1. Introduction
Autism Spectrum Disorder (ASD) is characterised by deficits in social reciprocity, communication impairments, and restricted, repetitive interests and behaviours (World Health Organization, 1993). Recent research suggests an approximate prevalence of 0.6-1.5% in the general population (Baron-Cohen et al., 2009; Schechter and Grether, 2008; Thomas et al., 2011; Zaroff and Uhm, 2011).
Numerous studies have reported differences in brain anatomy and function in individuals with ASD (e.g. see (Craig et al., 2007; Ecker et al., 2010; Hallahan et al., 2009)), but the underlying molecular basis of the condition remains unclear. This has led to a paucity of treatment targets. At present, therapeutic options for ASD are limited to medications used to alleviate specific symptoms, such as the licensed use of the antipsychotic risperidone for aggression and challenging behaviours (Matson et al., 2011)) and selective serotonin reuptake inhibitors in obsessive or repetitive behaviours (King et al., 2009). However, the efficacy of such pharmacotherapy has recently been questioned (McPheeters et al., 2011). There is, therefore, a need for better targeted, ASD-specific treatments, and this will only be possible on the basis of a better understanding of ASD pathophysiology.
ASD is now viewed as a heterogeneous set of disorders, which can be caused by various genetic, epigenetic and environmental factors, but emerging evidence suggests that an imbalance between excitatory glutamate and inhibitory gamma-amino-butyric acid (GABA) neurotransmission may form a final common pathway in ASD. In particular, defects in GABA transmission, leading to brain hyperexcitability, have been hypothesized to underlie the symptoms of ASD (Pizzarelli and Cherubini, 2011; Rubenstein and Merzenich, 2003; Yizhar et al., 2011).
GABA, the primary inhibitory neurotransmitter in the adult human brain, is synthesised from the excitatory neurotransmitter glutamate, via the action of glutamate decarboxylase (GAD) enzymes, GAD65 and GAD67. In the central nervous system, GABA is produced and released by inhibitory interneurons (Kubota et al., 2011; Tamamaki and Tomioka, 2010).
GABA acts on two main classes of membrane-bound receptors: ionotropic GABAA receptors (ligand-gated Cl-channels), and metabotropic (G protein-coupled) GABAB receptors. The GABAA receptor family is the predominant type in the brain, and is the site of action of drugs such as benzodiazepines and several anaesthetics (Reynolds et al., 2003).
The GABAA receptor is composed of five subunits arranged around a central pore (Nutt and Malizia, 2001). The subunits are diverse and different combinations of subunits give rise to GABAA receptors with specific properties. Most brain GABAA receptors contain α, β and γ subunits in a 2:2:1 stoichiometry (Tretter et al., 1997), although γ can be replaced by δ or ε subunits, and the σ subunit may substitute for the β subunit.
In man, Positron Emission Tomography (PET) has been critical in delineating the distribution and levels of GABAA receptors in a variety of neuropsychiatric disorders including anxiety related disorders, benzodiazepine dependence (Nutt and Malizia, 2001), alcoholism, and epilepsy (Malizia and Richardson, 1995).
Evidence for abnormal GABAA density in ASD comes from neuropathological studies. Blatt and colleagues reported reduced GABAA receptors and benzodiazepine binding sites in the hippocampus (Blatt et al., 2001), and the cingulate cortex (Oblak et al., 2009; Oblak et al., 2010) of individuals with ASD. Other studies found altered GAD expression in ASD (Yip et al., 2007, 2008) and reduced neuronal cell size and increased cell packing density in GABAergic hippocampal neurons and interneurons, subiculum, entorhinal cortex, amygdala, medial septal nucleus, and mammilary bodies in ASD compared to controls (Kemper and Bauman, 1998).
Converging evidence for a GABAA involvement in ASD comes from genetic studies. For instance, microduplications of the chromosome 15q11-13 locus have been observed in a proportion of people with ASD (Buxbaum et al., 2002; Cook et al., 1998; McCauley et al., 2004; Menold et al., 2001; Sebat et al., 2007; Shao et al., 2003). This region notably contains the genes coding for the GABAA α5, β3 and γ3 subunits, and although duplication might be expected to lead to over expression of these receptor proteins, in vitro studies of a human neuronal cell line carrying a 15q duplication showed that this variant actually leads to reduced GABRB3 expression (Meguro-Horike et al., 2011).
Abnormal patterns of expression of 15q11-13 locus genes have been reported even in ASD cases without the mutation: in 4 of 8 cases of idiopathic ASD, levels of GABAA α5, β3 and γ3 were reduced as expression of the maternally inherited copies of these genes predominated (Hogart et al., 2007). Finally, reduced frontal and temporal cortical expression of mRNA in a network of genes highly expressed in GABA interneurons was observed in two samples of ASD (Voineagu et al., 2011).
