Abstract
There are two main types of fluid in bone tissue, blood and interstitial fluid. The chemical composition of these fluids varies with time and location in bone. Blood arrives through the arterial system containing oxygen and other nutrients and the blood components depart via the venous system containing less oxygen and reduced nutrition. Within the bone, as within other tissues, substances pass from the blood through the arterial walls into the interstitial fluid. The movement of the interstitial fluid carries these substances to the cells within the bone and, at the same time, carries off the waste materials from the cells. Bone tissue would not live without these fluid movements. The development of a model for poroelastic materials with hierarchical pore space architecture for the description of blood flow and interstitial fluid flow in living bone tissue is reviewed. The model is applied to the problem of determining the exchange of pore fluid between the vascular porosity and the lacunar-canalicular porosity in bone tissue due to cyclic mechanical loading and blood pressure. These results are basic to the understanding of interstitial flow in bone tissue that, in turn, is basic to understanding of nutrient transport from the vasculature to the bone cells buried in the bone tissue and to the process of mechanotransduction by these cells.
Keywords: Interstitial fluid flow, blood flow, cortical bone
1. Introduction
Blood and interstitial fluid have many functions in bone. They transport nutrients to, and carry waste from, the bone cells (osteocytes) buried in the bony matrix. They are involved in the transport of minerals to the bone tissue for storage and the retrieval of those minerals when the body needs them. Interstitial flow is considered to have a role in bone's mechanosensory system. Bone deformation causes the interstitial flow over the cell processes of the osteocyte creating a drag on the fibers that connect the cell; the drag force created by the flowing interstitial fluid is sensed by the cell (Cowin et al., 1991, Weinbaum et al., 1994, Cowin et al., 1995, Sharma et al., 2007, Fritton and Weinbaum, 2009). A full physiological understanding of this mechanosensory system will provide insight into a number of important clinical problems, including the physiological mechanisms underlying osteoporosis, bone loss, osteonecrosis and the relationship between cardiovascular and musculoskeletal diseases.
Since one purpose of this work is to describe how these fluid systems work, consideration is limited to cortical bone in the mid-diaphysis of a long bone. Although most of what is described is also applicable to bone tissue at other anatomical sites, the discussion is more concise and direct if this limitation is stipulated.
The majority of the motive force for the blood flow is from the heart, but the contraction of muscles attached to bone and the mechanical loading of bone also contribute to this motive force. The majority of the motive force for the interstitial fluid flow is due to the mechanical loading of bone, but the contraction of muscles attached to bone and the heart also supply some of its motive force. The influence of the mechanical loading of a whole bone on the fluid systems’ maintenance of the bone tissue is critical. The fluid flow resulting from the mechanical loading is modeled by the theory of poroelasticity. This theory models the interaction of deformation and fluid flow in a fluid-saturated porous medium. The theory was proposed by Biot (1935, 1941) as a theoretical extension of soil consolidation models developed to calculate the settlement of structures placed on fluid-saturated porous soils. The theory has been widely applied to geotechnical problems beyond soil consolidation, most notably problems in rock mechanics. Certain porous rocks, marbles and granites have material properties that are similar to those of bone (Cowin, 1999). Some of the recent developments of this topic are reviewed here.
In this presentation the vascular system in bone is described first, then the interstitial fluid movement in bone as well as the factors that drive these flows and cause changes in the flow patterns associated with diseases, surgery and whole body movement. Thus the sections that follow immediately describe the arterial system, the microvascular network of marrow, the microvascular network of cortical bone and the venous drainage of bone. The connections between the vascular system and the interstitial fluid system are then described in a section on bone lymphatics and blood vessel trans-vessel-wall transport. Attention then turns to the spaces in bone tissue occupied by these two fluid systems, the vascular porosity (VP) and the lacunar-canalicular porosity (LCP) and the interfaces between the systems. The remainder of this work considers the poroelastic modeling of interstitial fluid flow, followed by a description of the most common pathologies associated with an impaired fluid flow in bone.
2. Arterial Supply
2.1 Overview of the arterial system in bone
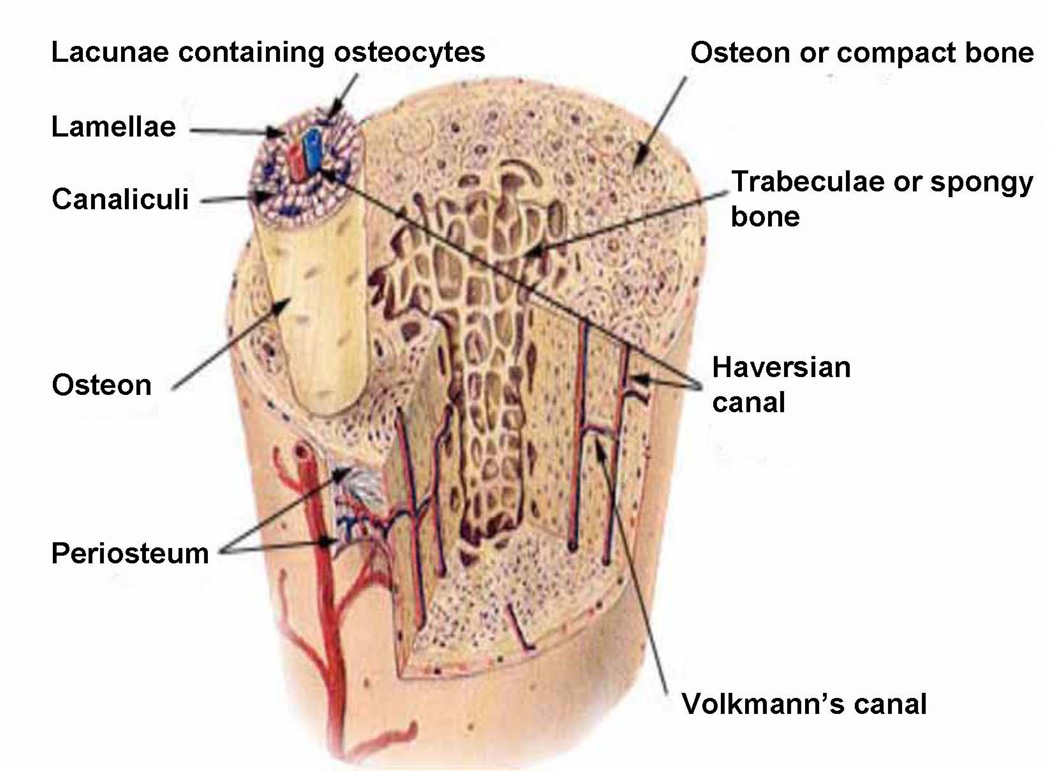
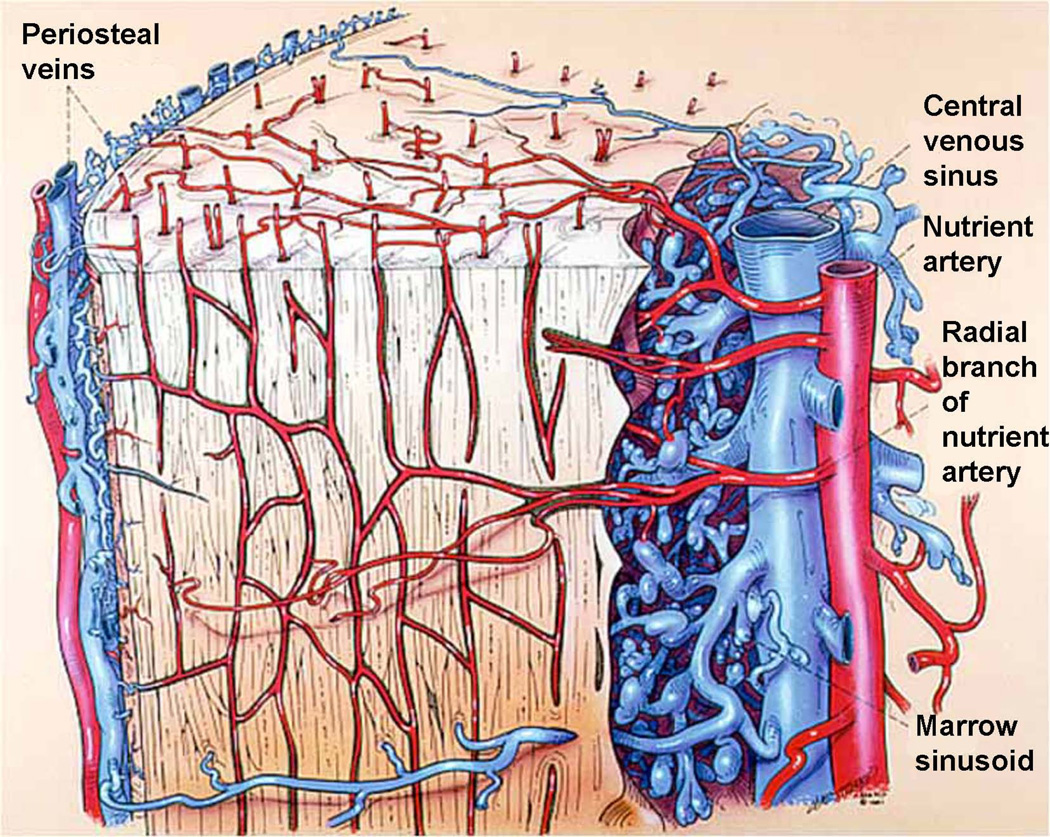
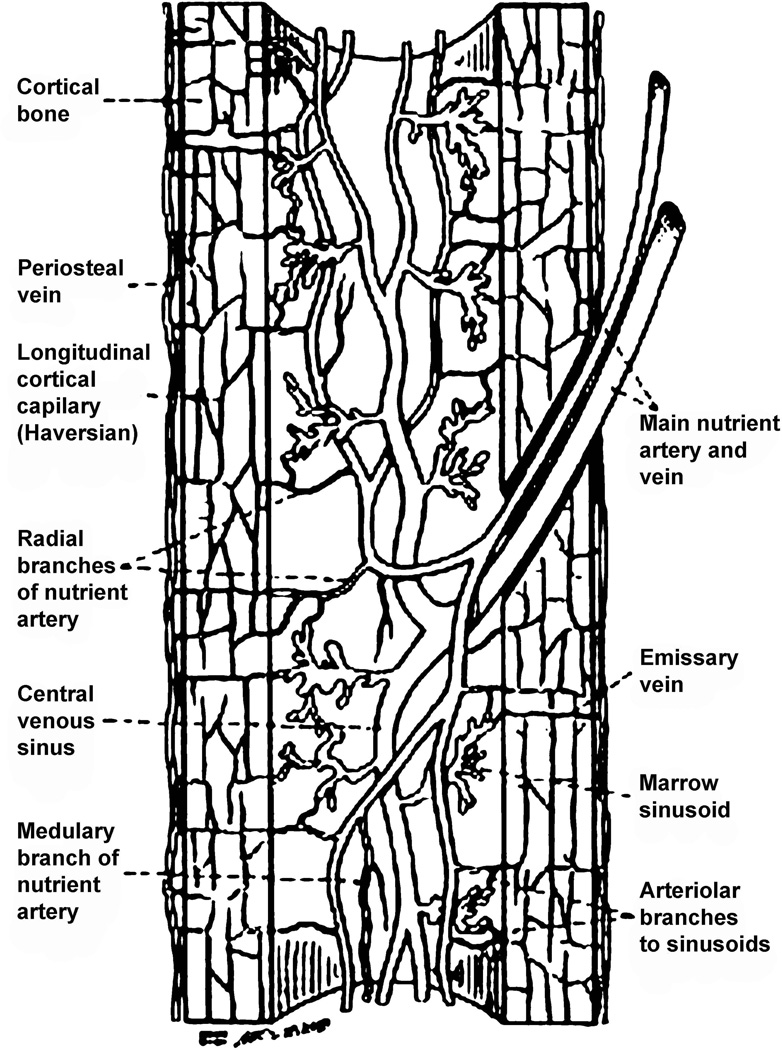
All elements of bone, including the marrow, perichondrium, epiphysis, metaphyses, and diaphyses, are richly supplied by vasculature. Mature long bones in all species have three sources of blood supply: (1) the multiple metaphyseal - epiphyseal vessel complex at the ends of the bones, (2) the “nutrient’’ artery entering the diaphyses (Fig. 1), and (3) the periosteal vessels (Fig. 1, Fig. 2). After entering the diaphyses, the nutrient artery divides into ascending and descending branches, which have further, radially orientated, branches streaming to the bone cortex (Fig. 1, Fig. 2). Usually a single nutrient artery enters the diaphyses of a long bone, though many human long bones such as the femur, tibia, and humerus often have two. When the nutrient artery enters bone, the vessel has a thick wall consisting of several cell layers, but within the medulla it rapidly becomes a thin-walled vessel with two cell layers and minimal supporting connective tissue (Yoffey, 1965). After reaching the medullary cavity the nutrient artery divides into an ascending and descending branch, which proceeds towards the metaphyseal bony ends (Fig. 1, Fig. 2). These branches approach the epiphyseal ends of bone, subdividing repeatedly along the way into branches, which pursue a helical course in the juxta-endosteal medullary bone. The terminal branches of the main ascending and descending branches supply the ends of the long bone and anastomose freely with the metaphyseal vessels. The vessels divide and subdivide to feed into a complex network of sinusoids (Fig. 1, Fig. 2). In the immature bone, the open cartilaginous epiphyseal growth plate separates the epiphyseal and metaphyseal vessel complexes.
