Abstract
A new fossil showing affinities with extant Laemanctus offers the first clear evidence for a casquehead lizard (Corytophanidae) from the Eocene of North America. Along with Geiseltaliellus from roughly coeval rocks in central Europe, the new find further documents the tropical fauna present during greenhouse conditions in the northern mid-latitudes approximately 50 million years ago (Ma). Modern Corytophanidae is a neotropical clade of iguanian lizards ranging from southern Mexico to northern South America.
Introduction
Fossil members of various animal, plant, fungal, and other clades currently confined to the tropics or subtropical areas are often found in the mid-to-high latitudes during warm periods in Earth history [1, 2]. Future global climate change may disproportionately affect tropical species [3, 4], so it is important to understand previous biotic responses to climate perturbation [5]. When extended to understanding some infectious disease (e.g., malaria) distributions, this importance is magnified by medical implications.
The mean average global temperature was approximately 9°C higher in the mid-Eocene than today [6]. Fossils from these and similarly aged strata suggest a lush, closed-canopy, tropical environment with a diverse reptile fauna along the eastern flank of the ancestral Rocky Mountains [7–9].
A new fossil corytophanid from the Bridger Formation (Fig 1) represents the sister-taxon to extant Laemanctus (Fig 2). Although modern corytophanids are restricted to Central and South America (Fig 2A and 2B), I posit that the group had a Euramerican/Laurasian origin and became restricted to lower latitudes after post-Eocene global cooling.
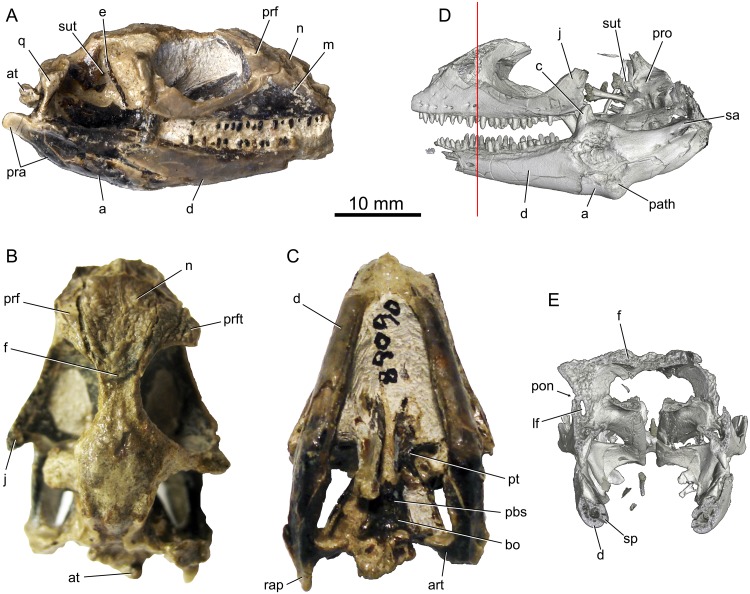
Fig 1. Holotype (UWBM 89090) specimen for Babibasiliscus alxi nov. taxon.
Photographs in (A) right lateral, (B) dorsal, and (C) ventral views. Digital reconstructions derived from HRXCT in (D) left lateral view and (E) transverse section. The vertical red line in (D) indicates the plane of section in (E).
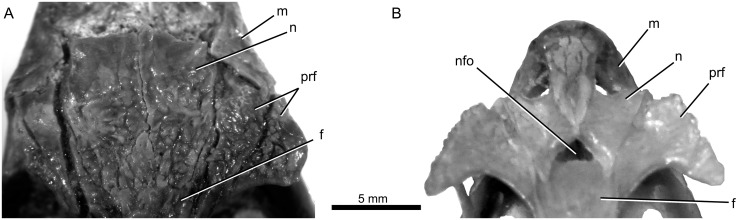
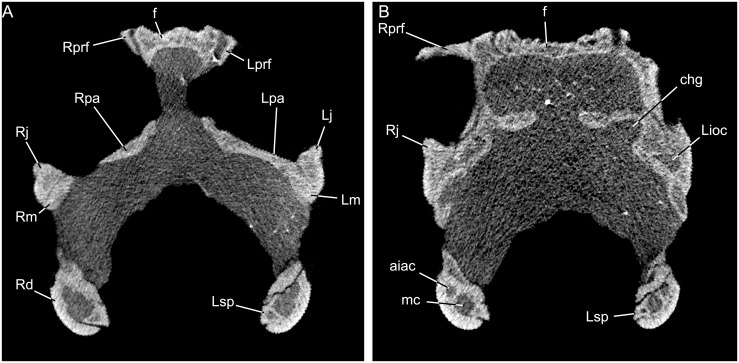
Fig 2. Comparison of the nasal and prefrontal regions of (A) Babibasiliscus alxi nov. taxon (UWBM 89090)and (B) Corytophanes hernandezi (AMNH R 147880) in dorsal view.
Materials and Methods
Institutional Abbreviations
AMNH, American Museum of Natural History (New York, NY); UWBM, Burke Museum of the University of Washington (Seattle, WA).
Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:F173E894-97D5-4C42-B760-BD7D7876656F. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central and LOCKSS.
Clade names follow recent phylogenetic definitions [10, 11].
Phylogenetics
The present phylogenetic analysis includes morphological data for 81 iguanomorph species and an anguimorph outgroup (Dataset A in S1 File). Characters and character states are largely from published analyses (Conrad et al. [12] and Gauthier et al. [13]), but with updates and modifications (Text B in S1 File). Supplementing the morphological characters are the molecular data included by Vieira et al. [14].
I used NEXUS Data Editor (NDE)[15] to assemble and manage the data matrix and performed an analysis using the New Technology Search in the computer program T.N.T: Tree analysis using new technology. One-thousand 1000 replicates were run with “ratchet” and “drift” options employed. I used PAUP* to reconstruct the Adams consensus.
Anatomical Abbreviations
a, angular; aiac, anterior internal alveolar canal; art, articular tubercle; bo, basioccipital; at, atlas; chg, choanal groove; d, dentary; e, epipterygoid; f, frontal; ioc, infraorbital canal; j, jugal; L, left; l, lacrimal; lf, lacrimal foramen; m, maxilla; n, nasal; nfo, nasofrontal fontanelle; pa, palatine; path, pathological area; pbs, parabasisphenoid; pon, preorbital notch; pra, prearticular; prf, prefrontal; prft, prefrontal tubercle; pro, prootic; pt, pterygoid; q, quadrate; R, right; rap, retroarticular process; sa, surangular; sp, splenial; sut, supratrigeminal process.