There is, therefore, mounting evidence implicating the GABA system and in particular GABAA receptors, generally in limbic areas, in ASD. However, there has only been one study which attempted to directly measure GABAA receptors in the living brain in ASD. This SPECT (Single Photon Emission Computed Tomography) study reported decreases in GABAA, especially in the frontal cortex in children with ASD (Mori et al., 2011). No study has yet examined adults with the condition.
We (Lingford-Hughes et al., 2002) have previously characterized the benzodiazepine receptor PET ligand [11C]Ro15-4513 (Halldin et al., 1992) in rats and humans and showed that its uptake primarily has a limbic distribution. High levels of uptake were described in the anterior cingulate cortex, hippocampus, insular cortex, septal region and amygdala, with lower levels seen in the occipital cortex and cerebellum than that observed with the traditional PET benzodiazepine ligand [11C]flumazenil (Lingford-Hughes et al., 2002).
These differences are explained by [11C]Ro15-4513 having relative selectivity, namely approximately 10-fold higher affinity for the α5 subtype of the GABAA receptor compared with the other receptor subtypes in both humans and rodents. In addition, recent refinements in the analysis of [11C]Ro15-4513 PET images have allowed us to further refine the signal, giving some discrimination between the high affinity α5 receptor subtype and the highly abundant α1 receptor subtype (Myers et al., 2012).
Therefore, we decided to use [11C]Ro15-4513 PET to measure α1 and α5 GABAA subtype receptor levels, with a particular interest in the α5 subtype in limbic regions that have been implicated in ASD: the amygdala, the hippocampus, and the nucleus accumbens. In the light of the previous work discussed above, our primary hypothesis was that, compared to controls, individuals with ASD have a significant reduction in α5 GABA receptor availability in these areas.
2. Methods
2.1. Participants
In this preliminary study, we included three male participants diagnosed with autism, meeting ICD-10 Research criteria, who scored above threshold for ASD in the Autism Diagnostic Observation Schedule (ADOS), and who had an IQ above 80. This IQ criterion was applied to avoid the possible confounding influence of IQ. We compared these individuals to data already acquired from three age-sex matched controls. All volunteers had capacity to consent to participation in the study; capacity was assessed by a qualified psychiatrist. English was the first language of all individuals. See Table 1 for participant clinical and demographic details.
Table 1.
Participant demographic and psychometric data.
| Group | Participant Number |
Gender | Age | ADOS* | Full Scale IQ |
|---|---|---|---|---|---|
| ASD | ASD1 | Male | 43 | 14 (5 + 7 + 2) | 117 |
| ASD2 | Male | 34 | 13 (3 + 7 + 3) | 123 | |
| ASD3 | Male | 41 | 11 (4 + 4 + 3) | 127 | |
| Control | HC1 | Male | 40 | n/a | n/a |
| HC2 | Male | 39 | n/a | n/a | |
| HC3 | Male | 37 | n/a | n/a |
ADOS = Autism Diagnostic Observation Schedule – Revised. Total scores are given first. Subscale scores are given in parentheses as follows: (Communication + Reciprocal Social Interaction + Stereotyped Behaviour/Restricted Interests). Note that ADOS and IQ scores were only available for ASD individuals.
We excluded people with a learning disability (mental retardation); people with diagnosed and treated ADHD, hyperkinesis or Tourette’s syndrome. We also excluded people who were taking psychotropic medication with possible effects on the GABA system i.e. antiepileptic drugs, benzodiazepines and antidepressants (Bhagwagar et al., 2004); a history of dependence to alcohol or substances of abuse (except nicotine); a major mental illness; or a medical or chromosomal disorder known to be associated with ASD such as Fragile X Syndrome).
All participants were able to tolerate MRI with no history of claustrophobia or presence of a cardiac pacemaker, other electronic medical implant, or ferromagnetic metal foreign bodies.
This study was approved by the North London Research Ethics Committee 3 (REC reference number 10/H0709/90) and by the Administration of Radioactive Substances Advisory Committee (ARSAC); it was also internally approved by the Institute of Psychiatry and the Maudsley Research and Development Office (R&D2011/014) and the Imperial College London and Imperial College Healthcare NHS trust Joint Research Office (JROHH0151).
