Abstract
Background
Over 25 drugs have been approved for the treatment of HIV-1 replication. All but one of these drugs is delivered as an oral medication. Previous studies have demonstrated that two drugs, decitabine and gemcitabine, have potent anti-HIV-1 activities and can work together in synergy to reduce HIV-1 infectivity via lethal mutagenesis. For their current indications, decitabine and gemcitabine are delivered intravenously.
Methods
As an initial step towards the clinical translation of these drugs for the treatment of HIV-1 infection, we synthesized decitabine and gemcitabine prodrugs in order to increase drug permeability, which has generally been shown to correlate with increased bioavailability in vivo. In the present study we investigated the permeability, stability and anti-HIV-1 activity of decitabine and gemcitabine prodrugs and selected the divalerate esters of each as candidates for further investigation.
Results
Our results provide the first demonstration of divalerate prodrugs of decitabine and gemcitabine that are readily permeable, stable and possess anti-HIV-1 activity.
Conclusions
These observations predict improved oral availability of decitabine and gemcitabine, and warrant further study of their ability to reduce HIV-1 infectivity in vivo.
Introduction
There are currently six classes of drugs approved to treat individuals infected with HIV-1. A combination of at least two to three classes of anti-HIV-1 drugs are typically used to suppress viral replication and decrease the emergence of drug-resistant virus. Despite the use of combination antiretroviral therapy, drug-resistant virus can emerge when active drug levels do not sufficiently suppress virus replication. Once drug resistance emerges, transmission of drug-resistant virus represents a significant public health issue. The presence and transmission of drug-resistant HIV-1 emphasizes the need for the development of novel anti-HIV-1 drugs that exploit new drug targets. One potential drug target that has yet to be exploited clinically is the mutation rate of HIV-1.
The high mutation rate of HIV-1 (3.4×10−5 mutations/base pair/replication cycle) [1] enables the virus to evade the immune system and to become resistant to drug therapy. However, this high mutation rate also leads to the production of a high proportion of non-infectious virus. Lethal mutagenesis is a strategy that uses chemotherapeutics to increase the mutation rate to the point where the virus is unable to replicate with enough fidelity to remain infectious [2,3].
We previously described the combination of two clinically approved nucleoside analogues, decitabine and gemcitabine, that appears to decrease viral infectivity by lethal mutagenesis in cell culture [4]. Decitabine is used clinically to treat myelodysplastic syndrome (MDS) [5], while gemcitabine is used to treat pancreatic, ovarian, lung and breast cancers [6]. Decitabine is used clinically for its ability to inhibit DNA methyltransferase activity, which alters DNA methylation and gene expression [7,8]. In contrast, gemcitabine inhibits ribonucleotide reductase, which reduces dNTP pools and can decrease cell proliferation [9–11].
We previously demonstrated that the combination of decitabine and gemcitabine inhibited viral replication at non-toxic doses in mice infected with LP-BM5 [12], a retrovirus that induces an AIDS-like syndrome in mice [13–15]. Furthermore, the doses of decitabine and gemcitabine required to demonstrate antiviral activity were well below those used clinically for the treatment of MDS and solid cancers, respectively. These data suggest that decitabine and gemcitabine could be repositioned as antiretroviral agents. However, due to poor bioavailability, decitabine and gemcitabine must be administered intravenously in order to achieve a plasma concentration high enough to treat their respective conditions. While it is possible that high doses of the drugs could be given orally multiple times per day in order to achieve a sustained antiviral effect, such a dosing regimen would likely lead to poor patient compliance which would facilitate the emergence of drug resistant virus. Thus, the low bioavailability of gemcitabine and decitabine limits their use as antiretroviral agents.
The low bioavailability of gemcitabine and decitabine has been attributed to low intestinal permeability, as well as their rapid deamination by cytidine deaminase, an enzyme that is highly expressed in the liver and intestines. Additionally, rapid phosphorylation of the 5′-hydroxyl group of gemcitabine in the liver tissues results in its accumulation in the liver and kidney thereby preventing its distribution in target tissues [16,17]. Several approaches have been used to improve the bioavail-ability and pharmacokinetic properties of decitabine and gemcitabine, including prodrugs [16–21]. Prodrug approaches have been used to increase intestinal permeability and to minimize phosphorylation and retention by the liver and kidneys [17]. In addition to prodrugs, tetrahydrouridine, an orally available inhibitor of cyti-dine deaminase, has been used to prevent deamination of decitabine and gemcitabine, which resulted in an increase in the bioavailability of decitabine [22] and an increase in the half-life of gemcitabine [23]. Despite the multiple approaches to improve the bioavailability and pharmacokinetic properties of decitabine and gemcitabine, prodrug derivatives of gemcitabine and decitabine have yet to be FDA approved.
