Abstract
Aim
Peripheral blood-derived endothelial cells (pBD-ECs) are an attractive tool for cell therapies and tissue engineering, but have been limited by their low isolation yield. We increase pBD-EC yield via administration of the chemokine receptor type 4 antagonist AMD3100, as well as via a diluted whole blood incubation (DWBI).
Materials & Methods
Porcine pBD-ECs were isolated using AMD3100 and DWBI and tested for EC markers, acetylated LDL uptake, growth kinetics, metabolic activity, flow-mediated nitric oxide production and seeded onto titanium tubes implanted into vessels of pigs.
Results
DWBI increased the yield of porcine pBD-ECs 6.6-fold, and AMD3100 increased the yield 4.5-fold. AMD3100-mobilized ECs were phenotypically indistinguishable from nonmobilized ECs. In porcine implants, the cells expressed endothelial nitric oxide synthase, reduced thrombin-antithrombin complex systemically and prevented thrombosis.
Conclusion
Administration of AMD3100 and the DWBI method both increase pBD-EC yield.
Keywords: AMD3100, biocompatible materials, cell Isolation, cell therapy endothelial cells, endothelial progenitor cells, swine, thrombosis, tissue engineering
Autologous endothelial progenitor cells (EPC), obtained from peripheral blood, are a potentially valuable source of endothelial cells for the treatment of various cardiovascular diseases, for example, limb ischemia [1]. In addition to their neovascularization potential, blood-derived EPCs hold promise for engineering solid organs that require an extensive blood supply. Peripheral blood-derived EPCs are now broadly categorized as either early-outgrowth EPCs [2] or late-outgrowth EPCs [2]. The latter are the most promising cells for ex vivo expansion and endothelial cell replacement. Late-outgrowth EPCs are highly proliferative, lack the leukocyte marker CD45, and have also been referred to as endothelial outgrowth cells (EOC) [2–4], endothelial colony-forming cells (ECFC) [5], and blood-derived endothelial cells (EC) [6]. We will use the term peripheral-blood derived endothelial cells (pBD-EC) in this study.
Despite their potential, the therapeutic application of pBD-ECs has been hindered by the difficulty to isolate sufficient numbers of pBD-ECs from blood for clinical- scale use. The average yield has been approximately 0.4 pBD-EC colonies per 20 ml human peripheral blood utilizing density gradient centrifugation – the ‘traditional method’ for isolation of progenitor cells [7,8].
We hypothesize that the yield of functional pBD-ECs for autologous cell therapies and tissue engineering can be increased by mobilization of progenitor cells using the chemokine receptor type 4 (CXCR4)-antagonist AMD3100, as well as by an isolation technique that reduces the number of steps and related cell loss. We recently developed the diluted whole blood incubation (DWBI) method in which diluted whole blood with the addition of human serum and growth factors is directly incubated on surfaces. This method avoids positive or negative selection steps that invariably lead to the loss of putative progenitor cells and resulted in yields of 7.0 ±2.5 EC colonies derived from 10 ml of human umbilical cord blood [6].
Although initially studied as a potential therapy for HIV, AMD3100 (plerixafor) was instead approved by the US FDA in 2008 for use in combination with G-CSF for the treatment of patients with non-Hodgkin’s lymphoma or multiple myeloma to mobilize hematopoietic stem cells (HSC) [9]. The mobilized HSCs are collected from the patient’s peripheral blood and used for autologous transplantation.
AMD3100 is a bicyclam small molecule that mobilizes HSCs from bone marrow by competitively binding the CXCR4 on HSCs. The CXCR4 on HSCs normally binds on the surfaces of bone marrow stromal cells expressed ligand CXCL12, aka SDF-1 [10]. SDF-1 binding causes CXCR4 G-protein activation, Ca2+ influx and receptor internalization, which directs HSCs to the bone marrow niche; disruption of that signal by AMD3100 allows those HSCs to instead enter the peripheral blood [11]. The most common side effects of AMD3100 observed in clinical trials include nausea and diarrhea in 10% of patients [12].
To date, it is unclear if the progenitor cells that give rise to pBD-ECs reside in bone marrow. Current evidence suggests that the late-outgrowth EPCs are derived from the vessel wall [7,13,14]. AMD3100-mobilized cells may thus constitute a cell population which is different from the progenitor cells that grow out pBD-ECs. To investigate any possible differences in these cell populations, we used rigorous tests under flow conditions ex vivo and in vivo to compare pBD-ECs isolated from late-outgrowth EPCs from pigs with or without AMD3100 treatment.
We chose pigs for our in vivo study because of the following reasons: they are the preferred large animal model for cardiovascular diseases [15], we have previously characterized pBD-ECs and found them to be phenotypically and functionally analogous to human blood-derived ECs [6,16,17], and pigs enabled us to transplant autologous cells into the pigs’ aortae and inferior vena cavae (IVCs) to test cell functionality under flow in vivo. In vivo studies presented here provide proof-of-concept for utilizing DWBI and AMD3100-mobilized pBD-ECs for autologous cell therapies by demonstrating that pBD-ECs remain functional under flow on the surface of implanted intravascular titanium (Ti) tubes, prevent thrombosis in the IVC locally and also reduce the coagulation response systemically.
Our results show that AMD3100 and the DWBI method independently and significantly increase the yield of pBD-ECs. pBD-ECs isolated from animals treated or untreated with AMD3100 were found to be phenotypically and functionally identical. If these pBD-ECs should indeed originate from blood vessels, our results cannot rule out the possibility that AMD3100 mobilizes pBD-ECs or their progenitor cells directly from vessel walls.
Materials & methods
AMD3100 crossover trial in swine
Animal experimentation was approved by the Duke University Institutional Animal Care and Use Committee. Female Yorkshire swine were used for all studies. To account for variability in the mobilization response between pigs, each pig served as its own control. Specifically, each of six pigs (~15 kg) sequentially received four subcutaneous gluteal injection of which two injections consisted of sham injections (controls) and two consisted of drug injections (test doses) with either a high dose (0.75 mg/kg) or a low dose (0.24 mg/kg) of AMD3100 (Sigma-Aldrich, MO, USA). The control injections consisted of phosphate buffered saline (PBS) solution and were followed by cell isolations with either 2% fetal bovine serum (FBS; Gemini Bio-Products, CA, USA) or 2% porcine serum (PS; Gemini Bio-Products). All drug injections were followed by cell isolations with 2% PS.