2.2. Imaging protocol
The PET tracer [11C]Ro15-4513 was synthesised (Halldin et al., 1992) and purified by reverse-phase high performance liquid chromatography (Phenomenex uLTRACARB 7 ODS, 250 × 10mm). A bolus of [11C]Ro15-4513 was administered through a cannula in the dominant vein of the antecubital fossa. There were no significant differences in dosimetry between the two groups (healthy mean ± s.d.: 40±5GBq/μmol in 4.14 ± 0.45μg of stable ligand; ASD mean ± s.d.: 33±11GBq/μmol in 5.19 ± 1.9845μg of stable ligand), compared using Student’s t-tests (specific activity: t=1.05, p>0.3; stable ligand: t=0.899, p>0.4).
Scans were carried out using a Siemens ECAT EXACT HR+ (CTI/Siemens, model 962), to acquire 63 transaxial planes, each a 2.42mm slice (Spinks et al., 2000). A 10 minute 137Cs transmission scan was acquired prior to each emission scan for correction for attenuation and scatter. Each [11C]Ro15-4513 scan was divided into 24 time frames (1 × 30, 4 × 15, 4 × 60, 2 × 150, 10 × 300, 3 × 600 seconds), reconstructed with filtered backprojection.
For the first 15 minutes of each scan, continuous measurement of blood radioactivity was sampled through a radial arterial cannula in each participant. Discrete blood samples were also taken approximately 4, 6, 8, 10, 20, 35, 50, 65, 80 and 90 minutes after [11C]Ro15-4513 injection, and plasma was analyzed for radiolabeled metabolites. The plasma [11C]Ro15-4513 concentration profile throughout the scan could then be estimated, using Clickfit, in-house software running in Matlab (The Mathworks, Natick, MA).
Each participant also underwent a T1-weighted MRI scan, with 1×1×1mm resolution, using a Philips 1.5T Gyroscan Intera scanner, for structural reference and localisation of any changes in PET signal.
2.3. Image Analysis
Reconstructed PET images were analysed on a Sun SPARC workstation (Sun Microsystems, Mountain View, CA, USA) using Analyze AVW version 9.0 (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN), Matlab (The Mathworks, Natick, MA) and SPM5 (available via http://www.fil.ion.ucl.ac.uk/spm/).
The structural MRI was coregistered, using a rigid-body technique, to the add-image, the weighted sum of all dynamic PET frames. Normalisation parameters were obtained by warping the coregistered structural MRI to ‘Montreal Neurological Institute’ space (International Consortium for Brain Mapping (ICBM)/MNI) using bias-corrected segmentation in SPM5. The inverse of these parameters was used to fit an object map of 83 regions of interest (ROIs) to each individual PET dynamic. Analyze 9.0 was used to check the goodness-of-fit of each object map, and to sample ROIs to collect time-activity curves (TACs) for each ROI.
Clickfit was used to carry out spectral analysis of each regional TAC collected from the original dynamic PET scans. TACs were described by a convolution of the plasma input function and a 1st-order polyexponential (Cunningham and Jones, 1993; Turkheimer et al., 1994). This analysis yields a spectrum of exponential parameters, each peak representing a component of the total signal from the tissue.
In order to allow us to distinguish between binding at the GABAA α1 and α5 subtypes, this spectrum of component exponential parameters was then divided into ranges representing the different two major binding compartments of [11C]Ro15-4513 (Myers et al., 2012).
In brief, the analysis relies on the fact that the temporal dynamics of binding at α1 and α5 differ; fast components are attributed to the rapidly equilibrating α1 subtype signal, between 0.0030 and 0.0040 sec−1. In the case of the slower α5 receptor subtype binding site, the range of exponential decay values is approximately 0.00063 – 0.0030 sec−1, and the VT (distribution volume) of the ligand binding at this site calculated using the integral of this range of the spectrum, using the equation VT = Σjk=i (αk / βk − λ), where αk is the kth peak height, βk is the kth peak position, λ the decay constant, and i and j are the limits of the ranges given above. For more details on this spectral fitting method, readers should consult (Myers et al., 2012).
2.4. Statistical Analysis
Scan data and age-matching were assessed using two-tailed Student’s t-statistics. A two-way repeated measures ANOVA was used to compare [11C]Ro15-4513 VT between ASD cases and healthy volunteers. Mean α5 VT estimates (see Section 2.3) were also compared using Student’s t-test in predefined ROIs: the left and right amygdala, hippocampus, and nucleus accumbens. All these are areas of the limbic system which are known to show high expression of the GABAA α5 subunit. All have been previously implicated in ASD; see (Amaral et al., 2008; Blatt et al., 2001; Dichter et al., 2010).
All statistical analyses were conducted using GraphPad Prism (version 4.02 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com).
