Abstract
The Mekong Delta in Vietnam has seen a rapid development and intensification of aquaculture in the last decades, with a corresponding widespread use of antibiotics. This study provides information on current antibiotic use in freshwater aquaculture, as well as on resulting antibiotic concentrations in the aquatic environment of the Mekong Delta. Two major production steps, fish hatcheries and mature fish cultivation, were surveyed (50 fish farm interviews) for antibiotic use. Different water sources, including surface water, groundwater and piped water (164 water samples) were systematically screened for antibiotic residues. To better understand antibiotic fate under tropical conditions, the dissipation behavior of selected antibiotics in the aquatic environment was investigated for the first time in mesocosm experiments. None of the investigated antibiotics were detected in groundwater and piped water samples. Surface water, which is still often used for drinking and domestic purposes by local populations, contained median concentrations of 21 ng L-1 sulfamethoxazole (SMX), 4 ng L-1 sulfadiazine (SDZ), 17 ng L-1 trimethoprim (TRIM), and 12 ng L-1 enrofloxacin (ENRO). These concentrations were lower than the predicted no effect concentrations (PNECs) and minimum inhibitory concentrations (MICs), suggesting limited antibiotic-related risk to aquatic ecosystems in the monitored systems. The dissipation half-lives of the studied antibiotics ranged from <1 to 44 days, depending on the availability of sunlight and sediment. Among the studied antibiotics TRIM was the most persistent in water systems. TRIM was not susceptible to photodegradation, while the dissipation of ENRO and SDZ was influenced by photolysis. The recorded dissipation models gave good predictions of the occurrence and concentrations of TRIM, ENRO and SDZ in surface water. In summary, the currently measured concentrations of the investigated antibiotics are unlikely to cause immediate risks to the aquatic environment, yet the persistence of these antibiotics is of concern and might lead to chronic exposure of aquatic organisms as well as humans.
Introduction
Vietnam, followed by Indonesia, Bangladesh, Thailand, Myanmar and the Philippines, was the number one producer of aquaculture products in Southeast Asia in 2010. With an export volume of US$5.1 billion in 2010, Vietnam became the fourth-largest exporter in the world [1]. The total area used for marine and freshwater aquaculture in Vietnam was ca. 1.04 million ha in 2012, delivering more than 5.7 million tons of aquaculture products for both domestic food demand and export. The lower Mekong Delta (Vietnam) contributed 57% of this output in 2011 [2]. However, the economic benefit of aquaculture production is affected by disease outbreaks caused by bacterial infection, viruses or parasites. Therefore, chemical and biological products, particularly antibiotics, have been applied to aquaculture ponds to prevent and treat diseases and to promote growth. It was estimated that about 0.15 kg of medicine containing antibiotics was used for each ton of fish produced [3]. At the same time, fish farms were reported to discharge wastewater directly into the rivers (63%), and primary canals (19%) [4]. In the absence of treatment facilities, antibiotics are likely to reach the water system, causing water pollution and probably contributing to the development of resistant strains of bacteria (e.g., [5,6])
Antibiotic pollution has been assessed in few studies in the Mekong Delta. In mangrove areas located in Thai Binh, Nam Dinh, and Ca Mau provinces and in Can Gio forests, the highest residue concentrations found in mud samples were 0.73 g kg-1 for the antibiotic trimethoprim, 0.82 g kg-1 for sulfamethoxazole, 2.62 g kg-1 for norfloxacin and 0.43 g kg-1 for oxolinic acid. Water samples collected from shrimp ponds contained residues at maximum concentrations of 1.04 mg L-1 trimethoprim, 2.39 mg L-1 sulfamethoxazole, 6.06 mg L-1 norfloxacin and 2.50 mg L-1 oxolinic acid [7]. In 2007, sulfamethazine, sulfamethoxazole, trimethoprim and erythromycin-H2O were reported to occur at concentrations from 7 to 360 ng L-1 in surface waters collected in the Mekong Delta [8]. The same study also documented the concentration range of sulfamethazine in pig farm wastewaters and in canals near chicken and pig farms with concentrations of up to 19.2 103 ngL-1. Knowing that inhabitants in rural areas frequently use surface water for domestic purposes and in some cases also for drinking (e.g., [9,10,11,12]), long-term chronic exposure to these antibiotics is likely to occur. So far, there are no guidelines for threshold concentrations of antibiotics in waters or soils.
After application, antibiotics can be transported through different pathways. Sorption has been considered as the major process governing the mobility and transport of antibiotics in environment [5], while in the meantime evidence is increasing that sequestration in particular, and bound-residue formation largely control the long-term storage of antibiotics in soils and sediments (e.g., [13,14,15]). The dissipation half-life of antibiotics may thus vary considerably, depending on the compound, degradation pathway and environmental media. Fluoroquinolones, for instance, maybe readily degraded by fungal cultures (e.g., [16,17]) or through photodegradation in aqueous media [18] but persist by strong sorption in soils for months [14]. Many of the antibiotic dissipation data stem from aerobic environments or laboratory studies that may not or only partially be translated to natural field conditions of aquaculture production systems (e.g., [19,20,21]) with varying water and air temperature, dilution by rainfall, presence of other microorganisms, and limited oxygen supply. Besides, most of those studies were conducted in temperate climate regions. The fate of oxytetracycline and oxolinic acid in an inland fish pond under tropical conditions was preliminary simulated by a modeling approach [22]. However, almost nothing is known about the dissipation at a natural field scale of antibiotics in a tropical climate, such as that prevailing in the aquaculture systems of large tropical deltas like in Vietnam.
Currently, information on antibiotic presence and concentration in the aquatic environment, particularly in drinking water sources, and the corresponding transport and dissipation characteristics have not been systematically studied, at least for tropical systems. Therefore, this research aimed to provide for the first time 1) a comprehensive overview of the use and environmental concentrations of common antibiotics being used in the two major production steps, fish hatcheries and mature fish, in the Mekong Delta, 2) assessment of the potential risk of antibiotic pollution to ecosystem and human health via different drinking water sources, and 3) empirical evidence of the fate of the selected target antibiotics sulfadiazine, trimethoprim and enrofloxacin under semi-field experimental conditions in a tropical climate.
Material and Methods
Study sites and sampling locations
The study was carried out in the Mekong Delta of Vietnam, specifically Can Tho City (in the middle of the delta) and An Giang Province (in the northern part of the delta) (Fig 1). The sampling sites were selected in order to cover different ranges of antibiotic use and potential environmental concentrations related to two major production steps in aquaculture: fish hatcheries and mature fish cultivation.