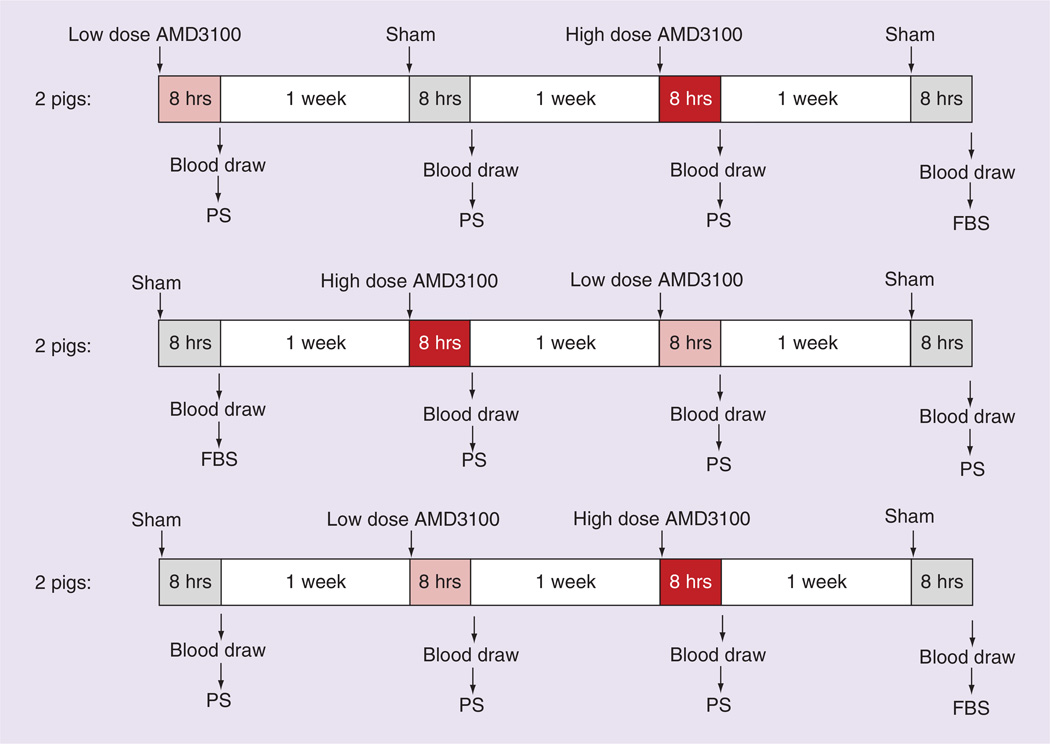
Each injection–blood draw sequence was separated by 1 week to ensure that there was no residual drug effect and that the blood volume was replenished. Eight hours following each injection, the pigs underwent blood draws as previously described [16] and 60 ml of blood was anticoagulated with 20 ml citrate dextrose solution (Pall Corporation, CA, USA). The six pigs were divided into three groups of two pigs, with the order of the four injections randomized for each group (Figure 1). Of the two sham injections for each pig, the pBD-ECs isolated following one of them was cultured in FBS and the other in PS.
Figure 1. Timeline of crossover trial testing AMD3100 injection prior to peripheral blood-derived endothelial cell isolation, as well as comparing porcine serum to fetal bovine serum.
Six pigs each received four different injections followed 8 h later by a 60 ml blood draw to isolate peripheral blood-derived endothelial cell (pBD-ECs) using the traditional method. For each pig, those four injection–blood draw sequences were separated by 1 week to ensure that there was no residual drug effect and that the blood volume was replenished. The four injections consisted of two phosphate buffered saline sham injections (control), a high dose (0.75 mg/kg) of AMD3100 and a low dose (0.24 mg/kg) of AMD3100. Pigs were divided into three groups of two pigs, with the order of the four injections randomized for each group as displayed in the figure. Of the two sham injections for each pig, the pBD-ECs isolated following one of them were cultured in FBS and the other in PS.
FBS: Fetal bovine serum; PS: Porcine serum.
In a separate experiment, seven more pigs were utilized to determine the optimal PS concentration in EC medium. EC medium was prepared by supplementing EBM-2 medium (Lonza, Basel, Switzerland) with EGM-2 SingleQuots Kit (Lonza), but substituting either 2% for the FBS contained in the SingleQuots Kit.
pBD-EC isolation with traditional density centrifugation method
The mononuclear cell (MNC)-containing layer of anticoagulated blood was collected via density centrifugation: anticoagulated blood was diluted 1:1 with Hank’s balanced salt solution (Lonza) and layered on equal volumes of Histopaque-1077 (Sigma-Aldrich), centrifuged (30 min, 740 g, low break) and the MNC-containing layer collected and diluted 1:1.5 in wash medium (MCDB-131, Invitrogen, CA, USA, with 2% PS, Gemini Bio-Products), then centrifuged × 2 (10 min, 515 g, low break). MNCs were then resuspended in full EC medium and incubated in collagen precoated 12-well tissue culture polystyrene plates (Corning Costar, MA, USA, with Type-1 calf skin collagen, Sigma-Aldrich, as previously described [6]) at 37°C, 5% CO2. Medium was changed every 24 h for the first 4 days, and subsequently every other day. All isolated pBD-ECs were cryopreserved in −80°C until used for experiments.
pBD-EC isolation with DWBI method
Porcine blood was harvested and anticoagulated with 20 USP heparin/ml blood (APP Pharmaceuticals, IL, USA) and then diluted 1:4 with full EC medium. Thirty milliliter of this final solution was added to a 100 mm × 15 mm polystyrene petri dish (Celltreat, MA, USA), which had been precoated with Type I collagen as described above. Following, diluted whole blood was incubated at 37°C and 5% CO2 and non-adherent cells and plasma were slowly removed every 24 h and replaced with 10 ml of new medium. On the 4th day, the adherent cells were gently washed once with medium and new medium was added every other day.
Traditional versus DWBI method trial in swine
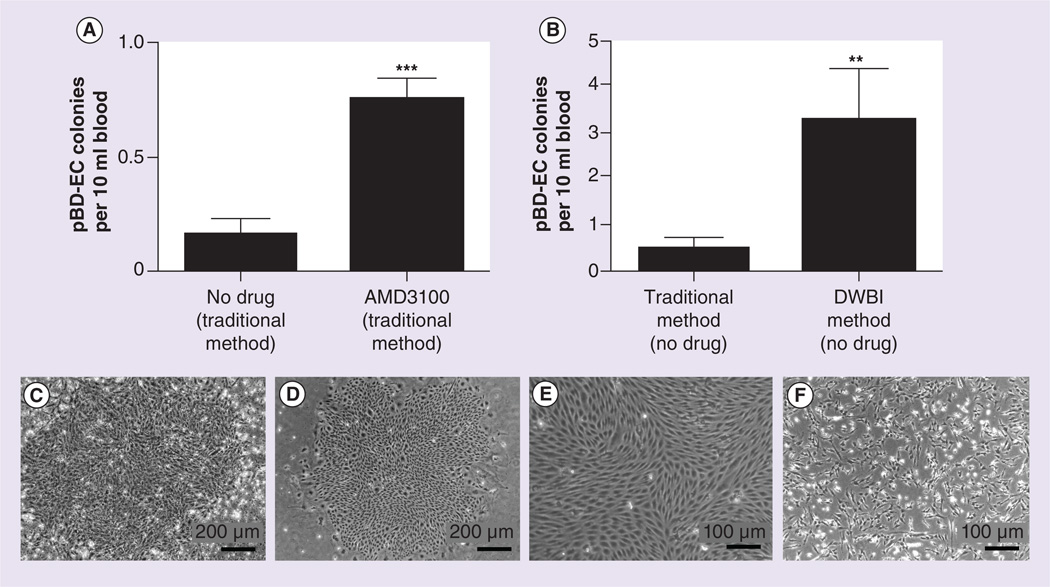
Twenty-two pigs (~47.5 kg) were used to collect 24 ml of blood per pig and 12 ml of each sample (anticoagulated as described above) was used for pBD-EC isolation according to the ‘traditional method’ and the other 12 ml for pBD-EC isolation according to the DWBI method. Seven days after isolation, the number of pBD-EC colonies was determined. A colony was defined as a group of at least 100 contiguously growing cells with pBD-EC morphology as determined by light microscopy. Examples of such colonies can be seen in Figure 2C&D.
Figure 2. Isolation of peripheral blood-derived endothelial cell colonies.