Results
Systematic paleontology
Squamata Oppel [16]
Iguania Cuvier [17]
Corytophanidae
Babibasiliscus gen. nov.
urn:lsid:zoobank.org:act:CB390F92-FDDC-4CD8-B6A5-4E4C12836686
Babibasiliscus alxi, gen. et sp. nov.
urn:lsid:zoobank.org:act:F9FB7055-76FE-4BBB-8DD6-7B795B01BB41
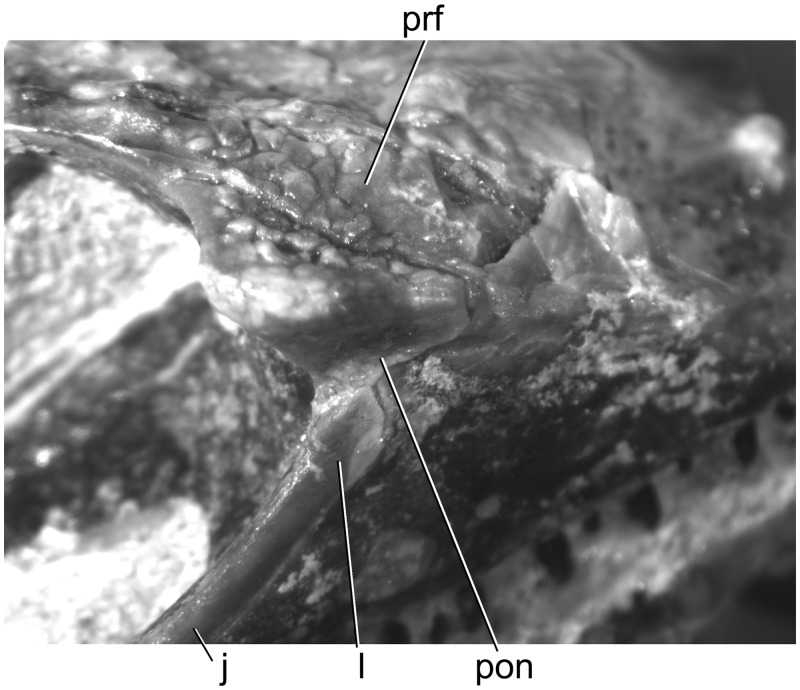
Fig 3. Right antorbital region of Babibasiliscus alxi nov. taxon (UWBM 89090) in dorsolateral view.
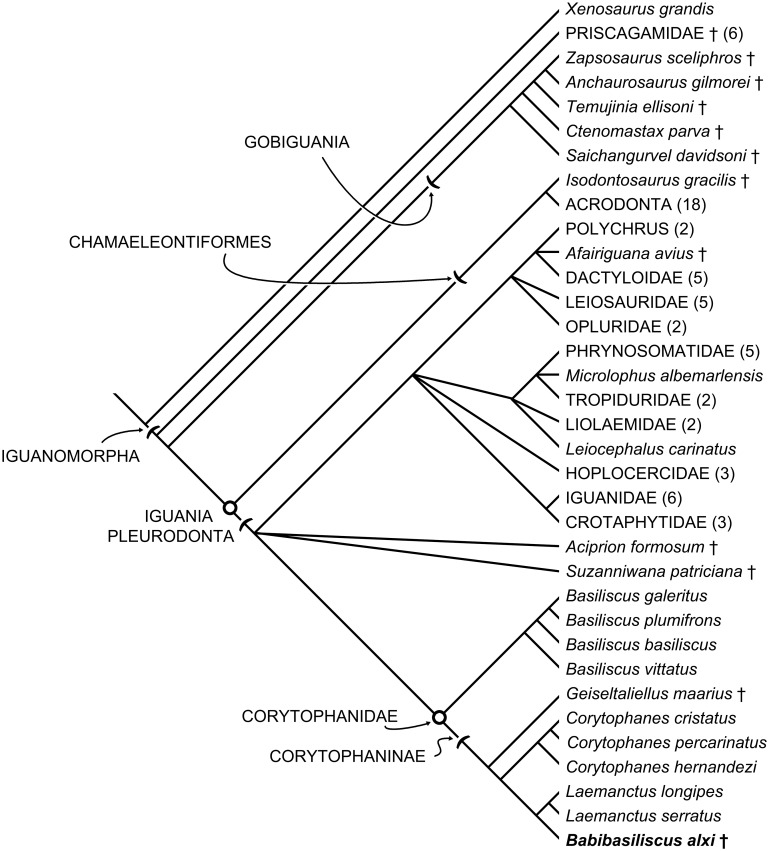
Fig 6. Phylogenetic hypothesis of Iguanomorpha based on the current analysis.
This is the Adam’s consensus of 98 trees. Suzanniwana patriciana and Aciprion formosum are the taxa with the most volatile positions within this analysis.
Holotype
UWBM 89090; a nearly complete skull with lower jaws and parts of first two cervical vertebrae (Fig 1).
Etymology
Babi- (Shoshoni) meaning “older male cousin” and Basiliscus, a corytophanid lizard. The generic name is meant to honor the Shoshone people who originally inhabited the areas in which the specimen was discovered and to refer to the relationship of the lizard with corytophanid lizards. At the request of Christian A Sidor, the species name honors John P. Alexander, who discovered the holotype.
Locality and Age
Lucky Lizard Locality (UWBM C1046), Uinta County, Wyoming. Blacks Fork Member of Bridger Formation (Bridger B), Green River Basin, late Early Eocene, approximately 48 Ma.
Diagnosis
UWBM C1046 is a corytophanid iguanian possessing the following combination of character states: Smoothly curved posterior nasal margins (Figs 1B and 2B); prefrontal-lacrimal groove (Fig 3); absence of a defined entocarotid fossa; and absence of a retroarticular process pit. UWBM C1046 differs from Geiseltaliellus in possessing dermal sculpturing on the prefrontal (Figs 1A, 1B, 2A and 3). It differs from Basiliscus and Laemanctus in possessing an extensive internasal contact. It differs from Laemanctus in lacking posteriorly elongated osseous external nares, and in possessing a jugal that lies mostly dorsal to the maxilla; from Corytophanes and Laemanctus in possessing a well-developed septomaxilla, a reduced crista prootica, and a splenial that terminates anterior to the coronoid apex; from Corytophanes in lacking a nasofrontal fontanelle (also present in Laemanctus lagnipes), absence of a contact between the prefrontal and the osseous external naris, in possessing distinct supratrigeminal process of the prootic, and in possessing a ventromedial process of the pterygoid (also absent in some Basiliscus). It differs from Basiliscus in possessing a medially flared palatine flange of the maxilla (Fig 4) and anterolateral processes of the frontal that terminate at about the same anterior level as the midline frontal process (Figs 1B and 2A; rather than extending anterior to that level or terminating posterior to that level).
Fig 4. Transverse sections through the (A) orbital region and (B) preorbital region of Babibasiliscus alxi nov. taxon (UWBM 89090) illustrating details of bones and their contacts.
Description and comparisons
Preservation
The specimen is an articulated, mostly complete skull with mandibles and the first two vertebrae preserved without evidence of plastic deformation (Fig 1). The premaxilla, posterior half of the frontal, parietal, postfrontals, postorbitals, squamosals, supratemporals, supraoccipital, dorsal half of each quadrate, and the anterior tips of both dentaries have been eroded away. The mandibles remain in articulation and are symmetrical in their alignment as preserved.
Cranial form
The preserved portion of the skull is approximately 42 mm from the anterior tip of the maxilla to the posterior margin of the occipital condyle. The complete skull would likely have been 2–3 mm longer.