Fig 1. Study sites in Can Tho City and An Giang Province.
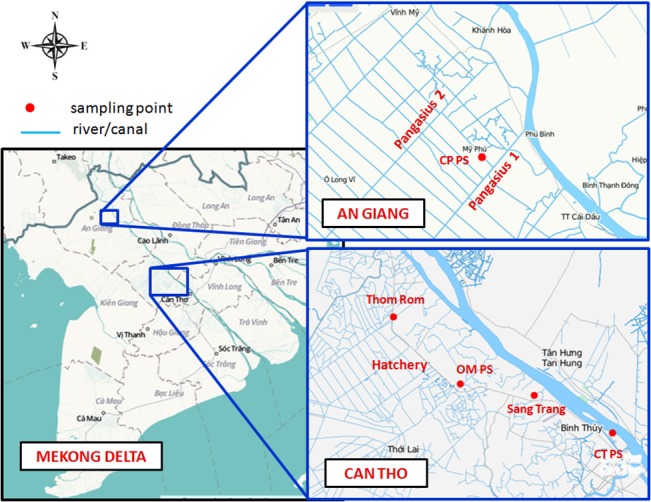
Map resource: http://www.openstreetmap.org/.
The first selected site was located close to the Hau (Bassac) River (one of the distributaries of the Mekong River) in Chau Phu district, An Giang Province. This site represented intensive cultivation of mature catfish. The total area of the study site was 140 hectares. During the time of this study (2011/2012), the area had 35 fish farms with an average farm size of 1.8 ha. Mature catfish were normally harvested after 8 to 10 months of cultivation, starting in March and harvested from October to November. Water was exchanged using electric pumps. The main domestic water sources in the area were piped water (30% interviewed households) and surface water (67%). Up to 33% of the respondents also reported using surface water for drinking and cooking (household survey result). Six sampling locations were identified in two parallel secondary canals (see Fig 1) named Pangasius 1 (P1a: 10° 36' 50.00" N, 105° 12' 16.30" E; P1b: 10° 36' 23.48" N, 105° 11' 56.34" E; P1c: 10° 36' 00.93" N, 105° 11' 36.91" E) where aquaculture wastewater was discharged to the rice fields before reaching the canal and Pangasius 2 (P2a: 10° 39' 03.41" N, 105° 10' 58.38" E; P2b: 10° 38' 36.54" N, 105° 10' 30.29" E; P2c: 10° 38' 08.51" N, 105° 10' 02.98" E), which received direct discharges from the catfish farms and also served as a domestic water source for local people.
The second site was located in Co Do, a rural district of Can Tho City. The site covered an area of 80 hectares with 10 fish farms with an average farm size of ca. 2.9 ha (including surface water area and dikes). The dominant aquaculture production step at this site was fish hatchery–mainly for carp, tench, tilapia, and catfish. The hatchery cycle lasted 2 to 3 months without a break between cycles. Nine of the ten farms discharged the wastewater directly into the adjacent canals. One farm had a lake to pre-treat wastewater before discharge. Paddy rice, upland crops and/or fruit trees surrounded the hatchery ponds. Groundwater and surface water were the main water sources for the inhabitants (70% and 40% of respondents, respectively) (household survey of this study, see also [23]). Three sampling locations were set in a secondary canal (see Fig 1) named Hatchery (H1: 10° 08' 21.21" N, 105° 33' 43.22" E, H2: 10° 08' 2.17" N, 105° 33' 55.84" E, and H3: 10° 07' 36.98" N, 105° 34' 11.54" E) where local people extracted water for domestic purposes.
Additionally, three sampling locations were set at the intake points of public pumping stations (PSs), which sourced water for a piped water supply station, located in 1) Hau river (CTPS: 10° 04' 14.99" N, 105° 46' 12.01" E) in Can Tho City, 2) O Mon river (OMPS: 10° 06' 54.96" N, 105° 37' 02.46" E) in O Mon district, (also Can Tho City), and 3) a secondary canal in Chau Phu district (An Giang Province) (CPPS, 10° 36' 16.85" N, 105° 11' 15.42" E). Moreover, two additional locations were selected in the two main canals in Can Tho City, i.e. the Sang Trang canal (ST: 10° 06' 07.97" N, 105° 41' 31.59" E) and the Thom Rom canal (TR: 10° 10' 58.67" N, 105° 33' 01.66" E), where the Sang Trang canal received industrial and domestic effluents while the Thom Rom canal received aquaculture wastewater and domestic effluents (expert interview).
Selected antibiotics and monitoring campaigns
Household interview
Questionnaire based household surveys were conducted in both study areas with the approval of the Department of Natural Resources and Environment (DONRE) of Can Tho City and the DONRE of An Giang Province. A semi-structured questionnaire was applied, which consisted of two main sections. The first section focused on the general demographics of the households, the water sources used for drinking, and general water consumption patterns. The second section focused on antibiotic use and fish farm characteristics. Twenty interviews were carried out at the Hatchery site (Can Tho)—in September 2011, and 30 at the Pangasius site (An Giang)—in March 2012. Respondents provided their written informed consent to participate in this study. Adequate information for the second section was, however, only received from 7 households at the Hatchery site and 10 households at the Pangasius sites. The fish pond owners hesitated in some cases to answer questions related to antibiotic use and farm operation.
Selection of studied antibiotics
Based on initial field survey results, the most common antibiotics used in aquaculture in these two study provinces were enrofloxacin (belonging to the fluoroquinolones, used in 47% of the farms), trimethoprim (diaminopyrimidine, 41%), sulfamethoxazole (sulfonamides, 41%), doxycycline (tetracyclines, 29%), florfenicol (phenicol, 29%), sulfadiazine (sulfonamides, 18%), amoxicillin (penicillins, 18%), ampicillin (penicillins, 12%), and oxytetracycline (tetracyclines, 12%). A minority of farmers reported using spectinomycine (aminoglycosides, 5.9%), sulfadimethoxine (sulfonamides, 5.9%), cephalexin monohydrate (penicillins, 5.9%), kanamycin sulfate (aminoglycosides, 5.9%), and chloramphenicol (phenicol, 5.9%). Even though tetracyclines were used frequently, their sampling and analysis requires avoiding glassware and using plastic apparatus due to their strong binding to borosilicate glassware [24], which prevented the monitoring and simultaneous determination of other antibiotics. Therefore, after consideration of usage frequency, physico-chemical properties (water solubility, octanol-water partition coefficient, soil organic carbon-water partition coefficient, acid dissociation constant, Table 1), and access to analytical equipment, sulfamethoxazole (SMX), sulfadiazine (SDZ), trimethoprim (TRIM) and enrofloxacine (ENRO) were selected for monitoring in water. For the fate study the same antibiotics except for SMX were considered. SMX has a similar mode of action as SDZ but it is less likely to cause harm to aquatic life (much higher median effective concentration (EC50) and median lethal concentration (LC50) concentrations compared to the other antibiotics, Table 2).