3. Results
Demographic and psychometric details for all six participants are shown in (Table 1). Participants were matched for age and gender
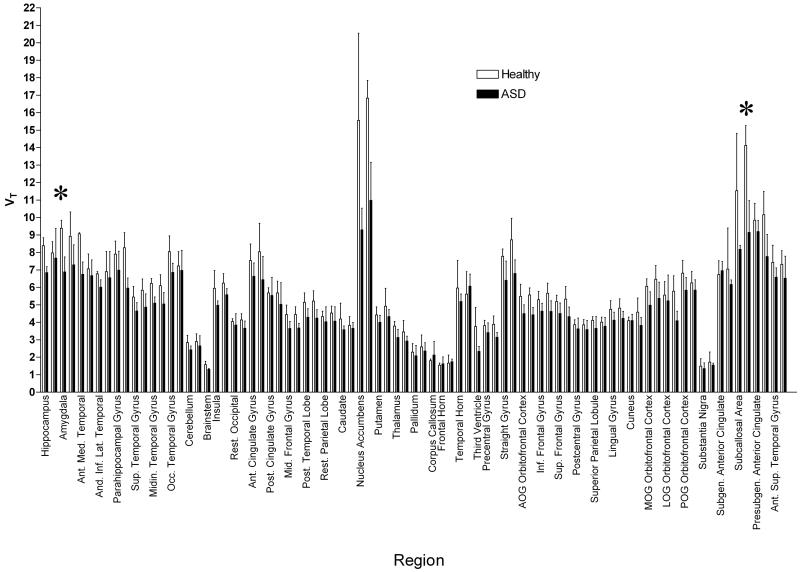
A two-way ANOVA showed significantly lower VT across 83 regions in the brains of participants with ASD as compared to controls (F=140.7, p<0.0001). See Figure 1. Four regions survived stringent Bonferroni correction for multiple comparisons, the right and left nucleus accumbens (t=8.61, p<0.001; t=8.03, p<0.001, respectively) and the right and left subcallosal area (t=4.62, p<0.001; t=6.84, p<0.001, respectively). (See Figure 1.)
Figure 1.
[11C]Ro15-4513 VT estimated by spectral analysis in 83 regions of the brain in 3 healthy volunteers and 3 ASD patients. Error bars show standard deviation; regions are divided left and right. VT is significantly lower in ASD patients than healthy volunteers across all regions, compared with a two-way ANOVA. Regions showing significant differences after Bonferroni correction for multiple comparisons are marked with an asterix *. These regions include the nucleus accumbens and the subcallosal area.
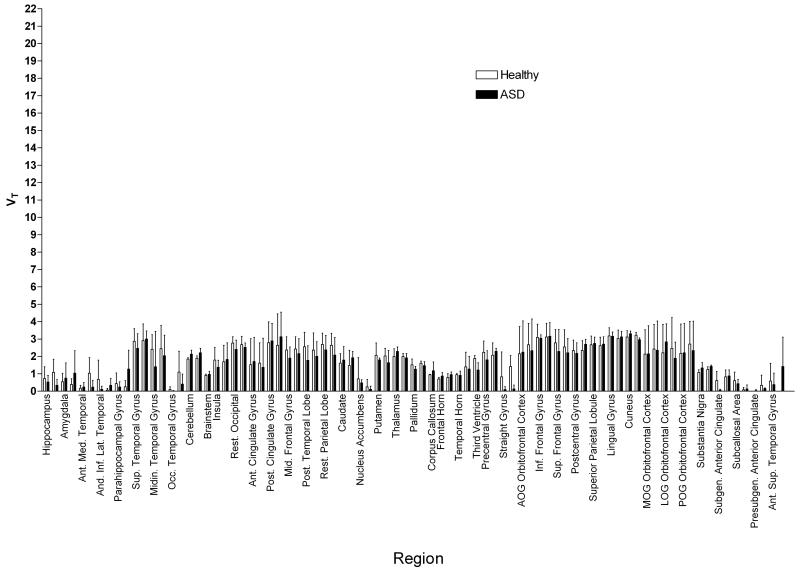
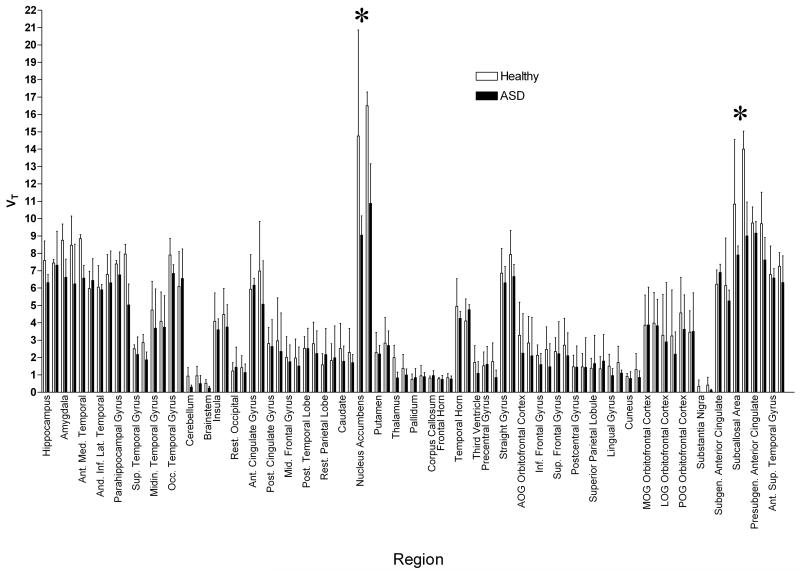
When the spectrum was analyzed in order to estimate VT for the α1 and α5 receptor subtypes separately (see section 2.3), the source of this difference was identified. A two-way ANOVA across all regions revealed no differences in the α1 subtype range (F=1.922, p>0.1). See Figure 2. There was, however, a highly significant difference in the estimated α5 subtype VT (F=32.27, p<0.0001). See Figure 3.