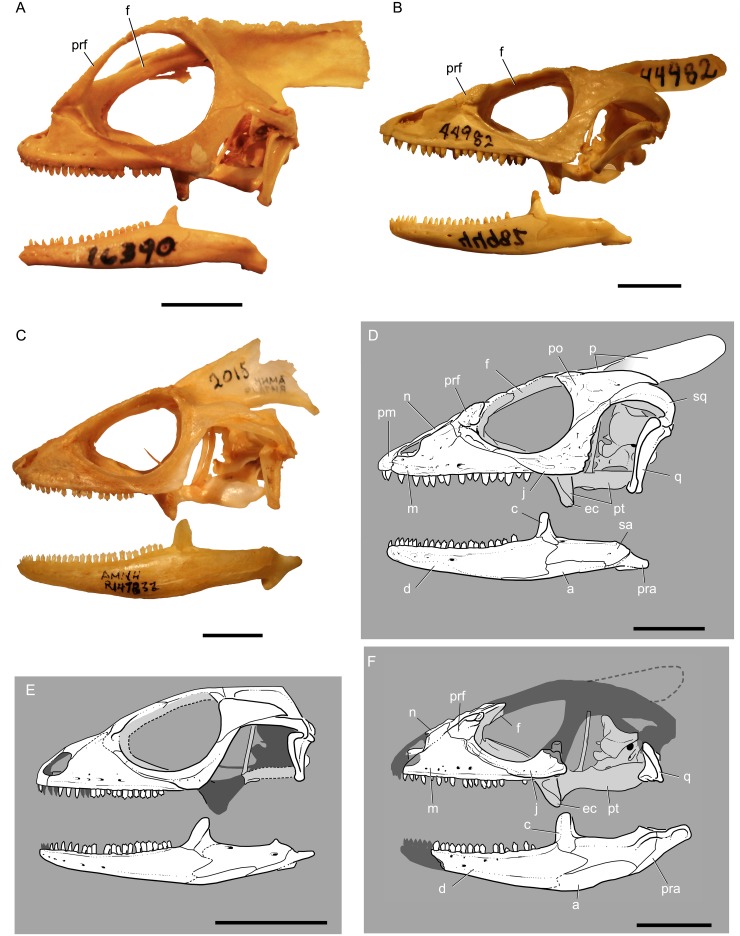
Three-dimensional preservation of the nearly complete skull allows a confident reconstruction (Fig 5F), although the presence or absence of a parietal crest like that of extant corytophanids (Fig 5A–5D) is uncertain.
Fig 5. Photographs (A-C) and line drawings (D-F) of the skulls of selected corytophanid species in left lateral view.
(A) Corytophanes cristatus (AMNH R 16390), (B) Laemanctus serratus (photograph; AMNH R 44982), (C) Basiliscus vittatus (AMNH R 147832), (D) Laemanctus serratus (line drawing), (E) Geiseltaliellus maarius, and (F) Babibasiliscus alxi taxon nov. (UWBM 89090). Note that it is unclear whether Babibasiliscus alxi taxon nov. had a parietal crest. Reconstructed areas are represented as semi-opaque areas and/or dotted lines. Scale bars equal 10mm.
Maxilla
The apex of the nasal process on the unsculptured maxilla is located anterior to the midpoint of the tooth row. The maxilla does not contact the vomer posterior to the vomeronasal opening.
There are seven labial ethmoidal foramina on the left maxilla and five on the right. Two additional, more dorsally located, ethmoidal foramina are preserved on the left maxilla and one is present on the right. These are present in many iguanians, including some Basiliscus and Laemanctus, but were not observed in available Corytophanes. A small posterolateral apex of the maxilla partly invades the anterior part of the prefrontal-lacrimal suture. Posterorventrally, the dentigerous part of the maxilla broadly underlies the orbit and lies completely lateral to the ectopterygoid. Thus, there is no lateral exposure of the ectopterygoid on the external skull surface.
The maxilla narrowly contributes to the anterolateral margin of the suborbital fenestra between the ectopterygoid and palatine. The small palatine flange extends dorsomedially from the medial part of the maxilla.
Nasal
The highly sculptured nasals are incomplete anteriorly, but preserve their contacts with the maxillae, prefrontals, and frontal. They have slight dorsal bowing and their posterior margins are U-shaped in dorsal view. The nasofrontal suture is W-shaped and there is no nasofrontal fontanelle like that seen in some other pleurodontan iguanians (e.g., Corytophanes hernandezi, Fig 2B).
Each nasal preserves an anterolateral process that partly underlies the maxillary nasal process. This is similar to the condition in Anolis carolinensis [18] and Laemanctus, but not Basiliscus or Corytophanes.
Prefrontal
The left prefrontal is damaged somewhat anterolaterally, but the right is complete. The prefrontals bear strong dermal rugosities.
The right prefrontal demonstrates the presence of a distinct tuberosity that is expanded posterolaterally (Figs 1B and 2A). Although many iguanians possess a dorsolateral tuberosity, that of Corytophanes, Basiliscus basiliscus, and some phrynosomatids (e.g., Phrynosoma asio;) is posterolaterally developed such that it forms a partial or complete lateral supraorbital arch [14]. Laemanctus and Babibasiliscus alxi lack such a lateral supraorbital arch, but show a more strongly developed tuberosity than in most squamates. This may be an incipient morphology (or a vestige) of the condition seen in some other corytophanids. Geiseltaliellus does not show development of this morphology.
A prefrontal-lacrimal groove, a corytophanid synapomorphy (reversed in Corytophanes;) extends anteriorly from the orbital margin (Fig 3).
Lacrimal
The lateral margins of the lacrimals are obscured because of partial fusion with the jugal, but the margins are visible posteriorly. The lacrimal forms the anterolateral and lateral margins of the lacrimal foramen, the lateral part of the anterodorsal margin of the palatine foramen, and the ventral part of the antorbital margin.
Jugal
Both jugals preserve the suborbital processes, but more of the postorbital process is preserved on the right jugal. The maxilla-jugal contact is a butt-joint lacking strong the tongue-in-groove contact (Fig 4) seen in some squamates [13]. The base of the postorbital process is laterally flattened and broader than the suborbital part, with some development of anterior and posterior flanges.
Frontal
Approximately the anterior one-third of the azygous frontal is preserved and bears extensive dermal ornamentation. The W-shaped nature of the nasofrontal suture suggests the possibility of nasal shelf of the frontal as in some other iguanians, but HRXCT imagery demonstrates the absence of this shelf as in modern corytophanids (e.g., [19, 20]). The prefrontal narrowly overlies the anterolateral process of the frontal. The median, internasal, process of the frontal extends to approximately the same anterior level as the anterolateral processes (Figs 1B and 2A). In contrast to some extant pleurodontans, such as dactyloids, the frontal lacks dorsoventral inflation (Fig 4).
Parietal
Although the parietal is not preserved, impressions on the matrix that originally underlied that bone reveal a few details. The parietal fossa was located at about the level of the dorsal end of the epipterygoid. The postfoveal crests were well-developed and approached posteriorly, but it is unclear if they converged. However, lack of preservation of the parietal precludes comparison to the crested anatomy seen in extant corytophanids (Fig 5).
Vomer
The broad, plate-like vomers are preserved, but lack their anterior tips. A parasagittal crest extends from the most anteromesial preserved tip of the vomer to a point near the posteromedial vomeropalatine contact. This crest defines a shallow, dorsally open groove on the dorsal surface of the vomer. The vomerine canal passes posterodorsally through the vomer. The vomers contact along their entire length and vomerine teeth are absent.