Table 1. Physicochemical properties of the studied antibiotics.
| Antibiotics | Class | Water solubility | LogKow | Koc | pKa | Use d |
|---|---|---|---|---|---|---|
| 20°C (mg L-1) | (L kg-1) | (%) | ||||
| Sulfamethoxazole a | Sulfonamides | 2800 | 0.89 | 219 | 5.8; 1.4 | 41 |
| Sulfadiazine | Sulfonamides | 77 b | -0.09 b | 124 c | 6.36 b | 17 |
| Trimethoprim a | Diaminopyrimidines | 1000 | 0.59 | 301 | 7.0 | 41 |
| Enrofloxacine a | Fluoroquinolones | 100 | 2.32 | 2179 | 6.4; 7.8 | 47 |
Table 2. Toxicity data for different aquatic organisms exposed to the studied antibiotics, PNEC, PEC, ratio of maximum quantification concentration and PNEC, and ratio of PEC and PNEC.
| Species | Test | Duration | Conc. | Sources | AF | PNEC | Max. quant. conc. | Max. quant. conc./PNEC | PEC | PEC/PNEC | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (h) | (mg L-1) | (µg L-1) | (ng L-1) | (µg L-1) | (µg L-1) | ||||||||
| Pangasius | Hatchery | Pangasius | Hatchery | ||||||||||
| SMX | Daphnia | EC50 | 96 | 177–204 | [27] | ||||||||
| Danio rerio (fish) | LC50 | 96 | >1000 | [27] | 1000 | 562.5 | 239 | 0.0004 | 64 | 21 | 0.11 | 0.04 | |
| Oryzias latipes (fish) | LC50 | 96 | 562.5 | [27] | |||||||||
| SDZ | Daphnia magna | EC50 | 48 | >57 | [28] | 100 | 10 | 108 | 0.0108 | 64 | 21 | 6.41 | 2.07 |
| Algae | NOEC | 96 | <1 | [28] | |||||||||
| TRIM | Daphnia magna | LC50 | 48 | 123 | [28] | ||||||||
| Oryzias latipes(fish) | LC50 | 96 | >100 | [28] | 100 | 255 | 330 | 0.0013 | 16 | 5 | 0.06 | 0.02 | |
| Algae | NOEC | 96 | 25.5 | [28] | |||||||||
| ENRO | Oryzias latipes (fish) | LC50 | 96 | 10 | [28] | ||||||||
| Oryzias latipes (fish) | NOEC | 21 days | 1 | [28] | 50 | 20 | 81 | 0.0040 | 36 | 11 | 1.78 | 0.57 | |
| Daphnia magna | NOEC | 21 days | 1 | [28] | |||||||||
EC50: median effective concentration; LC50: median lethal concentration; NOEC: no observed effect concentration; PNEC: predicted no effect concentration; PEC: predicted environmental concentration; AF: assessment factor
Antibiotic monitoring strategy
A total of 154 surface water samples were collected at 14 selected locations in eleven sampling events performed monthly from March 2012 to January 2013 with the permission of the DONRE of Can Tho City and the DONRE of An Giang Province. Focusing on drinking water quality, sampling locations and times were set to represent the surface water extraction routine of local households, e.g. at 3 m distance from the canal bank during high tide (when residents expect to extract water of better quality). Water samples taken to represent the water utilized by the water supply stations were collected close to the inlet of the pumping stations.
Field survey results showed that a larger proportion of piped water was consumed at the Pangasius sites (in An Giang) whereas a larger proportion of groundwater was consumed at the Hatchery site (in Can Tho). To initially screen piped water quality at the Pangasius sites and groundwater quality at the hatchery site, 5 piped water samples were collected from household taps and 5 groundwater samples from private drill wells. The water was purged for 5 minutes before sampling.
Samples (500 mL) were collected in pre-cleaned glass bottles, acidified (pH 2.5), transported to the laboratory under cool conditions and extracted within 24 hours. For each sampling event, basic water quality parameters including pH, dissolved oxygen (DO), electrical conductivity (EC), and temperature were measured in-situ by a WTW Multi 340i instrument (Weilheim, Germany) at every sampling location.
Experimental design of the fate study
The fate study was conducted under semi-field conditions. A secondary canal (10° 3' 17.27" N, 105° 42' 50.28" E) located in Binh Thuy district, Can Tho City was selected for the fate study. The canal was representative of the environmental conditions of the region, i.e. the water of the canal had a typically high electrical conductivity (EC) of 170 to 220 μS cm-1, while pH ranged from 6.8 to 7.2, and a low dissolved oxygen concentration (2 to 4 mg L-1) prevailed. Additionally, this site was surrounded by an area where no significant antibiotic pollution was expected from the region, i.e. the main land-use in this area was rice cultivation and fruit production, with no aquaculture or animal husbandry within a radius of 1 km. Moreover, it allowed for fast sample transportation to the laboratory (< 10 km distance). Finally, the site was located in an area where local authorities and farmers were willing to cooperate in setting up the experiment and to protect the microcosms against physical damage during the study period. The canal received water from a primary canal which was directly connected to the Hau River. The canal was influenced by tides. The difference between the lowest and highest water level during the experimental period was about 1.5 m.
