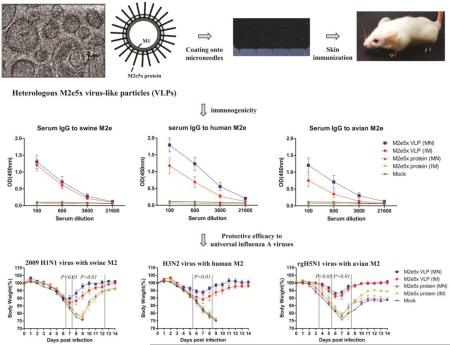
Abstract
A broadly cross-protective influenza vaccine that can be administrated by a painless self-immunization method would be a value as a potential universal mass vaccination strategy. This study developed a minimally-invasive microneedle (MN) patch for skin vaccination with virus-like particles containing influenza virus heterologous M2 extracellular (M2e) domains (M2e5x VLPs) as a universal vaccine candidate without adjuvants. The stability of M2e5x VLP-coated microneedles was maintained for 8 weeks at room temperature without losing M2e antigenicity and immunogenicity. MN skin immunization induced strong humoral and mucosal M2e antibody responses and conferred cross-protection against heterosubtypic H1N1, H3N2, and H5N1 influenza virus challenges. In addition, M2e5x VLP MN skin vaccination induced T-helper type 1 responses such as IgG2a isotype antibodies and IFN-γ producing cells at higher levels than those by conventional intramuscular injection. These potential immunological and logistic advantages for skin delivery of M2e5x VLP MN vaccines could offer a promising approach to develop an easy-to-administer universal influenza vaccine.
Keywords: M2e5x VLPs, Influenza universal vaccine, Microneedles, Cross-protection
Graphical Abstract
1. Introduction
Influenza virus is one of the most common causes of serious respiratory illness resulting in approximately 20,000 to 50,000 deaths annually worldwide [1-2]. The limitations of current influenza vaccines include the strain-specific protection, uncertainty in the prediction of the appropriate vaccine strains, and the inability to prevent a pandemic influenza. In 2009, a new influenza A strain emerged and spread globally at alarming speeds, which caused the first 21st century pandemic [3-5]. In order to effectively control a pandemic outbreak by overcoming strain-specific limitations, universal vaccine strategies have been reported by demonstrating protections against antigenically different influenza strains [6].
Although the extracellular domain of ion channel M2 protein (M2e) is relatively well conserved among influenza A viruses, M2e is intrinsically low immunogenic due to its small size, its low level of incorporation into virions, and shielding effects by larger viral surface proteins, hemagglutinin and neuraminidase [7]. Therefore, several strategies have been attempted to improve its immunogenicity including fusion of M2e peptides with carrier proteins or vehicles formulated in adjuvants such as cholera toxin subunits and flagellin [8-11]. Despite their ability to stimulate immune responses, these adjuvants would not be approved for human use because of potential toxicity. In addition, few amino acid (aa) changes are noted within the central M2e residues among human, swine, and avian influenza A viruses (aa10-13, human type: PIRN; swine type: PTRS; avian type: PTRN) which can cause antigenic difference to M2e [12].
Virus-like particles (VLPs) are similar to virus in structure and morphology but non-replicating. VLPs are effective in delivering vaccine antigens and in inducing immune responses probably due to their particulate nature. Recently, we developed a tandem repeat construct of M2e5x composed of human, swine, and avian influenza virus M2e sequences on VLP (M2e5x VLP) that conferred a broad range of cross protection after conventional intramuscular immunization [13]. However, studies on effective vaccination methods for a broad cross-protection are much limited. Thus, it is important to carry out comparative studies to evaluate immune responses and protective efficacy by M2e5x VLP vaccines depending on the routes of administration, i.e. IM and transdermal skin immunization.
The skin is not merely a physical barrier, but a promising alternative route of vaccination for inducing immune responses [14-16]. In addition to immunogenicity, skin administration using MN has been shown to be painless, simple to administer, and well accepted by patients and healthcare providers [17-18]. Here we hypothesized that this alternative skin vaccination with M2e5x VLP would be similar or better in producing M2e immunity and cross protection compared to conventional intramuscular (IM) needle injection immunization and soluble M2e5x protein vaccines. From the comparative evaluation of the immune response between skin route using MN patches and conventional IM route, this study demonstrated that skin vaccination of M2e5x VLP induced cross-protective immunity in mice, providing evidence of a proof-of-concept for its potential application as a simple-to-administer universal influenza vaccine for further development.
2. Materials and methods
2.1. Viruses and influenza M2e5x VLP
Influenza A viruses, A/California/04/09 (A/California, H1N1), A/Philippines/2/82 (A/Philippines, H3N2), and A/Mandarin Duck/Korea/PSC24-24/2010 (A/Mandarian duck, avian rgH5N1 containing HA with polybasic residues removed, NA and M genes from A/Mandarin Duck, and the remaining backbone genes from A/PR8 virus), were grown in 10-day-old chicken eggs at 37°C for 2 days. The viruses were inactivated using formalin [19]. M2e5x VLPs were produced as previously described [13]. The morphology of M2e5x VLP was analyzed by using cryogenic-transmission electron microscopy (cryo-TEM, JEOL 2200 FS; JEOL USA, Peabody, MA) at an acceleration voltage of 200 kV. Specimen was prepared by plunge-freezing thin aqueous films. Briefly, 7 μl of M2e5x VLP suspension in PBS (0.4 mg/ml) was placed on top of holey carbon grid, followed by blotting off excess solution. Samples were vitrified by rapidly plunging them into liquid ethane, transferred in liquid nitrogen, and loaded into a cryo-holder for analysis.