Palatine
The elongate, toothless palatines form the anterior margin of the pyriform recess. There is no palatine-ectopterygoid contact. A short, but well-defined, choanal groove is present (Fig 4). A short palatine canal is present.
The anteromedially tapering vomerine process has a broad contact with the vomer. The maxillary process dorsally and ventrally overlaps the palatine flange of the maxilla. The dorsomedially oriented prefrontal process is distinct, but relatively short, not approaching the frontal. Thus, there is a broad exposure of the prefrontal in the orbitonasal fenestra.
The pterygoid process has a narrow ventrolateral ramus that partly clasps the pterygoid. Palatine teeth generally are absent in all observed extant corytophanids, but have been found in at least one specimen of Laemanctus longipes.
Pterygoid
Each ptergyoid contacts the palatine, ectopterygoid, parabasisphenoid, and quadrate. The anteriorly attenuated palatine process forms much of the lateral margin of the pyriform recess and largely underlies the posterior part of the palatine. As with modern corytophanids, the transverse process is short. Its main body is dorsoventrally broad and gently anterolaterally oriented. A thin horizontal crest forms a sort of horizontal bony webbing between the transverse process and the palatine process. At the confluence of the palatine, transverse, and quadrate processes, the main body of the pterygoid is cylindrical anteriorly and then dorsally excavated in a columellar fossa. The dorsoventrally deep quadrate process is medially concave.
The body of the pterygoid bears a strongly developed basisphenoid buttress. No pterygoid teeth are preserved, but there are shallow, ovoid depressions that would have been tooth attachment points just lateral to a weak longitudinal ridge. Thus, modified pleurodont attachment of pterygoid teeth (sensu [21]) was present.
Ectopterygoid
The mediolaterally-oriented ectopterygoid is expanded at both ends and constricted in the middle. The lateral facet is the shape of an acute triangle facing anteriorly; thus the facet is anteroposteriorly broad. The medial (pterygoid) facet is dorsoventrally broader than its anteroposterior breadth. It clasps the anterior surface of the transverse process of the pterygoid.
Quadrate
About half of each quadrate is preserved, including the condylar part. Pterygoid flanges are, apparently, absent (Fig 1). The tympanic crest is robust as in extant corytophanids and Geiseltaliellus maarius.
Epipterygoid
The right epipterygoid is preserved in articulation with the pterygoid and demonstrates that the epipterygoid is somewhat posterodorsally inclined. The epipterygoid is elongate (Figs 1A, 1D and 5), not foreshortened as in Corytophanes (Fig 5A).
Parabasisphenoid
The parabasisphenoid is well-preserved except for the parasphenoid rostrum. It possesses robust basipterygoid processes and well-developed posterolateral processes that ventrolaterally overlie the anterior part of the basioccipital. The cristae trabecularis are proximally joined with the parasphenoid rostrum.
The basipterygoid processes are set at about 80 degrees from one another. Their articular surfaces are only moderately expanded such that the basipterygoid processes do not appear to have the extreme neck present in some squamates (e.g., Scincus scincus). The length of the basipterygoid process is subequal to its distal width, contrasting the condition seen in extant corytophanids wherein the basipterygoid processes are usually somewhat longer than their distal breadths.
The pituitary fossa is indistinct. The anterior openings of the Vidian canals lie immediately lateral to the cristae trabecularis. The posterior opening of the Vidian canal lies posterior to the level of the trigeminal notch of the prootic, just anterior to the level of the facial foramen. There is no entocarotid fossa.
Basioccipital
The basioccipital is complete and well preserved (Fig 1C). Although there is no fenestra basicranialis, the basioccipital is very thin anteromedially. The spheno-occipital tubercles are robust and posteroventrally-oriented with broad proximal bases and narrow distal tips. The ventral part of the mediolaterally broad crista interfenestralis lies near the anterodorsal part of the spheno-occipital tubercle. The basioccipital-otooccipital and basioccipital-prootic sutures occur ventrally within the occipital recess. The basioccipital apparently has no contribution to the inner ear or even the medial aperture of the recessus scalae tympani (sensu [22]). The basioccipital contribution to the occipital condyle is wedge-shaped in cross-section and is slightly larger than either of the otooccipital contribution. Dorsomedially, the basioccipital part of the occipital condyle is gently concave and the dorsolateral otooccipital sutures are ventrolaterally oriented.
Prootic
Each prootic is damaged dorsally and lack the supraoccipital contacts (Fig 1D), but are otherwise preserved in contact with the surrounding bones. A short, dorsally-oriented alar crest is present on the dorsal surface of the bulbous auditory bulla. Such a crest is uncommon amongst iguanians, but is also present in Basiliscus and Corytophanes. The shallow trigeminal notch (sensu [23]; the incisura prooticum of some authors) is dorally bordered by the auditory bulla and ventrally by a broad, ventromedially-oriented, inferior process. The prootic paroccipital process is elongate. The prootic forms the anterior and anterodorsal margins of the fenestra ovalis.
The broad prootic crest (crista prootica) originates near the posteroventral border of the trigeminal notch and extends posterolaterally onto the prootic paroccipital process. It possesses lateral and descending ventrolateral processes. The inferior process extends anterior to the level of the auditory bulla and alar crest. An elongate supratrigeminal process overhangs the inferior process (Fig 1A and 1D).
Otooccipital
Both otooccipitals are damaged laterally and their preserved contacts are partly obscured by fusion. However, a remnant of the basioccipital suture demonstrates that the otooccipital forms most of the margins of the occipital recess, and the posterior and ventral margins of the fenestra ovalis. Distinct vagus and hypoglossal foramina are present posteriorly. The occipital condyle parts of the otooccipitals are separated at midline by the basioccipital. Lack of clear sutural contacts caused by bone inter-growth make the exact nature of the otooccipital contributions to the inner ear cavities unclear.
The lateral margin of the crista interfenestralis is straight and extends from a point just dorsal to the spheno-occipital tubercle to the base of the paroccipital process immediately posterior to the fenestra ovalis. This contrasts the condition observed in extant corytophanids wherein the lateral margin of the crista interfenestralis is slightly medially concave.
The perilymphatic foramen is located anterodorsally and somewhat superficially within the occipital recess. The medial aperture of the recessus scalae tympani occurs dorsally within the occipital recess at about the level of the extreme ventral convexity of the spheno-occipital tubercle.
Dentary
Both dentaries are damaged anteriorly (Fig 1A, 1C and 1D). The preserved portion constitutes approximately 60 percent of the preserved mandible. When complete, the dentary probably constituted about two-thirds of the total mandibular length. There is no coronoid process of the dentary.
The right dentary has been damaged posteriorly such that the nature of its contact with the postdentary bones is unclear. The left dentary is pathological posteriorly (see below). A short dental shelf is present dorsal to the splenial contact. Meckel’s canal is open for the entire preserved length of the dentary and extends along the medial surface of the dentary, approaching (but not extending onto) the ventral mandibular surface. This contrasts the condition in extant Basiliscus and Corytophanes where the dentary partly closes Meckel’s canal.
Splenial
Both splenials are preserved in articulation and lack only their anterior tips. They close Meckel’s canal for its entire preserved length. Their preserved portions demonstrate that they are more elongate than the splenials of extant corytophanids. The splenial completely houses the inferior alveolar foramen and the anterior mylohyoid foramen. Posteriorly, the splenial-dentary contact narrowly overlies the anterior tip of the coronoid.