Here, we describe chemically stable and biologically active decitabine and gemcitabine prodrugs that demonstrate improved permeability in the Caco-2 model of oral availability compared to the parent drugs. Additionally, both prodrugs were stable at a wide pH range and retained biological activity. These observations predict improved oral availability of decitabine and gemcitabine and warrant further studies of these prodrugs in vivo.
Materials and methods
General chemistry methodologies
All commercial reagents (Sigma–Aldrich [St Louis, MO, USA], Acros [Geel, Belgium], Berry and 224 Associates [Dexter, MI, USA] and Carbosynth [Camp-ton, Berkshire, UK]) were used as provided unless otherwise indicated. Flash chromatography was performed with Ultra Pure silica gel (Silicycle [Quebec City, QC, Canada) with the indicated solvent system. Semi-preparative HPLC was performed on an Agilent Technologies (Santa Clara, CA, USA) 1200 series instrument with a Grace VisionHT C18 classic 5 μ column at a flow rate of 5 ml/min (Mobile Phase A: buffer 0.01 M ammonium formate, pH adjusted to 4.5 with formic acid; B: acetonitrile). Nuclear magnetic resonance spectra were recorded on a Varian 600 MHz (Varian Medical Systems, Inc. [Palo Alto, CA, USA]) with Me4Si or signals from residual solvent as the internal standard for 1H. Chemical shifts were reported in ppm, and signals were described as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), bs (broad singlet) and dd (double doublet). High-resolution mass spectra were recorded on an Agilent TOF/MS instrument equipped with a multimode ionization source.
Synthesis of decitabine and gemcitabine diester prodrugs
Decitabine was obtained from Moravek (Brea, CA, USA) and gemcitabine was purchased from Tocris (Bristol, UK). Decitabine or gemcitabine (1 mmol, 1 eq) was diluted into dry pyridine (5 ml/mmol, 5 ml) and cooled to 0°C (ice bath). An acid anhydride (2 mmol, 2 eq) was added drop wise and a catalytic amount of DMAP (dimethylaminopyridine) was added to the solution. The solution was stirred for three days at room temperature and the solvent was removed on a rotary evaporator. Silica gel chromatography (AcOEt) of the resulting crude gives the desired nucleoside diesters (60–70% yield) as white powders.
Cell lines and plasmids
The 293T and Caco-2 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: U373-MAGI-CXCR4CEM from Michael Emerman [24,25]. The vector containing and envelope-deficient HIV-1 (NL4-3) has been previously described [4,26].
Cell culture
293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal clone 3 (FC3) serum (HyClone, Logan, UT, USA) and penicillin/streptomycin at 37°C in 5% CO2. U373-MAGI-CXCR4CEM cells were maintained at 37°C in 5% CO2 in DMEM containing 10% FC3 serum, 1 μg/ml puromycin, 0.1 mg/ml hygromycin and 0.2 mg/ml neomycin. Caco-2 cells were cultured in DMEM with Glutamax, 10% FBS and supplemented with penicillin/streptomycin/fungizone and non-essential amino acids.
Caco-2 assay to assess permeability
The Caco-2 assay was performed by Cerep, Inc. (Seattle, WA, USA). Briefly, Caco-2 cells (passage 15) were cultured for 21–25 days to form a confluent monolayer on 96-well multiscreen plates. All samples were run apical (A) side to basolateral (B) side at 37°C with pH 7.4 buffer in both the donor and receiver chambers. Decitabine, gemcitabine or the prodrugs thereof were added to the donor chamber at 10 μM and samples were analysed at 0 and 60 min by HPLC-MS/MS. In addition to the parent analytes, the expected metabolites of all compounds were also monitored including the valerate and parent forms of each prodrug as well as the ring-opened forms of decitabine [27,28]. After permeability of the parent compounds and prodrugs were assessed, the monolayer integrity was assessed using fluorescein. Cell monolayers that demonstrated a fluorescein permeability of less than 1.5×10−6 cm/s were considered intact.
The permeability coefficient (Papp) was calculated from the following equation: Papp (cm/s) = (VR × CRend/Δt) × (1/(A × (CDmid – CRmid)).
Where VR is the volume of in the receiver chamber, CRend is the concentration of compound in the receiver chamber at the end of the time point, Δt is the incubation time (60 min), and A is the surface area of the cell monolayer. CDmid is the calculated midpoint concentration of the compound in the donor chamber and CRmid is the midpoint concentration of the test compound in the receiver chamber. Concentrations of the compounds were expressed as peak areas of the test compound.