2.2. M2e5x protein preparation
M2e5x protein was expressed from yeast. In brief, DNA fragment of M2e5x construct was cloned into the pPIC9 vector (Life technology, NY, USA) for secretory expression in the yeast. The recombinant plasmid was transformed into P. pastoris strain GS115, phenotype Mut+ (methanol utilization plus) by electroporation (Life Technologies). P. pastoris transformants were inoculated into BMGY medium (1% yeast extract, 2% peptone, 1.34% YNB, 1%glycerol, 100 mM potassium phosphate, pH 6.0) and incubated at 30°C for 48 h under vigorous agitation (240 rpm). For the induction of the M2e5x protein, the yeast transformants were transferred to BMMY medium (the same components as those of BMGY with glycerol replaced by 0.5% methanol). Methanol was added to a final concentration of 1% (v/v) on the second day and increased to 1.5% (v/v) on the third and fourth days. The culture was kept at 30°C with agitation for 72 h. Then the supernatants were recovered. M2e5x proteins were purified by ion exchange chromatography on Q-Sepharose (GE Healthcare, PA) followed by hydrophobic interaction chromatography on phenyl-Sepharose 6FF column (GE Healthcare, PA).
2.3. Preparation of microneedle (MN) patches
A vaccine patch with MNs was prepared by fabricating arrays of solid MNs and coating vaccine antigen on the surface of MNs as described previously [20-21]. Briefly, rows of solid metal microneedles were made by wet-etching photolithographically defined needle structures from stainless steel sheets (Tech Etch, Plymouth, MA). The resulting MNs measured 700 μm in length and 200 μm in width. To coat a layer of vaccine, MNs were first oxygen-plasma treated to make the MN surface more hydrophilic, dipped multiple times into coating solution containing M2e5x VLP or M2e5x proteins to load the vaccine dose designed for this study and air dried at room temperature (R.T.) [22]. The coating solution was composed of 1% (w/v) carboxymethyl cellulose (CMC) sodium salt (Carbo-Mer, San Diego, CA) as a viscosity enhancer and 15% (w/v) D-(+)-trehalose dehydrate (Sigma-Aldrich, St. Louis, MO) used as a stabilizer. A patch with an array of five MNs coated with 2 μg of influenza M2e5x VLPs or M2e5x proteins (total proteins) was used to vaccinate animals. Mock vaccination was carried out using microneedles without M2e5x VLPs or M2e5x proteins.
2.4. Stability of M2e5x VLP MN patches
The coated MNs were kept at 4°C and room temperature (R.T.) for stability test. In order to test the stability of M2e5x VLPs or M2e5x proteins after the coating process, MN patches coated with VLPs or proteins were dissolved in Na-Bicarbonate ELISA coating buffer. To determine M2e reactivity of M2e5x VLPs or proteins, the dissolved M2e5x VLPs or proteins were serially diluted four times, coated into ELISA immunoplate from 250 ng to 4 ng of total protein of M2e5x VLPs or proteins, and incubated at 4°C overnight. Monoclonal M2e antibody (14C2, Abcam) was used as primary antibody and HRP-conjugated goat anti-mouse IgG was used as secondary antibodies to determine total IgG antibody. Tetramethylbenzidine substrate (Sigma-Aldrich, St. Louis, Mo) and 1M H3PO4 were used to develop color and to stop color reaction, respectively. The optical density was read with an ELISA reader at 450 nm.
To test immunogenicity of M2e5x VLP coated MN patches stored at 4°C and R.T. for 8 weeks, six to eight-week-old female BALB/c mice (Charles River) were prime-immunized through the skin and boost-immunized at 4-weeks interval (5 mice per group, 2 μg of total protein). MN delivery to the skin was followed as described previously [23]. To compare M2e antibody response, sera were taken 3 weeks after each vaccination. Full details of this study and all animal experiments presented in this manuscript were approved by the IACUC review board. Approved IACUC protocols operate under the federal Animal Welfare Law (administered by the USDA) and regulations of the Department of Health and Human Services.
2.5. Immunization and challenge
Six to eight-week-old female BALB/c mice (Charles River) were immunized on the skin with MN arrays coated with 2 μg of total M2e5x VLPs or M2e5x proteins comparing to intramuscular (IM) injections using M2e5x VLPs dissolved in PBS with M2e5x VLP MN array. To compare M2e antibody responses, sera were collected 3 weeks after each vaccination. For challenge infections, mice lightly anesthetized with isoflurane were intranasally infected with a lethal dose of A/California/04/09 (H1N1) (2× 50% lethal dose [LD50]), A/Philippines/2/82 (H3N2) (4× LD50), or avian rgH5N1 (with mutant H5 HA, N1 NA, and M from A/Mandarian duck/Korea/PSC24-24/2010) (2× LD50) 6 weeks after boost vaccination. Mice were observed daily to monitor changes in body weight and to record mortality.
2.6. Antibody responses
M2e specific antibody responses were measured by ELISA using synthetic human, swine, or avian M2e peptides (2μg/ml) as previously described [13, 24-25].
2.7. Analysis of antibodies in bronchoalveolar lavages after challenge
Bronchoalveolar lavage fluids (BALF, N=4) were prepared for the analysis of antibody responses from mice at day 4 after challenge with 4× LD50 of influenza A/Philippines (H3N2) virus. BALF samples were collected via the trachea by infusing 1 ml of PBS into the lungs using a 25-gauge catheter (Exelint International Co., Los Angeles, CA)[13]. M2e specific or virus-specific antibody responses in BALF were measured by ELISA using synthetic M2e peptides as a coating antigen (2μg/ml) [13].
2.8. Inflammatory cytokine and lung viral titer
Inflammatory cytokines interleukin-6 (IL-6) in bronchoalveolar lavage fluid (BALF) collected at day 4 post-challenge was analyzed by the cytokine ELISA kit (eBioscience) according to the manufacturer's procedure as previously described [26]. Lung tissues were homogenized by mechanically grinding lung tissue using cell strainers with 1.5 ml of PBS per each lung and the lung extracts were collected. Ten-day-old embryonated chicken eggs were inoculated with serially diluted lung extracts and incubated at 37°C for 2 days, and hemagglutination activity was tested to determine viral titers as described [27].