Coronoid
Both coronoids are well preserved, although the left shows some deformation associated with a jaw pathology (Fig 1D). This pathology presents as an offset of the bones at the level of the coronoid with additional bone growth associated excess bone deposition. The coronoid forms the anterior margin of the adductor fossa (there is no exposed surangular-prearticular contact anterior to the adductor fossa). The coronoid is shaped like an inverted-V in lateral view, but also partly overrides the dorsal surface of the surangular at the level of the coronoid eminence.
The coronoid eminence is tall and anteroposteriorly narrow. Its dorsal margin is rounded. A broad labial flange is present, but does not extend far posteriorly.
Angular
Little can be determined regarding the morphology of the left angular (Fig 1D). The right angular is well preserved posteriorly, but somewhat eroded anteriorly. It spans approximately 40 percent of the mandibular height and tapers posteriorly. Anteriorly, it contributes a small part to the ventral margin of Meckel’s canal. The posterior mylohyoid foramen is present within the angular on the ventromedial surface of the mandible, posterior the anterior margin of the coronoid eminence.
Surangular
The well-preserved surangular is robust (Figs 1A, 1D, and 5F). It does not extend anteriorly to the level of the division between Meckel’s canal and the anterior alveolar canal. Posteriorly, there is a small buttress at the anterior margin of the mandibular glenoid. The surangular is narrowly exposed on the medial surface of the mandible between the anterior and posterior descending processes of the coronoid dorsally, and the angular ventrally. A posterolateral crest is well developed and extends laterally and dorsolaterally (Fig 5F).
The inferior alveolar canals passes anteriorly through the surangular from the posterior surangular foramen at the level of the glenoid fossa. The anterior surangular foramen is located on the dorsolateral surangular surface, just posterior to the coronoid apex. Its margins are formed exclusively by the surangular. The anterior surangular foramen and, therefore, the inferior alveolar canal connect with the posterior part of Meckel’s canal.
Prearticular and articular
The prearticular and articular form a fused unit hereafter referred to as the prearticular. It forms the ventral margin of the unexpanded mandibular fossa, the mandibular glenoid, and the retroarticular process. The mandibular glenoid is W-shaped in cross section with a weak mesial ridge and subequal medial and lateral facets. In dorsal view, the mandibular glenoid is rounded anteriorly with a straight posterior margin.
Most of the left retroarticular process is damaged and deformed, but the right is well preserved (Fig 1A and 1D). The retroarticular process is a little longer than the length of the mandibular glenoid. The posterior end of the retroarticular process is rounded and posteriorly directed with a slight posterodorsal incline. A robust, finger-like retroarticular process is present (Fig 1C).
Dentition
Eighteen tooth positions are evident on each dentary and between five and eight more tooth positions were likely present in a complete lower jaw. The teeth themselves are pleurodont with large resorption pits present at the tooth bases. The most anterior preserved teeth are conical. More posteriorly, the teeth develop weak shoulders. The last six tooth positions are weakly tricuspid with a strong central cusp and weakly developed anterior and posterior cusps. These cusps do not flare and there are no clear wear facets.
Mandibular pathology
The left mandible shows signs of injury and healing (Fig 1D). The posterior margin of the dentary shows extensive intergrowth with the more posterior mandibular elements. There is fusion with the angular. The coronoid is severely deformed. A large, bulbous mass of bone is present near the base of the coronoid. Posterior to the dentary and ventral to the coronoid, there are bone fragments that have been partly fused with the surangular dorsolaterally, perhaps representing a shattered posterior part of the dentary. Although the inferior alveolar canal appears to be intact and normal, the surangular foramina are enlarged and irregularly shaped. The angular shows excessive deposition of bone on the ventral mandibular surface such that the left angular is approximately six times the thickness of the right.
Atlas and Axis
The left atlantal arch, the atlas intercentrum, and the odontoid process of the axis are preserved. The atlas has a short and sharp lateral process and a short ventral keel.
Phylogeny
The phylogenetic analysis recovered 98 shortest-recovered trees of 3153 steps; the rescaled consistency index of 0.2288, and a retention index of 0.5157 (Fig 6). Babibasiliscus alxi is the sister taxon to extant Laemanctus in all of the recovered trees. Babibasliscus alxi and Laemanctus form a clade supported by three unambiguous synapomorphies. The jugal lies mostly dorsal to the maxilla, rather than medial to it as in other corytophanids (character 52, state 1). This is also presence in Aciprion formosum, sometimes considered to be a corytophanid. A ventromedial process is present on the pterygoid (character 117, state 1). Aciprion formosum and Basiliscus vittatus also possess this character state. There is no pit on the retroarticular process (character 208, state 1).
Babibasiliscus alxi, Laemanctus, and Corytophanes are united to the exclusion of all other taxa in this analysis (Figs 6 and 7) by four unambiguous synapomorphies. Dermal sculpturing is present on the prefrontal (character 9, state 1). The jugal possesses anterior and posterior flanges (character 49, state 1). This character cannot be confidently coded in Babibasiliscus alxi and has, therefore, been left coded as “?” in this matrix. Also, this character state is present in Basiliscus basiliscus and some specimens of Basiliscus vittatus. Rugosities are present on the postorbital process of the jugal (character 50, state 1). This character cannot be coded in Babibasiliscus alxi. The postorbital clasps the frontoparietal suture (character 89, state 1). This character cannot be coded in Babibasiliscus alxi because the relevant morphology is not preserved.
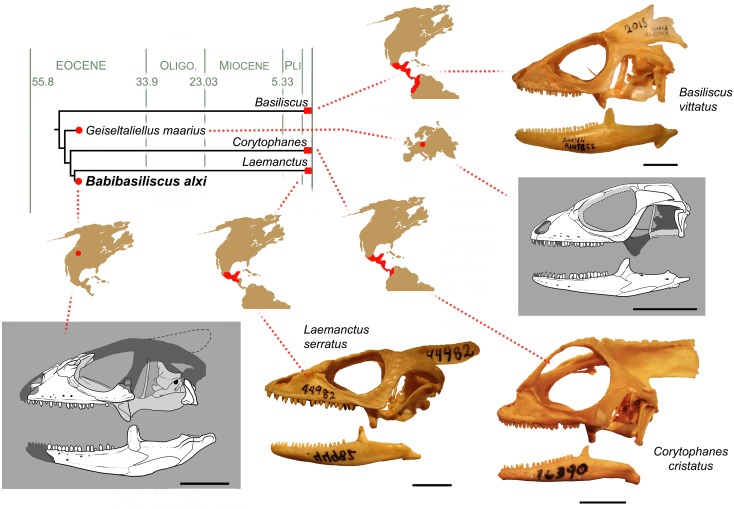
Fig 7. Phylogeny of corytophanids and distribution of anoles, para-anoles, corytophanids, and some malarial infection.
(A) Time-calibrated phylogeny of the corytophanid genera with representative illustrations of their skull morphologies.