Fig. 1.
Schematic diagram showing the vascular arrangement in the long bone diaphysis. Modified from Williams et al., (1984).
Fig. 2.
The capillary network within cortical bone. The major arterial supply to the diaphysis is from the nutrient artery. There is an abundant capillary bed throughout the bone tissue that drains outwards to the periosteal veins. Modified from Williams et al., (1984).
The blood supply to cortical bone may come from either the medullary canal (younger animals) or the periosteum (older humans, see below) (Fig. 2). The transcortical blood supply transits in the Volkmann canals and the longitudinal blood supply transits in Haversian systems or osteons. Haversian arteries run longitudinally in osteons (Haversian systems) oriented roughly about 15 degrees to the long axis of a bone (Fig. 3). Human cortical bone is largely Haversian at a rather young age compared to other animals. The thin-walled vessels in the cortical canals of Haversian and Volkmann canals are contained in hard unyielding canals in cortical bone and serve to connect the arterioles (the afferent system) with the venules (the efferent system), but unlike true capillaries, they apparently are not able to change diameter in response to physiologic needs (Rhinelander and Wilson, 1982). Diffusion from Haversian vessels to the bone cells buried in the bony matrix is insufficient to maintain their nutrition; convection driven by the interstitial fluid pressure gradients is necessary for the viability of these cells. Canaliculi serve to connect osteocytic processes (Doty, 1981). Increased distance from the vascular source (the Haversian artery) probably accounts for the finding that interstitial bone is more susceptible to ischemia than is Haversian bone (Kornblum, 1982).
Fig. 3.
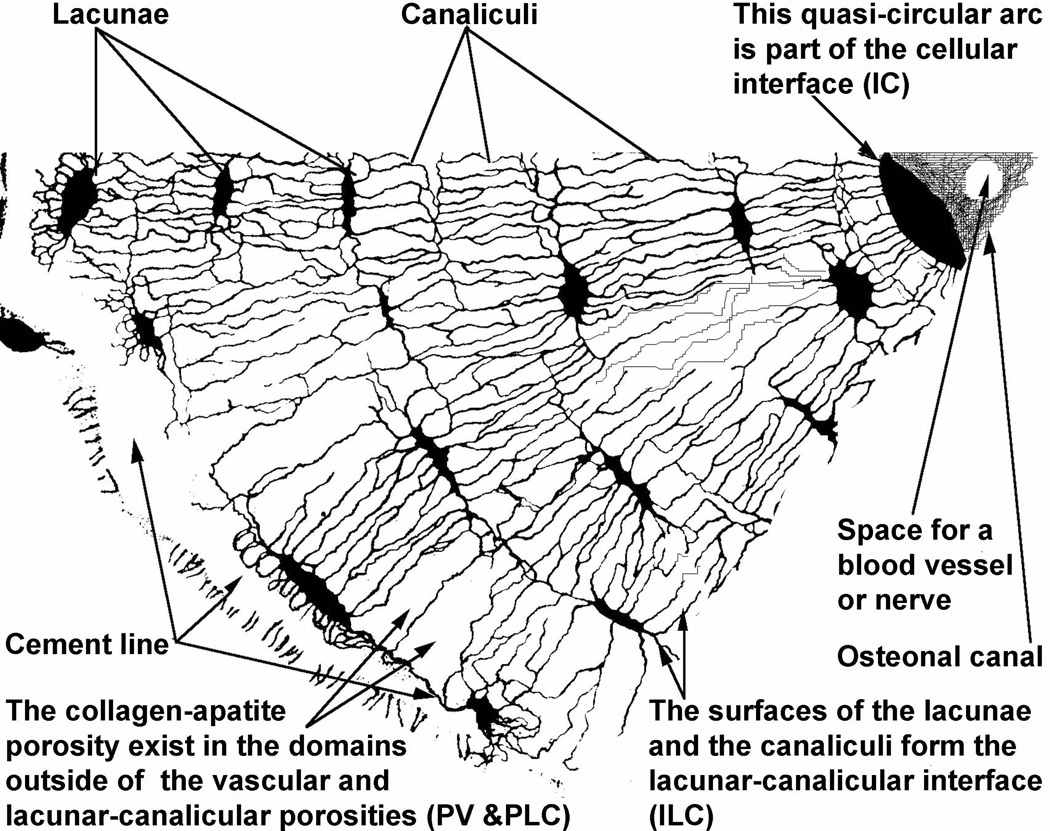
The osteon at the top of this figure is entirely PLC porosity except for its central lumen, called the osteonal canal or Haversian canal, which is part of the PV porosity. The PV porosity consists of the volume of all the tunnels in bones that contain blood vessels and includes all the osteonal canals and all the Volkmann canals, less the volume of the tunnels occupied by the blood vessels.
2.2 Dynamics of the arterial system
Important elements to be considered in the hemodynamics of any tissue are fluid and tissue pressure, fluid viscosity, vessel diameter, and the capillary bed. Blood vessels in bone are richly supplied with nerves and are intimately connected to vasomotor nerve endings; these nerves presumably exert a precise control over blood flow in bone (Herskovits et al., 1991). It is known that in most soft tissues the arteriolar mechanism reduces the blood pressure from 90 mmHg or more in arteries to about 35 mmHg at the arterial end of capillaries. Arterial vessels will close unless the transmural pressure is positive, that is to say, unless the blood pressure in the capillaries does not fall below that in the extravascular space, the interstitial fluid pressure. Note that transmural pressure (blood pressure minus the interstitial fluid pressure) must initially exceed osmotic pressure if filtration is to occur. Absorption of tissue fluid depends upon the transmural pressure being less than the osmotic pressure of the blood at the end of the sinusoid. Osmotic pressure is generally held to be about 20 mmHg. It follows that the pressure in the collecting sinuses of the diaphyseal marrow may be of the order of 55 mmHg. Note that one mmHg is 133.3 Pa or that 3 mmHg is approximately 400 Pa, 60 mmHg is approximately 8kPa. Bone fluids are interesting in that they exhibit metabolically produced differential diffusion gradients (Neuman and Neuman, 1958; Martin et al., 1958). They are sometimes limited in range, but well documented. Thus many ions, such as potassium, calcium, and phosphorus, exist in very different concentrations between blood and bone (Neuman, 1982).
2.3 Transcortical arterial hemodynamics
Bridgeman and Brookes (1996) have shown that aged bone cortex is supplied predominantly from the periosteum in contrast to the medullary supply in young human and animal bones, based on cross-sections through the mid-diaphyses. They argue that this change is attributed to increasingly severe medullary ischemia with age, brought on by arteriosclerosis of the marrow vessels. They note that an examination of the findings reported by investigators of animal bone blood supply in the past 40 years shows a large measure of agreement. Long standing controversy seems to be based on a failure to recognize that marrow ischemia accompanies natural senescence affecting transcortical hemodynamics and entraining an increasing periosteal supply for bone survival in old age. The change over from a medullary to a periosteal blood supply to bone cortex is the consequence of medullary ischemia and reduced marrow arterial pressure, brought about by medullary arteriosclerosis.
2.4 The arterial system in small animals may be different from that in humans
The marrow and cortical vascular networks in the rodent are thought to be in series while they are in parallel in the human. From perfusion studies on small mammals (guinea pig, rat, and rabbit) it has been concluded that the blood flow in long bones is such that the major blood supply to the bone marrow is transcortical (De Bruyn, et al., 1970). This means that the marrow and cortical vascular networks in the rodent are in series. Anatomic and perfusion studies in humans suggest that the circulations of the cortex and marrow are arranged in parallel from a longitudinally running nutrient artery (Lope-Curto et al., 1980). It was shown that the marrow sinusoids near the endosteal surface of bone typically receive a small arteriolar branch off the major conduit vessel as it enters the bone cortex.
3. Microvascular network of cortical bone
The vascularization of the medullary canal is illustrated in Figs. 1 & 2. The radial branches of the nutrient artery form a leash of arterioles that penetrate the endosteal surface to supply the bone capillary bed. Small arterioles from these radial branches supply the marrow sinusoids adjacent to the bone. In the adult dog, the marrow consists of adipose tissue (yellow marrow), which provides support for the lateral branches of the nutrient artery as they run toward the endosteal surface of the bone. However, in the immature animal, much of the marrow cavity is filled with active hemopoietic tissue (red marrow). The type of capillary varies between red and yellow marrow. Although it is easy to distinguish these types of marrow macroscopically, when seen microscopically, there is no clear-cut separation. The appearance can range from highly cellular to completely fatty. In active red marrow, the small vessels are thin-walled sinusoids, so called because they are many times the size of ordinary capillaries. Despite the thin walls of these vessels, Trueta and Harrison (1953) were not able to demonstrate open fenestrations between the endothelial cells. However, it should be noted that Zamboni and Pease (1961), using electron microscopy, considered the vessels in red marrow to consist of flattened reticulum cells with many fenestrations and no basement membrane. This would mean that there is minimal hindrance at the sinusoid wall for molecular exchange. In the fatty marrow, the capillaries are closed and continuous like those of other tissues such as muscle (Trueta and Harrison, 1953). This is supported by in vivo observations in the rabbit (Branemark, 1961) that the vessels varied according to the functional state of the marrow. It was estimated that the sinusoid was up to seven times the size of the marrow capillaries, which have a diameter of 8 microns.
Throughout the cortex of long bones there is a capillary network housed in small passages (Fig. 3). In immature bones, these are arranged rather haphazardly, but as bone remodels and matures, a more distinct pattern emerges. In the mature dog and the human, there are two basic systems, the Haversian canals, which run near longitudinally, and the Volkmann canals, which run nearly radially (Fig. 3). The two systems are intimately anastomosed to each other. The vessels within the Haversian canals of the human tibia have been examined by microscopy of decalcified sections (Nelson et al., 1960). The majority of the vessels were observed to be a single layer of endothelial cells. Occasionally, near the endosteal surface of the cortex, small arterioles with a muscular coat were seen, usually accompanied by a larger vein.
A comprehensive examination of the cortical bone of mature and immature dogs by electron microscopy has been reported in Cooper et al., (1966). This examination revealed considerable detail of the capillaries in bone. The Haversian canals ranged in size from 5 to 70 microns and contained either one or two vessels that had the ultrastructure of capillaries. On transverse section, they were lined by one or more endothelial cells, which were surrounded by a continuous basement membrane 400–600 Å thick. The junctions of the endothelial cells varied from simple juxtapositioning to a complex interlocking. These investigators found no smooth muscle cells in the walls of the vessels in the Haversian canals. This picture is supported by electron microscopy studies (Hughes and Blount, 1979) that showed that the cortical capillaries of the growing rat were similar to those found in skeletal muscle, although a basement membrane surrounding the capillaries could not be demonstrated. Thus it appears that the capillaries of bone are a closed tube formed from a single layer of endothelial cells. It has been suggested that transendothelial passage of substances involves two separate pathways; one through the intercellular clefts for hydrophilic substances and another across the endothelial cells themselves for lipophilic substances. If the intercellular capillary clefts are present, they are probably filled with material that makes their permeability low. This is suggested by the work of Cooper et al., (1966), who observed spaces of 175 Å between adjacent endothelial cells that were filled with an amorphous material seen by electron microscopy.