Half-life calculation for decitabine and gemcitabine divalerate prodrugs
Prodrugs (3 –4 mg) were diluted into a mixture of 10 ml of ethanol, 10 ml of water and 10 ml of appropriate buffer solution. Aliquots were analysed by HPLC at different times.
For pH 2.0, both decitabine divalerate and gemcitabine divalerate were examined at the following time points: 0, 55, 109, 163 and 218 min. For half-life calculations of decitabine divalerate at pH 4.65, 16 different time points were examined including: 0 h, 5 h, 10 h, 35 h and 45 h. To determine the half-life at pH 4.65, gemcitabine divalerate and metabolites thereof were examined at 10 different time points between 0–30 h including 0, 5, 10, 24 and 29 h. For half-life determination at pH 7.4, decitabine divalerate and gemcitabine divalerate were examined at 16 different time points between 0 and 35 h, including 0, 5, 10, 24 and 35 h.
HPLC conditions
The HPLC conditions include solvent A composed of an aqueous solution of 0.01 M of ammonium formate, the pH of the solution adjusted to 4.5 with formic acid. Solvent B was acetonitrile. The flow rate was 5 ml/min.The following method was used: from 0 to 1 min, 0 to 10% B; from 1 to 6 min, 10% B; from 6 to 10 min, 10 to 100% B; from 10 to 20 min, 100% B; from 20 to 21 min, 100 to 0% B; from 21 to 23 min, 0% B. The observed retention time for decitabine divalerate was 13.79 min and for gemcitabine divalerate was 14.28 min.
Production of virus stocks
293T cells (1.3×106) were plated on 10 cm culture dishes 24 h before transfection. The cells were transfected by calcium phosphate co-precipitation with 15 μg of the envelope-deficient HIV vector and 1.5 μg of a plasmid encoding the HIV envelope, pNL4-3env (obtained from Eric Freed, NIH, Frederick, MD, USA). The medium was replaced with 6 ml of DMEM containing 10% FC3 serum and penicillin/streptomycin 24 h after transfection. Cell supernatant containing virus was collected 24 h later and filtered through a 0.2 μm filter. Viral stocks were used immediately or frozen at −80°C.
Drug treatments and infection
U373-MAGI-CXCR4CEM cells (6.2×104/well) were plated in a 12-well culture dish 24 h prior to prodrug treatment. Cells were treated the prodrugs individually or in combination 2 h prior to infection. After the 2 h pretreatment, the viral stock (500 μl) was added to each well to give a final volume of 1 ml and a final drug concentration that is indicated in Figure 1. After infection (24 h), the medium was replaced. Cells were harvested for analysis by flow cytometry 48 h after infection.
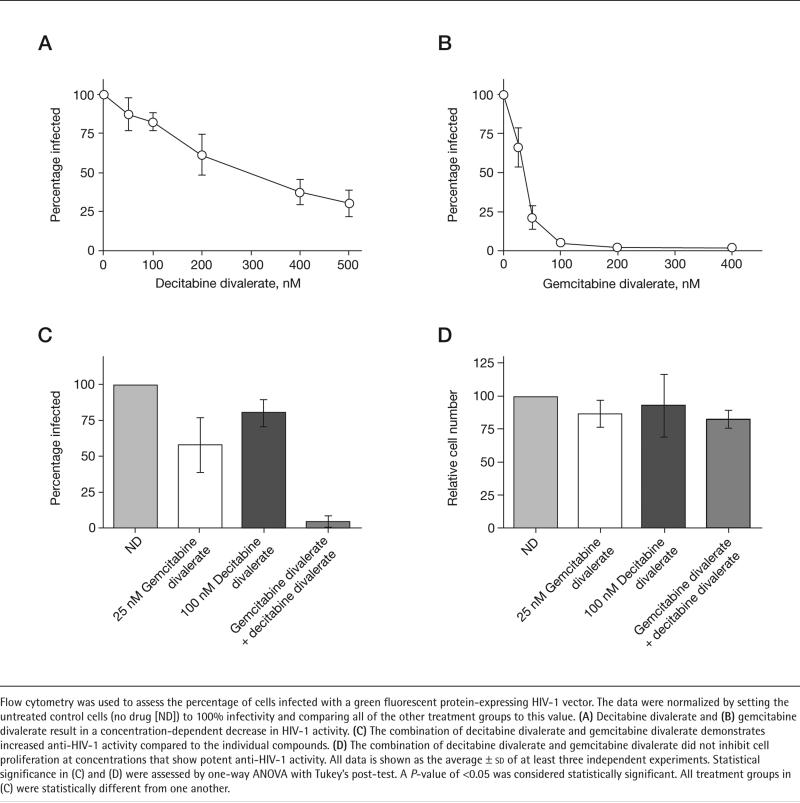
Figure 1. Effect of decitabine divalerate and gemcitabine divalerate on HIV-1 infectivity and cell proliferation.