(A) Number of pBD-EC colonies per 10 ml blood isolated using the traditional method with and without AMD3100 mobilization (high and low dose combined) in the crossover study of six pigs. Each blood draw collected 60 ml. Blood from pigs that received AMD3100 yielded significantly more pBD-EC colonies than controls (*** p < 0.0001, square root transformation followed by generalized estimating equation). (B) Number of pBD-EC colonies per 10 ml blood that were isolated using the traditional isolation method and the DWBI method. 24 ml of blood was drawn from each of 22 pigs; 12 ml of each blood draw underwent the traditional isolation method, while 12 ml underwent the DWBI isolation. A significantly greater number of pBD-EC colonies were observed with the DWBI method than with the traditional method (** p < 0.005, Wilcoxon signed rank sum test). Each error bar represents the SEM. (C) Colony observed at day 8 isolated from blood using the traditional method (without AMD3100 mobilization). (D) Colony observed at day 8 isolated from blood using the DWBI method (without AMD3100 mobilization). (E) Cells with representative endothelial cell (EC) morphology at day 8, isolated from blood using the traditional method (without AMD3100 mobilization). (F) Cells with representative non-EC morphology at day 8, isolated from blood using the traditional method (without AMD3100 mobilization).
DWBI: Diluted whole blood incubation; pBD-EC: Peripheral blood-derived endothelial cell.
pBD-EC characterization with flow cytometry/acetylated LDL uptake
Isolated colonies prior to cryopreservation were evaluated with flow cytometry for CD31, CD105 and CD146, as well as the leukocyte markers CD14 and CD45, and CD106. Cells were labeled with fluorescein isothiocyanate (FITC) directly conjugated mouse antiporcine CD14 (MCA1218F, Serotec, Oxford, UK), CD45 (MCA1222F, Serotec), CD31 (MCA1746F, Serotec), CD105 (ab53318, Abcam) CD106 (APG106F, Antigenix America, NY, USA) and CD146 (MCA214FT, Serotec) antibodies. Respective isotype controls were used (MCA691F and MCA928F, Serotec). A FACS-Calibur flow cytometer (Becton Dickinson, NJ, USA) was used for analysis as previously described [18].
pBD-ECs were further tested for uptake of DiI-labeled acetylated low-density lipoprotein (DiI-Ac-LDL, Biomedical Technologies, MA, USA) according to manufacturer’s instructions [6].
Redox assay
The metabolic activity of the four groups of pBD-ECs derived from animals in the AMD3100 crossover trial (high- or low-dose AMD3100, controls followed by cell isolation with 2% FBS or 2% PS) was compared with the alamarBlue redox assay (Invitrogen) as previously described [16].
Growth curves
Growth curves were created with pBD-ECs isolated from under four different conditions: traditional method and AMD3100-treated animals (from three pigs at passage 6); traditional method without AMD3100 (from three pigs at passage 5 and three pigs at passage 6); with the DWBI method and AMD3100-treated animals (from three pigs at passage 6); with the DWBI method without AMD3100-treated animals (from three pigs at passage 6 and three pigs at passage 9).
For all four conditions, cells were seeded into 6-well plates (Corning, MA, USA, 4×103 cells/cm2) and the number of cells per well was quantified in phase-contrast images (Leica DMI3000 B) with ImageJ software (NIH) after 24, 48 and 72 h of incubation (at 37°C, 5% CO2 in EC medium containing 2% PS). The population doubling time (PDT) was calculated as:
| (Equation 1) |
where PN and PN+1 are cell counts at different times after seeding, and t is the time between PN and PS.
Flow experiment & nitric oxide quantification
Prior to flow experiments, glass slides (Becton Dickinson) were cleaned in 2% sodium dodecyl sulfate, rinsed in deionized water, sterilized by steam autoclaving at 121°C for 30 min (Steris, Amsco Century, OH, USA) and precoated with fibronectin (Sigma-Aldrich, 0.25 µg/cm2 × 45 min at 21°C). pBD-ECs were then seeded (2.6 × 104 cells/cm2 in EC medium) onto two glass slides per experiment and incubated (37°C, 5% CO2, 24 h). Following, one of the two glass slides was designated as a static control and transferred to a quadriPERM cell culture vessel (Greiner Bio-One, Frickenhausen, Germany, in 10 ml of EC medium), and incubated. The other glass slide was inserted into a parallel plate flow chamber [19] in a flow circuit consisting of a 125 ml reservoir (Cole Parmer, Pyrex, IL, USA), peristaltic pump (L/S variable-speed economy drive and Easy Load Pump Head; Masterflex, IL, USA), and pulse dampener (Masterflex) in EC medium (total circuit volume 100 ml). pBD-ECs in our parallel plate flow chamber were exposed to a shear stress of 25 dynes/cm2 calculated according to
| (Equation 2) |
where τ denotes the wall shear stress (dynes/cm2), μ the medium viscosity at 37°C (0.01 g/cm/s), w the channel width (2.5 cm), h the channel height (0.04 cm), Q the volumetric flow rate (cm3/s) [19].
After 48 h, glass slides were fixed in 10% formalin (Medical Chemical Corporation, CA, USA), then stained for CD31 and nuclei with Hoechst dye. Four random images were taken per slide to measure cell angles (ImageJ) with respect to the direction of flow. In order to measure NO production under flow and static control, two replicate medium samples were collected from both the flow circuit (0.5 ml) and static control (0.3 ml) in the quadriPERM flask (Greiner Bio-One) at 0, 1, 24 and 48 h since the start of flow.
The concentration of the NO metabolite nitrite (NO2−) was measured by chemiluminescence with an Ionics/Sievers Nitric Oxide Analyzer (NOA 280; Sievers Instruments, CO, USA) as described previously [6,20,21]. The total amount of nitrite produced per EC was calculated using the following equation:
| (Equation 3) |
pBD-EC implantation into swine
pBD-ECs isolated with the DWBI method were seeded onto Ti tubes and implanted into each of the four pigs from which the cells had been derived. The cells were fluorescently labeled prior to implantation with PKH26 (Sigma-Aldrich) [17]. Four pigs received IVC implants (grade 2; Tico Titanium, MI, USA, pre-sectioned and reassembled as previously described) [17].
These Ti implants were 4.5 cm long, 1.27 cm in outer diameter, 0.94 cm in inner diameter, polished on the inside surfaces and presectioned. Ti tubes were directly inserted into the IVCs without use of an artificial graft following our established methods [17,22]. All four Ti tubes were seeded with autologous pBD-ECs (2.4 × 106 cells/ml in 4.5 ml of medium) derived with the DWBI method; however, in two of the four implants the cells had additionally been mobilized with AMD3100 (0.5 mg/kg). Four pigs received bare metal uncoated Ti implants as controls without pBD-ECs.
Systemic response of pBD-ECs in porcine model
We obtained blood samples from pigs’ ear veins immediately before surgery and 3 days postsurgery, and measured thrombin-antithrombin complex according to manufacturer’s instructions (Enzygnost TAT micro, Dade Behring, Germany) [23].
Immunohistochemistry/ microscopy
After explantation, Ti tubes and surrounding vessel segments were fixed in 3.7% paraformaldehyde (Sigma-Aldrich) and disassembled to evaluate for presence of prelabeled cells. Cell borders were additionally stained with antiporcine CD31 (APG311, Antigenix America) and secondary goat antirabbit antibody (AlexaFluor546, A-11035, Invitrogen). Adherent cells were tested for expression of endothelial nitric oxide synthase (eNOS) by permeabilizing with 0.1% Triton X (Sigma-Aldrich) followed by incubating with rabbit antipig eNOS antibody (ab5589; Abcam) and secondary goat antirabbit AlexaFluor488 (A-11008, Invitrogen). Nuclei were counterstained with DAPI (Vector Laboratories).