2.9. Determination of T cell responses
At day 4 post challenge, lung cells were isolated from corresponding tissue samples as described [28]. Interferon (IFN)-γ secreting cell spots were determined on Multi-screen 96 well plates (Millipore, Billerica, MA) coated with cytokine specific capture antibodies as described [13]. Briefly, 0.2×106 lung cells per well were cultured with challenged A/Philippines/2/82 virus (2 μg/ml) as an antigenic stimulator. After 36 h incubation, the spots of IFN-γ secreting T cells were counted using an ELISpot reader (BioSys, Miami, FL).
2.10. Statistical analysis
To determine the statistical significance, a two-tailed Student's t-test was used when comparing two different conditions. A P-value less than 0.05 was considered to be significant.
3. Results
3.1. Construction of recombinant M2e5x VLP or M2e5x protein and MN array coating
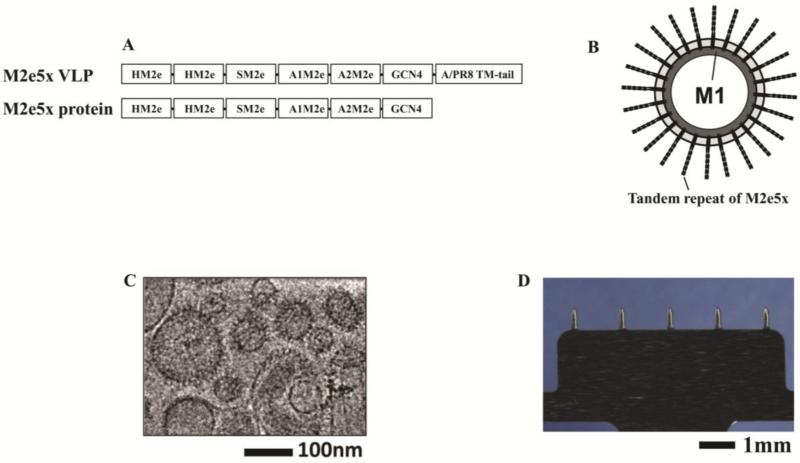
Constructs of tandem repeat M2e5x in M2e5x VLPs or M2e5x proteins were designed and generated in an effort to develop universal influenza vaccines (Fig. 1A). The tandem repeat of M2e5x construct is consisted of heterologous M2e sequences containing M2 extracellular domains (M2e) derived from human, swine, and avian species host influenza A viruses [13]. A schematic diagram of the M2e5x VLP vaccine is shown in Fig. 1B, exhibiting M2e5x protein on the surface and M1 matrix core proteins inside the particle. As shown in Fig. 1C, the prepared M2e5x VLP vaccine was found to resemble influenza virus particles in morphology and size. Especially, spike-like proteins can be clearly identified on the vaccine envelope in the cryo-TEM micrograph. The MNs used in this study were prepared as single rows containing five MNs each measuring 700 μm long, 200 μm wide at the base, and 50 μm in thickness. MN vaccines with dry coating of M2e5x VLPs showed a uniform dry coating on the surfaces of MN arrays (Fig. 1D). Similarly to M2e5x VLPs, a uniform coating of MN arrays with M2e5x proteins was obtained from MNs coated with M2e5x proteins (data not shown). Taken together, these results show that M2e5x VLPs, a particulate vaccine structurally similar to the influenza virus, were successfully coated onto MNs.
Fig. 1. MNs and M2e5x VLPs or M2e5x proteins for vaccination.
(A) Structure of M2e5x VLP or M2e5x proteins. HM2e: human M2e, SM2e: swine M2e (2009 pandemic flu), A1M2e: major avian M2e, A2M2e: minor avian M2e. (B) Schematic diagram of influenza M2e5x VLPs containing tandem repeat of heterologous M2e and matrix (M1) proteins. (C) Cryo-TEM (transmission electron microscopy) image of influenza M2e5x VLPs. (D) Microneedle array coated with M2e5x VLPs.
3.2. M2e5x VLPs coated onto MN arrays maintain M2e epitope integrity and are immunogenic
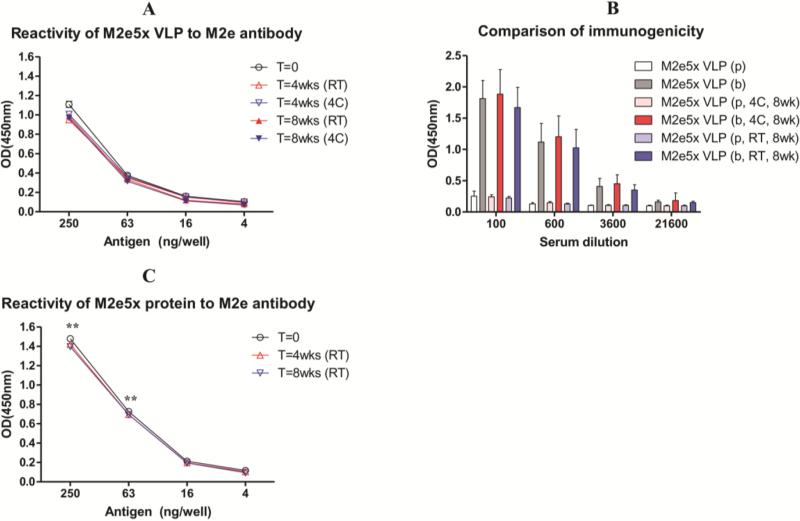
After coating MN arrays with a formulation containing M2e5x VLPs as antigen, stability of the M2e5x VLP vaccine was measured based on the reactivity of dissolved-off M2e5x VLPs against M2e monoclonal antibody (14C2). We compared M2e antibody (14C2) reactivity of coated M2e5x VLP vaccines with respect to different temperature and time of storage after drying coated M2e5x VLPs on solid metal MNs (Fig. 2A). No significant levels of time-and-temperature-dependent destabilization were observed from M2e5x VLPs coated onto MNs up to 8 weeks and at both 4°C and R.T., respectively. These results indicate that the stability of M2e5x VLP vaccines coated on MN arrays can be maintained for 8 weeks even at R.T. storage conditions without significant reduction in M2e epitope reactivity. Following in vitro characterization of M2e epitope reactivity, in vivo experiments were performed to better assess immunogenicity and efficacy of solid MNs coated with M2e5x VLP vaccines. Prime-boost skin vaccinations of mice with MN M2e5x VLPs stored at 4°C or R.T. for 8 weeks induced high levels of serum IgG antibodies specific for M2e peptides, comparable to those in mice immunized with freshly prepared M2e5x VLP MNs (Fig. 2B).