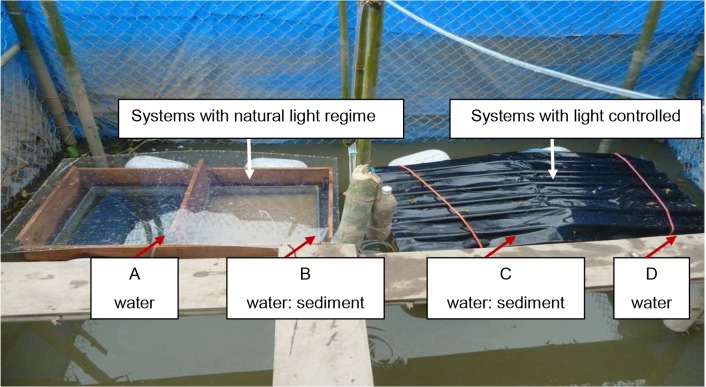
The semi-field experiment was designed based on a study by Laabs et al. [29] with 4 different microcosm systems (Fig 2):
- water system with natural light regime (system A),
- water:sediment system with natural light regime (system B),
- water:sediment with light control (system C),
- water system with light control (system D)
Fig 2. Experiment set up of the microcosms for assessing the dissipation of antibiotics in water and water:sediment systems.
Microcosms (60 x 60 x 54 cm) were constructed from glass plates of 5 mm thickness. Black glass plates were used for systems with light control. The systems were filled with filtered canal water (filtration by a stone layer and a sand layer) from the adjacent primary canal. The total water volume in the water systems was 194.4 L. The sediment to water ratio in sediment:water systems was 1:5 (v:v), corresponding to 9 cm sediment and 45 cm water layer (ca. 162 L of water). The sediment to set up the systems originated from an adjacent primary canal taken from a depth of 20–30 cm. The sediment was homogenized prior to use, and stones, leaves, and other materials were removed. The system was allowed 3 days to reach equilibrium, after which the basic characteristics of the water and sediment were analyzed (see S1 Table). Antibiotic solutions for initial spiking of the systems were prepared in a 50 mL methanol:water 50:50 (v:v) mixture, in which the initial concentration was ca. 20 103 ng L-1 for each antibiotic (20.09 103 ng L-1 of SDZ, 19.59 103 ng L-1 of ENRO, and 21.71 103 ng L-1 of TRIM). Accordingly, the volume of methanol introduced to each system after spiking was 6.25 mL (accounting for 0.00315%, which should not influence the dissipation of studied antibiotics in water). The water phase was stirred during the application of antibiotics but the sediment layer was not disturbed.
Wooden frames connected with plastic cans were constructed to keep the systems floating at the same water level as the canal water. Bamboo pillars and iron steel B40 nets were used as a fence to protect the systems against debris or water waves caused by boats. The concentration of dissolved oxygen, pH, electrical conductivity (EC) and water temperature were measured at 10 cm and 45 cm water depths in the test systems at every sampling date by a WTW Multi 340i instrument (Weilheim, Germany).Water loss due to evaporation during the fate study was compensated for with distilled water.
Samples were collected from each system in ten sampling events in three replicates. Sampling dates were: 0, 1, 2, 4, 8, 15, 29, 43, 57 and 85 days after application, starting on 14 May2012 and completed on 7 August 2012.
Antibiotic analysis
Chemicals and reagents
Antibiotic standards of sulfadiazine, sulfamethoxazole, trimethorprim and enrofloxacin were obtained from Sigma-Aldrich (Seelze and Schnelldorf, Germany). The internal standards including isotope-labeled 13C6-sulfadiazine were provided by the Institute of Environmental Biology and Chemodynamics of the RWTH Aachen University, while enrofloxacin hydrochloride (ethyl-d5), 13C6-sulfamethoxazole and 13C6-trimethoprim were supplied by LGC Standards (Wesel, Germany). All employed solvents were of HPLC grade. For accelerated solvent extraction (ASE), sea sand, salts, and acids were pro-analysis grade. A Millipore Synergy water treatment system was used to produce water.
Analytical method
Antibiotics in the water samples were analyzed according to modified methods developed by Gobel et al. [30]. Sample preparation was conducted in the Advanced Laboratory, Can Tho University, Vietnam. Solid-phase extraction was employed using Chromabond SB (SAX, Macherey & Nagel, Germany) in combination with Oasis HLB (Waters, USA) to reduce matrix effects and clogging. The cartridges were preconditioned with 4 mL methanol and 4 mL Millipore water at pH 2.5. A 500 mL water sample was filtered through a glass fiber filter (pore size 1 μm) before being applied to the cartridge at a flow rate of ca. 1.5 mL min-1. The cartridge was then washed with 5 mL Millipore water and dried in a nitrogen flow for 30 minutes. Dry cartridges were kept frozen at -40°C, which then were transferred frozen to the laboratory of the Division of Soil Science and Soil Ecology, Faculty of Agriculture, University of Bonn, Germany for elution and measurement. The analytes adsorbed on the solid phase of the Oasis HLB cartridge were eluted sequentially by 4 mL methanol, 4 mL acetonitrile and 4 mL acetonitrile + 0.1% HCl. The eluate was concentrated to ca. 500 μL by rotary evaporation and transferred to amber vials containing 25 ng of the internal standards 13C-SMX, 13C-SDZ, 13C-TRIM and ENRO-D5 hydrochloride. Amber vials were filled up to ca. 1 mL with 50 mM phosphoric acid:acetonitrile (80:20) solution and stored at -20°C until measurement.
The sediment samples from the dissipation study were stored at -20°C in the Advance Laboratory, Can Tho University, Vietnam and transferred frozen to the laboratory of the Division of Soil Science and Soil Ecology, Faculty of Agriculture, University of Bonn, Germany. Sediments were lyophilized and sieved to a grain size < 2 mm. Ten g of dry material of each sediment sample mixed with sea sand was extracted employing accelerated solvent extraction (ASE). Two different solutions for the extraction were combined to account for the different physico-chemical properties of the antibiotics (Table 1). Anaqueous 50 mM phosphoric acid:acetonitrile solution (50:50, v:v) adopted from Golet et al. [31] and a methanol:water solution (50:50, v:v) from Gobel et al. [30] were used. Twenty mL of ASE extract was diluted to 400 mL by Millipore water and adjusted to pH 2.5. The analytes were then extracted and eluted using the same procedure described above for the water samples.