Flow cytometry was used to assess the percentage of cells infected with a green fluorescent protein-expressing HIV-1 vector. The data were normalized by setting the untreated control cells (no drug [ND]) to 100% infectivity and comparing all of the other treatment groups to this value. (A) Decitabine divalerate and (B) gemcitabine divalerate result in a concentration-dependent decrease in HIV-1 activity. (C) The combination of decitabine divalerate and gemcitabine divalerate demonstrates increased anti-HIV-1 activity compared to the individual compounds. (D) The combination of decitabine divalerate and gemcitabine divalerate did not inhibit cell proliferation at concentrations that show potent anti-HIV-1 activity. All data is shown as the average ± sd of at least three independent experiments. Statistical significance in (C) and (D) were assessed by one-way ANOVA with Tukey's post-test. A P-value of <0.05 was considered statistically significant. All treatment groups in (C) were statistically different from one another.
Flow cytometry to assess anti-HIV effect of decitabine and gemcitabine divalerate prodrugs
The envelope-deficient HIV-1 vector used to generate virus expresses green fluorescent protein (GFP). Therefore, flow cytometry can be used to detect the percentage of cells infected by quantifying the percentage of cells expressing GFP. To do this, forward and side scatter gating was used to eliminate non-viable cells. 10,000 cells were then analysed for fluorescence at 488 nm to detect GFP signal. A histogram was used to determine the percentage of cells expressing GFP.
Cellular proliferation assay
Cell proliferation was examined using the CellTiter-Glo kit from Promega (Madison, WI, USA) according to the manufacturer's instructions. U373-MAGI-CXCR4CEM cells (4,500 cells/well) were plated in a 96-well dish 24 h prior to drug treatment. Cells were treated with the prodrugs individually or in combination for 24 h. After 24 h of exposure to the prodrugs, the media was changed and fresh media without prodrug was added. 24 h later (for a total of 48 h after drugs were added), proliferation was assessed as described in the manufacturer's instructions. Dimethyl sulfoxide (DMSO) was used as a control for the untreated cells. The data were converted to relative cell numbers by setting the value for untreated cells at 100 for each experiment and then multiplying the data for the other samples by the number used to convert the no-drug-treated cells to 100. This conversion was normalized for differences in luciferase activity among different experiments.
Statistical analyses
Data were analysed by calculating the mean ± standard deviation (sd). One-way analysis of variance (ANOVA) with Tukey's post-test was used to assess differences among treatment groups. A P-value of <0.05 was considered statistically significant.
Results
Effect of decitabine divalerate and gemcitabine divalerate on permeability
Decitabine and gemcitabine have poor bioavailability [23] which renders oral administration of these drugs suboptimal [17]. Therefore, a successful prod-rug approach should increase intestinal permeability and could make oral administration of these drugs practical. To examine the intestinal permeability of gemcitabine and gemcitabine prodrugs, we used the Caco-2 assay, a well-established in vitro system that models intestinal permeability and efflux liability of compounds [29]. Typical permeability values using the Caco-2 assay range from 5×10−8 cm/s to 5×10−5 cm/s. Permeabilities greater than 1×10−6 cm/s are associated with well absorbed compounds whereas permeabilities of less than 1×10−7 cm/s are associated with poor intestinal absorption [30]. A panel of 10 decitabine and gemcitabine prodrugs was examined for permeability using the Caco-2 assay. The divalerate prod-rugs demonstrated the most improved permeability compared to the parent compounds (data not shown and Figure 2). Specifically, the results indicate that the permeability of decitabine divalerate and gemcitabine divalerate were significantly improved compared to the parent compounds (Table 1). Specifically, the permeability of decitabine divalerate was fourfold higher than the permeability of decitabine (3.2×10−6 cm/s versus 0.8×10−6 cm/s). Similarly, the permeability of gemcitabine divalerate was 4.2-fold higher than the permeability of gemcitabine (2.1×10−6 cm/s versus 0.5×10−6 cm/s). These permeability values predict that both decitabine divalerate and gemcitabine divalerate would be well absorbed. In contrast, the permeability values of decitabine and gemcitabine are typical of compounds that would be predicted to have low to moderate intestinal absorption.