Figure 2.
[11C]Ro15-4513 VT at the GABAA α1 subtype, estimated by integrating spectral analysis between frequencies of approximately 0.0030 to 0.0050 sec−1, in 83 regions of the brain in 3 healthy volunteers and 3 ASD participants. Error bars show standard deviation; regions are divided left and right. A two-way ANOVA shows no significant differences between the two subject groups.
Figure 3.
[11C]Ro15-4513 VT at the GABAA α5 subtype, estimated by integrating spectral analysis between frequencies of approximately 0.0006 to 0.0030 sec−1, in 83 regions of the brain in 3 healthy volunteers and 3 ASD participants. Error bars show standard deviation; regions are divided left and right. A two-way ANOVA shows significantly lower VT across all regions in ASD participants compared with controls. Regions showing significant differences after Bonferroni correction for multiple comparisons are marked with an asterix *. These regions include the nucleus accumbens and subcallosal area.
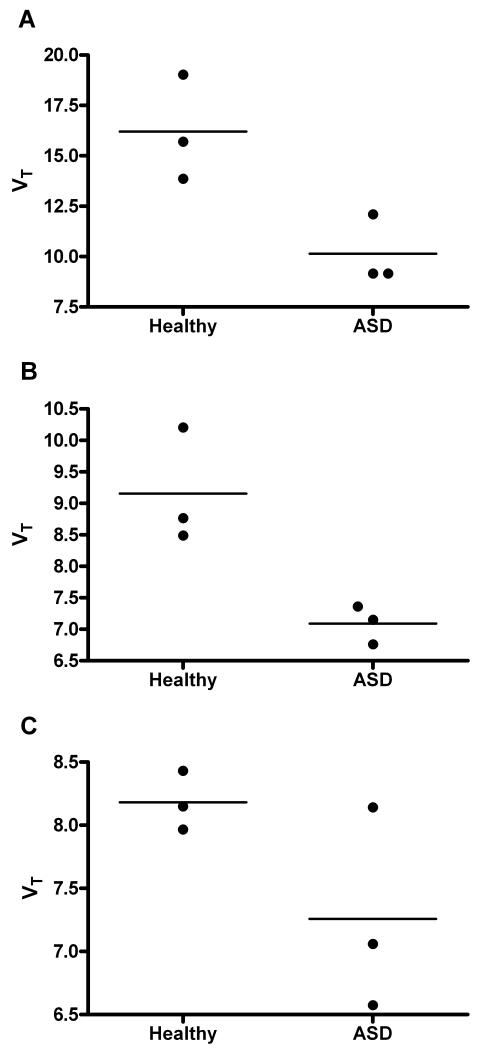
The three a priori selected limbic brain regions (see 2.4) were used to compare total VT in ASD cases and healthy controls to further confirm the hypothesis. Two showed significant differences with Student t-tests, the nucleus accumbens (t=3.360, p<0.05) and the amygdala (t=3.684, p<0.05). See Figure 4. The hippocampus showed no significant differences, although GABAA was non-significantly lower in the ASD group (t=1.916, p<0.07).
Figure 4.
Three regions were selected a priori for analysis by Student’s t-test, the nucleus accumbens (A), amygdala (B) and hippocampus (C). VT estimated by spectral analysis in 3 healthy volunteers was compared with that in 3 ASD participants. In the nucleus accumbens and amygdala, there was a significantly lower VT. The hippocampus showed no significant differences but GABAA was non-significantly lower in the ASD group.