The three species of Corytophanes are united to the exclusion of Laemanctus and Babibasiliscus alxi in this analysis (Fig 6) by 16 unambiguous synapomorphies. The snout is relatively short, making up less than 30 percent of the basicranial length (character 1, state 0). This character cannot be coded in Babibasiliscus alxi. The nasals contact for less than one-half of their total length (character 21, state 1). This condition is also present in some specimens of Basiliscus vittatus, and it cannot be scored based on available data for Geiseltaliellus maarius (character 24, state 1). The prefrontal extends anteriorly to the external naris, blocking the maxilla from contact with the nasal (character 38, state 0). Corytophanes species possess no lateral expansions of the parietals at the frontoparietal sutures (character 72, state 0). This character cannot be confidently coded in Babibasiliscus alxi. The pineal foramen occurs within the frontal in Corytophanes (character 77, state 1). This character state also occurs in Basiliscus. It is considered a convergence between Corytophanes and Basiliscus because pineal foramen occurs at the frontoparietal suture in the corytophanines Laemanctus and Geiseltaliellus maarius. This character cannot be scored in Babibasiliscus alxi. There is no transverse margin of the parietal between the supratemporal processes (character 82, state 1). Because the parietal is not preserved in Babibasiliscus alxi, this character cannot be coded in that taxon. The postorbital is elongate, extending for more than three-quarters of the length of the supratemporal fenestra, in Corytophanes (character 96, state 2). This state is also present in Basiliscus, but is interpreted as having evolved convergently in that taxon because the postorbital is relatively shorter in Laemanctus and Geiseltaliellus maarius. This character cannot be coded in Babibasiliscus alxi. Meckel’s canal is closed and fused anteriorly/medially in Corytophanes (character 181, state 2). This character state is also present in Basiliscus basiliscus. The anterior inferior alveolar foramen is not present as a distinct and separate opening in Corytophanes (character 183, state 3). There are no distinct surangular and angular dentary processes in Corytophanes (character 185, state 0). The clavicles are rod-like, rather than possessing expanded proximal ends, in Corytophanes (character 260, state 0). This character cannot be coded in Babibasiliscus alxi. The pelvis is strongly sutured, but the individual bones remain partly discernible in Corytophanes (character 284, state 1). Most corytophanids possess pelves in which the sutures are obliterated by intergrowth of the bones in adults. This character was not coded in Basiliscus galeritus, Babibasiliscus alxi, or Geiseltaliellus maarius. It was coded as polymorphic for Basiliscus plumifrons and Basiliscus vittatus. The presence of a complete secondary dorsolateral orbital margin formed by the union of the posterolateral process of the prefrontal with the postorbital (character 373, state 2) is recovered as an unambiguous synapomorphy of Corytophanes here. Even so, there is variation within Corytophanes hernandezi and that species is coded as polymorphic. A broad jugal-squamosal contact (character 389, state 1) is a Corytophanes synapomorphy. This character could not be coded in Babibasiliscus alxi or Geiseltaliellus maarius. There is no ventral squamosal process (character 390, state 2) in Corytophanes. This character could not be coded in Babibasiliscus alxi or Geiseltaliellus maarius.
I apply the name Corytophaninae to the stem-based clade containing all taxa sharing a more recent common ancestor with Corytophanes cristatus than with Basiliscus basiliscus (Fig 6). In the present analysis, that clade includes Geiseltaliellus maarius, Corytophanes, Laemanctus, and Babibasiliscus alxi, and is united by four unambiguous synapomorphies. The jugal is angulated (character 47, state 0) rather than curved as in Basiliscus plumifrons and Basiliscus galeritus. Even so, the other two species of Basiliscus. There is no postorbital tubercle (character 90, state 0), as there is in all Basiliscus basiliscus. This character cannot be scored in Babibasiliscus alxi. Absence of an anterior groove associated with the anterior surangular foramen (character 174, state 0) is recovered as an unambiguous corytophanine synapomorphy in this analysis. This means that this character is reversed in Laemanctus and convergent in Basiliscus basiliscus. The splenial extends for less than one-half of the dentary tooth row (character 189, state 0). This character is reversed in Babibasiliscus alxi, which has a relatively elongate splenial.
Discussion
Iguania (the crown node for Iguanomorpha) has a basal dichotomy (Fig 6) between chameleons, agamas, and their relatives (Chamaeleontiformes), and iguanas, basilisks, anoles, fence lizards, and their relatives (Pleurodonta). Modern iguanians are widely distributed, but the basal phylogenetic dichotomy is reflected in the biogeography of the group, with chamaeleontiforms occurring in the Old World and pleurodontans occurring only in the Americas (except Opluridae from Madagascar and Brachylophus from Fiji) [24].
Iguania probably originated in mid- to high-latitudes in Eurasia before dispersal. Modern iguanians occur on every continent except Antarctica and range from southern Canada and central Russia in the north to the southern tip of South America in the south, but their greatest species richness occurs in the tropics [24–26]. The earliest known iguanians come from Cretaceous deposits in Asia [27, 28] and North America [29, 30]. Although a variety of Cretaceous squamates are known from North America [31–34], only a few iguanians have been found there. By contrast, many Cretaceous iguanians or iguanomorphs are known from Asia [10, 27, 28]. Conrad and Norell [27] suggested a rapid iguanomorph diversification during the Cretaceous.
By the Eocene, crown-group pleurodontans must have been relatively diverse as evinced by the presence of the nested Afairiguana avius and Babibasiliscus alxi, as well as less phylogenetically well-resolved Suzanniwana patriciana and Aciprion formosum (Fig 6). The Americas have no extant chamaelontiform iguanians, but fragmentary remains demonstrate their presence during the Eocene, suggesting their dispersal across Beringia during the Paleocene and/or Eocene [35].
Perhaps an early pulse of diversification resulted in many basal radiations of Iguanomorpha occurring in Asia, with a few forms dispersing to North America. Subsequent diversification may have led to the origin of many modern pleurodontan groups during the Paleogene. This may have happened primarily in North America based on modern distributions.
The fossil record demonstrates that some terrestrial animals currently confined to the tropics were present in mid- to high-latitudes during warm periods in Earth history [36]. Varanids and shinisaurids are tropics loving taxa that occur primarily in the Old-World [25, 37, 38]. Even so, Saniwa ensidens (Varanidae) [31, 39, 40] and Bahndwivici ammoskius (Shinisauridae) [41] demonstrate dispersal of these clades into North America before the mid-Eocene. Necrosaurus cayluxi, ‘Necrosaurus’ eucarinatus, and Necrosaurus feisti (=Saniwa feisti) appear to be European Eocene varanids [11, 12]. These dispersals may also have been via Beringia, indicating that at least varanids, chamaeleontiforms, and shinisaurids possessed much broader Paleogene ranges than they currently enjoy. This may be related to the warmer global temperatures during the Paleogene [42], and their modern (more restricted) ranges may be a product of tropical/equatorial crowding associated with lower global temperatures.
Babibasiliscus alxi represents the first conclusive record of a crown-group corytophanid from extratropical North America. Phylogenetic analyses excluding fossils have uniformly proposed a tropical origin for the group [43–45]. This is unsurprising since all extant corytophanids range from equatorial western South America north through most of tropical Central America (Fig 7A and 7B). Consequently, the corytophanid range might be expected to have never been more extensive than the range it currently inhabits, but discovery of Babibasiliscus alxi from North America offers additional complexity for the biogeographic history of this clade. The Eocene corytophanids Geiseltaliellus and Babibasiliscus alxi suggest an ancestral distribution in extratropical Euramerica.