Fig 2. Stability and immunogenicity of M2e5x vaccines coated onto MNs.
M2e5x vaccines coated onto MN were preserved in 4°C or R.T. for 4 or 8 weeks. Stability of M2e5x VLPs was measured by reactivity to M2e monoclonal antibody (14C2) using enzyme-linked immunosorbent assay (ELISA). (A) Stability of M2e5x VLP coated onto MNs stored at different temperatures. (B) Immunogenicity of M2e5x VLP coated MNs stored at different temperatures. BALB/c mice (N=5) were immunized by delivering M2e5x VLP MNs to the skin. Sera were collected 3 weeks after prime (p) and boost (b) vaccination. (C) Stability of M2e4x proteins coated onto MNs. Asterisk indicates significant differences between M2e5x VLP and M2e5x protein (*: <0.01). Error bars indicate mean ± SEM.
Stability and immunogenicity of M2e5x proteins coated onto MNs were determined in similar experimental conditions to M2e5x VLP. M2e antibody, 14C2, reactivity to M2e5x proteins coated onto MNs was measured as an indicator of MN vaccine stability was found to be maintained after storage for 8 weeks at R.T., similar to that of M2e5x proteins coated onto MN arrays a day earlier (Fig. 2C). Based on 14C2 reactivity, M2e epitope contents into M2e5x protein MN vaccines were approximately 2 fold higher than that in M2e5x VLP MN vaccines at the concentration of 63ng/well of total coated proteins. Despite higher 14C2 reactivity, prime-boost MN vaccination after coating MN with M2e5x proteins did not induce M2e specific antibody responses (data not shown). These results suggest that M2e5x VLPs coated onto metal MN arrays in solid formulation maintain M2e epitope integrity during storage for over 8 weeks at R.T. and are able to induce potent M2e antibody responses after delivery to the skin of mice.
3.3. MN skin immunization with M2e5x VLP is effective in inducing IgG2a isotype antibody responses
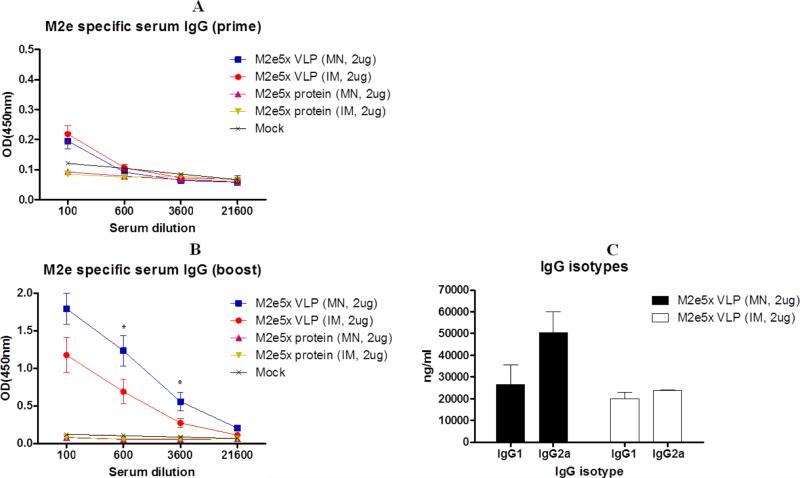
It is important to compare immunogenicity of tandem repeat of M2e epitopes presented on VLPs (M2e5x VLP) and in a soluble form (M2e5x protein) after MN delivery through the skin and conventional IM immunization. Groups of mice (N=12) were immunized with M2e5x VLPs or M2e5x protein (2 μg of total protein) using solid MNs through the skin or syringes by IM injection by using coating buffer as a mock control group at weeks 0 and 4 (Figs. 3A and B). After prime immunization, the M2e-specific antibody levels were induced at a low level, which are similar in both groups of MN skin and IM immunization (Fig. 3A). After boost, M2e antibody levels were induced at approximately 4-fold higher levels by MN skin immunization with M2e5x VLP than those by IM injection with significant difference in 600 or 3600-fold diluted sera (Fig. 3B). The M2e5x protein group did not induce M2e-specific antibody responses even after boost immunization through the MN skin or IM route. These results suggest that dry-coated M2e5x VLP MN skin immunization would be an alternative route that might be better than the conventional IM route.
Fig. 3. M2e specific antibody responses after MN skin or IM immunization with M2e5x VLP or M2e5x protein vaccines.
BALB/c mice (N=12) were immunized by MN skin or IM route with M2e5x protein or M2e5x VLPs. Sera were collected 3 weeks after prime (p) and boost (b) vaccination. The IgG or IgG isotype antibody levels were detected using human M2e peptide as an ELISA coating antigen. (A) Serum IgG antibody responses specific for human M2e peptide after prime. (B) Serum IgG antibody responses specific for human M2e peptide after boost. (C) IgG isotype antibody responses to human M2e peptide between the M2e5x VLP MN and M2e5x VLP IM groups. Asterisk indicates significant differences between the MN and IM groups of M2e5x VLPs (*: <0.05). Error bars indicate mean ± SEM. MN: microneedle skin immunization, IM: intramuscular immunization using syringe-needle injection.
