice, and deficiency of Coronin-1A results in CID. Although some immunological manifestations of Coronin-1A deficiency differed between the patients described so far, absence of Coronin-1A affected the T cell compartment in all patients. B cell numbers were lower than normal and antibody responses were impaired. Variable NK cell defects associated with absent Coronin-1A to date will require detailed analysis of further patients. HCT was curative for patients with Coronin-1A deficiency when the disease was diagnosed early, before onset of irreversible complications arising from infections and EBV associated malignancy.
With new evidence about the potential of anti-Coronin-1A monoclonal antibodies to treat B cell malignancies and T cell-mediated auto-inflammatory diseases, Coronin-1A can now be said to be involved in the overall regulation of the immune system, and inappropriate expression can lead to either immune deficiency or autoimmunity.
References
- 1.Milner JD, Holland SM. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nature reviews Immunology. 2013;13(9):635–48. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- 2.Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers in immunology. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liston A, Enders A, Siggs OM. Unravelling the association of partial T-cell immunodeficiency and immune dysregulation. Nature reviews Immunology. 2008;8(7):545–58. doi: 10.1038/nri2336. [DOI] [PubMed] [Google Scholar]
- 4.Notarangelo LD. Functional T cell immunodeficiencies (with T cells present) Annual review of immunology. 2013;31:195–225. doi: 10.1146/annurev-immunol-032712-095927. [DOI] [PubMed] [Google Scholar]
- 5.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, et al. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nature immunology. 2008;9(11):1307–15. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM. Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clinical immunology. 2009;131(1):24–30. doi: 10.1016/j.clim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moshous D, Martin E, Carpentier W, Lim A, Callebaut I, Canioni D, et al. Whole-exome sequencing identifies Coronin-1A deficiency in 3 siblings with immunodeficiency and EBV-associated B-cell lymphoproliferation. The Journal of allergy and clinical immunology. 2013;131(6):1594–603. doi: 10.1016/j.jaci.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouwette M, Noben JP, Van Horssen J, Van Wijmeersch B, Hupperts R, Jongen PJ, et al. Identification of coronin-1a as a novel antibody target for clinically isolated syndrome and multiple sclerosis. Journal of neurochemistry. 2013;126(4):483–92. doi: 10.1111/jnc.12335. [DOI] [PubMed] [Google Scholar]
- 9.Xu XJ, Tang YM, Zhao HZ, Guo L, Wang ZJ. ZCH-2B8a, an antibody targeting actin-binding protein coronin-1a, is a potential therapeutic agent for B-lineage malignancies. Journal of drug targeting. 2014 doi: 10.3109/1061186X.2014.888072. [DOI] [PubMed] [Google Scholar]
- 10.de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. The EMBO journal. 1991;10(13):4097–104. doi: 10.1002/j.1460-2075.1991.tb04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki K, Nishihata J, Arai Y, Honma N, Yamamoto K, Irimura T, et al. Molecular cloning of a novel actin-binding protein, p57, with a WD repeat and a leucine zipper motif. FEBS letters. 1995;364(3):283–8. doi: 10.1016/0014-5793(95)00393-n. [DOI] [PubMed] [Google Scholar]
- 12.Jayachandran R, Gatfield J, Massner J, Albrecht I, Zanolari B, Pieters J. RNA interference in J774 macrophages reveals a role for coronin 1 in mycobacterial trafficking but not in actin-dependent processes. Molecular biology of the cell. 2008;19(3):1241–51. doi: 10.1091/mbc.E07-07-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130(1):37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 14.Mueller P, Massner J, Jayachandran R, Combaluzier B, Albrecht I, Gatfield J, et al. Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nature immunology. 2008;9(4):424–31. doi: 10.1038/ni1570. [DOI] [PubMed] [Google Scholar]