Antibiotic concentrations were analysed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS, Quantum Ultra; Thermo Electron Corporation, Dreieich, Germany), equipped with a heated electro spray ionisation source (ESI) in the positive ion mode with an injection volume of 10 μL. Antibiotic classes were separated by XBridge C18 collumn (3.5 μm, 2.1x150 mm, Waters, USA) with the following mobile phases: solvent A: acetonitrile + 0.1% HCOOH, solvent B: 1 mM ammonium acetate in millipore water + 0.1% HCOOH with 400 μL min-1 flow. The initial condition was 5% A and 95% B, changed to 60% A over 5 min, then to 80% A over 10 min, then to 100% A over 1 min, hold 100% A for 2 min and return to the initial condition, hold initial condition for 7 min. The current was set at 4000 V, the vaporizer temperature was 390°C and the capillary temperature was 217°C.
Routine limit of quantification (RLOQ = lowest concentration of standard used) was calculated at 1 ng L-1 water and 50 ng kg-1 sediment dry weight for all studied antibiotics.
The recovery rates of antibiotics from water samples (n = 5) were 63 (± 6.6) % for ENRO, 77.6 (± 4.5) % for SDZ, SMX and TRIM. In the dissipation study, the recovery rates for spiked sediment samples (n = 3) ranged from 32% to 42% for ENRO, and from 76% to 93% for SDZ and TRIM. Monitored concentrations were not corrected for recovery rates.
Statistical methods
IBM SPSS Statistics version 20.0 (IBM Corp, Armonk, New York, USA) and Sigma Plot version 11.0 softwares (Systat Software Inc, San Jose, California, USA) were used to perform the statistical analysis. The Kolmogorov–Smirnov test was applied to test the normal distribution of the data (p = 0.05). If the data was normally distributed, one way ANOVA was run to find significant differences between sites. Otherwise non-parametric tests were used. The Kruskal-Wallis H test was applied to find differences between sites. The Mann–Whitney U tests were used to identify differences within sites.
Statistics for fate study
Exponential decay equations were fitted to antibiotics dissipation data:
Ct: the concentration of antibiotic at time t (ng L-1 or ng kg-1)
C0: the initial concentration (ng L-1)
k: the dissipation rate constant (d-1)
t: the elapsed time (d)
Dissipation half-lives (DT50: the time required for 50% of the initial concentration to dissipate) were calculated from the above equation: DT50 = ln(2) k-1. For persistence comparisons, 90% and 99% dissipation times were also calculated.
Results and Discussion
Fish farming practices and antibiotic use at the study sites
The interviews revealed that farmers at the Hatchery site (Can Tho) cultured ca. 2.6 million fingerlings per hectare with an average production yield from each cycle of ca. 40 tons ha-1 (ca. 30 g weight at the time of harvest; Table 3). At the mature Pangasius sites (An Giang), average fish density at the stocking stage was lower than at the Hatchery site (Table 3). At the time of harvesting, production yield was ca. 250 tons ha-1 per season (from 800 to 1000 g weight at the time of harvest). Mortality at the hatcheries was typically high (40%–50% per crop) due to the susceptibility of fingerlings to diseases. Overall, around 30% of the mature catfish was lost. This loss predominantly took place at the early stages of a cycle (own survey, and [4]). Prevalent fish bacterial diseases recorded at both sites were 1) red spot disease, with the occurrence of hemorrhages on head, mouth and fins, 2) necrosis, causing spots on liver, kidney, spleen. These were considered the most common and severe diseases of catfish in Vietnam (e.g., [32,4]). Fish farmers at both sites assumed that mainly specific weather conditions, e.g. the floods and heavy rain which take place annually from August to November were responsible for the spread of diseases. Local farmers buried or even sold the dead fish to local dealers, which likely contributed to the spread of the diseases [4]. High loss rates caused by diseases in combination with high (but also fluctuating) market demand were thus mentioned by farmers as reasons for antibiotic use. Additionally, antibiotics were also applied prophylactically (own survey, and [33]).
Table 3. Fish farming and antibiotic use in the Hatchery and Pangasius sites.
| Hatchery site | Pangasius sites | |
|---|---|---|
| (n = 7) | (n = 10) | |
| ( * )Ave. farm size (ha) | 2.9 | 1.8 |
| Ave. fish weight at harvest time (g) | 30 | 960 |
| Ave. initial stock density (individuals ha-1) | 2,700,000 | 374,000 |
| Ave. production (tons ha-1) per crop | 40 | 250 |
| Loss rate per crop (%) | 40–50 | 30 |
| Water exchange frequency (days) | 35 | Everyday, partial exchange |
| Pond depth (m) | 1–1.5 | 2.5–5 |
| Antibiotic use technique | Instruction on label, Antibiotic retailers | Aquaculture extensionists |
| Farmer/worker directly exposed to antibiotics (dermal exposure) | 100% | 100% |
| Av. number of different types of antibiotics used per season | 2 | 3 |
| Overdose application (based on farmer´s report) | 40% | Farmers did not answer |
(*) Each fish farm could have one or more fish ponds
Among the 14 currently used antibiotics at the two study sites, ENRO, SMX, TRIM, doxycycline and florfenicol were most often used (see also Selection of studied antibiotics). Notably, ENRO and chloramphenicol are prohibited in aquaculture production according to Vietnam's Circular 15/2009/TT-BNN promulgated by the Ministry of Agriculture and Rural development [34]. Farmers at both sites used their unprotected hands to mix antibiotics with food or water (in case of bath treatments). In general, two different antibiotic active ingredients were applied per hatchery farm and three at the mature Pangasius farm. At the Hatchery site, 40% of farmers reported applying a higher dosage than the recommendation on the product's label. No information could be collected on the antibiotic dosage applied at the mature Pangasius sites because local fish farmers refused to answer and asserted that they had completely followed the instructions of aquaculture extension officers. The same situation was also reported by Tuan and Munekage [7] who faced difficulties when attempting to collect information on the dosage of antibiotics used in the case of shrimp farmers. Despite the information gaps, the interview results demonstrated a tendency of inadequate or at least uninformed antibiotic handling.
At the Hatchery farms, the water depths in ponds were low and varied from 1 to 1.5 m. The farms discharged water from the ponds every 35 days on average. Water was taken in or discharged out from the same canal (where sampling locations were set). Water treatment before discharge was only reported at one farm, in which wastewater was drained to a lake for sedimentation prior to lime application. This low rate of water treatment was also reported in the study of Lam et al. [4], in which only 11.2% of investigated catfish farms applied chlorine or lime before discharging wastewater. At the mature Pangasius farms, pond depth ranged from 2.5 to 5 m. Ponds were surrounded by dikes, which were built to prevent the loss of fish during the flooding season or heavy rainfall events (household interview, and [35]). Pond water was partly refreshed daily. Similar practices were also mentioned by Carballo et al. [36] and Lam et al. [4]. The wastewater from these mature fish farms was normally discharged to the surrounding fields or in most of the cases at the site Pangasius 2 to the adjacent canals.