4. Venous drainage of bone
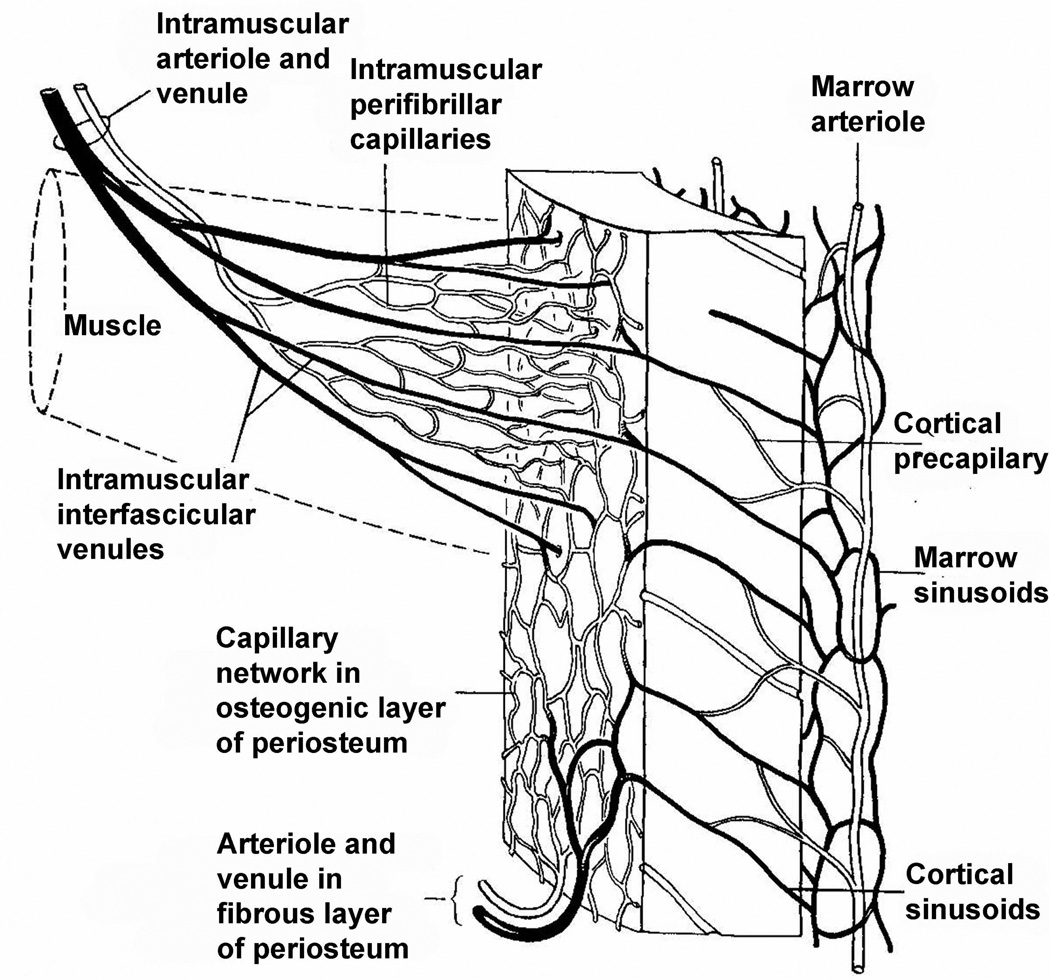
The venous complexes draining a long bone parallel those of the arteries. Many workers have commented (Trueta and Harrison, 1953; Nelson et al., 1960) on the extreme thinness of their walls. In the marrow, the venous sinusoids drain into a large, single-cell-walled, central venous sinus, which in turn drains into the "nutrient" veins of the diaphyses. In the adult dog, this thin-walled "nutrient" vein accounts for only 10% of the drainage from the diaphysis (Cuthbertson et al., 1965). The multiple, penetrating, venous radicles in the metaphyses and epiphysis are also thin-walled and run a more tortuous course than the arteries (Trueta and Harrison, 1953). The major share of the venous blood leaving long bones has been shown by phlebography to travel by this route (Cuthbertson et al., 1965). The abundantly anastomosing periosteal network of veins is considered by some workers to drain the diaphyseal bone cortex completely under normal conditions (Brooks and Revell, 1998). Many of the veins leaving the long bone pass through muscles, in particular the calf muscle in the case of the lower limb. The alternate contraction and release of the muscles containing the veins is effectively a pump returning the blood towards the heart and away from the bone and decreasing the inter medullary pressure. The inter medullary pressure can be reduced by exercise of the muscles of the calf. This arrangement in the case of the calf muscles is illustrated in Fig. 4. It is clear that the long bone as a whole has multiple venous pathways, the relative importance of which can vary with time and circumstance.
Fig. 4.
The vascular connection between bone marrow, cortex, periosteum and attached muscle. From Fig. 9.37 of Brooks and Revell (1998).
Impaired venous circulation (venous stasis) has been shown to stimulate periosteal bone formation or increase bone mass in the young dog (Kelly and Bronk, 1990), the young goat (Welch et al., 1993) and in a disuse, hind limb suspended, rat model (Bergula, et al., 1999). Venous stasis was induced in the experimental animals by applying tourniquets or vein ligation that lasted from 10 days, with additional 30 days recovery (Lilly and Kelly, 1970) or up to 42 days (Kelly and Bronk, 1990) before the bones were examined. There are many other studies demonstrating similar effects: (Lilly and Kelly, 1970; Arnoldi et al., 1971; Green and Griffin, 1982; Liu and Ho, 1991). A hypothesis for the underlying mechanism of the periosteal bone formation induced by venous stasis has been presented (Wang et al., 2003).
5. Bone lymphatics and blood vessel trans-arterial-wall transport
The purpose of this section is to indicate where the interstitial fluid can flux from a blood vessel and where it may flux back into a blood vessel. Interstitial fluid can flux from an arterial blood vessel with nutritional solutes that are used to nourish the cells in bone. Interstitial fluid may flux back into venous blood vessels with wastes as these vessels leave the bone; this becomes a possibility because of evidence (see below) of a lack of lymphatics in the periosteum.
The vascular porosity occupied by interstitial fluid is the space outside the blood vessels and nerves in the Volkmann and Haversian canals. This bone interstitial fluid freely exchanges with the vascular fluids because of the thin capillary walls of the endothelium, the absence of a muscle layer, and the sparse basement membrane. There is both outward filtration due to a pressure gradient and inward reabsorption due to the osmotic pressure. The function of these flows is to deliver nutrients to, and remove wastes from, the bone interstitial fluid. Lymph is the fluid that is formed when interstitial fluid enters the conduits of the lymphatic system. The lymphatic system has three interrelated functions. It is responsible for the removal of interstitial fluid from tissues. It absorbs and transports fatty acids and fats as chyle to the circulatory system. The last function of the lymphatic system is the transport of antigen presenting cells, such as dendritic cells, to the lymph nodes where an immune response is stimulated. The lymph is not pumped through the body like blood, it is moved mostly by the contractions of skeletal muscles (Causey et al., 2012).
The existence of lymphatic vessels in bone remains unclear. On physiologic evidence, some sort of lymph circulation must be present. Large molecules, such as albumin (mol wt 68,000) and horseradish peroxidase (mol wt 40,000), have been shown to leak out of bone capillaries into the interstitial fluid (Doty and Schofield, 1972; Owen and Triffitt, 1976), and they must have a pathway to return to the general circulation. Kolodny (1925) demonstrated that two weeks after India ink was injected into the medullary cavity of long bones, carbon particles were found in the regional lymph nodes.
However, attempts to demonstrate discrete lymphatic vessels within marrow and bone tissue have been consistently unsuccessful. It has been shown with injection studies using thorotrast (Seliger, 1970) that this substance leaks from the capillaries of cortical bone into the perivascular fluid and that eventually it can be seen in the periosteal lymphatic vessels. A similar finding has been observed in cortical bone after the use of India ink (Williams et al., 1984). The indirect conclusion seems to be that, although there are no demonstrable lymphatic channels in bone tissue, the perivascular fluid as a whole circulates toward the periphery of the bone, carrying with it substances such as large proteins and carbon particles to be taken up by a mechanism at or near the periosteum.
Anderson (1960) noted that high arterial pressure in the bone marrow probably correlates with an absence of lymphatics in bone marrow and cortex. We note that lymphatic circulation is unlikely to play a role in bone fluid transport in normal bone because lymphatic vessels are absent in bone. A chapter on the physiology of blood circulation in a book entitled Blood Vessels and Lymphatics In Organ Systems (Abramson and Dobrin, 1984) contains no description of the bone lymphatic system. It has been shown using immunohistochemistry that lymphatics were not present in normal bone (Vittas and Hainau, 1989; Edwards et al., 2008).
6. The levels of bone porosity and their bone interfaces
There are three levels of bone porosity containing blood or interstitial fluid within cortical bone and within the trabeculae of cancellous bone. A section of a long bone indicating the vascular structure is shown in Fig. 1 and more detailed views of the local bone structure are shown in Figs. 2 and 3. The three levels of bone porosity include the vascular porosity (VP) associated with the Volkmann canals (Figs. 2 and 3) and the Haversian or osteonal canals (Figs. 2 and 3), which are of the order of 20 µm in radii; the lacunar-canalicular porosity (LCP) associated with the fluid space surrounding the osteocytes and their processes (Fig. 5), which are of the order 0.1 µm in radii; and the collagen-hydroxyapatite porosity (CAP) associated with the spaces between the crystallites of the mineral hydroxyapatite (order 0.01 µm radius). The total volume of the bone fluid vascular porosity is about one half or less than that of the lacunar-canalicular porosity (Ciani et al., 2007, 2008; Cardoso et al., 2013; Palacio-Mancheno et al., 2014).
Figure 5.
A transverse cross-section of a pie-shaped section of an osteon. The osteonal canal is on the upper right, the cement line to the left. The osteonal canal is part of the vascular porosity, the lacunae and the canaliculi are part of the lacunar-canalicular porosity and the material in the space that is neither vascular porosity nor lacunar-canalicular porosity contains the collagen-apatite porosity. The three interfaces, the cement line, the cellular interface and the lacunar-canalicular interface are each indicated. The radius of an osteon is usually about 100 mm to 150 mm, and the long axis of a lacuna is about 15 mm. Using this information it should be possible to establish the approximate scale of the printed version of this illustration.
6.1 The vascular porosity (VP)
The vascular porosity (VP) occupied by bone fluid is the space outside the blood vessels and nerves in the Volkmann and Haversian canals. The typical pore size (20 µm in radii) of the vascular porosity channels is not the blood vessel pore size; rather it is the size of the tubular tunnels (Haversian systems or osteons and Volkmann canals) containing the blood vessels, the arterioles and the venules, with the actual dimensions of these vessels subtracted from the volume of the tubular tunnels.
The vascular porosity is a low-pressure reservoir that can interchange fluid with the lacunar-canalicular porosity (LCP). This is the case because the lineal dimension associated with the bone fluid VP is two orders of magnitude larger than the lineal dimension associated with the LCP, and the vascular porosity is typically at blood pressure, which is low in bone. The total volume of the VP is, however, considerably less than that of LCP (Morris et al., 1982; Ciani et al., 2007; Ciani et al., 2008).
The measurement of the permeability of the vascular porosity is somewhat difficult, primarily because of the topological intertwining of the vascular porosity with the lacunar-canalicular porosity. This difficulty and others were discussed in some detail in (Johnson, 1984) where it is noted that experiments reported (Rouhana et al., 1981) bovine cortical bone permeabilities on the order of 10−14 m2. Factors presented in (Johnson, 1984) suggest that the actual permeability could be larger than that value. The vascular porosity permeabilities reported in the past are thought to be underestimates because these previous values represent a lacunar-canalicular porosity and vascular porosity lumped measurement rather than a vascular porosity measurement alone. However since, as noted in the section following, the ratio of the permeabilities of the vascular porosity to those of the lacunar-canalicular porosity are thus of the order of 108, the permeability of the lacunar-canalicular porosity is insignificant compared to the permeability of vascular porosity.
6.2 The lacunar-canalicular porosity (LCP)
The lacunar-canalicular porosity (LCP) consists of the fluid spaces surrounding the osteocytes and their processes (Fig. 5), less volume of the soft tissue structures in these fluid spaces. The status of the research on the assessment of bone porosity, permeability and interstitial fluid flow has been recently reviewed in (Cardoso et al., 2013). The pore size estimate of the effective radii is of the order 50 to 200 nm in radii.
These lumped measurements also compromise the measurement of the permeability of the lacunar-canalicular porosity as pointed out (Beno et al., 2006) in the discussion of the reported LCP permeabilities (Dillaman et al., 1991; Steck et al., 2003). A new method for the experimental determination of the permeability of a small sample of a fluid-saturated hierarchically structured porous material was described and applied to the determination of the lacunar–canalicular permeability in osteonal bone (Benalla et al., 2012, 2013). While the permeability of LCP has been estimated to be anywhere from 10−17 m2 to 10−25 m2 (Cardoso et al., 2013), it was shown by Benalla et al., (2013), in a combined experimental and analytical, that the LCP permeability is on the order of 10−22 m2 for human osteonal bone. It was found that the permeability of this space has a linear dependence on loading frequency, decreasing at a rate of 2 × 10−14 m2/Hz from 1 to 100 Hz, and using the proposed model, the porosity alone was able to explain 86% of the permeability of this space variability.