4. Discussion
We found significantly lower [11C]Ro15-4513 VT across all 83 regions in the brain of participants with ASD compared with healthy volunteers(p<0.0001). Four regions survived stringent Bonferroni correction for multiple comparisons: the right and left nucleus accumbens and the right and left subcallosal area.
Further analysis suggested that these results were primarily due to lower levels of the α5 receptor subtype, rather than α1 subtype. Therefore, the results of this preliminary PET study in ASD, is consistent with evidence that ASD is characterised by a defect in GABA signalling via limbic GABAA receptors (see the Introduction).
Although these results are preliminary, we selected healthy volunteers that were matched in age and gender. We also took care not to include anyone with a history of substance use disorder, except for nicotine, or those taking medications that could modulate GABA or GABAA receptors (Bhagwagar et al., 2004). None had a history of seizures which have also been associated with alterations in GABAA (Laufs et al., 2011), and none suffered from mental retardation (intellectual disability).
To our knowledge, only one previous study has quantified GABAA density in the living ASD brain: Mori et al (Mori et al., 2011) found reduced accumulation of the GABAA-benzodiazepine ligand [123I]iomazenil (which binds to the α1, 2, 3 and 5 subtypes in frontal cortex) using SPECT (Single Photon Emission Computed Tomography).
However, there are a number of important differences between our study and that of Mori et al. Firstly, Mori et al studied children with ASD (mean age, 7.3 ± 3.5 years), while we recruited adults only. Secondly, the patients in this study were sedated using triclofos, while all of our participants were medication free. Thirdly, most of the ASD children had a low IQ, with only 9 of the 24 ASD subjects having an IQ above 85. Finally, similarly to [11C]flumazenil, it is likely that [123I]iomazenil binding reflects the α1 subtype with limited discrimination of any other subtype. The advantage of using [11C]Ro15-4513 is that we were able to discriminate α1 from α5, and hence probe the α5 subtype, which is present in high levels in the limbic cortex where dysregulation in ASD is thought to occur.
The α5 subunit-containing receptors constitute approximately 25% of GABAA receptors in the hippocampus, and are also highly expressed in the anterior cingulate cortex and the ventral striatum. There is increasing evidence that these brains regions are critically implicated in the symptomatology of ASD (Ecker et al., 2012; McAlonan et al., 2005). The α5 subtype is localized primarily to extrasynaptic regions, on the cell bodies and apical dendrites of glutamatergic pyramidal neurons in the CA1 and CA3 hippocampal regions (Brunig et al., 2002); and mediate tonic inhibitory currents - rather than synaptic, transient, neurotransmission (Caraiscos et al., 2004). They have been implicated in reward, learning and memory (Nutt, 2006; Nutt et al., 2007).
The differences in GABAA α5 levels found in this study may therefore be related to some of the social and emotional difficulties observed in people with ASD. For example, we observed significantly reduced GABAA α5 in the nucleus accumbens, which is part of the ventral striatum. Ventral striatal abnormalities reported in previous studies of ASD include: abnormal anatomical developmental trajectories in the nucleus accumbens (Langen et al., 2009); impaired NAcc functional responses to social rewards (Scott-Van Zeeland et al., 2010); and abnormalities in white matter tracts connecting the frontal cortex to the nucleus accumbens (Langen et al., 2011). Since α5-containing GABAA receptors mediate tonic inhibition, a deficit in these receptors might lead to hyperexcitability and impaired information processing (Pizzarelli and Cherubini, 2011; Rubenstein and Merzenich, 2003). However, it is also possible that the reduced GABAA α5 levels are an adaptation to, or downstream consequence of, other neurobiological changes, rather than being directly responsible for the symptoms of ASD. Further work is needed to examine this possibility.
This study has a number of limitations. Owing to the closure of the PET facility we were only able to collect data from a small number of individuals for this preliminary report, but we believe it is scientifically and ethically important to present these data in the public domain. Methodologically, we are confident that our analytical approach can dissociate α1 from α5 benzodiazepine receptor subtypes (Myers et al 2012), however given the small number of participants together with low signal to noise ratio of these methods of estimating VT at the lower affinity α1-containing subtype, we remain cautious. However, the lack of changes in receptor kinetics at either subtype suggests the differences in binding are probably due to down-regulation of receptor expression, supporting previous findings in gene assays (Fatemi et al., 2009).
Due to the small sample size, we could not examine possible correlations between GABAA binding and particular symptoms of ASD, age, IQ, or symptoms of comorbidities frequently associated with ASD, such as anxiety disorders, OCD and depression. We were also unable to address the effects of possible neuroanatomical differences between people with ASD and controls, which might lead to partial volume effects in PET studies. However, the modest magnitude of the volumetric differences seen in most studies of high-functioning ASD (Ecker et al., 2012; McAlonan et al., 2005) suggests that it is unlikely that these could fully explain the present findings.