In general, these farming practices in combination with untreated discharge flows from fish ponds containing excess feed, dead fish and residues of veterinary medicines including antibiotics suggested a risk of pollution of the surrounding aquatic environments.
Occurrence of antibiotics in surface water
As shown in Table 4, almost all surface water samples were contaminated by at least one antibiotic (91.6%), while in 55.2% of the samples, a mixture of 3 or 4 antibiotics co-occurred. Among the two studied sulfonamides, SMX was more frequently detected in surface water than SDZ (82% vs. 58%, respectively). Detected concentrations of SMX ranged from 1 to 239 ng L-1 (median 21 ng L-1). These concentrations were lower than the concentrations reported by Managaki et al. [8], where median concentrations of SMX were 80 ng L-1. The concentrations of SDZ were lower compared with that of the other antibiotics (Table 4). This is in line with interview results showing that SDZ was less frequently used than the other antibiotics (Table 1). The highest SDZ concentration of 108 ng L-1 was observed in the sample from the Hatchery site in June 2012. In terms of veterinary medicine for fish, SMZ and TRIM or SMX and TRIM are normally mixed in commercial products, e.g. in "Trimesul" and "Cotrim" (field survey) since the effectiveness of sulfonamides is enhanced when combined with TRIM [37]. Hence, TRIM was also abundant and occurred in 87% of monitored water samples (Table 4). This diaminopyrimidine compound was quantified with a median concentration of 17 ng L-1 which was comparable to the median concentration (20 ng L-1) reported by Managaki et al. [8], suggesting that the concentrations found here are in a typical range. SMX and TRIM residues in water taken from shrimp farms in the South of Vietnam [7] were one order of magnitude higher. In Taiwan, concentrations of SMX and TRIM in aquaculture wastewater were higher (median 229 ng L-1 and 85 ng L-1, respectively) [38]. ENRO is forbidden and thus has the lowest detection frequency. Nevertheless, 38% of surface water samples contained this compound with median concentration of 12 ng L-1 (Table 4). Low water solubility in combination with strong adsorption to soil of the fluoroquinolone group in general (e.g., [31,14]) and of ENRO in particular (Table 1) were the main factors limiting the detection of ENRO in water.
Table 4. Occurrence of SMX, TRIM, SDZ, ENRO ng L-1 in collected surface water samples in 2012.
| Hatchery | Pangasius 1 | Pangasius 2 | Pumping stations (PSs) | Main canals | TOTAL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling locations ( * ) | H1 | H2 | H3 | P1a | P1b | P1c | P2a | P2b | P2c | CP PS | OM PS | CT PS | Sang Trang | Thom Rom | ||
| ENRO | Mar | 7 | - | - | - | - | - | - | - | - | 5 | - | - | - | 6 | |
| (n = 154) | Apr | - | 12 | 4 | - | 1 | 4 | 6 | 4 | 7 | 8 | 10 | 6 | 6 | 5 | |
| May | - | - | 17 | - | - | 5 | - | - | 6 | 5 | - | 5 | - | 10 | ||
| Jun | 26 | 14 | 12 | 22 | 28 | 16 | 59 | 18 | 25 | 34 | 28 | 40 | 22 | 55 | ||
| Jul | 34 | 30 | - | 15 | 11 | 17 | 20 | 43 | 20 | 15 | 9 | 20 | 12 | - | ||
| Aug | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Sep | - | - | - | - | - | - | - | - | - | - | - | 5 | 5 | - | ||
| Oct | - | - | - | 24 | - | - | - | 19 | 5 | 49 | 17 | - | - | 15 | ||
| Nov | - | - | - | 5 | - | 11 | - | - | - | - | - | - | 8 | - | ||
| Dec | - | - | - | - | - | - | - | - | 3 | - | - | - | - | - | ||
| Jan ( * ) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Median | 14 | 13 | 18 | 11 | 13 | 6 | 8 | 10 | 12 | |||||||
| Quant. freq (%) | 27.3 | 36.4 | 39.4 | 54.5 | 36.4 | 45.5 | 45.5 | 45.5 | 38.3 | |||||||
| SDZ | Mar ( *** ) | |||||||||||||||
| (n = 126) | Apr ( *** ) | |||||||||||||||
| May | 3 | 4 | 6 | - | 2 | 2 | 4 | 8 | 4 | 2 | 4 | 11 | 14 | 2 | ||
| Jun | 108 | 22 | 90 | 2 | - | 3 | 6 | 10 | 10 | 6 | 48 | 17 | 36 | 26 | ||
| Jul | - | 4 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Aug | 6 | 6 | 13 | 2 | - | - | 25 | 9 | 2 | 4 | 1 | - | 21 | 3 | ||
| Sep | 14 | 4 | 7 | - | - | - | 2 | 2 | 2 | - | 3 | 4 | 12 | 3 | ||
| Oct | 2 | 2 | 2 | 17 | - | 1 | 4 | 10 | 4 | 15 | 11 | 2 | 3 | 10 | ||
| Nov | 1 | - | - | 3 | 2 | 7 | 3 | - | 3 | 1 | 3 | 3 | 63 | 8 | ||
| Dec | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Jan ( * ) | - | - | - | - | - | - | - | - | - | - | - | - | 12 | - | ||
| Median | 6 | 3 | 5 | 4 | 4 | 4 | 14 | 6 | 4 | |||||||
| Quant. freq (%) | 63.0 | 37.0 | 63.0 | 55.6 | 66.7 | 55.6 | 77.8 | 66.7 | 57.9 | |||||||
| SMX | Mar | 10 | 2 | 16 | 15 | 27 | 10 | 40 | 94 | - | 22 | 22 | 80 | 148 | 6 | |
| (n = 154) | Apr | - | 10 | - | 81 | - | 3 | 6 | 6 | 16 | - | 4 | 6 | - | - | |
| May | 46 | 104 | 42 | 20 | 27 | 32 | 48 | 44 | 92 | 40 | 25 | 80 | 143 | 3 | ||
| Jun | 30 | 21 | 24 | 14 | 34 | 38 | 74 | 88 | 88 | 76 | 21 | 34 | 55 | 39 | ||
| Jul | 19 | 34 | 15 | 17 | 12 | 11 | 13 | 8 | 25 | 18 | 6 | 8 | 5 | 156 | ||
| Aug | 38 | 29 | 58 | 20 | 17 | 14 | 24 | 17 | 4 | 21 | 16 | - | 239 | 43 | ||