Statistical analysis
The number of pBD-EC colonies in the AMD3100 crossover trial was compared with a Generalized Estimating Equation (GEE, SAS version 9.3, NC, USA). To account for unequal variance, a square root transformation was performed. The fraction of colonies with EC morphology when isolated using 2% PS versus 2% FBS was compared using Fisher’s exact test (GraphPad Prism 5, CA, USA). The metabolic activity of groups evaluated with the alamar-Blue assay was compared with one-way ANOVA (SAS). To compare the pBD-EC isolation counts with the traditional isolation method to the DWBI isolation method, the Wilcoxon signed rank sum test was used (SAS). The angles of cell orientation respective to the direction of flow were evaluated with the Kolmogorov–Smirnov test (OriginPro 9, MA, USA). The longitudinal data of PDTs as well as NO production under static and flow conditions were analyzed with the between estimator of a panel data model (R 3.0.2, Vienna, Austria) [24]. The Wilcoxon rank sum test was used to analyze TAT values for bare uncoated Ti implants and pBD-EC-coated implants in the IVC. A significance level of 0.05 was assumed for all tests.
Results
AMD3100 crossover trial in swine
Subcutaneous administration of AMD3100 in the crossover trial of six swine significantly increased the number of pBD-EC colonies as compared with PBS control injections into the same pigs (0.750 ±0.047 colonies per 10 ml blood vs 0.167 ±0.021 colonies per 10 ml blood, p < 0.0001, GEE, Figure 2A). Although the dose-dependent effect of AMD3100 did not reach statistical significance, more colonies were isolated with the higher dosage of AMD3100 (0.75 mg/kg) than with the lower dosage (0.24 mg/kg) for each pig (0.972 ±0.104 colonies per 10 ml blood vs 0.528 ±0.073 colonies per 10 ml blood, p = 0.234, square root transformation followed by GEE).
The type of serum (PS vs FBS) did not significantly impact the absolute number of pBD-EC colonies isolated for a given volume of blood (0.250 ±0.055 colonies per 10 ml blood vs 0.083 ±0.015 colonies per 10 ml blood, p = 0.478, square root transformation followed by GEE), nor the percentage of colonies with EC morphology. Although 9 of 30 colonies had EC morphology when using 2% PS while only 3 of 33 colonies had EC morphology when using 2% FBS, that difference was not statistically significant (p = 0.053, two-tailed Fisher’s exact test).
Traditional versus DWBI method trial in swine
When comparing the DWBI method of pBD-EC isolation to the traditional method using blood isolated from 22 pigs, we found that the DWBI method yielded more pBD-EC colonies (3.26 ±1.09 colonies per 10 ml blood) than the traditional method (0.49 ±0.20 colonies per 10 ml blood, p < 0.005, Wilcoxon signed rank sum test, Figure 2B).
Characterization of pBD-ECs under static conditions
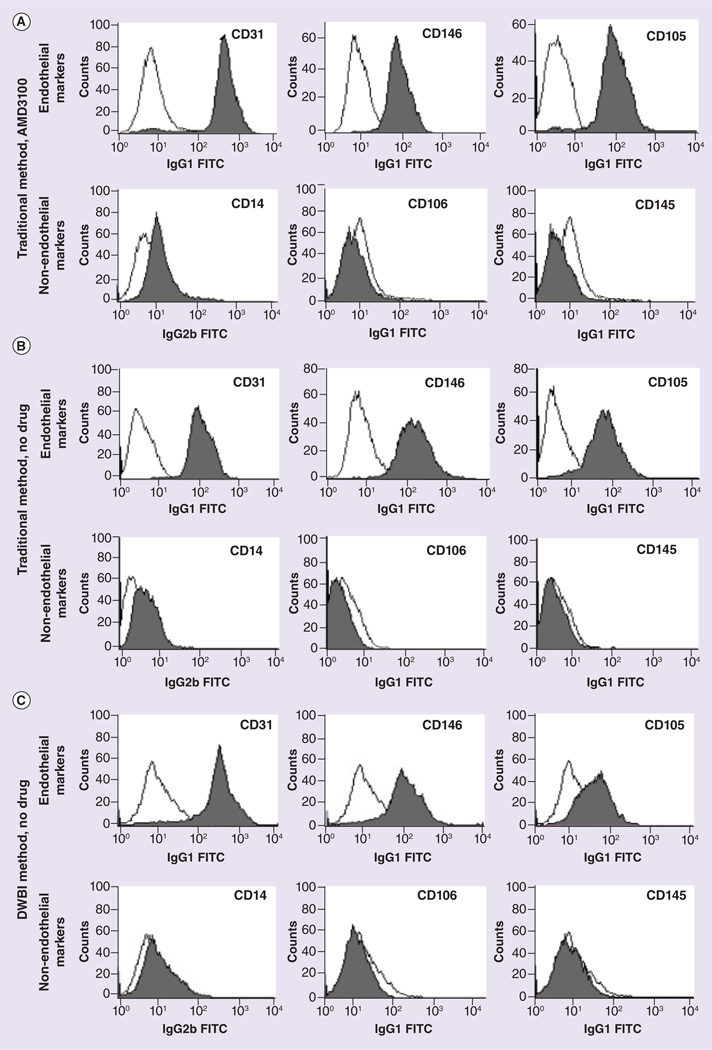
Flow cytometry confirmed the presence of CD31, CD146, CD105 and the absence of CD14, CD45 and CD106 in pBD-ECs isolated from animals treated or untreated with AMD3100 (Figure 3). pBD-ECs also equally took up DiI-Ac-LDL, whether isolated with or without AMD3100 mobilization, or using the traditional or DWBI methods (Figure 4A–C).
Figure 3. Flow cytometry results (see facing page).
(A) Flow cytometry results of peripheral blood-derived endothelial cell (pBD-EC) isolated from animals treated with AMD3100 using the traditional method. (B) Flow cytometry results of pBD-ECs isolated from animals without AMD3100 treatment, using the traditional method. (C) Flow cytometry results of pBD-ECs isolated from animals untreated with AMD3100 using the DWBI method.
DWBI: Diluted whole blood incubation.
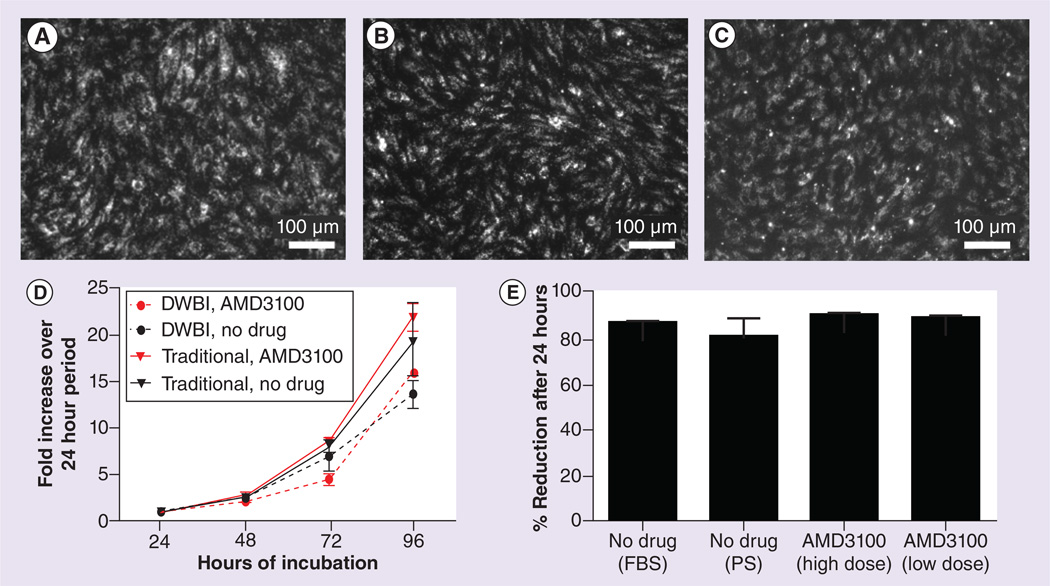
Figure 4. Characterization of peripheral blood-derived endothelial cells isolated under various conditions.