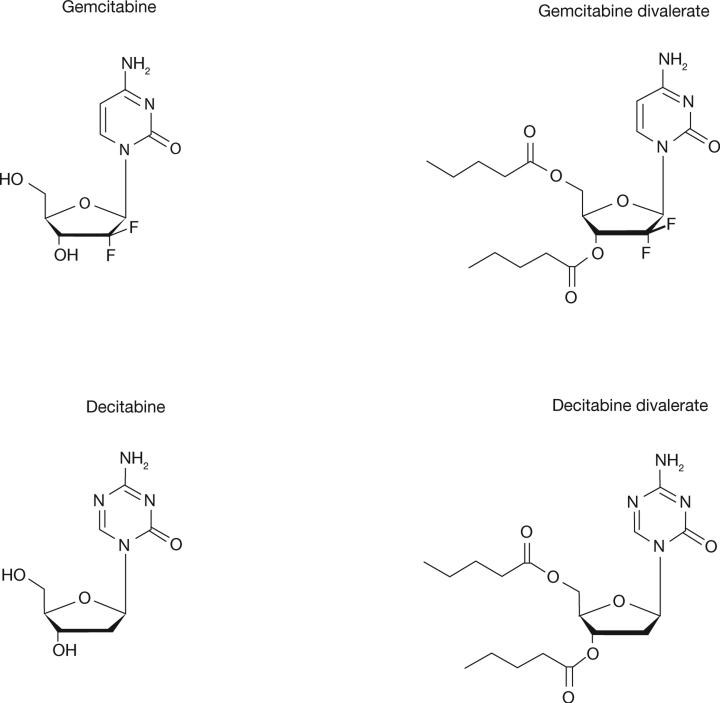
Figure 2.
Structure of gemcitabine, decitabine and the divalerate prodrugs
Table 1.
Permeability and recovery of decitabine, gemcitabine and the divalerate prodrugs of decitabine and gemcitabine
| Compound | Permeability, 10−6 cm/s | Percentage recovery |
|---|---|---|
| Decitabine | 0.8 | 91 |
| Gemcitabine | 0.5 | 111 |
| Decitabine divalerate | 3.2 | 16 |
| Gemcitabine divalerate | 2.1 | 18 |
The half-life of decitabine divalerate and gemcitabine divalerate at pH 2.0, 4.65 and 7.4
The results of the Caco-2 assay indicate that the decitabine and gemcitabine divalerate prodrugs have improved intestinal permeability. To examine if the prodrugs demonstrate sufficient stability, we examined the half-life of the prodrugs at pH 2.0, which is consistent with the pH of the stomach; pH 4.65, which is consistent with the pH of the duodenum near the pylorus; and pH 7.4, which is consistent with the pH of the plasma and near the pH of the terminal ileum [31,32]. Stability at pH greater than 7.4 was not examined as these prodrugs are expected to be absorbed in the gastrointestinal tract prior to reaching alkaline conditions. The estimated half-life of decitabine divalerate at pH 7.4 was 28 h (Table 2), a value that is significantly greater than 12.9 ±0.9 h reported for the parent compounds at the same pH [33]. The estimated half-life of decitabine divalerate was 17 h at pH 2.0 and 36 h at pH 4.65 (Table 2). These results indicate that decitabine divalerate is stable at a wide range of pH. The estimated half-life of gemcitabine divalerate at pH 7.4 was 135 h, which is similar to that reported for gemcitabine at pH 7.4 [34]. As expected, gemcitabine divalerate was stable at both pH 2.0 and pH 4.65, as demonstrated by a half-life of 85 h and 247 h, respectively.
Table 2.