The current distribution of Corytophanidae may represent relicts of southwardly spreading clades (Basiliscus, Corytophanes, and Laemanctus; Fig 7A and 7B). Perhaps some northern iguanian clades went extinct (e.g., Priscagamidae) at the Cretaceous-Tertiary boundary, and others were pushed southward with the global cooling of the Late Tertiary (e.g., Opluridae and Chamaeleontiformes in the Old World; the remainder of Pleurodonta in the Americas).
When viewed in the context of other Eocene fossils representing typically tropics-living squamates, Babibasiliscus alxi adds important new data regarding modern distributions of those clades. Corytophanids, along with shinisaurids and varanids, may have been in mid- to high-latitudes because those environments were warmer than today, but also because they were cooler than the (possibly) prohibitively hot equatorial ages in the early Paleogene [46]. Later members of those clades were likely pushed toward the Equator as that area became more suitable and the higher latitudes cooled.
Supporting Information
Dataset A, Morphology Character-By-Taxon Matrix. Here is the full morphological data matrix. Text A, Comparative material. Observations on the following specimens and publications were used for this study. Institutional abbreviations: AMNH, American Museum of Natural History, New York, NY; FMNH, The Field Museum, Chicago, IL; IGM, Institute of Geology, Mongolian Academy of Sciences, Ulan Bator, Mongolia; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, MA; REE, Richad E Etheridge collection; UCMP, University of California Museum of Paleontology, Berkeley, CA; UC MVZ, Museum of Comparative Zoology, University of California, Berkeley, CA; UF, Florida State Museum, University of Florida, Gainesville, FL; USNM, United States National Museum of Natural History, Smithsonian Institution, Washington, DC; UWBM, Burke Museum of Natural History and Culture, University of Washington, Seattle, WA; YPM, Yale Peabody Museum, New Haven, CT; ZPAL, Zakład Paleobiologii, Polska Akademmia Nauk (Paleobiological Institute, Polish Academy of Sciences), Warsaw, Poland. Text B, Descriptions of Added Morphological Characters. Morphological characters and character-states here newly added to the phylogenetic analysis.
(DOCX)
Acknowledgments
The specimen was discovered as part of an expedition led by C.A. Sidor; it was discovered by J. Alexander, for whom it is named. I am indebted to C.A. Sidor for allowing me to describe this specimen and for many important conversations. I am thankful to A.M. Balcarcel who facilitated the HRXCT data collection of UWBM 89090 at the microscopy facility at AMNH with the help of J. Thostenson. I am grateful to T.E. Macrini for assistance with processing of earlier, lower-resolution CT scans. Comparative specimen access was provided and facilitated by M. Norell, C.M. Mehling, D. Frost, D. Kizirian, R. Pascocello, M. Borsuk-Białynicka, J. Rosado, A. Wynn, P. Barrett, M. Carrano, B. Simpson, A. Resetar, G. Watkins-Colwell, J. Gauthier, and D. Brinkman. Helpful advice and discussions were provided by A.M. Balcarcel, C.M. Mehling, J. McCartney. I am grateful to all of these individuals. Any errors herein are, of course, my own.
Data Availability
All relevant data are within the paper, its Supporting Information files, and the Harvard Dataverse through the following link: http://dx.doi.org/10.7910/DVN/FTQPXR.
Funding Statement
Jack L. Conrad received funding through startup funds from the Anatomy Department at NYIT College of Osteopathic Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grande L. Paleontology of the Green River Formation, with a review of the fish fauna. Geol Surv Wyo, Bull. 1984; 63:1–133. [Google Scholar]
- 2. Estes R, Hutchison JH. Eocene lower vertebrates from Ellesmere Island, Canadian Arctic Archipelago. Palaeogeogr Palaeoclimatol Palaeoecol. 1980; 30:325–47. [Google Scholar]
- 3. Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, et al. Impacts of climate warming on terrestrial ectotherms across latitude. PNAS. 2008; 105:6668–72. 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Alvarez Pérez HJ, et al. Why tropical forest lizards are vulnerable to climate warming. Proc R Soc London, Ser B. 2009; 276:1939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wing SL, Harrington GJ, Smith FA, Bloch JI, Boyer DM, Freeman KH. Transient floral change and rapid global warming at the Paleocene-Eocene boundary. Science. 2005; 310:993–6. [DOI] [PubMed] [Google Scholar]
- 6. Greenwood DR, Wing SL. Eocene continental climates and latidunal temperature gradients. Geol. 1995; 23(11):1044–8. [Google Scholar]
- 7. Rose KD. The Beginning of the Age of Mammals. Baltimore: The Johns Hopkins University Press; 2006. [Google Scholar]
- 8. Alexander JP, Burger JB. Stratigraphy and taphonomy of Grizzly Buttes, Bridger Formation, Middle Eocene of Wyoming In: Gunnell GF, editor. Eocene Biodiversity: unusual occurrences and rarely sampled habitats. New York, NY: Kluwer Academic/Plenum Publishing; 2001. p. 165–96. [Google Scholar]
- 9. Sullivan RM. Revision of the Paleogene genus Glyptosaurus (Reptilia, Anguidae). Bull Am Mus Nat Hist. 1979; 163(1):1–72. [Google Scholar]
- 10. Conrad JL. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull Am Mus Nat Hist. 2008; 310:1–182. [Google Scholar]
- 11. Conrad JL, Ast JC, Montanari S, Norell MA. A combined evidence phylogenetic analysis of Anguimorpha (Reptilia: Squamata). Cladistics. 2011; 27:230–77. [DOI] [PubMed] [Google Scholar]
- 12. Conrad JL, Balcarcel AM, Mehling CM. Earliest example of a giant monitor lizard (Varanus, Varanidae, Squamata). PLoS One. 2012; 7(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gauthier JA, Kearney M, Maisano JA, Rieppel O, Behlke DB. Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bull of the Peabody Mus Nat Hist. 2012; 53(1):3–308. [Google Scholar]
- 14. Vieira GHC, Colli GR, Báo SN. Phylogenetic relationships of corytophanid lizards (Iguania, Squamata, Reptilia) based on partitioned and total evidence analyses of sperm morphology, gross morphology, and DNA data. Zool Scr. 2005; 34:604–25. [Google Scholar]
- 15. Page RDM. NDE: Nexus Data Editor for Windows. Glasgow: Page, R. D. M.; 2001. [Google Scholar]
- 16. Oppel M. Die Ordnungen, Familien, und Gattungen der Reptilien als Prodrom einer Naturgeschichte derselben. München: Joseph Lindauer; 1811. 86 p. [Google Scholar]
- 17. Cuvier G. Le regne animal distribué d'apès son organisation, pour servir de base l'histoire naturelle des animaux et d'introduction a l'antomie comparée. Paris: 1817. [Google Scholar]
- 18.The Deep Scaly Project. Anolis carolinensis Austin: Digital Morphology; 2006 [cited 2013]. Available: http://digimorph.org/specimens/Anolis_carolinensis.
- 19.The Deep Scaly Project. Basiliscus basiliscus Austin: Digital Morphology; 2006 [cited 2014]. Available: http://digimorph.org/specimens/Basiliiscus_basiliscus.
- 20.The Deep Scaly Project. Corytophanes cristatus Austin: Digital Morphology; 2008 [cited 2014]. Available: http://digimorph.org/specimens/Corytophanes_cristatus.