| Sep | 44 | 11 | 32 | 9 | 5 | 33 | 8 | 12 | 10 | 36 | 9 | 20 | 3 | 14 | ||
| Oct | 47 | 80 | 135 | 10 | 6 | 10 | 92 | 73 | 75 | 10 | 70 | 51 | 57 | 185 | ||
| Nov | 21 | - | 20 | 3 | 7 | 28 | 22 | 19 | 14 | 10 | 13 | 17 | 19 | 31 | ||
| Dec | - | - | - | 9 | 2 | 3 | 11 | 9 | 45 | - | 3 | - | 34 | - | ||
| Jan ( * ) | - | - | - | 3 | - | 8 | - | - | - | - | - | - | - | - | ||
| Median | 31 a | 12 b | 22 a | 21 | 15 | 27 | 55 | 35 | 21 | |||||||
| Quant. freq (%) | 72.7 | 93.9 | 87.9 | 72.7 | 90.9 | 81.8 | 81.8 | 72.7 | 82.5 | |||||||
| TRIM | Mar ( *** ) | |||||||||||||||
| (n = 126) | Apr ( *** ) | |||||||||||||||
| May | 29 | 24 | 6 | 24 | 26 | 26 | 36 | 30 | 50 | 17 | 19 | 40 | 41 | 24 | ||
| Jun | 69 | 23 | 309 | 12 | 16 | 26 | 41 | 42 | 46 | 49 | 144 | 104 | 111 | 78 | ||
| Jul | 71 | 330 | 22 | 13 | 8 | 12 | 32 | 29 | 46 | 10 | 37 | 24 | 45 | 163 | ||
| Aug | 14 | 11 | 16 | 18 | 6 | 5 | 11 | 12 | - | 10 | 13 | - | 45 | 20 | ||
| Sep | 18 | 23 | 63 | 7 | 1 | 19 | 11 | 14 | 13 | 21 | 9 | 9 | 11 | 23 | ||
| Oct | 13 | 16 | 15 | 7 | 5 | 30 | 37 | 25 | 16 | 13 | 20 | 14 | 21 | 21 | ||
| Nov | 13 | - | 20 | 7 | 9 | 18 | 14 | 16 | 17 | 8 | 17 | 15 | 23 | 20 | ||
| Dec | - | - | - | 5 | - | - | - | - | 1 | - | 6 | 1 | 11 | - | ||
| Jan( * ) | - | - | - | 14 | 13 | 7 | 9 | 13 | 6 | 3 | 2 | 1 | 3 | - | ||
| Median | 0.021 a | 0.012 b | 17 a | 12 | 17 | 14 | 23 | 23 | 17 | |||||||
| Quant. freq (%) | 74.1 | 92.6 | 88.9 | 88.9 | 100.0 | 88.9 | 100.0 | 77.8 | 87.3 | |||||||
(*): Sampling locations: see section "Study sites and sampling locations" for details
(**): January 2013
(***): not monitored
-: no detection
a and b: median concentrations of individual antibiotics with different letter superscript indicate significant differences between study sites (p < 0.001)
Antibiotic residues in water at the fish farm sites
Spatial variation: Significantly lower median concentrations of SMX and TRIM were found (p < 0.001) at the site Pangasius 1 (12 ng L-1 for both antibiotics) in comparison with the site Pangasius 2 (median 22 ng L-1 for SMX and 17 ng L-1 for TRIM) and the Hatchery site (median 31 ng L-1 for SMX and 21 ng L-1 for TRIM). The results suggest that antibiotic contamination in water was partly mitigated by draining wastewater to the rice fields (see Pangasius 1) prior to discharge to surrounding aquatic environments. The drainage removed excess nutrients from aquaculture effluents effectively, while serving as a fertilizer in rice production (e.g., [39,40]).
SMX and TRIM occurred more often in water samples collected from Pangasius than from Hatchery sites (Table 4). This might partially be due to the more frequent effluent discharge at the Pangasius sites than at the Hatchery site (everyday vs. every 35 days, Table 3). Quantification frequency was similar for these two antibiotics at each site. This result is in line with the survey data showing that these two compounds have been applied in a mixture to enhance treatment efficiency.
As already reported for the surface waters, SDZ and ENRO residue levels were lower than that of the other antibiotics at both the Hatchery site and the Pangasius sites, ranging from 1 to 108 ng L-1 for SDZ and from 1 to 59 ng L-1 for ENRO, respectively (Table 4). The reasons are again related to lower application amounts (for SDZ) and the lower water solubility of both compounds (Table 1; see also discussion above).
Temporal variation: At both Hatchery and Pangasius sites ENRO was most frequently detected from April to July (79.4% of the contaminated water samples were sampled in these months). Almost 90% of the samples with ENRO concentrations higher than the median value (12 ng L-1), fell within this period. ENRO was almost completely absent in the samples taken between August 2012 and January 2013. The pattern for SDZ, SMX and TRIM was partly different. The median values (4 ng L-1 for SDZ, 21 ng L-1 for SMX, and 17 ng L-1 for TRIM) were exceeded in the water samples from May to November. An exception was the case of SDZ in July, in which the median value was not exceeded in any analyzed sample. Antibiotics were detected at low frequency and concentration in December 2012 and January 2013 (Table 4). This could be partly explained by the climate of the Mekong Delta. The period from May to October corresponds to the rainy season, with hot, humid and rainy weather (75% of total rain water fell in this season in 2012 (Ca Mau station, [41])). These climatic conditions generally facilitate diseases outbreaks and transmission, forcing fish farmers to use more antibiotics.
Antibiotic residues at the inlet of public pumping stations and in two main canals in Can Tho
At the investigated pumping stations, sampling points were set right at the inlet of the stations. The surface water extracted served as the source for piped water supply. For extracted water, treatments included flocculation, deposition, filtration, and chlorine disinfection in the water plant. These treatment methods are known to be able to remove several organic compounds [42].