The half-life of decitabine divalerate and gemcitabine divalerate at pH 2.0, 4.65 and 7.4
| Compound | Half-life at pH 2.0, h | Half-life at pH 4.65, h | Half-life at pH 7.4, h |
|---|---|---|---|
| Decitabine divalerate | 17 | 36 | 28 |
| Gemcitabine divalerate | 85 | 247 | 135 |
Effect of decitabine divalerate and gemcitabine divalerate on HIV-1 infectivity and cell proliferation
The anti-HIV-1 activity of decitabine divalerate and gemcitabine divalerate was assessed using an assay that enables detection of infected cells through quantification of a GFP marker gene expressed in the HIV-1 vector used to generate virus. The assay, which we have previously described [4,26], uses an HIV-1 vector that expresses GFP, but does not express the HIV-1 envelope. Therefore, vector virus was made by transfecting 293T cells with both the HIV-1 vector plasmid DNA as well as a plasmid that encodes for the HIV-1 envelope. Virus made from 293T cells was then used to infect cells that are pre-treated with solvent (DMSO) or one of the prodrugs. Infected cells are detected as GFP-expressing cells by flow cytometry. As shown in Figure 1, treatment with either gemcitabine divalerate or decitabine divalerate produced a concentration-dependent decrease in HIV-1 infectivity. Furthermore, when used in combination the prodrugs show a decrease in infectivity (Figure 1) that suggests an interaction which is more than additive, indicating that the combined antiretroviral effect is similar to what was observed with the parent compounds [4]. Furthermore, the decrease in infectivity did not correlate with a corresponding decrease in cell proliferation (Figure 1) as determined by cellular ATP levels. The concentration of gemcitabine divalerate needed to inhibit proliferation by 50% (IC50) was greater than 5 μM, whereas decitabine divalerate showed less than 5% decrease in cell proliferation at 5 μM (data not shown). Since previous studies have shown that ATP levels are sensitive to both cytotoxic and cytostatic compounds [35], these results indicate that the prodrugs are neither cytotoxic nor cytostatic.
Discussion
Drug-resistant HIV-1 is a significant public health problem that emphasizes the need for the development of new anti-HIV-1 therapies that exploit new drug targets. Although the mutation rate has been proposed as a rational drug target, no compounds that lethally mutagenize HIV-1 have been approved for clinical use. We previously described two nucleoside analogues, decitabine and gemcitabine that, when used in combination, decrease HIV-1 replication through lethal mutagenesis. Decitabine and gemcitabine are clinically approved to treat MDS [36,37] and various solid cancers, respectively [38]. In addition to their anti-MDS, anti-cancer, and anti-HIV-1 activities, decitabine and gemcitabine have been shown to have antiviral activity against other retroviruses including feline leukaemia virus [39] and murine leukaemia virus [12,40]. Additionally, gemcitabine exerts antiviral activity against influenza A [41], a member of Orthomyxoviridae. The antiviral activities of decitabine and gemcitabine warrant further investigation into the potential to reposition these drugs as antiviral drugs. However, due to their low intestinal permeability, deamination by cytidine deaminase, and rapid phosphorylation in the liver and kidneys, decitabine and gemcitabine have poor bioavailability and must be administered intravenously. Since repeated administration is not feasible, we used a prodrug approach to increase the intestinal permeability of decitabine and gemcitabine.
Prodrug approaches have been used to increase the bioavailability of several approved nucleoside analogues including tenofovir disoproxil fumarate, valacyclovir, adefovir and famciclovir. In general, prodrug approaches are used to increase intestinal absorption and to enhance drug transport into cells. Here, we describe the divalerate prodrug of decitabine and gemcitabine. The valerate moieties were selected to serve multiple purposes. First, the lipophilicity of the valerate moieties should increase intestinal permeability, which should correlate with an increase in bioavailability. Second, the valerate moiety on the 5′-hydroxyl group should delay phosphorylation in the liver which should improve its tissue distribution. Third, the ester cytosine prodrugs are reported to be resistant to deamination which should improve the ability to attain physiologically relevant concentrations by oral administration [42]. Finally, the valerate moieties should be readily hydrolysed by carboxyesterases that are ubiquitously expressed [43,44]. Abundant expression of carboxyesterase in the liver would be expected to hydrolyse the divalerate prodrugs during first pass metabolism [45]. Hydrolysis of the prod-rugs releases the free nucleosides, which must then be phosphorylated by cellular kinases in order to become biologically active.
This study has found that decitabine divalerate and gemcitabine divalerate have increased intestinal permeability when compared to the parent compounds (Table 1), which typically correlates with an increase in bioavail-ability. The half-life data predicts prodrug stability in the acidic environment of the gastrointestinal tract. Furthermore, both prodrugs demonstrated potent anti-HIV-1 activity at concentrations that were not cytostatic or cytotoxic. To exert antiviral activity, the prodrugs must be hydrolysed to release the parent nucleoside, and this hydrolysis step is dependent on adequate expression of carboxyesterases in the cell line that was used to assess antiviral activity. However, in an in vivo system, hydrolysis of the prodrugs is expected to be carried out by human carboxyesterase-1, which is abundantly expressed in the liver on first pass metabolism [45]. Since the prodrugs are expected to be hydrolysed by the liver, the target cells are not expected to play a significant role in the generation of the active drug. Therefore, comparing the antiviral activity of the prodrug to the parent drug in an in vitro system is unlikely to reveal relevant in vivo information.