To further understand types of immune responses to M2e, we analyzed IgG isotypes (IgG1 or IgG2a) of serum antibodies after boost immunization (Fig. 3C). In conventional IM route, the level of M2e specific IgG2a antibody was found to be similar to that of IgG1, which seems to be effective in inducing balanced T helper type I (Th1) and 2 (Th2). In contrast, through the skin route using MN, an average level of M2e-specific IgG2a was approximately 2 fold higher than that of IgG1, which seems to be more effective in inducing Th1 type immune responses. These results provide evidence that delivery of M2e5x VLP to the skin using solid MN arrays could shape the host immune responses to antigen in a direction to a Th1 type response.
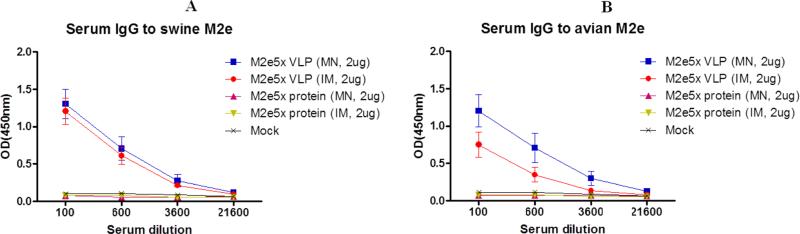
3.4. MN M2e5x VLP skin immunization induces antibodies specific for swine and avian M2e antigens
The central sequence of M2 ectodomain epitopes has a few residue changes among human, swine, or avian influenza A strains. Thus, we tested whether immune sera would be reactive with M2e peptide sequences of swine or avian influenza A viruses (Fig. 4). M2e5x VLP immune sera showed the highest levels of antibodies specific for a swine or avian M2e antigen, while M2e5x protein immune mice did not induce M2e antibodies at a detectable level. MN skin and IM injected boost immune sera from M2e5x VLP immunization showed similar levels of antibody reactivity to swine type M2e peptide (Fig. 4A). On the other hand, MN skin immune sera showed a higher level of antibodies reactive to an avian type M2e peptide antigen than that of IM injection (Fig. 4B). These results suggest that M2e5x VLP MN skin immunization induces antibodies specific for human, swine, and avian M2e antigens, which is comparable to or better than that of IM injection.
Fig. 4. Serum M2e antibody responses to swine and avian M2e antigens.
BALB/c mice (N=12) were immunized by MN skin or IM route with M2e5x protein or M2e5x VLPs. Sera were collected at 3 weeks after boost vaccination. The IgG antibody levels were detected using swine M2e or avian M2e peptides as an ELISA coating antigen. (A) Serum IgG antibody responses to swine M2e peptide. (B) Serum IgG antibody responses to avian M2e peptide. Error bars indicate mean ± SEM.
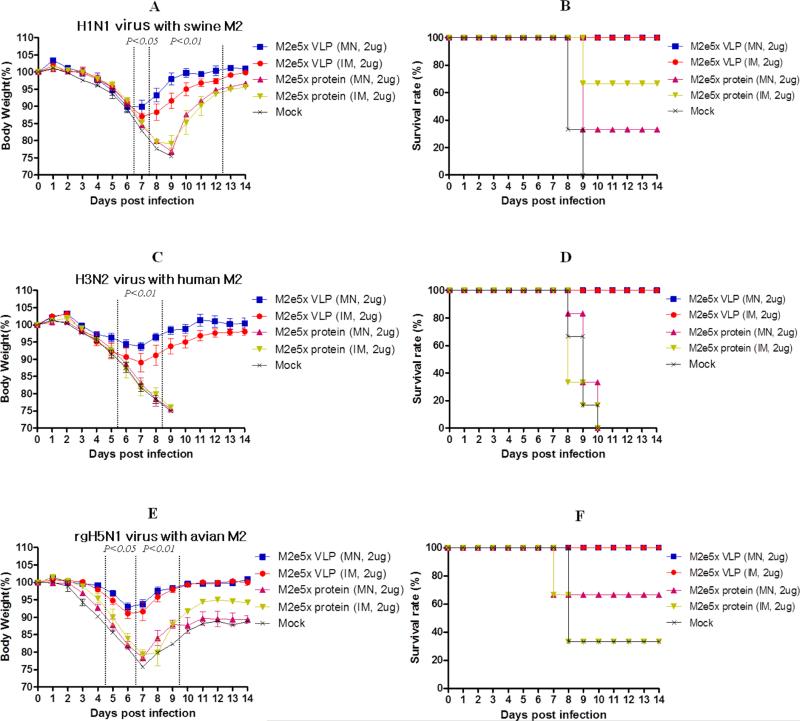
3.5. Skin immunization of M2e5x VLP using MNs provides cross protection
To determine the efficacy of MN skin immunization on conferring cross protection against lethal challenge infection, the groups of immunized mice were intranasally challenged with a lethal dose of A/California (H1N1), A/Philippines (H3N2), or avian rgH5N1 (with M2 from A/Mandarian duck) virus at 6 weeks after skin or intramuscular boost immunization (Fig. 5). All mice immunized with M2e5x VLPs showed body weight loss less than 13%, resulting in 100% protection. In comparison with IM injection, MN M2e5x VLP skin immunization showed a less loss (approximately 10, 6, 6%) in body weight against A/California, A/Philippines, and avian rgH5N1, respectively.
Fig. 5. Skin immunization of M2e5x VLPs using MNs provides heterosubtypic cross protection.