Peripheral blood-derived endothelial cells (pBD-EC) isolated using the traditional method from animals receiving no drug (A), using the traditional method from animals receiving AMD3100 (B) and using the DWBI method from animals receiving no drug (C), all incorporated DiI-labeled acetylated low-density lipoprotein (DiI-Ac-LDL). (D) Growth kinetics of the four pBD-EC groups. The four cell groups shown are pBD-ECs isolated with: the DWBI method and AMD3100 injection; the DWBI without AMD3100 injection; the traditional method and AMD3100 injection; the traditional method without AMD3100 injection. The population doubling times (PDT) for the four groups were: 0.83 ±0.13; 1.14 ±0.20; 0.68 ±0.04; and 0.90 ±0.15 days. The differences between the PDTs with and without AMD3100 were not significant (p = 0.12, between estimator of panel data), and the differences between PDTs of pBD-ECs isolated with the DWBI method and traditional method were not signficiant (p = 0.21, between estimator of a panel data model). Each error bar represents the SEM. (E) Measure of pBD-ECs’ metabolic activity. There were no significant differences in the percent reduction of alamarBlue between pBD-ECs isolated with AMD3100 and pBD-ECs in the control groups cultured in either FBS or PS (p = 0.438, one-way ANOVA).
DWBI: Diluted whole blood incubation; FBS: Fetal bovine serum; PS: Porcine serum.
A study of the growth kinetics revealed that cells isolated under the four different conditions – with the traditional method from animals treated or untreated with AMD3100, and with the DWBI method from animals treated or untreated with AMD3100 – had similar growth patterns (Figure 4D). The cells isolated with the traditional method from animals treated or untreated with AMD3100 had PDTs of 0.68 ±0.04 days and 0.90 ±0.15 days, respectively, while the PDTs for cells isolated with the DWBI method from animals treated or untreated with AMD3100 were 0.83 ±0.13 days and 1.14 ±0.20 days, respectively.
Any differences between the PDTs of these groups were not significant, whether comparing isolation using the DWBI and traditional methods (p = 0.21, between estimator of panel data), or whether the pBDECs were mobilized by AMD3100 or not mobilized (p = 0.12, between estimator of panel data).
The alamarBlue redox assay (Invitrogen) demonstrated that there was no significant difference in metabolic activity in pBD-ECs, regardless of whether they were isolated with a high dose or a low dose of AMD3100, or if the isolation was achieved from animals untreated with AMD3100 in medium containing either PS or FBS (p = 0.438, one-way ANOVA, n = 3, Figure 4E).
Characterization of pBD-ECs under fluid flow conditions
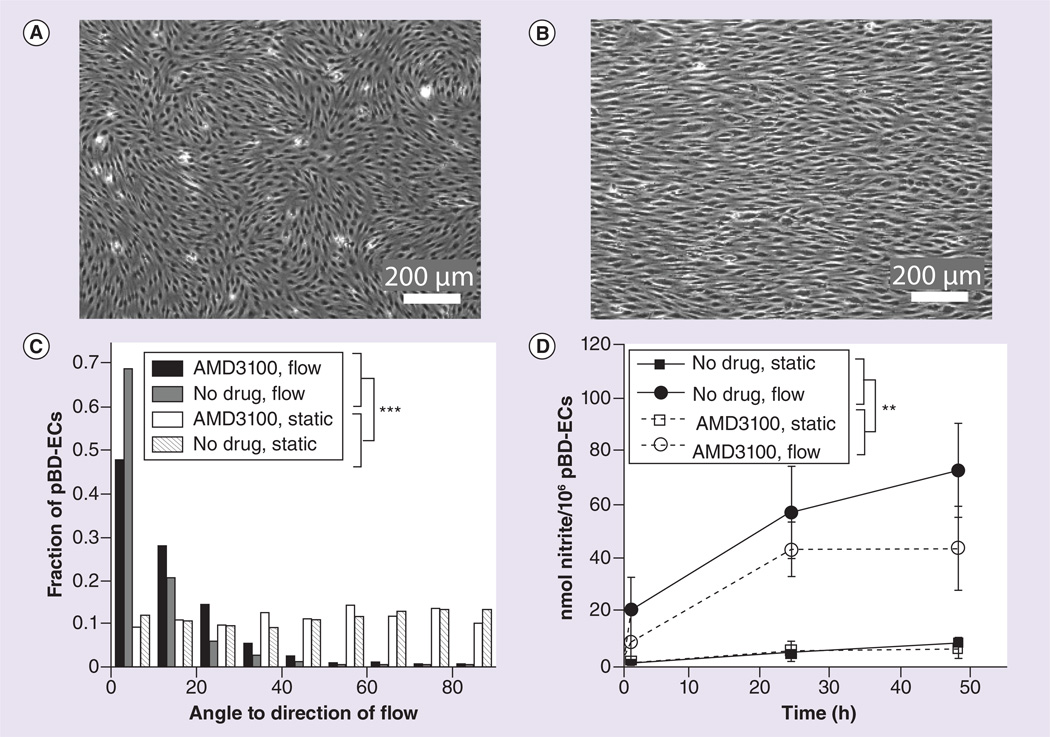
pBD-ECs that are grown to confluence on the surface of a fibronectin-coated microscope slide are initially randomly oriented (Figure 5A). However, exposure to 25 dynes/cm2 of fluid shear stress for 48 h aligned the major axes of the cells in the direction of the flow, consistent with an endothelial cell phenotype (Figure 5B). This effect was seen for pBD-ECs isolated from animals treated or untreated with AMD3100 via the traditional method (cells from animals treated with AMD3100: 14.3 ±13.6°, cells from untreated animals: 9.5 ±10.8°, Figure 5C) as compared with their corresponding static controls (cells from animals treated with AMD3100: 46.6 ±25.0°, p < 0.0001, Kolmogorov–Smirnov test; cells from untreated animals: 47.2 ±26.7°, p < 0.0001, Kolmogorov–Smirnov test, Figure 5C). In addition to alignment under flow, pBD-ECs from both groups also increased NO production approximately tenfold after 18 h at 25 dynes/cm2 under shear stress (p < 0.01, between estimator of panel data model, Figure 5D). At 24 h, nitrite production/106 pBD-ECs was 4.57 ±1.56 (for no drug, static), 56.9 ±34.6 (for no drug, flow), 5.23 ±6.40 (for AMD3100, static) and 43.1 ±20.3 (for AMD3100, flow). At 48 h, nitrite production/106 pBDECs was 8.16 ±2.55 (for no drug, static), 72.6 ±35.1 (for no drug, flow), 6.27 ±6.59 (for AMD3100, static) and 43.7 ±31.4 (for AMD3100, flow). pBD-ECs isolated from animals treated or untreated with AMD3100 exhibited no discernible functional differences in their production of NO (p = 0.362, between estimator of panel data model).