The increase in prodrug stability and permeability compared to the parent compounds suggest that decitabine divalerate and gemcitabine divalerate are likely to show improved bioavailability and pharmacokinetic properties while maintaining biological activity. Further studies are warranted to examine the bioavailability of decitabine divalerate and gemcitabine divalerate in an in vivo system.
Acknowledgements
This research was supported by NIH grant R01 GM56615 and a University of Minnesota Academic Health Center Translational Research Grant.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dapp MJ, Patterson SE, Mansky LM. Back to the future: revisiting HIV-1 lethal mutagenesis. Trends Microbiol. 2013;21:56–62. doi: 10.1016/j.tim.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeb LA, Essigmann JM, Kazazi F, et al. Lethal mutagenesis of HIV with mutagenic nucleoside analogs. Proc Natl Acad Sci U S A. 1999;96:1492–1497. doi: 10.1073/pnas.96.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clouser CL, Patterson SE, Mansky LM. Exploiting drug repositioning for discovery of a novel HIV combination therapy. J Virol. 2010;84:9301–9309. doi: 10.1128/JVI.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tohyama K. Utility of DNA methyltransferase inhibitors for the treatment of myelodysplastic syndromes. Curr Pharm Des. 2012;18:3190–3197. doi: 10.2174/1381612811209023190. [DOI] [PubMed] [Google Scholar]
- 6.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(Suppl 5):v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 7.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 8.Jüttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci U S A. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinemann V, Xu YZ, Chubb S, et al. Cellular elimination of 2′,2′-difluorodeoxycytidine 5′-triphosphate: a mechanism of self-potentiation. Cancer Res. 1992;52:533–539. [PubMed] [Google Scholar]
- 10.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17:v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 11.Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 12.Clouser CL, Holtz CM, Mullett M, et al. Activity of a novel combined antiretroviral therapy of gemcitabine and decitabine in a mouse model for HIV-1. Antimicrob Agents Chemother. 2012;56:1942–1948. doi: 10.1128/AAC.06161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay SK, Morse HC, III, Makino M, Ruscetti SK, Hartley JW. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1989;86:3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chattopadhyay SK, Sengupta DN, Fredrickson TN, Morse HC, III, Hartley JW. Characteristics and contributions of defective, ecotropic, and mink cell focus-inducing viruses involved in a retrovirus-induced immunodeficiency syndrome of mice. J Virol. 1991;65:4232–4241. doi: 10.1128/jvi.65.8.4232-4241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosier DE, Yetter RA, Morse HC., III. Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS). J Exp Med. 1987;165:1737–1742. doi: 10.1084/jem.165.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbruzzese JL, Grunewald R, Weeks EA, et al. A Phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 17.Moysan E, Bastiat G, Benoit JP. Gemcitabine versus modified gemcitabine: a review of several promising chemical modifications. Mol Pharm. 2013;10:430–444. doi: 10.1021/mp300370t. [DOI] [PubMed] [Google Scholar]
- 18.Francia G, Shaked Y, Hashimoto K, et al. Low-dose metronomic oral dosing of a prodrug of gemcitabine (LY2334737) causes antitumor effects in the absence of inhibition of systemic vasculogenesis. Mol Cancer Ther. 2012;11:680–689. doi: 10.1158/1535-7163.MCT-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koolen SL, Witteveen PO, Jansen RS, et al. Phase I study of oral gemcitabine prodrug (LY2334737) alone and in combination with erlotinib in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6071–6082. doi: 10.1158/1078-0432.CCR-11-0353. [DOI] [PubMed] [Google Scholar]
- 20.Lavelle D, Saunthararajah Y, Vaitkus K, et al. S110, a novel decitabine dinucleotide, increases fetal hemoglobin levels in baboons (P. anubis). J Transl Med. 2010;8:92–99. doi: 10.1186/1479-5876-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao XM, Wang JC, Wang JB, et al. Enhanced anticancer activity of gemcitabine coupling with conjugated linoleic acid against human breast cancer in vitro and in vivo. Eur J Pharm Biopharm. 2012;82:401–409. doi: 10.1016/j.ejpb.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Lavelle D, Vaitkus K, Ling Y, et al. Effects of tetrahydrouridine on pharmacokinetics and pharmacodynamics of oral decitabine. Blood. 2012;119:1240–1247. doi: 10.1182/blood-2011-08-371690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beumer JH, Eiseman JL, Parise RA, et al. Modulation of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. Clin Cancer Res. 2008;14:3529–3535. doi: 10.1158/1078-0432.CCR-07-4885. [DOI] [PubMed] [Google Scholar]