Groups of mice that were immunized via MN skin or IM injection were intranasally challenged with a lethal dose of influenza viruses, A/California (H1N1, N=3, 2× LD), A/Philippines (H3N2, N=6, 4× LD), and A/Mandarian duck (rgH5N1, N=3, 2× LD), 6 weeks after boost vaccination. (A) H1N1 virus with swine M2: Body weight changes after A/California challenge. (B) H1N1 virus with swine M2: Survival rates after A/California challenge. (C) H3N2 virus with human M2: Body weight changes after A/Philippines challenge. (D) H3N2 virus with human M2: Survival rates after A/Philippines challenge. (E) rgH5N1 virus with avian M2: Body weight changes after avian rgH5N1 A/Mandarian duck challenge. (F) rgH5N1 virus with avian M2: Survival rates after avian rgH5N1 A/Mandarian duck challenge. Body weight and survival rates were monitored for 14 days. P value indicates significant difference between M2e5x VLP (MN) and M2e5x protein (MN or IM). Error bars indicate mean ± SEM. LD, lethal dose.
Moreover, all mice survived lethal challenge infection (Fig. 5). Meanwhile, mice immunized via IM injection showed more loss (approximately 13, 11, 8%) in body weight than those in MN M2e5x VLP skin-immunized mice after infection with A/California, A/Philippines, or avian rgH5N1 influenza virus, respectively. Both M2e5x VLP MN skin and IM groups showed 100% protection against lethal challenge with H1N1 pandemic, H3N2, or avian rgH5N1 influenza virus (Fig. 5). In contrast, mice immunized with M2e5x proteins showed severe body weight loss and low or no protection against influenza virus. All naïve mice lost over 25% in body weight and were not protected. Therefore these results demonstrate that M2e5x VLP vaccine is superior to soluble M2e5x protein in conferring protection and delivery of M2e VLP vaccine to the skin using solid MN arrays is better than IM injection in conferring cross protection.
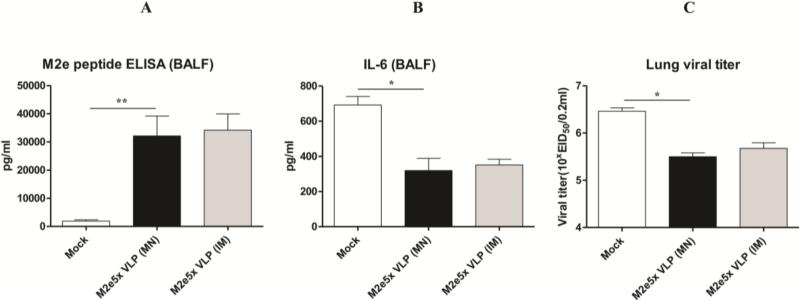
3.6. M2e5x VLP delivery to the skin contributes to inducing M2e mucosal antibodies and lowering inflammatory cytokine levels and lung viral titers
In another set of mouse challenge experiments, M2e-specific IgG antibody responses were determined in bronchoalveolar lavage fluids (BALF) collected from mice sacrificed at day 4 after challenge with A/Philippines (H3N2) virus (Fig. 6). Significantly higher levels of IgG antibody responses specific for M2e antigen were observed in mice immunized through either skin or IM route (Fig. 6A). It is well known that pro-inflammatory cytokines are involved in causing tissue damage, contributing to lethal disease. As expected, higher levels of IL-6 were observed in BALF from the mock control group than those from M2e5x VLP immunized mice through skin or IM injection (Fig. 6B). The level of IL-6 in BALF from mice immunized with M2e5x VLP using MN was similar to that of mice immunized by IM injection. To better assess the protective efficacy against A/Philippines (H3N2) virus, lung viral titers were determined at day 4 after challenge. The group of mice immunized with M2e5x VLP vaccine by skin or IM routes showed approximately 10-fold lower lung viral titers compared to those in the mock control group (Fig. 6C). The viral titer from mice immunized with M2e5x VLP using MN was similar to that of mice immunized using IM injection. These results suggest that M2e specific immune responses in systemic and mucosal sites can effectively contribute to controlling virus replication. Therefore, it is likely that inhibition of inflammatory cytokine production as a result of better lung viral control by M2e5x VLP immunization through the skin delivery plays a role in improving cross protection upon lethal challenge.
Fig. 6. M2e-specific IgG antibodies and inflammatory IL-6 cytokine in bronchoalveolar lavage fluids and viral titers in lungs.
Levels of IgG antibodies, inflammatory IL-6 cytokine, and viral lung titers were determined at day 4 post-challenge with 4× LD50 of A/Philippines (H3N2) virus from mice (N=4) that were immunized with M2e5x VLP via MN skin or IM route. (A) M2e specific IgG antibody response. IgG antibody responses were determined by ELISA using human M2e peptide as a coating antigen (4 μg/ml). (B) Inflammatory IL-6 cytokine in bronchoalveolar lavage fluids (BALF). IL-6 cytokine was determined by a commercial cytokine ELISA IL-6 kit. (C) Lung viral titers. Lung viral titers were determined by an egg inoculation assay. Asterisks indicate significant differences (*: <0.05, **: <0.01). Data represent mean ± SEM. BALF, bronchoalveolar lavage fluid; EID, egg infectious dose; IL, interleukin; pg, picogram.
3.7. M2e5x VLP delivery to the skin enhances IFN-γ secreting T cell responses
To determine the induction of T-cell responses by skin immunization and its correlation with cross protection, we analyzed IFN-γ secreting cellular responses in lung cells (Fig. 7). Lung cells were collected at day 4 post-challenge and IFN-γ cytokine ELISPOT assay was performed. Low levels of IFN-γ spots were observed in lung cells of the mock control group (Fig. 7). However groups of mice immunized with M2e5x VLPs vaccines via skin or IM route showed significantly higher levels of IFN-γ secreting cell spots in lung cells. Interestingly, the group of mice immunized with M2e5x VLP vaccines using MNs showed significantly higher levels of IFN-γ secreting cells to the challenged virus in lung cells than those in mice immunized by IM route. These results provide evidence that delivery of M2e5x VLP to the skin using MNs induces INF-γ secreting T-cells to influenza virus antigens in lungs, a major infection site.