Figure 5. Characterization of peripheral blood-derived endothelial cells under fluid shear stress conditions.
(A) Representative image of random pBD-EC orientation under static conditions (cells mobilized by AMD3100 shown here). (B) Representative image of pBD-EC alignment following 48 h of 25 dynes/cm2 fluid shear stress (cells mobilized by AMD3100 shown here). (C) Angles with respect to the direction of flow for pBD-ECs under both flow and static conditions. Static cells showed no bias, while cells under flow showed alignment. (D) Nitrite production was measured as surrogate marker for nitric oxide (NO). Cells under flow exhibited increased NO production relative to static control groups. Each error bar represents the SEM. The effect of shear stress was significant (** p < 0.01, between estimator of panel data model); however, there was no difference in NO production among pBD-ECs isolated from animals treated or untreated with AMD3100 (p = 0.362, between estimator of panel data model).
pBD-EC: Peripheral blood-derived endothelial cell.
Short-term inferior vena cava (IVC) implantation study
pBD-ECs were isolated with the DWBI method after AMD3100 mobilization and implanted into the IVC of those pigs from which the cells had been derived. Three days after implantation, all four Ti tubes coated with autologous pBD-ECs were clot-free (Figure 6A). On the other hand, two control implants with bare, uncoated Ti tubes in two other pigs were completely or partially clotted (Figure 6B).
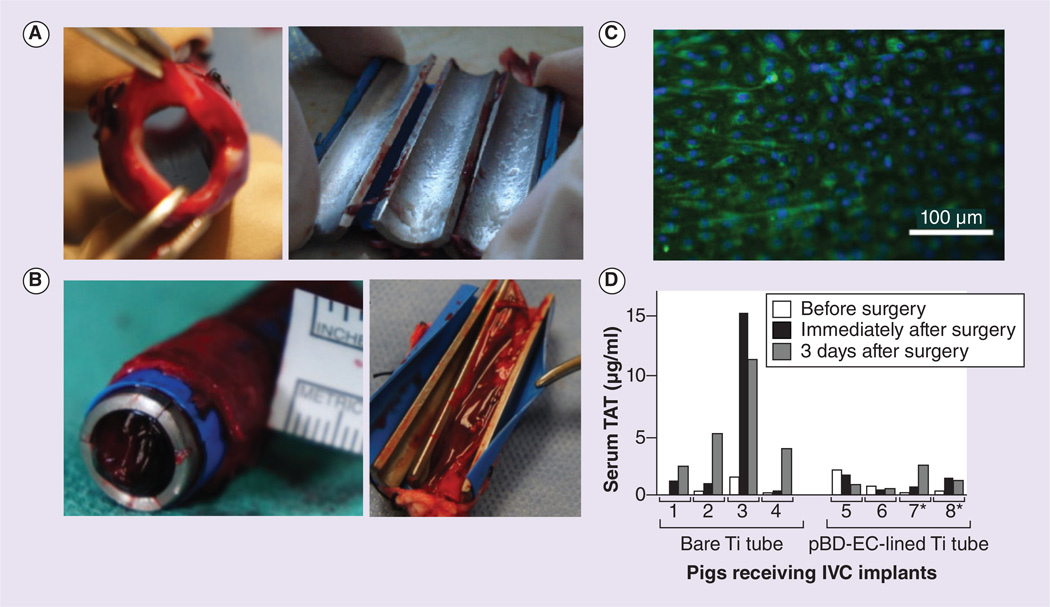
Figure 6. Inferior vena cava implanation study.
(A) Four Ti tubes coated with pBD-ECs remained clot free after removal from pig IVCs three days following implantation. Those pBD-ECs were implanted in the same pigs from which they were derived; all the cells were isolated using the diluted whole blood incubation method were combined, while two of the pigs received pBD-ECs isolated following AMD3100 mobilization (shown here), and the other two received pBD-ECs that were isolated with no mobilization. (B) Bare Ti tubes implanted into the IVCs of four pigs were completed or partially clotted. (C) Expression of endothelial nitric oxide synthase (eNOS, green) by pBD-ECs (nuclei are blue) on Ti tubes after 3 days of IVC implantation. (D) TAT levels for each of the eight pigs receiving implants (four lined by pBD-ECs, four bare metal) immediately before surgery, immediately after surgery, and 3 days postsurgery. * Indicates pBD-ECs isolated following injection of AMD3100.
IVC: Inferior vena cava; pBD-EC: Peripheral blood-derived endothelial cell; TAT: Thrombin-antithrombin complex; Ti: Titanium.
CD31 and eNOS staining of the ECs on the cell-coated Ti tubes was performed after explanation and confirmed that the ECs spread to confluency and maintained their endothelial cell phenotype (Figure 6C).
Systemic response to autologous pBD-ECs in porcine model
TAT was measured by an enzyme immunoassay (Enzygnost TAT micro) in all pigs receiving IVC implants immediately before surgery and 3 days post-surgery. Over that time period, the pigs with bare metal Ti implants exhibited a significantly greater increase in TAT (5.18 ±1.62 µg/mg) than the pigs with pBD-EC-coated implants (0.40 ±0.75 µg/mg, p < 0.05, Wilcoxon rank sum test, Figure 6D).
Discussion
One of the limitations of utilizing autologous pBD-ECs is the low yield of isolation. Our results show that this problem may be overcome by improving the isolation technique through removal of centrifugation steps and instead directly plating diluted whole blood. Additionally, progenitor cells may be mobilized with AMD3100, which has been shown in previous studies to mobilize hematopoietic stem cells for clinical applications [25,26]. AMD3100 is an attractive agent for translation of pBD-EC therapies into clinical practice because its maximal mobilization is achieved approximately 9 h after administration of a single subcutaneous injection in humans [27]. Consequently, Shepherd et al. tested if AMD3100 would mobilize human pBD-ECs in a well-designed clinical study and reported a 10.2-fold increase of pBD-ECs following AMD3100 administration [28]. These results were interpreted as bone-marrow mobilization of progenitor cells giving rise to pBD-ECs. Similarly, Pitchford et al. concluded that AMD3100 causes the egress of progenitor cells from bone marrow after conducting isolated murine hind limb perfusion experiments [29]. In their system, the femoral artery and vein were cannulated in order to isolate the tibia bone marrow from other tissues. However, this experimental setup did not exclude the vasculature and other studies suggest a vascular origin of pBD-ECs. Lin et al. collected peripheral blood from sex-mismatched bone marrow transplant patients and found that approximately 95% of pBD-ECs grown out from blood initially expressed the recipient genotype, but after 4 weeks this percentage was reversed with the vast majority of cells expressing the donor genotype [30]. It was concluded that most pBD-ECs were vessel-derived but highly proliferative rare cells existed in bone marrow. Recently, Tura et al. sought to grow out pBD-ECs from peripheral blood and bone marrow, the latter of which did not yield any pBD-ECs and the authors concluded that pBD-ECs are not derived from bone marrow [14]. If true, this conclusion leads to the question of how AMD3100 could mobilize progenitor cells from bone marrow that grow out pBD-ECs. Two conceivable explanations are as follows: first, AMD3100-mobilized cells belong to a different population than nonmobilized cells in which case the pBD-ECs grown out from two different populations should exhibit functional differences, which we could not detect in our study, or second, AMD3100 might mobilizes pBD-ECs or their progenitor cells from the vessel walls. The latter theory has not been investigated in the literature but receives support through several facts: pBD-ECs express the CXCR4 receptor [31], its ligand SDF-1 is expressed in the media of blood vessels [32] and intravascularly administered stem cells with high concentrations of CXCR4 preferentially home to areas of vascular injury [33]. We believe that further research into the mechanisms of progenitor cell mobilization with mobilizing agents may reveal the much-debated origin of pBD-ECs.