The ratio of the permeabilities of the vascular porosity to those of the lacunar-canalicular porosity are thus of the order of 108. This 108 ratio reflects a ratio of 167 of the diameters of the pores of these two porosities and highlights the significant size difference, pore pressure difference and relaxation times in these two distinct pore size porosities.
6.3 The collagen-hydroxyapatite porosity (CAP)
The collagen-hydroxyapatite porosity (CAP) has the smallest pore size (approximately 10 nm diameter) (Holmes et al., 1964; Cowin, 1999). The interstitial pore fluid in the collagen-apatite porosity (CAP) has been shown (Wehrli and Fernández-Seara, 2005) to be bound to the solid structure, and the CAP is not of interest in the present considerations of interstitial flow for that reason. This portion of the interstitial fluid is considered to be part of the collagen-hydroxyapatite structure.
6.4 The interfaces between the levels of bone porosity
There are two external boundaries to the several porosity domains of interest in bone, the periosteum and endosteum. These are, mechanically and biologically, very different structures. The endosteum is mechanically insignificant while the periosteum is like a pre-tensioned, stiff, relatively impermeable fiber stocking attached to the exterior surface of bone. It has been reported that the periosteum acts as a barrier to bone fluid flow (Li et al., 1987). The endosteum is simply a monolayer of cells. Multiple layers of cells at various stages of differentiation lie under the periosteum.
There are two interfaces between the three levels of bone porosity within cortical bone and within the trabeculae of cancellous bone, the vascular / lacunar-canalicular porosity interface and the lacunar-canalicular / collagen-hydroxyapatite porosity interface. Topologically, all the lacunar-canalicular / collagen-hydroxyapatite porosity interface is completely contained within the vascular / lacunar-canalicular porosity interface.
The first important point concerning these interfaces, and the porosities described above, is that they change rapidly after birth, being quite porous at birth and subsequently reducing their porosity as the bone tissue becomes fully mineralized (Li et al., 1987; Soares et al., 1992). Experimental permeability studies clearly show time dependent changes in the interstitial pathways as bone matures. At the earliest times the unmineralized collagen-proteoglycan bone matrix is porous to large solutes. A study (Dillaman, 1984) with ferritin (10 nm in diameter) in two-day old chick embryo shows a continuous halo around primary osteons five minutes after the injection of this tracer. The halo passes right through the lacunar-canalicular system suggesting that, before complete mineralization, pores of a larger size can exist throughout the bone matrix. It was later demonstrated (Wang et al., 2004; Ciani et al., 2005) that such halos were very likely an artifact of histological processing and could be eliminated by shortened fixation methods (Fritton and Weinbaum, 2009). These studies found that ferritin was confined exclusively to the vascular canals and blood vessels and did not enter the lacunar-canalicular porosity. The porosity in puppies is 3.5 times higher than that of dogs (Li et al., 1987). In the present work only the adult or fully mineralized situation is described. The vascular / lacunar-canalicular porosity interface, which separates the mineralized tissue from the vascular channels, is considered first.
The region interior to the vascular / lacunar-canalicular porosity is called the "milieu intérieur" and existence of a "bone membrane" that would coincide with what we call the vascular / lacunar-canalicular porosity interface was suggested (Neuman, 1969). This interface is a continuous layer of bone lining cells (Miller and Jee, 1992); all the surfaces of the Haversian canals and the Volkmann canals are part of this interface as is the endosteum. There is a report of tight junctions occurring in the bone lining cells on the interface (Soares et al., 1992). In (Neuman and Neuman, 1958) it was noted that the bone fluid of the vascular porosity (serum) and the bone fluid of the lacunar-canalicular porosity (extracellular fluid) were nearly equivalent in composition (pH, Ca++, Na+, etc.), but it was argued that there must be some diffusion barrier, some ion gradient or ion pump, between the two fluid compartments, a view also later revised (Neuman, 1982). It has been analytically demonstrated that there are high transient pressure gradients across the interface that will serve to move the bone water across the interface (Zhang et al., 1998a,b). The bone lining cells with tight junctions will not form a significant barrier to the transport of bone water across this interface. During each cycle of bone loading, the bone fluid of the vascular porosity (serum) will briefly mix with the bone fluid of the lacunar-canalicular porosity (extracellular fluid). As a first approximation it appears reasonable to assume that the permeability of this interface is equal to the permeability of the lacunar-canalicular porosity.
The lacunar-canalicular / collagen-hydroxyapatite porosity interface is considered next. The evidence suggests that this interface is generally impermeable. Again, the evidence is from tracer studies. This conclusion is supported by the studies in the alveolar bone of five-day-old rats using the small tracer microperoxidase (MP) (2 nm) (Tanaka and Sakano, 1985). These studies clearly showed that the MP only penetrated the unmineralized matrix surrounding the lacunae and the borders of the canaliculi (see Fig. 13 of that study) and was absent from the mineralized matrix. Using more mature rats another study confirmed the failure of the small (2 nm) MP tracer to penetrate the mineralized matrix tissue from the bone fluid compartments (Asasaka et al., 1992). Further confirmation comes from studies that observed tracers of ruthenium red (MW 860, 1.13 nm in largest dimension) and procion red (MW 300–400) did not penetrate the bone mineral porosity, but were present in the lacunar-canalicular porosity (Doty, 1997; Knothe Tate, 1998).
An important physiological consideration arises from the fact that bone serves as a reservoir for calcium and phosphorus, and these mineral reserves should be connected to the circulation. Clearly these minerals must cross the vascular / lacunar-canalicular porosity interface, but must they cross the lacunar-canalicular / collagen-hydroxyapatite porosity interface? That is to say, can the necessary minerals be supplied by the bone lining cells from the bone matrix they are situated upon, or must the osteocytes be involved in this process. Estimates show that sufficient mineral can be supplied by the bone lining cells, consistent with the suggestions above that the lacunar-canalicular / collagen-hydroxyapatite porosity interface is generally impermeable and that the permeability of the vascular / lacunar-canalicular porosity interface is equal to the permeability of the lacunar-canalicular porosity. However, the possibility that the interface permeability between the lacunar-canalicular porosity and the collagen-hydroxyapatite porosity might be changed by physiological demands is worthy of consideration. The exact method of mineral retrieval and redeposition lies at the root of many studies (Soares et al., 1992; Williams and McCarthy, 1986; McCarthy and Yang, 1992; Martin, 1994).
7. Interstitial fluid flow
7.1 The different fluid pressures in long bones (blood pressure, interstitial fluid and intramedullary pressure)
Since the blood is encased in very thin-walled blood vessels that are contained within the VP, the interstitial fluid pressure is less than the blood pressure. The difference between the blood pressure and the interstitial fluid pressure is the transmural pressure. The VP is a vast low-pressure reservoir for interstitial fluid that can interchange that fluid with the LCP. This is the case because the lineal dimension associated with the bone fluid vascular porosity is two orders of magnitude larger than the lineal dimension associated with the LCP, and the interstitial fluid pressure in the vascular porosity is typically lower than the blood pressure within the blood vessels.
The intramedullary pressure in normal bone is the pressure of blood in a local pool of hemorrhage from ruptured intraosseous vessels obtained by drilling into the marrow cavity through the cortex to insert a steel cannula through which the marrow cavity pressure is measured. This was pointed out by Azuma (1964) and supported by Shim et al., (1972). Therefore, the measurable marrow cavity pressure varies to some extent by the size and type of vessels ruptured as well as by the vasomotor action in the marrow cavity under a condition of anticoagulation. The differences in the intramedullary pressure from region to region in a given bone, from bone to bone, and from animal to animal in the same and different species were noted by Shim et al., (1972). If the femoral vein was occluded, the intramedullary pressure was elevated and nutrient venous outflow increased, an indication of venous congestion of bone. If the nutrient or femoral artery was occluded, there was an immediate fall in the intramedullary pressure and a profound decrease in nutrient venous outflow. The intramedullary pressure can be increased by mechanical loading of the bone and by venous ligature, and the intramedullary pressure can be reduced by exercise of the muscles of the calf (Fig. 4).
The pore fluid pressures in these two pore size bone porosities are distinct and vary very differently with time under mechanical loading of the whole bone. Under physiologically possible rapid rise-time loadings of bone, the pore fluid pressure may rise considerably in the LCP (Zhang et al., 1998a,b). The decay time for this pore pressure rise is much larger in the LCP than it is in the VP (Johnson, 1984), (Zhang et al., 1998a,b). The VP is a low-pore-fluid-pressure domain because the VP permeability is sufficiently large to permit a rapid decay of a pressure pulse. This must be the case because the VP contains thin-walled blood vessels carrying blood with a pressure of 40 to 60 mm of Hg; a pore-fluid-pressure significantly greater than 40 to 60 mm of Hg will collapse these blood vessels and a prolonged increase in the pore-fluid-pressure significantly above 40 to 60 mm of Hg would deprive the tissue of oxygen and nutrients.
7.2 Interstitial flow and mechanosensation
Since bone fluid in the porosity with the largest lineal dimension, the VP, is always at a low pressure, the middle porosity - the LCP - appears to be the most important porosity for the consideration of mechanical and mechanosensory effects in bone. The interstitial pore fluid pressure in the LCP can be, transiently, much higher. The LCP is the primary porosity scale associated with the relaxation of the excess pore pressure due to mechanical loading. This relaxation of the interstitial pore fluid pressure was illustrated in (Wang et al., 1999). In this work the interstitial pore fluid pressure distributions across a bone are calculated using an idealized bone microstructural model consisting of six abutting osteons with osteonal canals. It is the porosity associated with the osteocytes that are the prime candidates for the mechanosensory cell in bone. A detailed theoretical model of the contents of the lacunar-canalicular porosity was given in Weinbaum et al., (1994) and Cowin et al., (1995).
In addition to mechanosensation a function of these flows is to deliver nutrients to, and remove wastes from, the osteocytes housed in the lacunae buried in bone matrix (Fig. 5). An osteocyte left in vitro without nutrient exchange for 4 hours will die (James et al., 1986). This observation makes sense given the estimate that osteonecrosis in vivo is significant if bone is ischemic for 6 hours or more (Catto, 1976).
The lacunar-canalicular porosity is the porosity associated with the osteocytes that are the prime candidates for the mechanosensory cell in bone because of the fluid movement induced by the interstitial pore fluid pressure gradients (Weinbaum et al., 1994; Cowin et al., 1995; Sharma et al., 2007; Fritton and Weinbaum, 2009). The bone fluid in the smallest porosity, the collagen-hydroxyapatite porosity, is considered to be immovable under normal conditions because it is bound to the collagen-hydroxyapatite structure.
Over the last forty years many researchers have used tracers to document bone fluid transport; see (Knothe Tate et al., 1998) for a summary of the tracers employed. A recent summary of these efforts is given in (Fritton and Weinbaum, 2009). These tracers show that the normal bone fluid flow is from the marrow cavity to the periosteal lymphatic vessels through the Volkmann and Haversian canals. The flow passes from the Haversian canal into the lacunar canalicular porosity to the cement line of the osteon.
7.3 The poroelastic model for cortical bone
Poroelasticity is a well-developed theory for the interaction of fluid and solid phases of a fluid saturated porous medium. It is widely used in geomechanics and it has been applied to bone by many authors in the last fifty years. A review of the literature related to the application of poroelasticity to bone fluid was presented in (Cowin, 1999). Described in that work were the specific physical and modeling considerations that establish poroelasticity as an effective and useful model for deformation-driven bone fluid movement in bone tissue. The application of poroelasticity to bone differs from its application to soft tissues in two important ways. First, the deformations of bone are small while those of soft tissues are generally large. Second, the bulk compressibility of the mineralized bone matrix is about seven times stiffer than that of the fluid in the pores while the bulk compressibilities of the soft tissue matrix and the pore water are almost the same. Poroelasticity and electrokinetics can be used to explain strain-generated potentials in wet bone. It is noted that strain-generated potentials can be used as an effective tool in the experimental study of local bone fluid flow, and that the knowledge of this technique will contribute to the answer of a number of questions concerning bone mineralization and the bone mechanosensory system.
A poroelastic model for the interstitial fluid flow space in bone tissue, with a reasonably accurate anatomical model for the architecture of its pore space structure, is presented in Cowin et al., (2009). In order to characterize the special type of porous material pore structure considered, the phrase “hierarchical” was used as an adjective to modify “poroelasticity.” Alternatively it could be described as a set of nested porosities like a set of Russian nested dolls or babushka (matryoshki) is a set of dolls of decreasing sizes placed one inside another; each doll but the smallest may be opened to reveal another doll of the same sort inside. The idea of a smaller structure within a larger, similarly shaped, structure is the idea that is to be transferred from a set of babushka to sets of different pore structures in a porous material. The body fluids in tissues reside in such nested, topologically similar, pore structures with different pore sizes in bone, and other tissue types (Gailani and Cowin, 2011). Examples of these porosities in bone tissue are the VP, the LCP and CAP.