- 21. Mahler DL, Kearney M. The palatal dentition in squamate reptiles: morphology, development, attachment, and replacement. Integr Comp Biol. 2006:126 (meeting abstracts). [Google Scholar]
- 22. Conrad JL, Norell MA. High-resolution x-ray computed tomography of an Early Cretaceous gekkonomorph (Squamata) from Öösh (Övörkhangai; Mongolia). Hist Biol. 2006; 18(4):405–31. [Google Scholar]
- 23. Oelrich TM. The anatomy of the head of Ctenosaura pectinata (Iguanidae). Misc Publ: Mus Zool, University of Michigan. 1956; 94:1–122. [Google Scholar]
- 24. Frost DR, Etheridge R. A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata). Univ Kans Mus Nat Hist, Misc Publ. 1989; 81:1–65. [Google Scholar]
- 25. Pianka ER, Vitt LJ. Lizards: windows to the evolution of Diversity. Berkeley: University of California Press; 2003. 346 p. [Google Scholar]
- 26. Zug GR, Vitt LJ, Caldwell JP. Herpetology: an introductory biology of amphibians and reptiles. London, United Kingdom: Academic Press; 2001. 630 p. [Google Scholar]
- 27. Conrad JL, Norell MA. A well-preserved iguanian (Squamata: Reptilia) from the Cretaceous of the Gobi and identification of a new clade of Iguania. Amer Mus Novit. 2007; 3584:1–47. [Google Scholar]
- 28. Gao K-Q, Norell MA. Taxonomic composition and systematics of Late Cretaceous lizard assemblages from Ukhaa Tolgod and adjacent localities, Mongolian Gobi Desert. Bull Am Mus Nat Hist. 2000; 249:1–118. [Google Scholar]
- 29. Gao K-Q, Fox RC. Taxonomy and evolution of Late Cretaceous lizards (Reptilia: Squamata) from western Canada. Bull of Carnegie Mus Nat Hist. 1996; 33:1–107. [Google Scholar]
- 30. DeMar DG Jr., Varricchio DJ, Head JJ, Moore JR, Wilson GP. A nearly complete fossil iguanian from the Upper Cretaceous (Campanian) Two Medicine Formation of western Montana. J Vert Paleontol. 2012; 32 (electronic supplement):86. [Google Scholar]
- 31. Estes R. Sauria terrestria, Amphisbaenia. New York: Gustav Fischer Verlag; 1983. 249 p. [Google Scholar]
- 32. Nydam RL. Lizards of the Mussentuchit local fauna (Albian-Cenomanian boundary) and comments on the evolution of the Cretaceous lizard fauna of North America. J Vert Paleontol. 2002; 22(3):645–60. [Google Scholar]
- 33. Nydam RL, Voci GE. Teiid-like scincomorphan lizards from the Late Cretaceous (Campanian) of southern Utah. J Herpetol. 2007; 41(2):211–9. [Google Scholar]
- 34. Nydam RL, Cifelli RL. Lizards from the Lower Cretaceous (Aptian-Albian) Antlers and Cloverly Formations. J Vert Paleontol. 2002; 22(2):286–98. [Google Scholar]
- 35. Smith KT. On the phylogenetic affinity of the extinct acrodontan lizard Tinosaurus In: Shuchmann K-L, editor. Tropical Vertebrates in a Changing World. Bonn, Switzerland: Zoologisches Forshungsmuseum, Alexander Koenig; 2011. p. 9–27. [Google Scholar]
- 36. Markwick PJ. Fossil crocodilians as indicators of Late Cretaceous and Cenozoic climates: implications for using paleontological data in reconstructing paleoclimate. Palaeogeogr Palaeoclimatol Palaeoecol. 1998; 137:205–71. [Google Scholar]
- 37. Zug GR. The distribution and patterns of the major arteries of the iguanids and comments on the intergeneric relationships of iguanids (Reptilia: Lacertilia). Smithson Contrib Zool. 1971; 83:1–23. [Google Scholar]
- 38. Conrad JL, Head JJ, Carrano MT. Unusual soft-tissue preservation of a crocodile lizard (Squamata, Shinisauria) from the Green River Formation (Eocene) and shinisaur relationships. Anat Rec. 2014; 297:545–59. 10.1002/ar.22868 [DOI] [PubMed] [Google Scholar]
- 39. Gilmore CW. Fossil lizards of North America. Mem Natl Acad Sci. 1928; 22(3):1–197. [Google Scholar]
- 40. Conrad JL, Rieppel O, Grande L. Re-assessment of varanid evolution based on new data from Saniwa ensidens Leidy, 1870 (Squamata, Reptilia). Amer Mus Novit. 2008; 3630:1–15. [Google Scholar]
- 41. Conrad JL. An Eocene shinisaurid (Reptilia, Squamata) from Wyoming, U.S.A. J Vert Paleontol. 2006; 26(1):113–26. [Google Scholar]
- 42. Greenwood DR, Wing SL. Eocene continental climates and latitudinal temperature gradients. Geology. 1995; 23:1044–8. [Google Scholar]
- 43. Savage JM. The origins and history of the Central American herpetofauna. Copeia. 1966; 1966(4):719–66. [Google Scholar]
- 44. Vanzolini PE, Heyer WR. The American herpetofauna and the interchange In: Strehli FG, Webb SD, editors. The Great American Biotic Interchange. New York: Plenum Press; 1985. p. 475–87. [Google Scholar]
- 45. Vieira GHC, Colli GR, Báo SN. Phylogenetic relationships of corytophanid lizards (Iguania, Squamata, Reptilia) based on partitioned and total evidence analyses of sperm morphology, gross morphology, and DNA data. Zool Scr. 2005; 34(6):605–25. [Google Scholar]
- 46. Head JJ, Bloch JI, Hastings AK, Bourque JR, Cadena EA, Herrera FA, et al. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature. 2009; 457:715–8. 10.1038/nature07671 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset A, Morphology Character-By-Taxon Matrix. Here is the full morphological data matrix. Text A, Comparative material. Observations on the following specimens and publications were used for this study. Institutional abbreviations: AMNH, American Museum of Natural History, New York, NY; FMNH, The Field Museum, Chicago, IL; IGM, Institute of Geology, Mongolian Academy of Sciences, Ulan Bator, Mongolia; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, MA; REE, Richad E Etheridge collection; UCMP, University of California Museum of Paleontology, Berkeley, CA; UC MVZ, Museum of Comparative Zoology, University of California, Berkeley, CA; UF, Florida State Museum, University of Florida, Gainesville, FL; USNM, United States National Museum of Natural History, Smithsonian Institution, Washington, DC; UWBM, Burke Museum of Natural History and Culture, University of Washington, Seattle, WA; YPM, Yale Peabody Museum, New Haven, CT; ZPAL, Zakład Paleobiologii, Polska Akademmia Nauk (Paleobiological Institute, Polish Academy of Sciences), Warsaw, Poland. Text B, Descriptions of Added Morphological Characters. Morphological characters and character-states here newly added to the phylogenetic analysis.
(DOCX)
Data Availability Statement
All relevant data are within the paper, its Supporting Information files, and the Harvard Dataverse through the following link: http://dx.doi.org/10.7910/DVN/FTQPXR.