We conducted a crossover trial in a large animal model with each animal receiving two test and two control injections (high- and low-dose AMD3100) in order to minimize any variability in the mobilization response between different pigs. We found that a significantly increased number of pBD-EC colonies are isolated with subcutaneous AMD3100 administration and that these cells are phenotypically and functionally indistinguishable from pBD-ECs without mobilizing agent: pBD-ECs with or without mobilizing agent expressed typical EC markers CD31, CD105 and CD146. CD146 has also been referred to as the ‘gold standard’ for the selection of ECs [34]. Moreover, the absence of CD14 and CD45 in our pBD-ECs confirms that the isolated cells are not part of the monocyte, macrophage or lymphocyte lineages [35,36]. We also ruled out the possibility that ECs are in an activated, proinflammatory state by demonstrating the absence of the inflammatory marker CD106 that mediates leukocyte-endothelial cell adhesion.
pBD-ECs with or without AMD3100 mobilization exhibited similar levels of DiI-Ac-LDL uptake, metabolic activity and growth patterns under static culture. Since healthy ECs are constantly exposed to flow that is exerting a tangential shear stress on the cell surface in vivo, rigorous functional analyses should include assessment of pBD-EC function under flow conditions. We found that pBD-ECs with or without AMD3100 mobilization aligned in the direction of fluid flow shear stress when exposed to a shear stress of 25 dynes/cm2, mimicking the magnitude of arterial shear stress. There was no statistical significant difference in the time-dependent production of NO between cells isolated from animals that were treated with AMD3100 or untreated. As expected, pBD-ECs under fluid flow shear stress secreted significantly more NO under flow as compared with static culture conditions (approximately tenfold more NO production after 48 h at 25 dynes/cm2, p < 0.01). These results are analogous to our prior studies with porcine pBD-ECs isolated with the traditional method (approximately sevenfold more NO production after 48 h at a lower shear stress of 15 dynes/cm2) [16] and almost identical to human umbilical cord blood-derived ECs with the DWBI method (approximately tenfold increase in NO production after 48 h at 25 dynes/cm2) [6].
Whereas our previous study with human umbilical cord blood demonstrated an approximate 20-fold increase of EC isolations with the DWBI method [6], we were able to increase the yield from porcine blood approximately sevenfold. These differences may be due to a larger number of available progenitor cells in human cord blood as compared with adult pig blood. Nevertheless, we confirmed that cells isolated with the DWBI method are functional, based on their ability to spread and adhere under blood flow in pig IVCs.
In the current study the local and systemic effects of autologous pBD-ECs derived with the DWBI-method from animals treated or untreated with AMD3100 were evaluated in a large animal model. The porcine model was chosen rather than a rodent model because the large animal model more closely approximates potential human studies with autologous cells for personalized cell therapy.
We implanted pBD-EC-lined Ti tubes into the IVCs of four pigs, two of which received pBD-ECs mobilized by AMD3100, while the other two received pBD-ECs isolated without mobilization. In all four pigs, CD31 staining of the cells after explantation confirmed that the pBD-ECs spread to confluence under flow conditions in vivo and maintained their endothelial cell phenotype. Additionally, pBD-EC function was confirmed 3 days after implantation into the IVC by positive staining for eNOS, indicating the presence of the EC-specific NO secreting enzyme in vivo (Figure 6D). Since pBD-ECs had been prelabeled with PKH26 before implantation, we were able to rule out that adherent pBD-ECs were derived from possible circulating ‘fallout’ cells that attached after implantation of Ti tubes into the animals.
In accordance with secretion of antithrombotic markers such as NO, the pBD-EC-seeded implants remained free of clot (Figure 6A). In contrast, four bare metal Ti tubes were either fully or partially thrombosed after 3 days (Figure 6B).
The in vivo systemic effects of pBD-ECs were evaluated by quantifying TAT production. TAT complexes are formed when the coagulation system is activated [37–39]. The coagulation activity was significantly reduced in pigs that received pBD-EC-coated Ti implants as compared with pigs that received bare metal Ti implants (Figure 6D). These results demonstrate that autologous pBD-ECs not only reduce clot formation locally, but also exert beneficial effects systemically.
Our study has several limitations. We were unable to establish a significant benefit of the use of allogenic serum (PS) as compared with xenogenic serum (FBS); however, we noticed a trend with allogenic serum producing lower numbers of ‘contaminating cells’ with non-EC morphology (see Figure 2F, colony representative of cells with non-EC morphology). We speculate that this observation may be due to different growth factors in xenogenic serum and proceeded to only utilize PS for our experiments in pigs.
In our analysis, the 1.8-fold increase in number of pBD-EC colonies with higher doses of AMD3100 as compared with lower doses was not statistically significant (p = 0.234), likely because of the limited number of six pigs in the AMD3100 crossover trial. We also did not quantify the increased yield if AMD3100 mobilization and the DWBI method are combined; rather we found that the DWBI method increased yield approximately 6.6-fold (p < 0.005) and AMD3100 mobilization approximately 4.5-fold (p < 0.0001), independent of each other.
Further improvements in isolation technique in conjunction with the use of mobilizing agents may solve the low-yield problem of utilizing pBD-ECs for the purpose of tissue engineering replacement organs and in the clinical setting. Future studies should expand the established animal model to long-term in vivo studies and to clinical studies focusing on patient groups that would benefit most from autologous EC therapies.
Conclusion
To increase pBD-EC isolation yield to the scale necessary for clinical cell therapies, we administered AMD3100 prior to blood draw and developed a novel DWBI method for cell culture. Those interventions increased the isolation yield of pBD-ECs from porcine peripheral blood 4.5-fold and 6.6-fold, respectively. The isolated pBD-ECs were fully functional as demonstrated through in vitro and in vivo experiments. Our findings augment the therapeutic potential of pBD-ECs for cardiovascular diseases as we have demonstrated their ability to prevent thrombosis when seeded onto titanium implants.
Future perspective
pBD-ECs are a promising tool for cell therapies. Aided by improved yield during isolation due to AMD3100-induced mobilization and DWBI, we predict that autologous pBD- ECs will be utilized for cell therapies to repair vascular damage, as well as lining the blood-contacting surfaces of implants such as stents, grafts, and ventricular assist devices to prevent thrombosis and improve biocompatibility. In addition, the neovascularization capability of pBD-EC also holds significant potential for the engineering of solid organs that require an extensive blood supply.
Executive summary.
Background
Peripheral blood-derived endothelial cells (pBD-ECs) are an attractive tool for treatment of cardiovascular diseases, but such therapies have been hindered by low cell isolation yields.
Results
Isolation yield of porcine pBD-ECs is increased by AMD3100 administration before blood draw as well as culturing the cells using a novel diluted whole blood incubation method. Isolated pBD-ECs were fully functional and antithrombotic, as demonstrated through in vitro and in vivo experiments.
Discussion
AMD3100 administration and the diluted whole blood incubation cell culture method enhance the therapeutic potential of pBD-ECs for cardiovascular diseases.