The animal vascular tree is an example of a pore structure with two such nested systems that are connected. In a microcirculatory bed blood flows from arteries to arterioles, then to capillaries and on to venules and into the veins; in each of these pore structures the pore size is relatively uniform, but it varies monotonically between levels of porosity characterized by their pore size. The arterial system consists of the capillaries nested within the arterioles that are nested within the arteries. The venous system consists of the capillaries nested within the venules that are nested within the veins. The capillary plexuses of the two nested systems are connected.
Body fluids in tissues reside in such nested, topologically similar, pore structures with different pore sizes in bone, tendon, meniscus and possibly other tissue types. The nesting or ordering criterion is the porosity pore size. The nested porosities are connected so the pore fluid may flow easily through each and across the boundaries between the two nearest neighbor porosities, but any particular pore size porosity may only interchange its pore fluid with the next larger pore size porosity and the next smaller pore size porosity. The flow of interstitial fluid in tissues like bone, tendon, meniscus and possibly other tissue types is similar to blood flow in the vascular system in the sense that the different pore size porosities are nested, but unsimilar in three important aspects; (1) there are only two levels of pore size porosity important for bone fluid flow, (2) there is no flow out of, or in from, the smaller pore size porosity into any even smaller pore size porosity, only into, or in from, a larger pore size porosity and (3) the fluid flow direction reverses in the normal physiological function of these tissues.
With only the exception of the animal vascular tree, the applications of poroelasticity to fluid movement in biological tissues have simply transferred to tissues the models of the pore structure employed in geomechanics. Existing theories of the poroelasticity of materials with multiple connected porosities with different characteristic sizes and therefore different permeabilities do not address the case of nested porosities. These existing theories are appropriate for their intended use, fractured porous geological structures, but they are not appropriate for the biological tissues of interest. The nested porosities in biological tissue are hierarchical based on the average diameters of their fluid transport channels while the multiple porosity poroelasticity theories for fractured geological structures are democratic. Their fluid transport channels of a particular size may exchange fluid with transport channels of any pore size. The primary objective of this work is to provide a model of a poroelastic pore structure appropriate for bone tissue; it is a model that is easily extended to other tissues such as the tendon and the meniscus. Concerning bone, a principal focus is the modeling of the mechanical and blood pressure load-driven movement of the interstitial bone fluid flow.
The absence of the assumption of incompressible constituents is a significant difference between the version of poroelasticity theory employed in (Cowin et al., 2009) and the poroelasticity theory used for previous published solutions involving soft tissues. The assumption of incompressible constituents, while appropriate for soft tissues, is inaccurate for hard tissues. The solution for the unconfined compression of an annular, transversely isotropic, linear poroelastic hollow cylinder with compressible constituents was developed by Gailani and Cowin (2008). The total load generated on a specimen by an arbitrary strain history can be determined. Alternatively, if the load history is prescribed, then the equation can be used to determine the strain history. The displacement field and the pore pressure field may be evaluated for an imposed load history, as opposed to imposed displacement history. On the basis of this solution a protocol was devised for an experimental test procedure to determine tissue permeabilities for the smallest nested bone porosity, the osteonal lumen wall, and the osteonal cement line. This extends to bone tissue an experimental technique that has been very effective in determining soft tissue poroelastic properties (Gailani et al., 2009).
As noted above, current theoretical and experimental evidence suggests that the bone cells in the lacunae (pores) of the lacunar-canalicular porosity are the principal mechanosensory cells of bone, and that they are activated by the induced drag from fluid flowing through the lacunar-canalicular porosity (Weinbaum et al., 1994; Cowin et al., 1995; Sharma et al., 2007; Fritton and Weinbaum, 2009). The movement of bone fluid from the region of the bone vasculature through the canaliculi and the lacunae of the surrounding mineralized tissue accomplishes three important tasks. First, it transports nutrients to the cells in the lacunae buried in the mineralized matrix. Second, it carries away the cell waste. Third, the bone fluid exerts a force on the cell process, a force that is large enough for the cell to sense. This is thought to be the basic mechanotransduction mechanism in bone, the way in which bone senses the mechanical load to which it is subjected. Understanding bone mechanotransduction is fundamental to the understanding of how to treat osteoporosis, how to cope with microgravity in long term manned space flight and how to design prostheses that are implanted in bone tissue to function for longer periods.
7.4 Interchange of interstitial fluid between the vascular and lacunar canalicular porosities
Using the hierarchical scheme described in the previous subsection a model was formulated in (Cowin et al., 2009) for the transport of bone interstitial fluid between the VP and LCP porosity levels in osteonal cortical bone. A section of this bone is illustrated in Fig. 3. The osteon at the top of this figure is entirely LCP porosity except for its central lumen, called the osteonal canal or Haversian canal, which is part of the VP porosity. The VP porosity consists of the volume of all the tunnels in bones that contain blood vessels and includes all the osteonal canals and all the Volkmann canals, less the volume of the tunnels occupied by the blood vessels.
The VP and the LCP are both modeled as poroelastic hollow circular cylinders. The poroelastic hollow circular cylinder model of the LCP connects through its inner cylindrical wall to the VP; the hollow part of this cylinder is actually part of the VP. The inner surface of the cylinder representing the LCP is the surface across which the two porosities exchange pore fluids. The LCP is assumed to permit flow across its inner radial boundary, but not its outer radial boundary. While other assumptions are possible, an earlier study (Wang et al., 1999) showed that this is a reasonable assumption. The VP is assumed to permit flow across both of its radial boundaries. The LCP hollow cylinder is the osteon of Figure 3. The VP hollow cylinder is the entire bone of Figure 3 with central lumen of the whole bone, the medullary canal, constituting the hollow part of the VP model. Both of these models are continuum models and the transport connection between is the outflow-influx across the osteonal or Volkmann inner wall between the LCP and the VP (Fritton et al., 2001). In the domain between the inner surface and outer surface of the VP cylinder there are areal sources-sinks that permit interchange of fluid between the two continuum models representing the VP and LCP.
The pore fluid movement from the perivascular region or VP in bone to the bone cells housed in the lacunae of the mineralized matrix or LCP was evaluated in (Cowin et al., 2009). Knowledge of this fluid movement is necessary for the determination of the nutrient transport and the effect of the fluid drag on the cell processes of osteocytes in the canaliculi, which is thought to be a principal mechanotransduction mechanism. The problem of determining the exchange of pore fluid between the VP and the LCP in bone tissue due to cyclic mechanical loading and blood pressure oscillations was solved. A formula for the volume of fluid that moves between the LCP and VP in a cyclic loading was obtained as a function of the cyclic mechanical loading and blood pressure oscillations. Formulas for the fluid pore pressure in both the LCP and the VP were obtained as a function of the two driving forces, the cyclic mechanical straining and the blood pressure, both with specified amplitude and frequency. A general observation is that the VP and the LCP are greatly different. The LCP is a relatively high-pressure domain characterized by a relatively small pore size and no vascular vessels. On the other hand the VP is a relatively low-pressure domain characterized by a relatively large pore size that contains vascular vessels. The model shows that, although the two porosities exchange fluids, their influence on one another in terms of pressure change is small.
The results of (Cowin et al., 2009) suggest that the VP and LCP function almost independently, the prime mechanical influences for the two porosities being very different as are the time scales of their response. The mechanical loading of the whole bone moves the bone fluid in the LCP. When the bone is compressed the bone fluid is driven from the LCP into the low-pressure VP and when the bone is in tension, or the compression is reduced, the bone fluid is sucked from the VP into the LCP. These drainage and imbibing processes occur on a pressure time scale that is much larger than the short pressure adjustment relaxation time for the VP and therefore have minimum influence on the pressure in the VP. The change in interstitial pore fluid pressure in the VP due to inflow or outflow of bone fluid from the LCP is insignificant because of the time period of pressure adjustment that is much shorter (estimates of these time periods are in Zhang et al., 1999a,b) than the pressure adjustment time period for the LCP. While the bone fluid in the LCP is significantly affected for a longer time by the mechanical loading of the whole bone, the bone fluid pressure in the VP is hardly affected because the VP relaxes the pressure pulse by diffusion so rapidly.
8. Blood flow, interstitial fluid flow and bone pathology
8.1 Osteoporosis
An increasing number of studies indicate that cardiovascular disease (CVD) and osteoporosis are significantly related (Table 1 in Warburton et al., 2007). Bone mineral density has been inversely associated with coronary and/or aortic calcification (Uyama et al., 1997; Barengolts et al., 1998; Bagger et al., 2006), and directly associated with high-density lipoprotein cholesterol, also known as the good cholesterol (Yamaguchi et al., 2002). Recent clinical data has revealed an association between accelerated bone loss, diabetes (Schwartz et al., 2005) and cardiovascular pathology (Romney and Lewanczuk 2001; McGavock et al., 2004). In cases of asymmetric vascular disease in the lower limbs (i.e. vascular function is affected differentially on opposite sides of the body), the side that has the greatest vascular dysfunction also displays the lowest bone mineral content (Laroche et al., 1994; Laroche et al., 2003). The blood vessels in the proximal femur are often atherosclerotic in elderly who has suffered femoral head fractures (Bocchi et al., 1985). The bone loss rate at the hip is greater in women who have the greatest reduction in blood flow (Vogt et al., 1997). Interestingly, there is an increased risk of cardiovascular-related and stroke mortality for each standard deviation decrease in bone mass (Browner et al., 1991; von der Recke et al., 1999; Kado et al., 2000). Fatty involution of the hematopoietic marrow and loss of arterial capillaries has been associated with osteoporosis (Burkhardt, 1971). Estrogen reduction after ovariectomy results in increased osteoclastic resorption and increased intraosseous vascularization (Laroche, 2002).
8.2 Bone remodeling
New vessels are involved in both intramembranous and endochondral osteogenesis (Collin-Osdoby, 1994; Streeten and Brandi 1990; Trueta and Buhr, 1963) as well as in pathologic increases in bone remodeling (Burkhardt et al., 1984). The coupling between bone resorption and formation during bone remodeling is coordinated by the vascular bud at the center of every bone multicellular unit (Parffit, 2000). The endothelial cells at the tip of this capillary are considered to respond to a number of stimuli including tensile forces and growth factors, in order to produce the signals that allow osteoclast precursors, and pericytes and/or osteoblast precursors to exit the capillary through its wall during bone remodeling (Parffit, 2000). Alterations in intraosseous vascularization seem to affect oxygen consumption, bone remodeling, and particularly, bone formation (Reilly et al., 1998; Francklin and Kelly, 1998; Laroche, 2002).
8.3 Osteonecrosis
Osteonecrosis is a common skeletal disorder that may result in bone fracture, pain and loss of function. It is also known as avascular necrosis, ischemic necrosis, subchondral avascular necrosis, and aseptic necrosis of bone. Osteonecrosis is characterized by focal regions of dead bone, with empty osteocyte lacunae and lack of other bone cells (i.e. osteoblasts and bone lining cells), which occur as a consequence of a number of conditions leading to an impairment of blood supply to the bone tissue. The etiology of osteonecrosis is known in conditions such as caisson disease and sickle cell disease, but it is mostly unknown for many other conditions, such as systemic lupus erythematosus, corticosteroid-related osteonecrosis, alcoholism and some other idiopathic forms. An intrinsic factor contributing to the development of osteonecrosis is the impaired blood flow and vascularity of fat marrow in comparison with haemopoietic marrow. Impaired blood flow can occur as a consequence of vascular compression, trauma or occlusion of blood vessels by nitrogen bubbles (e.g. caisson disease) or rigid sickle cells (e.g. sickle cell anemia). Non-traumatic osteonecrosis seems to be associated with vessel infarction, stenosing arteritis, arteriosclerotic disease, extraosseous arterial involvement, extraosseous venous abnormality, hypercoagulability and hypofibrinolysis. Osteonecrosis-related infarcts in long bones are generally asymptomatic and remain clinically undetected, until a fracture may occur. Bone infarcts are generally adjacent to a joint, mainly in the femoral head (Cushner and Friedman, 1988), humeral head (Frostick and Wallace, 1989), knee (Aglietti et al., 1983), foot and hand bones (Taniguchi et al., 2003; Karlakki and Bindra, 2003; Suzuki et al., 2003; Strokon et al., 2003), and the vertebrae (Yu et al., 2007).