Fig. 7. Mucosal IFN-γ secreting cell spots after viral challenge.
Lung cells were isolated from mice day 4 post-challenge (N=4). IFN-γ secreting cells were detected in the presence of challenged A/Philippines/2/82 (H3N2) virus as a stimulator (2 μg/ml). IFN-γ cytokine-producing cells were counted by ELISPOT reader. Asterisk indicates significant differences (*: <0.05, **: <0.01). Data represent mean ± SEM. IFN: interferon, M2e5x VLP: M2e5x virus-like particle.
Discussion
Recently, human infection by H5N1 and H7N9 avian influenza virus has brought real concerns and alert, emphasizing the priority of developing a broadly cross protective vaccine and an effective vaccination method [29-30]. M2e, a target protein for universal influenza vaccines, has very low immunogenicity and, although M2e is highly conserved among human influenza viruses, there are host species specific amino acids within the central M2e epitope from swine and avian influenza viruses. Delivery of influenza antigen is also important for effectively targeting the antigens to antigen presenting cells. Therefore, we hypothesized that presenting tandem repeat of heterologous M2e epitopes on VLPs (M2e5x VLP) as well as delivery of M2e5x VLPs to the skin using MN arrays would enhance the cross-protective efficacy. In this experiment, M2e5x proteins were not immunogenic in the absence of conjugating immunogenic carrier proteins and adjuvants. The present study showed that a simple-to-administer universal M2e5x VLP influenza vaccine using MN arrays without adjuvants would induce high levels of M2e specific IgG and IgG2a isotype antibodies as well as improve cross-protective efficacy against heterosubtypic swine and avian influenza viruses. It is expected that MN skin immunization with M2e5x VLP as a universal influenza vaccine might provide a new strategy of vaccination, potentially reducing morbidity and mortality of a new emerging influenza pandemic.
We found that M2e5x VLP MN vaccine delivered to the skin was more effective than those administered by IM injection in enhancing M2e antibody levels and recall cellular response in lungs, which is consistent with a previous study on influenza hemagglutinin VLP MN vaccination [26]. The mechanisms of microneedle vaccination comparing to IM immunization are unknown. It has been suggested that vaccine antigens through the skin are more likely to be captured by antigen-presenting cells, such as dermal dendritic cells and Langerhans cells, and transported to the lymph nodes for activation of T and B cells [31-33]. In contrast, antigens delivered into muscular tissues might be passively drained to surrounding tissues with antigen presenting cells or the regional lymph nodes via the lymphatic conduits or bloodstream, resulting in less efficiently capturing antigens by immune cells [34-35]. In this experiment, skin delivery using M2e5x VLP might have dose-sparing efficacy since an average level of M2e-specific antibody by skin injection was approximately 6-fold higher than that by intramuscular injection. Thus, it is likely that M2e5x VLP vaccine antigens delivered to the skin might have an easy access to dermal dendritic cells and Langerhans cells which present antigens T cells and stimulate B cells.
It is well known that the stability of influenza vaccines in liquid formulation is dependent on temperature. Vaccines in the liquid state are suggested to be kept at 4°C because of their unstability when stored at higher temperatures [36-38]. In contrast to liquid formulation, a number of different carbohydrate excipients were tested as stabilizers for pharmaceutical vaccine compounds during the formulation of a dry powder and trehalose was found to be most effective [39]. The addition of trehalose prevented physical changes in secondary and tertiary structure of influenza HA protein during a freezing process [40-41] and significantly improved the stability of influenza VLPs [23, 37]. MN coating with influenza M2e5x VLPs involves a drying process that accompanies a change of vaccine formulation from liquid to solid. The stability of influenza M2e5x VLPs using trehalose as a coating buffer was maintained for 8 weeks, as evidenced by the reactivity to M2e monoclonal antibody and immunogenicity in mice. Influenza M2e5x VLP vaccine coated with trehalose was equally stable at 4°C and R.T. during storage up to 2 months, which is a significant advantage for mass vaccination in resource-limited countries.
Previously, we observed high humoral antibody responses by IM immunization with M2e5x VLPs which resulted in a balanced IgG2a/IgG1 antibody ratio in mice, indicating both Th1 and Th2-associated immune responses. In contrast, MN delivery of M2e5x VLPs through the skin showed high humoral antibody titers and induced Th1-biased immune responses with an IgG2a dominant IgG subtype profile and IFN-γ secreting cells in lungs compared to IM route. A previous study confirmed that MN vaccination in the absence of trehalose stabilization induced IgG1 as a dominant isotype antibody probably due to deformed HA proteins but there was a switch to IgG2a dominance using trehalose-stabilized influenza vaccines [42]. Here, we also found that skin vaccination using influenza M2e5x VLPs with trehalose was effective in inducing IgG2a isotype antibodies as a representative of Th1 immune responses. Although the underlying mechanisms for preferential induction of IgG2a isotype antibodies by MN skin delivery still remain unknown, an effective uptake of M2e5x VLPs by dendritic cells under the skin after MN delivery is likely to be a contributing factor in addition to the antigenic stability. In support of this speculation, IgG1 dominant antibody responses by influenza VLP vaccination were induced in mutant mice that are deficient of MyD88 a common innate adaptor molecule for toll-like receptor signaling on antigen presenting cells[43]. The induction of IgG2a antibodies has been demonstrated to be effective in clearing virus infection through multiple mechanisms, including activation of the complement system, stimulation of antibody-dependent cellular cytotoxicity, and clearance of opsonized virus by macrophages [44-46]. Therefore, this study suggests that the M2e epitope integrity of M2e5x VLP MN vaccines using trehalose and delivery to the skin might be promoting effective interactions with B cells, dendritic cells, and/or other cells to stimulate the innate immune system and to induce IgG2a-dominant-type immune responses.