Acknowledgements
The authors would like to acknowledge the following contributions: conception and design: RM Jamiolkowski, SD Kang, NR Haley, O Alzate, F-H Lin, G Truskey and HE Achneck; collection and/or assembly of data: RM Jamiolkowski, SD Kang, AK Rodriguez, JM Haseltine, LJ Galinat, AE Jantzen, TA Carlon, MD Darrabie, AJ Arciniegas, JG Mantilla, JD Allen, TV Stabler, JW Frederiksen, LG Keil, S Liu, F-H Lin and HE Achneck; data analysis and interpretation: RM Jamiolkowski, SD Kang, AK Rodriguez, JG Mantilla, M Noviani, O Alzate and HE Achneck; manuscript writing: RM Jamiolkowski, SD Kang, AK Rodriguez, M Noviani, G Truskey and HE Achneck; final approval of manuscript: G Truskey and HE Achneck.
Subsequent to the submission, review, and acceptance of this manuscript, HE Achneck accepted an employment position at Hemostemix, Inc. This study was supported by grants from the NIH (R21 HL109897–01) and AHA (12BGIA11070002, Microenvironment to enhance EPC outgrowth, proliferation and function) to HE Achneck.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest
- 1. Fan CL, Gao PJ, Che ZQ, Liu JJ, Wei J, Zhu DL. Therapeutic neovascularization by autologous transplantation with expanded endothelial progenitor cells from peripheral blood into ischemic hind limbs. Acta Pharmacol. Sin. 2005;26(9):1069–1075. doi: 10.1111/j.1745-7254.2005.00168.x. ; Demonstrates the clinical promise of peripheral blood-derived endothelial cells in the treatment of peripheral vascular disease.
- 2.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler. Thromb. Vasc. Biol. 2007;27(7):1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 3.Eirin A, Zhu X-Y, Li Z, et al. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler. Thromb. Vasc. Biol. 2013;33(5):1006–1013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce WG, Zanette C, Caplice NM, Mackrill JJ. Calcium signalling in adult endothelial outgrowth cells. Biochem. Biophys. Res. Commun. 2012;417(1):358–363. doi: 10.1016/j.bbrc.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 5.Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J. Cell. Mol. Med. 2009;13(1):87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang SD, Carlon TA, Jantzen AE, et al. Isolation of Functional human endothelial cells from small volumes of umbilical cord blood. Ann. Biomed. Eng. 2013;41(10):2181–2192. doi: 10.1007/s10439-013-0807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105(7):2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 8. Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. • First early characterization of peripheral blood-derived endothelial cells, as well as ‘traditional’ methods of cell isolation and culture.
- 9.Fricker SP. Physiology and pharmacology of plerixafor. Transfus. Med. Hemother. 2013;40(4):237–245. doi: 10.1159/000354132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nervi B, Link DC, Dipersio JF. Cytokines and hematopoietic stem cell mobilization. J. Cell. Biochem. 2006;99(3):690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 11. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24–31. doi: 10.1038/leu.2012.254. • Authors sought to grow out endothelial cells (ECs) from peripheral blood and bone marrow, the latter of which did not yield any ECs These results support the conclusion that peripheral blood-derived ECs are not derived from bone marrow
- 12.Wagstaff AJ. Plerixafor: in patients with non-Hodgkin’s lymphoma or multiple myeloma. Drugs. 2009;69(3):319–326. doi: 10.2165/00003495-200969030-00007. [DOI] [PubMed] [Google Scholar]
- 13. Yoder MC. Is Endothelium the Origin of Endothelial Progenitor Cells? Arterioscler. Thromb. Vasc. Biol. 2010;30(6):1094–1103. doi: 10.1161/ATVBAHA.109.191635. • Demonstrates porcine peripheral blood-derived endothelial cell phenotype when grown on titanium for in vitro conditions, while establishing the in vitro flow circuit techniques used in this study.
- 14. Tura O, Skinner EM, Barclay GR, et al. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells. 2013;31(2):338–348. doi: 10.1002/stem.1280. • Demonstrates porcine peripheral blood-derived endothelial cell phenotype when grown on the blood-contacting surfaces of titanium implants inserted into pig vasculature, establishing the in vivo techniques used in this study.
- 15.Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, et al. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achneck HE, Jamiolkowski RM, Jantzen AE, et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti implants. Biomaterials. 2011;32(1):10–18. doi: 10.1016/j.biomaterials.2010.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jantzen AE, Lane WO, Gage SM, et al. Use of autologous blood-derived endothelial progenitor cells at point-of-care to protect against implant thrombosis in a large animal model. Biomaterials. 2011;32(33):8356–8363. doi: 10.1016/j.biomaterials.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroncek JD, Grant BS, Brown MA, Povsic TJ, Truskey GA, Reichert WM. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng. Part A. 2009;15(11):3473–3486. doi: 10.1089/ten.tea.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane WO, Jantzen AE, Carlon TA, et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. J. Vis. Exp. 2012;(59) doi: 10.3791/3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide-Biol. Chem. 2009;20(4):231–237. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achneck HE, Jamiolkowski RM, Jantzen AE, et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells EPC-seeded antithrombotic Ti Implants. Biomaterials. 2011;32(1):10–18. doi: 10.1016/j.biomaterials.2010.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jantzen AE, Lane WO, Gage SM, et al. Autologous endothelial progenitor cell-seeding technology and biocompatibility testing for cardiovascular devices in large animal model. J. Vis. Exp. 2011;(55):e3197. doi: 10.3791/3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velik-Salchner C, Schnurer C, Fries D, et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb. Res. 2006;117(5):597–602. doi: 10.1016/j.thromres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao C. Analysis of Panel Data (2nd Edition) New York, NY, USA: Cambridge University Press; 2003. [Google Scholar]
- 25.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 27.Bakanay SM, Demirer T. Novel agents and approaches for stem cell mobilization in normal donors and patients. Bone Marrow Transplant. 2012;47(9):1154–1163. doi: 10.1038/bmt.2011.170. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd RM, Capoccia BJ, Devine SM, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108(12):3662–3667. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4(1):62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smadja DM, Bieche I, Uzan G, et al. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler. Thromb. Vasc. Biol. 2005;25(11):2321–2327. doi: 10.1161/01.ATV.0000184762.63888.bd. [DOI] [PubMed] [Google Scholar]
- 32.Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2007;27(2):263–265. doi: 10.1161/01.ATV.0000256727.34148.e2. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Shibata R, Ishii M, et al. Therapeutic reendothelialization by induced pluripotent stem cells after vascular injury–brief report. Arterioscler. Thromb. Vasc. Biol. 2013;33(9):2218–2221. doi: 10.1161/ATVBAHA.113.301313. [DOI] [PubMed] [Google Scholar]
- 34.Mund JA, Estes ML, Yoder MC, Ingram DA, Jr, Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2012;32(4):1045–1053. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, Le Beau MM. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988;239(4839):497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Bailey MA, Griffin KJ, Sohrabi S, et al. Plasma thrombin-antithrombin complex, prothrombin fragments 1 and 2, and D-dimer levels are elevated after endovascular but not open repair of infrarenal abdominal aortic aneurysm. J. Vasc. Surg. 2013;57(6):1512–1518. doi: 10.1016/j.jvs.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 38.Ataga KI, Brittain JE, Desai P, et al. Association of coagulation activation with clinical complications in sickle cell disease. PLoS ONE. 2012;7(1):e29786. doi: 10.1371/journal.pone.0029786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerman MP, Green D, Gilman-Sachs A, et al. Coagulation changes in individuals with sickle cell trait. Am. J. Hematol. 2002;69(2):89–94. doi: 10.1002/ajh.10021. [DOI] [PubMed] [Google Scholar]