8.4 Other pathologies
In addition to osteoporosis, bone remodeling and osteonecrosis, impairment of blood circulation in bone seems linked to other diseases such as reflex sympathetic dystrophy, Paget disease, delayed fracture repair, and bone metastases. Also, primary hyperparathyroidism has been associated with increased vascularization, increased bone resorption and excessive marrow cellularity (Burkhardt et al., 1984, 1987). Bone apposition has been correlated to intraosseous blood flow rate measured using a strontium clearance method (Reeves et al., 1988). Moreover, use of antiresorptive bone therapy based on bisphosphonates (e.g. tiludronate) inhibited excessive resorption without an increase in vascularization (Laroche et al., 1996).
8.5 Possible mechanisms
Chronic inflammation, proinflammatory cytokines (interleukin-6) and tumor necrosis factor-apha (TNF-α) have been associated with vascular disease (Blake and Ridker, 2001) and increased bone resorption (Cohen-Solal et al., 1993). Activation of the inducible nitric oxide synthase isoform (iNOS) pathway contributes to inflammation-mediated osteoporosis via suppressed bone formation and osteoblast apoptosis (Armour et al., 2001b). Endothelial nitric oxide synthase isoform (eNOS) knock-out mice has shown reduced bone volume, bone formation rates, BMD, osteoblast numbers (Aguirre et al., 2001, Armour et al., 2001a, Samuels et al., 2001), and exogenous estrogens. Osteoblast-like cells under pulsatile fluid flow have increased eNOS production (Klein-Nulend et al., 1998). Overall, sex steroids (e.g. estrogens) have been shown to affect both cardiovascular and musculoskeletal systems (Stevenson, 2004; Rossouw, 2005; Baldini et al., 2005). Hypervascularization is also seen in regenerative anemia and in the bone marrow response to inflammation (Burkhardt et al., 1987). The cytokines and growth factors that regulate angiogenesis or vasomotility (e.g. IGF, PTH or PTHrp, NO, VEGF) are also involved in bone remodeling.
Calcified atherosclerotic plaque has numerous cellular and molecular elements that participate in bone formation including bone morphogenic protein-2, collagen-1, osteonectin, osteopontin, matrix Gla proteins, osteocalcin and osteoprotegerin (Doherty et al., 2003; Witney et al., 2004; Hamerman, 2005). Several plausible biological pathways have been proposed in which decreased cardiovascular health can influence musculoskeletal health and vice versa (Witney et al., 2004; Doherty et al., 2003; Hamerman 2005; Rajzbaum and Bezie, 2006).
Osteoprotegerin, a member of the TNF receptor superfamily, serves as a bone resorption inhibitor by blocking the RANK/RANK-ligand interaction (Kiechl et al., 2004; Rajzbaum and Bezie, 2006). High levels of osteoprotegerin were found to be an independent risk factor for the progression of atherosclerosis and the development of CVD (Browner et al., 2001; Kiechl et al., 2004). Serum lipids and accumulation of oxidized lipids in the endothelium are well-established risk factors for CVD, promoting arterial calcification and inhibiting bone mineral formation (Parhami et al., 2000) while affecting osteoblasts (Parhami et al., 1997, 2000, 2002) and increasing osteoclastic bone resorption (Tintut et al., 2004). However, the role of lipids in the relationship between CVD and osteoporosis remains controversial because many other studies have found the opposite effect (Adami et al., 2004) or no contribution of lipids on bone loss (Bagger et al., 2007). Such discrepancies suggest that the obstructive vascular disease, rather than the lipids itself, is responsible for the bone loss, indicating that a decrease in peripheral blood flow and supply could suppress bone cell function (Rubin and Silverberg, 2004).
8.6 Interstitial fluid flow in the lacunar-canalicular system
Mechanical loading of bones leads to deformation of the bone matrix material, generating interstitial fluid flow (IFF) in the lacunar-canalicular porosity. The IFF is the stimulus by which osteocytes in the LCP initiate the mechanotransduction process (Weinbaum et al., 1994). Fluid flow has been shown necessary for mechanotransduction and maintenance of bone mass in vitro (Klein-Nulend et al., 1995; McAllister et al., 2000; You et al., 2001; Kim et al., 2006; You et al., 2008; Arnsdorf et al., 2009; Kwon et al., 2010). Also, the mechanosensory function of osteocytes was proved in vivo in a murine model of osteocyte ablation (Tatsumi et al., 2007), and apoptosis of osteocytes was shown to be a controlling step of activation of intracortical resorption (Cardoso et al., 2009). In addition to IFF in the LCP, IFF in the vascular porosity can also prevent bone loss and lead to bone formation, as shown by in vivo studies in which fluid flow was decoupled from bone matrix strains (Qin et al., 2003; 2009; Kwon et al., 2010). More recently, it has been shown that intramedullary-driven IFF in the vascular system can also prevent bone loss and lead to bone formation, even in the absence of osteocytes, suggesting that bone cells other than osteocytes (i.e. bone lining cells or quiescent osteoblasts) can be responsive to IFF in the vascular porosity (Kwon et al., 2010, 2012). The IFF mechanisms for stimulation of bone cell mechanotransduction and prevention of bone loss in the VP and the LCP seem to be decoupled since the fluid pressure, relaxation time, and lineal dimension of their porosities are several orders of magnitude different.
9. Summary
The vascular system in bone and the transcortical arterial hemodynamics were described in detail. The reviewed literature shows that with ageing, the vascular supply in cortical bone changes from medullary to periosteal, as a consequence of medullary ischemia and reduced marrow arterial pressure produced after arteriosclerosis. Throughout the cortex of long bones there is a microvascular network of capillaries running within anastomosed Haversian and Volkman canals. The fluid in this network is drained into the venous sinusoid complexes, central venous sinus and the nutrient veins with the help of skeletal muscle contractions. Impaired venous circulation leading to venous stasis stimulates periosteal bone formation. The existence of lymphatic vessels in bone remains controversial, and more research at this regard is needed. The characteristics of the three different levels of bone porosity and their bone interfaces were also described. The bone porosity includes the vascular porosity associated with the Volkmann canals and the Haversian or osteonal canals; the lacunar-canalicular porosity is associated with the fluid space surrounding the osteocytes and their processes; and the collagen-hydroxyapatite porosity is associated with the spaces between the crystallites of the mineral hydroxyapatite.
The anastomosed VP and LCP are the two bone porosities involved in interstitial fluid flow and mechanotransduction. Blood is encased in very thin-walled blood vessels that are contained within the VP, a vast low-pressure reservoir for interstitial fluid. The interstitial fluid pressure in the vascular porosity is typically lower than the blood pressure to avoid collapsing blood vessels in the VP. The intramedullary pressure can be increased by mechanical loading of the bone and by venous ligature, and it can be reduced by exercise of the muscles surrounding bone. The lineal dimension associated with the bone fluid vascular porosity is two orders of magnitude larger than the lineal dimension associated with the LCP. The total volume of the VP is, however, considerably less than that of LCP. The ratio of the permeabilities of the vascular porosity to those of the lacunar-canalicular porosity are of the order of 108, the permeability of the lacunar-canalicular porosity is insignificant compared to the permeability of vascular porosity.
Lumped measurements in cortical bone compromise the measurement of the permeability of the lacunar-canalicular porosity because of being intertwined with the vascular porosity. A hierarchical poroelastic model for the interstitial fluid flow space in bone tissue was used to determine the permeability of the LCP without the influence of the intertwined VP. The model shows that, although the two porosities exchange fluids, their influence on one another in terms of pressure change is small. The drainage and imbibing processes occur on a pressure time scale that is much larger than the short pressure adjustment relaxation time for the VP and therefore have minimum influence on the pressure in the VP. Recent studies in single isolated osteons with this hierarchical poroelastic model allowed the assessment of LCP permeability alone.
Finally, a direct relationship between vascular disease and bone mineral density measurements has been found in several clinical studies after adjusting the data for many common risk factors (e.g. age, years after menopause, BMI, level of education, smoking, physical inactivity), suggesting both chronic diseases may be linked by common pathophysiological mechanisms; however, a causal link between CVD and osteoporosis has not been proved yet. The mechanisms responsible for the relationship between cardiovascular disease and musculoskeletal pathologies remain to be determined.
Acknowledgments
This work is dedicated to the memory of Rik Huiskes who contributed to the research, education of future researchers and the international socialization of biomechanics.
This work was supported by NSF (PHY-0848491, CMMI-1333560, MRI-0723027, and MRI-1229449), NIH (NIA AG34198, NIDDK DK103362) and the PSC-CUNY Research Award Program of the City University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors state that they have no conflicts of interest.
References
- Abramson DI, Dobrin PB, editors. Blood Vessels and Lymphatics In Organ Systems. Orlando: Academic Press; 1984. [Google Scholar]
- Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, Battaglia E. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004 Feb;74(2):136–142. doi: 10.1007/s00223-003-0050-4. Epub 2003 Dec 15. [DOI] [PubMed] [Google Scholar]
- Aglietti P, Insall JN, Buzzi R, et al. Idiopathic osteonecrosis of the knee. Aetiology, prognosis and treatment. J Bone Joint Surg. 1983;65B:588–597. doi: 10.1302/0301-620X.65B5.6643563. [DOI] [PubMed] [Google Scholar]
- Aguirre J, Buttery L, O'Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukkanen M, Huang P, MacIntyre I, Polak J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. 2001 Jan;158(1):247–257. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW. Studies of the lymphatic pathways of bone and bone marrow. Journal of Bone and Joint Surgery. 1960;42A:716–717. [Google Scholar]
- Armour KE, Armour KJ, Gallagher ME, Gödecke A, Helfrich MH, Reid DM, Ralston SH. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001a Feb;142(2):760–766. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- Armour KJ, Armour KE, van't Hof RJ, Reid DM, Wei XQ, Liew FY, Ralston SH. Activation of the inducible nitric oxide synthase pathway contributes to inflammation-induced osteoporosis by suppressing bone formation and causing osteoblast apoptosis. Arthritis Rheum. 2001b Dec;44(12):2790–2796. doi: 10.1002/1529-0131(200112)44:12<2790::aid-art466>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Arnoldi CC, Lemperg R, Linderholm H. Immediate effect of osteotomy on the intramedullary pressure in the femoral head and neck in patients with degenerative osteoarthritis. Acta Orthopaedica Scandinavica. 1970;42:454–455. [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009 Feb 15;122(Pt 4):546–553. doi: 10.1242/jcs.036293. Epub 2009 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asasaka N, Kondo T, Goto T, Kido MA, Nagata E, Tanaka T. Differences in the transport systems between cementocytes and osterocytes in rats using microperoxidase as a tracer. Archives of Oral Biology. 1992;37:363–368. doi: 10.1016/0003-9969(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Azuma H. Intraosseous pressure as a measure of hemodynamic changes in bone marrow,”. Angiology. 1964;15:396–406. doi: 10.1177/000331976401500903. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tankó LB PERF study group. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007 Apr;18(4):505–512. doi: 10.1007/s00198-006-0255-2. Epub 2006 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C Prospective Epidemiological Risk Factors Study Group. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006 Jun;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Baldini V, Mastropasqua M, Francucci CM, D'Erasmo E. Cardiovascular disease and osteoporosis. J Endocrinol Invest. 2005;28(10 Suppl):69–72. [PubMed] [Google Scholar]
- Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998 Mar;62(3):209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- Benalla M, Cardoso L, Cowin SC. Analytical basis for the determination of the lacunar-canalicular permeability bone using cyclic loading, Biomechanics and Modeling in Mechanobiology. 2012;11:767–780. doi: 10.1007/s10237-011-0350-y. [DOI] [PubMed] [Google Scholar]
- Benalla M, Palacio-Mancheno PE, Fritton SP, Cardoso L, Cowin SC. Dynamic permeability of the lacunar-canalicular system in human cortical bone, Biomechanics and Modeling in Mechanobiology. 2013 Oct 22; doi: 10.1007/s10237-013-0535-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beno T, Yoon YJ, Cowin SC, Fritton SP. Estimation of bone permeability using accurate microstructural parameters. Journal of Biomechanics. 2006;39:2378–2387. doi: 10.1016/j.jbiomech.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Bergula AP, Huang W, Frangos JA. Femoral vein ligation increases bone mass in the hindlimb suspended rat. Bone. 1999;24:171–177. doi: 10.1016/s8756-3282(98)00165-3. [DOI] [PubMed] [Google Scholar]
- Biot MA. Le problème de la consolidation des matières argileuses sous une charge. Annales de la Société Scientifique de Bruxelles. Série. 1935;B55:110–113. [Google Scholar]
- Biot MA. General theory of three-dimensional consolidation. Journal of Applied Physics. 1941;12:155–164. [Google Scholar]
- Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001 Oct 26;89(9):763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- Bocchi L, Orso CA, Passarello F, Lio R, Petrelli L, Tanganelli P, Weber G. Atherosclerosis of the microcirculation in the femoral head: based on a study by optical and electron microscopy of femoral heads removed at operation. Ital J Orthop Traumatol. 1985 Sep;11(3):365–370. [PubMed] [Google Scholar]
- Branemark PI. Experimental investigation of microcirculation in bone marrow. Angiology. 1961;12:293–305. [Google Scholar]
- Bridgeman G, Brookes M. Blood supply to the human femoral diaphysis in youth and senescence. Journal of Anatomy. 1996;188:611–621. Part3. [PMC free article] [PubMed] [Google Scholar]
- Brooks M, Revell WJ. Bloods Supply of Bone. London: Springer; 1998. [Google Scholar]
- Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001 Feb;86(2):631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991 Aug 10;338(8763):355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- Burkhardt R, Bartl R, Frisch B. In Bone Circulation. Baltimore: Williams and Wilkins; 1984. The structural relationship of bone forming and endothelial cells of bone marrow; pp. 2–14. [Google Scholar]
- Burkhardt R, Kettner G, Bohn W, Schmidmeir M, Schlap R, Frisch B, et al. Changes in trabecular bone, hematopoiesis and marrow vessels in aplastic anaemia, primary osteoporosis and old age. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- Burkhardt R. Vascularisation de l’os et de la moelle osseuse. In: Masson, editor. Atlas d’Histopathologie clinique de la moelle osseuse et des os. Paris: 1971. pp. 28–35. [Google Scholar]
- Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009 Apr;24(4):597–605. doi: 10.1359/JBMR.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC. Advances in assessment of bone porosity, permeability, and interstitial fluid flow. Journal of Biomechanics. 2013;46:253–265. doi: 10.1016/j.jbiomech.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catto M. In: Pathology of aseptic bone necrosis. Davidson JK, editor. Aseptic Necrosis of Bone Amsterdam, Nederlands Excerpta Medic app; 1976. pp. 3–100. [Google Scholar]
- Causey L, Cowin SC, Weinbaum S. A quantitative model for predicting lymph formation and muscle compressibility in skeletal muscle during contraction and stretch. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(23):9185–9190. doi: 10.1073/pnas.1206398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani C, Doty SB, Fritton SP. Mapping bone interstitial fluid movement: displacement of ferritin tracer during histological processing. Bone. 2005;37:379–387. doi: 10.1016/j.bone.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani C, Doty SB, Fritton SP. Osteopenia Increases Osteocyte Lacunar-Canalicular Porosity in Rat Metaphyseal Bone; Transactions of the 54th Meeting of the Orthopaedic Research Society; 2008. p. 344. [Google Scholar]
- Ciani C, Ramirez Marin PA, Doty SB, Fritton SP. High-Resolution Measurement of Cortical Bone Porosity in Normal and Osteopenic Rats; Transactions of the 53rd Meeting of the Orthopaedic Research Society; 2007. p. 263. [Google Scholar]
- Cohen-Solal ME, Graulet AM, Denne MA, Gueris J, Baylink D, de Vernejoul MC. Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokines. J Clin Endocrinol Metab. 1993 Dec;77(6):1648–1653. doi: 10.1210/jcem.77.6.8263153. [DOI] [PubMed] [Google Scholar]
- Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J Cell Biochem. 1994 Jul;55(3):304–309. doi: 10.1002/jcb.240550306. Review. [DOI] [PubMed] [Google Scholar]
- Cooper RR, Milgram JW, Robinson RA. Morphology of the osteon: an electron microscopy study. Journal of Bone and Joint Surgery. 1966;48A:1239–1271. [PubMed] [Google Scholar]
- Cowin SC. Bone Poroelasticity. Journal of Biomechanics. 1999;32:218–238. doi: 10.1016/s0021-9290(98)00161-4. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Gailani G, Benalla M. Hierarchical Poroelasticity: Exchange of interstitial fluid between porosity levels in bones, Philosophical Transactions of the Royal Society. 2009;A367:3401–3444. doi: 10.1098/rsta.2009.0099. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Weinbaum S, Zeng Yu. A case for bone canaliculi as the anatomical site of strain-generated potentials. Journal of Biomechanics. 1995;28:1281–1296. doi: 10.1016/0021-9290(95)00058-p. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. Journal of Biomechanical Engineering-Transactions of the Asme. 1991;113(2):191–197. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- Cushner FD, Friedman RJ. Osteonecrosis of the femoral head. Orthopedic Reviews. 1988;17:29–34. [PubMed] [Google Scholar]
- Cuthbertson EM, Siris E, Gilfillan RS. The femoral diaphyseal medullary venous system as a venous collateral channel in the dog. Journal of Bone and Joint Surgery. 47A:965–974. [PubMed] [Google Scholar]
- De Bruyn PPH, Breen PC, Thomas TB. The microcirculation of the bone marrow. Anatomical Record. 1970;168:55–68. doi: 10.1002/ar.1091680105. [DOI] [PubMed] [Google Scholar]
- Dillaman RM. Movement of Ferritin in the 2 Day-Old Chick Femur. Anatomical Record. 1984;209:445–453. doi: 10.1002/ar.1092090404. [DOI] [PubMed] [Google Scholar]
- Dillaman RM, Roer RD, Gay DM. Fluid movement in bone: theoretical and empirical. Journal of Biomechanics. 1991;24:163–177. doi: 10.1016/0021-9290(91)90386-2. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Asotra K, Fitzpatrick LA, Qiao JH, Wilkin DJ, Detrano RC, Dunstan CR, Shah PK, Rajavashisth TB. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11201–11206. doi: 10.1073/pnas.1932554100. Epub 2003 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty SD. 1997 Private communication. [Google Scholar]
- Doty SB. Morphological evidence of gap junctions between bone cells. Calcified Tissue International. 1981;33:509–512. doi: 10.1007/BF02409482. [DOI] [PubMed] [Google Scholar]
- Doty SD, Schofield BH. Metabolic and structural changes within osteocytes of rat bone,”. In: Talmage RV, Munson PL, editors. Calcium, Parathyroid Hormone and the Calcitonins. Amsterdam: Excerpta Medica; 1972. pp. 353–364. [Google Scholar]
- Edwards JR, Williams K, Kindblom LG, Meis-Kindblom JM, Hogendoorn PCW, Hughes D, Forsyth RG, Jackson D, Athanasou NA. Lymphatics and bone. Human pathology. 2008;39:49–55. doi: 10.1016/j.humpath.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Francklin HS, Kelly PJ. Relationship of bone remodeling, oxygen consumption and blood flow in bone. J. Bone Joint Surg. 1998;52A:1377–1389. [PubMed] [Google Scholar]
- Fritton SP, Wang L, Weinbaum S, Cowin SC. Interaction of Mechanical Loading, Blood Flow, and Interstitial Fluid Flow in Osteonal Bone. Proceedings of the Bioengineering Conference, BED. 2001;50:341–342. [Google Scholar]
- Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annual Review of Fluid Mechanics. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostick SP, Wallace WA. Osteonecrosis of the humeral head. Clinical Rheumatology. 1989;3:651–657. doi: 10.1016/s0950-3579(89)80014-7. [DOI] [PubMed] [Google Scholar]
- Gailani G, Benalla M, Mahamud R, Cowin SC, Cardoso L. Experimental determination of the permeability in the lacunar-canalicular porosity of bone. Journal of Biomechanical Engineering. 2009;131(10):101007. doi: 10.1115/1.3200908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailani G, Cowin SC. The unconfined compression of a porous annular cylindrical disk. Journal of Mechanics of Materials and Structure. 2008;40(6):507–523. [Google Scholar]
- Gailani G, Cowin SC. Ramp loading in Russian doll poroelasticity. Journal of the Mechanics and Physics of Solids. 2011;53:103–120. [Google Scholar]
- Green NE, Griffin PP. Intra-osseous venous pressure in Legg-Perthes disease. Journal of Bone and Joint Surgery. 1982;64A:666–671. [PubMed] [Google Scholar]
- Hamerman D. Osteoporosis and atherosclerosis: biological linkages and the emergence of dual-purpose therapies. QJM. 2005 Jul;98(7):467–484. doi: 10.1093/qjmed/hci077. Epub 2005 Jun 13. [DOI] [PubMed] [Google Scholar]
- Herskovits MS, Singh IJ, Sandhu HS. Innervation of bone. In: Matrix Bone, Hall BK., editors. Bone: A Treatise. Vol. 3. New Jersey: Telford Press; 1991. [Google Scholar]
- Holmes JM, Davies DH, Meath WJ, Beebe RA. Gas adsorption and surface structure of bone mineral. Biochemistry. 1964;3:2019–2024. doi: 10.1021/bi00900a042. [DOI] [PubMed] [Google Scholar]
- Hughes S, Blount M. The structure of capillaries in cortical bone. Annals of The Royal College of Surgeons of England. 1979;61:312–315. [Google Scholar]
- James J, Steijn-Myagkaya Death of osteocytes: Electron microscopy after in vitro ischemia. Journal of Bone and Joint Surgery. 1986;68B:620–624. doi: 10.1302/0301-620X.68B4.3733842. [DOI] [PubMed] [Google Scholar]
- Johnson MW. Behavior of fluid in stressed bone and cellular stimulation. In: Cowin SC, Lanyon LE, editors. Calcified Tissue International. Vol. 36. 1984. pp. S72–S76. 1984. Functional Adaptation in Bone Tissue Supplement to Volume 36, Calcified Tissue International. [DOI] [PubMed] [Google Scholar]
- Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000 Oct;15(10):1974–1980. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]
- Karlakki SL, Bindra RR. Idiopathic avascular necrosis of the metacarpal head. Clin Orthop Relat Res. 2003;406:103–108. doi: 10.1097/01.blo.0000030168.56585.cb. [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Bronk JT. Venous pressure and bone formation. Microvascular Research. 1990;39:364–375. doi: 10.1016/0026-2862(90)90049-w. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004 May 11;109(18):2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. Epub 2004 Apr 26. [DOI] [PubMed] [Google Scholar]
- Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006 Nov;39(5):1043–1047. doi: 10.1016/j.bone.2006.05.017. Epub 2006 Jul 24. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun. 1998 Sep 8;250(1):108–114. doi: 10.1006/bbrc.1998.9270. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995 Dec 14;217(2):640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- Kolodny A. Archives of surgery. Vol. 11. Chicago: 1925. The relation of the bone marrow to the lymphatic system: its rôle in the spreading of carcinomatous metastases throughout the skeleton; pp. 690–707. [Google Scholar]
- Kornblum SS. Graduate thesis. University of Minnesota: 1962. The micro radiographic morphology of bone from ischemic limbs. [Google Scholar]
- Kwon RY, Meays DR, Meilan AS, Jones J, Miramontes R, Kardos N, Yeh JC, Frangos JA. Skeletal adaptation to intramedullary pressure-induced interstitial fluid flow is enhanced in mice subjected to targeted osteocyte ablation. PLoS One. 2012;7(3):e33336. doi: 10.1371/journal.pone.0033336. Epub 2012 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon RY, Meays DR, Tang WJ, Frangos JA. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res. 2010 Aug;25(8):1798–1807. doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche M, Barbier A, Ludot I, Thiechart M, Viguier G, Mazières B. Effects of Tiludronate on bone remodelling and vascularization in ovariectomised rats. Osteoporosis Int. 1996;6:126–129. [Google Scholar]
- Laroche M, Moulinier L, Leger P, Lefebvre D, Mazières B, Boccalon H. Bone mineral decrease in the leg with unilateral chronic occlusive arterial disease. Clin Exp Rheumatol. 2003 Jan-Feb;21(1):103–106. [PubMed] [Google Scholar]
- Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazieres B. Comparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic disease. Clin Rheumatol. 1994 Dec;13(4):611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- Laroche M. Intraosseous circulation from physiology to disease. Joint Bone Spine. 2002 May;69(3):262–269. doi: 10.1016/s1297-319x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- Li G, Bronk JT, An KN, Kelly PJ. Permeability of cortical bone of canine tibiae. Microcirculation Research. 1987;34(302) doi: 10.1016/0026-2862(87)90063-x. 1987. [DOI] [PubMed] [Google Scholar]
- Lilly AD, Kelly PJ. Effects of venous ligation on bone remodeling in the canine tibia. Journal of Bone and Joint Surgery. 1970;52A:515–520. [PubMed] [Google Scholar]
- Liu SL, Ho TC. The role of venous hypertension in the pathogenesis of Legg-Perthes disease. A clinical and experimental study. Journal of Bone and Joint Surgery. 1991;73A:194. [PubMed] [Google Scholar]
- Lopez-Curto JA, Bassingthwaighte JB, Kelly PJ. Anatomy of the microvasculature of the tibial diaphysis of the adult dog. Journal of Bone and Joint Surgery. 1980;62A:1362–1369. [PMC free article] [PubMed] [Google Scholar]
- Martin B. Mathematical model for the mineralization of bone. Journal of Orthopaedic Research. 1994;12:375–383. doi: 10.1002/jor.1100120310. [DOI] [PubMed] [Google Scholar]
- Martin GR, Firschein HE, Mulryan BJ, Neuman WF. Concerning the mechanisms of action of parathyroid hormone. II. Metabolic effects. Journal of the American Chemical Society. 1958;80:6199–6201. [Google Scholar]
- McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000 Apr 13;270(2):643–648. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- McCarthy ID, Yang L. A distributed model of exchange processes within the osteon. Journal of Biomechanics. 1992;25:441–450. doi: 10.1016/0021-9290(92)90263-z. [DOI] [PubMed] [Google Scholar]
- McGavock J, Mandic S, Lewanczuk R, Koller M, Muhll IV, Quinney A, Taylor D, Welsh R, Haykowsky M. Cardiovascular adaptations to exercise training in postmenopausal women with type 2 diabetes mellitus. Cardiovasc Diabetol. 2004 Mar;15(3):3. doi: 10.1186/1475-2840-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Jee WSS. Bone lining cells, in Bone. In: Hall BK, editor. Bone Metabolism and Mineralization. Vol. 4. Boca Raton: CRC Press; 1992. pp. 1–19. [Google Scholar]
- Morris MA, Lopez-Curato JA, Hughes SPF, An KN, Bassingthwaighte JB, Kelly PJ. Fluid spaces in canine bone and marrow. Microvascular Research. 1982;23:188–200. doi: 10.1016/0026-2862(82)90064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GG, Kelly PJ, Lowell FA, Peterson LFA, Janes JM. Blood supply of the human tibia. Journal of Bone and Joint Surgery. 1960;42A:625–635. [PubMed] [Google Scholar]
- Neuman MW. Bone Blood Equilibrium. Calcified Tissue International. 1982;34:117–120. doi: 10.1007/BF02411220. [DOI] [PubMed] [Google Scholar]
- Neuman WF. The milieu intérieur of bone: Claude Bernard revisited. Federation of American Societies for Experimental Biology. 1969;28:1846–1850. [PubMed] [Google Scholar]
- Neuman WF, Neuman MW. The Chemical Dynamics of Bone. Chicago: University of Chicago Press; 1958. [Google Scholar]
- Owen M, Triffitt JT. Extravascular albumin in bone tissue. Journal of Physiology. 1976;257:293–307. doi: 10.1113/jphysiol.1976.sp011369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000 Apr;26(4):319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002 Oct 4;91(7):570–576. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. 2000 Nov;20(11):2346–2348. doi: 10.1161/01.atv.20.11.2346. [DOI] [PubMed] [Google Scholar]
- Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997 Apr;17(4):680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003 Oct;36(10):1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Qin YX, Lam H. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J Biomech. 2009 Jan 19;42(2):140–145. doi: 10.1016/j.jbiomech.2008.10.018. Epub 2008 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajzbaum G, Bézie Y. Postmenopausal osteoporosis and atheroma. Joint Bone Spine. 2006 Dec;73(6):661–666. doi: 10.1016/j.jbspin.2006.02.009. Epub 2006 Sep 5. [DOI] [PubMed] [Google Scholar]
- Reeves J, Arlot M, Wootton R, Edouard C, tellez M, Hesp R, et al. Skeletal blood flow, iliac crests histomorphometry and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rate. J. Clin. Endocrinol. Metab. 1988;66:1124–1131. doi: 10.1210/jcem-66-6-1124. [DOI] [PubMed] [Google Scholar]
- Reilly TM, Seldes R, Lucchitti W, Brighton CT. Similarities in the phenotypic expression of pericytes and bone cells. Clin Orthop Related Res. 1998;342:95–103. [PubMed] [Google Scholar]
- Rhinelander FW. Blood supply to developing, mature and healing bone. In: Summer-Smith G, Wilson JW, editors. Bone in Clinical Orthopaedics. Philadelphia: W. B. Saunders; 1982. pp. 81–158. [Google Scholar]
- Romney JS, Lewanczuk RZ. Vascular compliance is reduced in the early stages of type 1 diabetes. Diabetes Care. 2001 Dec;24(12):2102–2106. doi: 10.2337/diacare.24.12.2102. [DOI] [PubMed] [Google Scholar]
- Rossouw JE. Coronary heart disease in menopausal women: implications of primary and secondary prevention trials of hormones. Maturitas. 2005 May 16;51(1):51–63. doi: 10.1016/j.maturitas.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Rouhana SW, Johnson MW, Chakkalakal DA, Harper RA. Permeability of the osteocyte lacuno-canalicular compact bone. Proceedings of the Joint ASME-ASCE Conference Biomechanics Symposium Applied Mechanics Division. 1981;43:169–172. [Google Scholar]
- Rubin MR, Silverberg SJ. Vascular calcification and osteoporosis--the nature of the nexus. J Clin Endocrinol Metab. 2004 Sep;89(9):4243–4245. doi: 10.1210/jc.2004-1324. [DOI] [PubMed] [Google Scholar]
- Samuels A, Perry MJ, Gibson RL, Colley S, Tobias JH. Role of endothelial nitric oxide synthase in estrogen-induced osteogenesis. Bone. 2001 Jul;29(1):24–29. doi: 10.1016/s8756-3282(01)00471-9. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Strotmeyer ES, Tylavsky FA, Feingold KR, Resnick HE, Shorr RI, Nevitt MC, Black DM, Cauley JA, Cummings SR, Harris TB Health ABC Study. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005 Apr;20(4):596–603. doi: 10.1359/JBMR.041219. Epub 2004 Dec 13. [DOI] [PubMed] [Google Scholar]
- Seliger WG. Tissue fluid movement in compact bone. Anatomical Record. 1969;166:247–255. doi: 10.1002/ar.1091660213. [DOI] [PubMed] [Google Scholar]
- Sharma U, Mikos AG, Cowin SC. Mechanosensory mechanisms in bone. In: Lanza R, Langer R, Vacanti JP, editors. Textbook of Tissue Engineering. 3rd edition. Elsevier/Academic Press; 2007. [Google Scholar]
- Shim S, Hawk H, Yu W. The Relationship between blood flow and marrow cavity pressure of bone. Surgery, Gynecology and Obstetrics. 1972;135:353–360. [PubMed] [Google Scholar]
- Soares AMV, Arana-Chavez VE, Reid AR, Katchburian B. Lanthanum tracer and freeze-fracture studies suggest that compartmentalization of early bone matrix may be related to initial mineralization. Journal of Anatomy. 1992;181:343–356. [PMC free article] [PubMed] [Google Scholar]
- Steck R, Niederer P, Knothe Tate ML. A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. Journal of Theoretical Biology. 2003;220:249–259. doi: 10.1006/jtbi.2003.3163. [DOI] [PubMed] [Google Scholar]
- Stevenson JC. Hormone replacement therapy: review, update, and remaining questions after the Women's Health Initiative Study. Curr Osteoporos Rep. 2004 Mar;2(1):12–16. doi: 10.1007/s11914-004-0009-z. [DOI] [PubMed] [Google Scholar]
- Streeten EA, Brandi ML. Biology of bone endothelial cells. Bone Miner. 1990 Aug;10(2):85–94. doi: 10.1016/0169-6009(90)90084-s. Review. [DOI] [PubMed] [Google Scholar]
- Strokon A, Loneragan R, Workman GS, et al. Avascular necrosis of the talus. Clin Nucl Med. 2003;28:9–13. doi: 10.1097/00003072-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Tanaka Y, Omokawa S, et al. Idiopathic osteonecrosis of the first metatarsal head: a case report. Clin Orthop Relat Res. 2003;415:239–243. doi: 10.1097/01.blo.0000092971.12414.7e. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Sakano A. Differences in permeability of microperoxidase and horseradish peroxidase into the alveolar bone of developing rats. Journal of Dental Research. 1985;64:870–876. doi: 10.1177/00220345850640060201. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Yoshida M, Iwasaki H, et al. Kienbock's disease in elderly patients. J Hand Surg [Am] 2003;28:779–783. doi: 10.1016/s0363-5023(03)00299-5. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007 Jun;5(6):464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Morony S, Demer LL. Hyperlipemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004 Feb;24(2):e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. Epub 2003 Dec 11. [DOI] [PubMed] [Google Scholar]
- Trueta J, Buhr AJ. The vascular contribution to osteogenesis. V. The vasculature supplying the epiphysial cartilage in rachitic rats. J Bone Joint Surg Br. 1963 Aug;45:572–581. [PubMed] [Google Scholar]
- Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in the adult man. Journal of Bone and Joint Surgery. 1953;35B:442–461. doi: 10.1302/0301-620X.35B3.442. [DOI] [PubMed] [Google Scholar]
- Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997 Sep;28(9):1730–1732. doi: 10.1161/01.str.28.9.1730. [DOI] [PubMed] [Google Scholar]
- Vittas D, Hainau B. Lymphatic capillaries of the periosteum: do they exist? Lymphology. 1989;22:173–177. [PubMed] [Google Scholar]
- Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997 Feb;12(2):283–289. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999 Mar;106(3):273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone's interstitial fluid pathway in vivo. Bone. 2004;34:499–509. doi: 10.1016/j.bone.2003.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fritton SP, Cowin SC, Weinbaum S. Fluid pressure relaxation mechanisms in osteonal bone specimens: modeling of an oscillatory bending experiment. Journal of Biomechanics. 1999;32:663–672. doi: 10.1016/s0021-9290(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Fritton SP, Weinbaum S, Cowin SC. On bone adaptation due to venous stasis. Journal of Biomechanics. 2003;36:1439–1451. doi: 10.1016/s0021-9290(03)00241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DER, Nicol CW, Gatto SN, Bredin SSD. Cardiovascular disease and osteoporosis: Balancing risk management. Vasc Health Risk Manag. 2007 Oct;3(5):673–689. [PMC free article] [PubMed] [Google Scholar]
- Wehrli FW, Fernández-Seara MA. Nuclear magnetic resonance studies of bone water. Annals of Biomedical Engineering. 2005;33:79–86. doi: 10.1007/s10439-005-8965-8. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Yu. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. Journal of Biomechanics. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Welch RD, Johnston CE, Waldron MJ, Poteet B. Bone changes associated with intraosseous hypertension in the caprine tibia. Journal of Bone and Joint Surgery. 1993;75A:53–60. doi: 10.2106/00004623-199301000-00008. [DOI] [PubMed] [Google Scholar]
- Whitney C, Warburton DE, Frohlich J, Chan SY, McKay H, Khan K. Are cardiovascular disease and osteoporosis directly linked? Sports Med. 2004;34(12):779–807. doi: 10.2165/00007256-200434120-00001. [DOI] [PubMed] [Google Scholar]
- Williams EA, Fitzgerald RH, Kelly PJ. The physiology and pharmacology of the microcirculation. Vol. 2. Academic Press; 1984. Microcirculation of Bone. [Google Scholar]
- Williams SM, McCarthy ID. Distributed model of blood-bone exchange. Journal of Biomechanical Engineering. 1986;8:235–243. doi: 10.1016/0141-5425(86)90090-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002 Apr;49(2):211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- Yoffey JM, Hudson G, Osmond DG. The lymphocyte in guinea-pig bone marrow. Journal of Anatomy. 1965;99:841–860. [PMC free article] [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001 Apr 20;276(16):13365–13371. doi: 10.1074/jbc.M009846200. Epub 2001 Jan 26. [DOI] [PubMed] [Google Scholar]
- You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008 Jan;42(1):172–179. doi: 10.1016/j.bone.2007.09.047. Epub 2007 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CW, Hsu CY, Shih TT, et al. Vertebral osteonecrosis: MR imaging findings and related changes on adjacent levels. AJNR Am J Neuroradiol. 2007;28:42–47. [PMC free article] [PubMed] [Google Scholar]
- Zamboni L, Pease DC. The vascular bed of red bone marrow. Journal of Ultrastructure Research. 1961;5:65–85. doi: 10.1016/s0022-5320(61)80006-3. [DOI] [PubMed] [Google Scholar]
- Zhang D, Weinbaum S, Cowin SC. Estimates of the peak pressures in bone pore water. Journal of Biomechanical Engineering. 1998a;120(6):697–703. doi: 10.1115/1.2834881. [DOI] [PubMed] [Google Scholar]
- Zhang D, Weinbaum S, Cowin SC. On the calculation of bone pore water pressure due to mechanical loading. International Journal of Solids and Structures. 1998b;35(34–35):4981–4997. [Google Scholar]