These preliminary results suggest that potentiation of GABAA signalling, especially at GABAA α5-subunit containing receptors, might potentially be a novel therapeutic target for ASD. Unselective GABAA agonists and positive allosteric modulators, such as benzodiazepines, have undesirable features such as abuse potential and tolerance, but more selective modulators might avoid such limitations (Rudolph and Knoflach, 2011). Further research should extend this work in a larger sample of ASD individuals. It would also be interesting to use PET with the ligand [11C]Ro15-4513 to measure GABAA in disorders of known etiology characterised by ASD symptoms, such as Fragile X Syndrome (Rossi et al., 1995) and 15q11-13 duplication (Buxbaum et al., 2002; Cook et al., 1998; McCauley et al., 2004; Menold et al., 2001; Sebat et al., 2007; Shao et al., 2003).
5. Conclusion
In summary, we present preliminary evidence of reduced GABAA α5 expression in adult males with ASD, consistent with the hypothesis that ASD is characterised by a defect in GABA signalling.
Acknowledgements
We wish to thank the volunteers for their patience and collaboration with the study, and we are grateful for the technical support and assistance provided by Hammersmith Imanet. This project was supported by a program grant from National Institute for Health Research (NIHR) and the Biomedical Research Centre. The MRI component of this project was funded by the Medical Research Council AIMS Network and also by a grant from the Wellcome Trust (Reference 091300). The PET scans were funded by a Medical Research Council, UK grant to Dr Oliver Howes (Grant code: MC-A656-5QD30).
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
References
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, Brayne C. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr., Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Jr., Courchesne RY, Cox NJ, Lord C, Gonen D, Guter SJ, Lincoln A, Nix K, Haas R, Leventhal BL, Courchesne E. Linkage-disequilibrium mapping of autistic disorder, with 15q11-13 markers. Am J Hum Genet. 1998;62:1077–1083. doi: 10.1086/301832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Zaman SH, Daly EM, Cutter WJ, Robertson DM, Hallahan B, Toal F, Reed S, Ambikapathy A, Brammer M, Murphy CM, Murphy DG. Women with autistic-spectrum disorder: magnetic resonance imaging study of brain anatomy. Br J Psychiatry. 2007;191:224–228. doi: 10.1192/bjp.bp.106.034603. [DOI] [PubMed] [Google Scholar]
- Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. doi: 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2010;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Marquand A, Mourao-Miranda J, Johnston P, Daly EM, Brammer MJ, Maltezos S, Murphy CM, Robertson D, Williams SC, Murphy DG. Describing the brain in autism in five dimensions--magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci. 2010;30:10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJ, Williams SC, Murphy DG. Brain Anatomy and Its Relationship to Behavior in Adults With Autism Spectrum Disorder: A Multicenter Magnetic Resonance Imaging Study. Arch Gen Psychiatry. 2012;69:195–209. doi: 10.1001/archgenpsychiatry.2011.1251. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras P. GABAA Receptor Downregulation in Brains of Subjects with Autism. J Autism Dev Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Daly EM, McAlonan G, Loth E, Toal F, O’Brien F, Robertson D, Hales S, Murphy C, Murphy KC, Murphy DG. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med. 2009;39:337–346. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- Halldin C, Farde L, Litton JE, Hall H, Sedvall G. [11C]Ro 15-4513, a ligand for visualization of benzodiazepine receptor binding. Preparation, autoradiography and positron emission tomography. Psychopharmacology (Berl) 1992;108:16–22. doi: 10.1007/BF02245279. [DOI] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, Donnelly CL, Anagnostou E, Dukes K, Sullivan L, Hirtz D, Wagner A, Ritz L. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective Coexpression of Multiple Chemical Markers Defines Discrete Populations of Neocortical GABAergic Neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Dell’acqua F, Durston S, Murphy DG. Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex. 2011 doi: 10.1016/j.cortex.2011.05.018. Epub Ahead Of Print. [DOI] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, van Engeland H, Durston S. Changes in the developmental trajectories of striatum in autism. Biol Psychiatry. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Laufs H, Richardson MP, Salek-Haddadi A, Vollmar C, Duncan JS, Gale K, Lemieux L, Loscher W, Koepp MJ. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 2011;77:904–910. doi: 10.1212/WNL.0b013e31822c90f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Hume SP, Feeney A, Hirani E, Osman S, Cunningham VJ, Pike VW, Brooks DJ, Nutt DJ. Imaging the GABA-benzodiazepine receptor subtype containing the alpha5-subunit in vivo with [11C]Ro15 4513 positron emission tomography. J Cereb Blood Flow Metab. 2002;22:878–889. doi: 10.1097/00004647-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Malizia AL, Richardson MP. Benzodiazepine receptors and positron emission tomography: ten years of experience: a new beginning? J Psychopharm. 1995;9:355–368. doi: 10.1177/026988119500900411. [DOI] [PubMed] [Google Scholar]