- 24.Harrington RD, Geballe AP. Cofactor requirement for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vodicka MA, Goh WC, Wu LI, et al. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology. 1997;233:193–198. doi: 10.1006/viro.1997.8606. [DOI] [PubMed] [Google Scholar]
- 26.Dapp MJ, Clouser CL, Patterson S, Mansky LM. 5-Azacytidine can induce lethal mutagenesis in human immunodeficiency virus type 1. J Virol. 2009;83:11950–11958. doi: 10.1128/JVI.01406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin KT, Momparler RL, Rivard GE. High-performance liquid chromatographic analysis of chemical stability of 5-aza-2′-deoxycytidine. J Pharm Sci. 1981;70:1228–1232. doi: 10.1002/jps.2600701112. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Marcucci G, Byrd JC, et al. Characterization of decomposition products and preclinical and low dose clinical pharmacokinetics of decitabine (5-aza-2′-deoxycytidine) by a new liquid chromatography/tandem mass spectrometry quantification method. Rapid Commun Mass Spectrom. 2006;20:1117–1126. doi: 10.1002/rcm.2423. [DOI] [PubMed] [Google Scholar]
- 29.Press B, Di Grandi D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr Drug Metab. 2008;9:893–900. doi: 10.2174/138920008786485119. [DOI] [PubMed] [Google Scholar]
- 30.Ziemba A, Hayes E, Freeman BB, III, Ye T, Pizzorno G. Development of an oral form of azacytidine: 2′3′5′triacetyl-5-azacytidine. Chemother Res Pract. 2011;2011:965826–965835. doi: 10.1155/2011/965826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans DF, Pye G, Bramley R, et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- 33.Rogstad DK, Herring JL, Theruvathu JA, et al. Chemical decomposition of 5-aza-2′-deoxycytidine (decitabine): kinetic analyses and identification of products by NMR, HPLC, and mass spectrometry. Chem Res Toxicol. 2009;22:1194–1204. doi: 10.1021/tx900131u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Xue X, Li G, et al. Synthesis and biological evaluation of oral prodrugs based on the structure of gemcitabine. Chem Biol Drug Des. 2012;80:479–488. doi: 10.1111/j.1747-0285.2012.01422.x. [DOI] [PubMed] [Google Scholar]
- 35.Obajimi O, Melera PW. The depletion of cellular ATP by AG2034 mediates cell death or cytostasis in a hypoxanthine-dependent manner in human prostate cancer cells. Cancer Chemother Pharmacol. 2008;62:215–226. doi: 10.1007/s00280-007-0593-6. [DOI] [PubMed] [Google Scholar]
- 36.Leone G, Teofili L, Voso MT, Lubbert M. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica. 2002;87:1324–1341. [PubMed] [Google Scholar]
- 37.Pinto A, Zagonel V. 5-Aza-2′-deoxycytidine (decitabine) and 5-azacytidine in the treatment of acute myeloid leukemias and myelodysplastic syndromes: past, present and future trends. Leukemia. 1993;7(Suppl 1):51–60. [PubMed] [Google Scholar]
- 38.Carmichael J, Fink U, Russell RC, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greggs WM, III, Clouser CL, Patterson SE, Mansky LM. Discovery of drugs that possess activity against feline leukemia virus. J Gen Virol. 2012;93:900–905. doi: 10.1099/vir.0.039909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clouser CL, Holtz CM, Mullett M, et al. Analysis of the ex vivo and in vivo antiretroviral activity of gemcitabine. PLoS ONE. 2011;6:e15840. doi: 10.1371/journal.pone.0015840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denisova OV, Kakkola L, Feng L, et al. Obatoclax, saliphenylhalamide, and gemcitabine inhibit influenza a virus infection. J Biol Chem. 2012;287:35324–35332. doi: 10.1074/jbc.M112.392142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song X, Lorenzi PL, Landowski CP, et al. Amino acid ester prodrugs of the anticancer agent gemcitabine: synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol Pharm. 2005;2:157–167. doi: 10.1021/mp049888e. [DOI] [PubMed] [Google Scholar]
- 43.Imai T, Ohura K. The role of intestinal carboxylesterase in the oral absorption of prodrugs. Curr Drug Metab. 2010;11:793–805. doi: 10.2174/138920010794328904. [DOI] [PubMed] [Google Scholar]
- 44.Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- 45.Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]