- 15.Yagi H, Matsumoto M, Nakamura M, Makino S, Suzuki R, Harada M, et al. Defect of thymocyte emigration in a T cell deficiency strain (CTS) of the mouse. Journal of immunology. 1996;157(8):3412–9. [PubMed] [Google Scholar]
- 16.Haraldsson MK, Louis-Dit-Sully CA, Lawson BR, Sternik G, Santiago-Raber ML, Gascoigne NR, et al. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008;28(1):40–51. doi: 10.1016/j.immuni.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foger N, Rangell L, Danilenko DM, Chan AC. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science. 2006;313(5788):839–42. doi: 10.1126/science.1130563. [DOI] [PubMed] [Google Scholar]
- 18.Mace EM, Orange JS. Lytic immune synapse function requires filamentous actin deconstruction by Coronin 1A. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(18):6708–13. doi: 10.1073/pnas.1314975111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stray-Pedersen A, Jouanguy E, Crequer A, Bertuch AA, Brown BS, Jhangiani SN, et al. Compound Heterozygous CORO1A Mutations in Siblings with a Mucocutaneous-Immunodeficiency Syndrome of Epidermodysplasia Verruciformis-HPV, Molluscum Contagiosum and Granulomatous Tuberculoid Leprosy. Journal of clinical immunology. 2014;34(7):871–90. doi: 10.1007/s10875-014-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Hostos EL. The coronin family of actin-associated proteins. Trends in cell biology. 1999;9(9):345–50. doi: 10.1016/s0962-8924(99)01620-7. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97(4):435–47. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 22.Nal B, Carroll P, Mohr E, Verthuy C, Da Silva MI, Gayet O, et al. Coronin-1 expression in T lymphocytes: insights into protein function during T cell development and activation. International immunology. 2004;16(2):231–40. doi: 10.1093/intimm/dxh022. [DOI] [PubMed] [Google Scholar]
- 23.Appleton BA, Wu P, Wiesmann C. The crystal structure of murine coronin-1: a regulator of actin cytoskeletal dynamics in lymphocytes. Structure. 2006;14(1):87–96. doi: 10.1016/j.str.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Gatfield J, Albrecht I, Zanolari B, Steinmetz MO, Pieters J. Association of the leukocyte plasma membrane with the actin cytoskeleton through coiled coil-mediated trimeric coronin 1 molecules. Molecular biology of the cell. 2005;16(6):2786–98. doi: 10.1091/mbc.E05-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rybakin V, Clemen CS. Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. BioEssays : news and reviews in molecular, cellular and developmental biology. 2005;27(6):625–32. doi: 10.1002/bies.20235. [DOI] [PubMed] [Google Scholar]
- 26.Uetrecht AC, Bear JE. Coronins: the return of the crown. Trends in cell biology. 2006;16(8):421–6. doi: 10.1016/j.tcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, et al. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. The Biochemical journal. 1997;328 (Pt 1):105–12. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphries CL, Balcer HI, D’Agostino JL, Winsor B, Drubin DG, Barnes G, et al. Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. The Journal of cell biology. 2002;159(6):993–1004. doi: 10.1083/jcb.200206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodal AA, Sokolova O, Robins DB, Daugherty KM, Hippenmeyer S, Riezman H, et al. Conformational changes in the Arp2/3 complex leading to actin nucleation. Nature structural & molecular biology. 2005;12(1):26–31. doi: 10.1038/nsmb870. [DOI] [PubMed] [Google Scholar]
- 30.Mueller P, Liu X, Pieters J. Migration and homeostasis of naive T cells depends on coronin 1-mediated prosurvival signals and not on coronin 1-dependent filamentous actin modulation. Journal of immunology. 2011;186(7):4039–50. doi: 10.4049/jimmunol.1003352. [DOI] [PubMed] [Google Scholar]