- Matson JL, Sipes M, Fodstad JC, Fitzgerald ME. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597–606. doi: 10.2165/11591700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DG, Chua SE. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:51–59. doi: 10.1002/ajmg.b.30038. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127:e1312–1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Meguro-Horike M, Yasui DH, Powell W, Schroeder DI, Oshimura M, Lasalle JM, Horike SI. Neuron-specific impairment of inter-chromosomal pairing and transcription in a novel model of human 15q-duplication syndrome. Hum Mol Genet. 2011;20:3798–3810. doi: 10.1093/hmg/ddr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Ravan SA, Abramson RK, Wright HH, Delong GR, Cuccaro ML, Pericak-Vance MA, Gilbert JR. Association analysis of chromosome 15 gabaa receptor subunit genes in autistic disorder. J Neurogenet. 2001;15:245–259. doi: 10.3109/01677060109167380. [DOI] [PubMed] [Google Scholar]
- Mori T, Mori K, Fujii E, Toda Y, Miyazaki M, Harada M, Hashimoto T, Kagami S. Evaluation of the GABAergic nervous system in autistic brain: (123)I-iomazenil SPECT study. Brain Dev. 2011 doi: 10.1016/j.braindev.2011.10.007. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Myers JF, Rosso L, Watson BJ, Wilson SJ, Kalk NJ, Clementi N, Brooks DJ, Nutt DJ, Turkheimer FE, Lingford-Hughes AR. Characterisation of the contribution of the GABA-benzodiazepine alpha1 receptor subtype to [(11)C]Ro15-4513 PET images. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2011.177. Epub Ahead Of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D. GABAA receptors: subtypes, regional distribution, and function. J Clin Sleep Med. 2006;2:S7–11. [PubMed] [Google Scholar]
- Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol’s amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53:810–820. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Reduced GABA(A) receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2010;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011;2011 doi: 10.1155/2011/297153. 297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P. EEG features and epilepsy in patients with autism. Brain Dev. 1995;17:169–174. doi: 10.1016/0387-7604(95)00019-8. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter R, Grether JK. Continuing increases in autism reported to California’s developmental services system: mercury in retrograde. Arch Gen Psychiatry. 2008;65:19–24. doi: 10.1001/archgenpsychiatry.2007.1. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Cuccaro ML, Hauser ER, Raiford KL, Menold MM, Wolpert CM, Ravan SA, Elston L, Decena K, Donnelly SL, Abramson RK, Wright HH, DeLong GR, Gilbert JR, Pericak-Vance MA. Fine mapping of autistic disorder to chromosome 15q11-q13 by use of phenotypic subtypes. Am J Hum Genet. 2003;72:539–548. doi: 10.1086/367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks TJ, Jones T, Bloomfield PM, Bailey DL, Miller M, Hogg D, Jones WF, Vaigneur K, Reed J, Young J, Newport D, Moyers C, Casey ME, Nutt R. Physical characteristics of the ECAT EXACT3D positron tomograph. Phys Med Biol. 2000;45:2601–2618. doi: 10.1088/0031-9155/45/9/313. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Tomioka R. Long-Range GABAergic Connections Distributed throughout the Neocortex and their Possible Function. Front Neurosci. 2010;4:202. doi: 10.3389/fnins.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Zahorodny W, Peng B, Kim S, Jani N, Halperin W, Brimacombe M. The Association of Autism Diagnosis With Socioeconomic Status. Autism. 2011:218–228. doi: 10.1177/1362361311413397. [DOI] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer F, Moresco RM, Lucignani G, Sokoloff L, Fazio F, Schmidt K. The use of spectral analysis to determine regional cerebral glucose utilization with positron emission tomography and [18F]fluorodeoxyglucose: theory, implementation, and optimization procedures. J Cereb Blood Flow Metab. 1994;14:406–422. doi: 10.1038/jcbfm.1994.52. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . ICD-10 International statistical classification of diseases and related health problems. Tenth Edition 1993. [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaroff CM, Uhm SY. Prevalence of autism spectrum disorders and influence of country of measurement and ethnicity. Soc Psychiatry Psychiatr Epidemiol. 2011;47:395–398. doi: 10.1007/s00127-011-0350-3. [DOI] [PubMed] [Google Scholar]