In addition to the immunologic advantages of M2e5x VLP MN vaccination, MN patch can also offer desirable logistic features compared to conventional IM injection. M2e5x VLP MN vaccination may be a significant advance that enables reliable and simple skin delivery for animal research and clinical use. In addition to conferring broad cross-protection, M2e5x VLP MN skin vaccination provides potential advantages over conventional IM injection due to the expected speed, simplicity, and potential dose-sparing efficacy [23, 47]. The small package size of a MN patch can simplify vaccine storage, transportation and disposal, as well as reduce the risk of needle re-use and needle-stick injury [48]. These characteristics of MNs using M2e5x VLPs would increase the coverage of seasonal and pandemic influenza vaccination by facilitating school-based vaccination of children and easy access to vaccination in elder-care facilities. These results indicate that vaccination using M2e5x VLP MNs is not inferior to IM injection in inducing protective host immune responses specific for universal antigenic target M2e. Thus, MN skin delivery can offer a promising alternative method to simplify influenza M2e5x VLP vaccination for improving cross protective efficacy.
In conclusion, protective efficacy with MN delivery of M2e5x VLPs to the skin could be comparable to or better than conventional IM injection. Immunity to M2e could confer cross protection potentially overcoming the hemagglutinin-based strain-specific protection by current vaccination. Alternatively, M2e5x VLPs MN patch vaccines can be developed as a supplementary vaccine providing immunity to a conserved M2e immunity in addition to current hemagglutinin vaccines. With the potential advantages for administration, safety and storage, skin vaccination using MN-based delivery of the M2e5x VLPs could be developed as a promising approach for an easy-to-administer universal influenza vaccine.
Acknowledgements
This work was supported by NIH/NIAID grants AI105170 (S.M.K.) and AI093772 (S.M.K.). The authors thank Hui Qian at the National Institute for Nanotechnology for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viboud C, Simonsen L. Does seasonal influenza vaccination increase the risk of illness with the 2009 A/H1N1 pandemic virus? PLoS Med. 2010;7:e1000259. doi: 10.1371/journal.pmed.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterholm MT. Preparing for the next pandemic. N Engl J Med. 2005;352:1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- 3.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 5.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 6.Subbarao K, Matsuoka Y. The prospects and challenges of universal vaccines for influenza. Trends Microbiol. 2013;21:350–358. doi: 10.1016/j.tim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schotsaert M, De Filette M, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev Vaccines. 2009;8:499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliasson DG, El Bakkouri K, Schon K, Ramne A, Festjens E, Lowenadler B, Fiers W, Saelens X, Lycke N. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine. 2008;26:1243–1252. doi: 10.1016/j.vaccine.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, Adler-Moore JP, Fujii G. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH. High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine. 2004;23:366–371. doi: 10.1016/j.vaccine.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang BZ, Gill HS, He C, Ou C, Wang L, Wang YC, Feng H, Zhang H, Prausnitz MR, Compans RW. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release. 2014;178:1–7. doi: 10.1016/j.jconrel.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005;7:171–177. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5:e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debenedictis C, Joubeh S, Zhang G, Barria M, Ghohestani RF. Immune functions of the skin. Clin Dermatol. 2001;19:573–585. doi: 10.1016/s0738-081x(00)00173-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Prow TW, Crichton ML, Jenkins DW, Roberts MS, Frazer IH, Fernando GJ, Kendall MA. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139:212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28:95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 18.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. Journal of virology. 2008;82:1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147:326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen HS, Wang SP. Mouse adaptation of the Asian influenza virus. The Journal of infectious diseases. 1959;105:9–17. doi: 10.1093/infdis/105.1.9. [DOI] [PubMed] [Google Scholar]
- 28.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei L, Song P, Tang Q, Shan K, Tobe RG, Selotlegeng L, Ali AH, Cheng Y, Xu L. Changes in and shortcomings of control strategies, drug stockpiles, and vaccine development during outbreaks of avian influenza A H5N1, H1N1, and H7N9 among humans. Biosci Trends. 2013;7:64–76. [PubMed] [Google Scholar]
- 30.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 31.Dubois B, Massacrier C, Caux C. Selective attraction of naive and memory B cells by dendritic cells. J Leukoc Biol. 2001;70:633–641. [PubMed] [Google Scholar]
- 32.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 33.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 34.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 35.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, Corbett HJ, Primiero CA, Ansaldo AB, Frazer IH, Brown LE, Kendall MA. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152:349–355. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res. 2011;28:135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coenen F, Tolboom JT, Frijlink HW. Stability of influenza sub-unit vaccine. Does a couple of days outside the refrigerator matter? Vaccine. 2006;24:525–531. doi: 10.1016/j.vaccine.2005.07.081. [DOI] [PubMed] [Google Scholar]
- 39.Leslie SB, Teter SA, Crowe LM, Crowe JH. Trehalose lowers membrane phase transitions in dry yeast cells. Biochim Biophys Acta. 1994;1192:7–13. doi: 10.1016/0005-2736(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 40.Amorij JP, Huckriede A, Wilschut J, Frijlink HW, Hinrichs WL. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25:1256–1273. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jonge J, Amorij JP, Hinrichs WL, Wilschut J, Huckriede A, Frijlink HW. Inulin sugar glasses preserve the structural integrity and biological activity of influenza virosomes during freeze-drying and storage. Eur J Pharm Sci. 2007;32:33–44. doi: 10.1016/j.ejps.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 42.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang SM, Yoo DG, Kim MC, Song JM, Park MK, O E, Quan FS, Akira S, Compans RW. MyD88 plays an essential role in inducing B cells capable of differentiating into antibody-secreting cells after vaccination. J Virol. 2011;85:11391–11400. doi: 10.1128/JVI.00080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 45.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352:418–426. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 48.Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]