- 31.Kueh HY, Charras GT, Mitchison TJ, Brieher WM. Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers. The Journal of cell biology. 2008;182(2):341–53. doi: 10.1083/jcb.200801027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhi M, Achard V, Blanchoin L, Goode BL. Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Molecular cell. 2009;34(3):364–74. doi: 10.1016/j.molcel.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan KT, Creed SJ, Bear JE. Unraveling the enigma: progress towards understanding the coronin family of actin regulators. Trends in cell biology. 2011;21(8):481–8. doi: 10.1016/j.tcb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brieher WM, Kueh HY, Ballif BA, Mitchison TJ. Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. The Journal of cell biology. 2006;175(2):315–24. doi: 10.1083/jcb.200603149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro-Castro A, Ojeda V, Barreira M, Sauzeau V, Navarro-Lerida I, Muriel O, et al. Coronin 1A promotes a cytoskeletal-based feedback loop that facilitates Rac1 translocation and activation. The EMBO journal. 2011;30(19):3913–27. doi: 10.1038/emboj.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieters J, Muller P, Jayachandran R. On guard: coronin proteins in innate and adaptive immunity. Nature reviews Immunology. 2013;13(7):510–8. doi: 10.1038/nri3465. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa D, Mizuno M, Yamamura T, Miyake S. GRAIL (gene related to anergy in lymphocytes) regulates cytoskeletal reorganization through ubiquitination and degradation of Arp2/3 subunit 5 and coronin 1A. The Journal of biological chemistry. 2011;286(50):43465–74. doi: 10.1074/jbc.M111.222711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchang VS, Mekker A, Siegmund K, Karrer U, Pieters J. Diverging role for coronin 1 in antiviral CD4+ and CD8+ T cell responses. Molecular immunology. 2013;56(4):683–92. doi: 10.1016/j.molimm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Jayachandran R, Liu X, Bosedasgupta S, Muller P, Zhang CL, Moshous D, et al. Coronin 1 regulates cognition and behavior through modulation of cAMP/protein kinase A signaling. PLoS biology. 2014;12(3):e1001820. doi: 10.1371/journal.pbio.1001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7(4):248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moshous D, de Villartay JP. The Expanding Spectrum of Human coronin 1A deficiency. Current allergy and asthma reports. 2014;14(12):481. doi: 10.1007/s11882-014-0481-1. [DOI] [PubMed] [Google Scholar]
- 42.Parvaneh N, Filipovich AH, Borkhardt A. Primary immunodeficiencies predisposed to Epstein-Barr virus-driven haematological diseases. British journal of haematology. 2013;162(5):573–86. doi: 10.1111/bjh.12422. [DOI] [PubMed] [Google Scholar]
- 43.Mugnier B, Nal B, Verthuy C, Boyer C, Lam D, Chasson L, et al. Coronin-1A links cytoskeleton dynamics to TCR alpha beta-induced cell signaling. PloS one. 2008;3(10):e3467. doi: 10.1371/journal.pone.0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogquist KA. Immunodeficiency: when T cells are stuck at home. Nature immunology. 2008;9(11):1207–8. doi: 10.1038/ni1108-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. The New England journal of medicine. 2008;358(7):667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 46.Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(41):17076–81. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaminski S, Hermann-Kleiter N, Meisel M, Thuille N, Cronin S, Hara H, et al. Coronin 1A is an essential regulator of the TGFbeta receptor/SMAD3 signaling pathway in Th17 CD4(+) T cells. Journal of autoimmunity. 2011;37(3):198–208. doi: 10.1016/j.jaut.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Siegmund K, Zeis T, Kunz G, Rolink T, Schaeren-Wiemers N, Pieters J. Coronin 1-mediated naive T cell survival is essential for the development of autoimmune encephalomyelitis. Journal of immunology. 2011;186(6):3452–61. doi: 10.4049/jimmunol.1003491. [DOI] [PubMed] [Google Scholar]
- 49.Rouwette M, Somers K, Govarts C, De Deyn PP, Hupperts R, Van Wijmeersch B, et al. Novel cerebrospinal fluid and serum autoantibody targets for clinically isolated syndrome. Journal of neurochemistry. 2012;123(4):568–77. doi: 10.1111/jnc.12005. [DOI] [PubMed] [Google Scholar]
- 50.Xu XJ, Tang YM. Coronin-1a is a potential therapeutic target for activated T cell-related immune disorders. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2014 doi: 10.1111/apm.12277. [DOI] [PubMed] [Google Scholar]