During the eleven sampling campaigns from March 2012 to January 2013, SMX and TRIM were again detected at a higher frequency at all sampling points than SDZ and ENRO (Table 4). Except for the median concentration of SMX (21 ng L-1), which was significantly higher (p < 0.05) than that of SDZ (4 ng L-1) at CPPS, there were no statistically significant differences between occurrences of studied antibiotics within the inlet points of the monitored stations, or of specific antibiotics between stations. The prevalence of antibiotics at the CPPS (in a secondary canal in An Giang Province) and in the Thom Rom canal (a main canal in Can Tho City) could be caused by wastewater discharged from the surrounding intensive aquaculture farms. However, even though it was expected that less pollution would occur at the CTPS (located at the Hau River, one tributary of the Mekong River with high dilution capacity), antibiotics were also frequently detected (Table 4). The source of pollution at this sampling point could be attributed to the discharges from cage aquaculture cultivation along the upstream banks of the Hau River. The high solubility of antibiotics and the high water flows of the river would facilitate the transportation of antibiotics downstream to this pumping station. In the cases of the OMPS and Sang Trang canal, the field survey recorded a predominance of industrial and residential land-use. Therefore, the possible pollution sources could be from human pharmaceutical use or veterinary products from livestock farms. Since similar concentrations of antibiotics were found at all pumping stations, regardless of local differences in industry and land use, it seems likely that the captured concentrations of antibiotics in both incoming and discharging water sources are typical steady-state concentrations, and that the main sources of antibiotics in the region, including inland aquaculture, cage aquaculture, veterinary and human pharmaceutical products, have contributed to a region-wide occurrence of antibiotics that was detected in every waterway monitored.
Antibiotics were not detected in any of the collected ground- or piped water samples. However, the year-round prevalence of the studied antibiotics in surface water, together with the use of surface water for domestic and drinking water still gives rise to concerns as to whether—at least locally—antibiotic residues pose a risk for ecosystem and human health.
Risk assessment
To assess the potential risk associated with antibiotic residues in the aquatic environment, the monitoring results were compared with Predicted No Effect Concentrations (PNEC) for aquatic organisms (e.g. [43]), and Minimum Inhibitory Concentration (MIC) for bacteria strains. Worst-case scenario was also conducted by calculating the Predicted Environmental Concentrations (PECs) of individual antibiotics based on the prescribed dosages from veterinary producers and water volumes in fish ponds and then compared to PNEC [44] and MIC values.
In terms of PNEC assessment, concentrations lower than the PNEC are assumed to pose no risk to the environment [45]. The toxicity data of individual studied antibiotics consisting of different EC (effect concentration) or LC (lethal concentration) values and NOECs (no effect concentrations) are shown in Table 2. To reflect the degree of uncertainty when extrapolating from available laboratory toxicity tests to effects in environment, so-called assessment factors (AFs) were used. Selection of these AF varied from 1000 to 1, based on European Commission guidelines ([45], see S2 Table for details). Where possible, the NOEC was used, since it is the more conservative value. PNEC value was calculated by dividing the lowest toxicity concentration of an antibiotic by the respective AF. In the case of SMX, since there was no NOEC value available, an AF of 1000 was selected. Meanwhile, only one NOEC for algae was found for SDZ and TRIM, thus the respective AF was 100. Of the four monitored antibiotics, ENRO was considered as the most toxic antibiotic to aquatic living organisms as there were at least two NOEC values available (see S2 Table). Therefore, the selected AF for ENRO was 50 (Table 2).
With regard to MIC data, there is a strong variation in the sensitivity of different bacterial strains to a certain antibiotic; hence, the reported MIC values of each studied antibiotic varied across abroad range (Table 5).
Table 5. Minimum inhibitory concentrations (MICs) for ENRO, SMX, TRIM and SDZ against some bacterial strains, ratio of max.
quantification concentrations and MIC50, and ratio of PEC and MIC50.
| MIC | Source | Max. quant. conc. | Max. quant. conc./MIC50 | PEC/MIC50 | |||||
|---|---|---|---|---|---|---|---|---|---|
| (mg L-1) | (ng L-1) | Pangasius | Hatchery | ||||||
| E. coli | Gram possitive bacteria (231) | Gram neggative bacteria (98) | |||||||
| ENRO | MIC50 | < 0.03 | 0.13–1 | 0.06–1 | [46] | 81 | 0.003 | 1.19 | 0.038 |
| MIC90 | 0.13 | 0.25–2 | 0.06–1 | ||||||
| Anaerobic bacteria (119) | |||||||||
| SMX | MIC50 | 2.4–53 | 239 | 0.0001 | 0.03 | 0.01 | |||
| MIC90 | 5.3 - > 128 | [47] | |||||||
| TRIM | MIC50 | 0.3–51 | 330 | 0.001 | 0.05 | 0.02 | |||
| MIC90 | > 19.5–128 | ||||||||
| Neisseria Meningitidis strains | |||||||||
| SDZ | MIC | 0.005–2 | [48] | 108 | 0.022 | 12.82 | 4.14 | ||
See Table 2 for PEC values; MIC50 (MIC90): MIC at which 50% (90%) of the strains were at or below
As shown in Table 2, the ratios of the maximum quantified concentrations to the PNEC values were lower than 1. Thus, the occurrence of the four studied antibiotics did not pose a risk to the aquatic environment in the studied systems. Moreover, quantified antibiotic concentrations in all collected water samples were approximately three or four orders of magnitude lower than the respective MIC values. However, the calculated PEC values for all four studied antibiotics were three orders of magnitude higher compared to the median quantified concentrations (Table 4). The PEC/PNEC ratios as well as the PEC/MIC ratios of SDZ and ENRO were higher than 1, stressing the importance of detailed investigations of the environmental fate of SDZ and ENRO for further risk assessment [44].
In summary, the studied antibiotics were found nearly ubiquitously in surface water, but as the background levels were low, they are unlikely to cause an overall risk to aquatic organisms, according to the current state of knowledge. However, detected background levels were influenced by dilution processes (rainfall, mixing of water sources with other water sources) and particularly by dissipation processes i.e. the monitoring could not cover maximum antibiotic loads immediately after application. Dissipation of organic pollutants like pesticides in tropical ecosystems may occur very rapidly (e.g., [49,50]). Since PEC/PNEC ratios of SDZ and ENRO were greater than 1, we conducted dissipation studies to better understand the fate of the antibiotics under tropical aquaculture conditions.
Dissipation of SDZ, TRIM and ENRO in different semi-field experiment systems
Persistence of SDZ, TRIM and ENRO
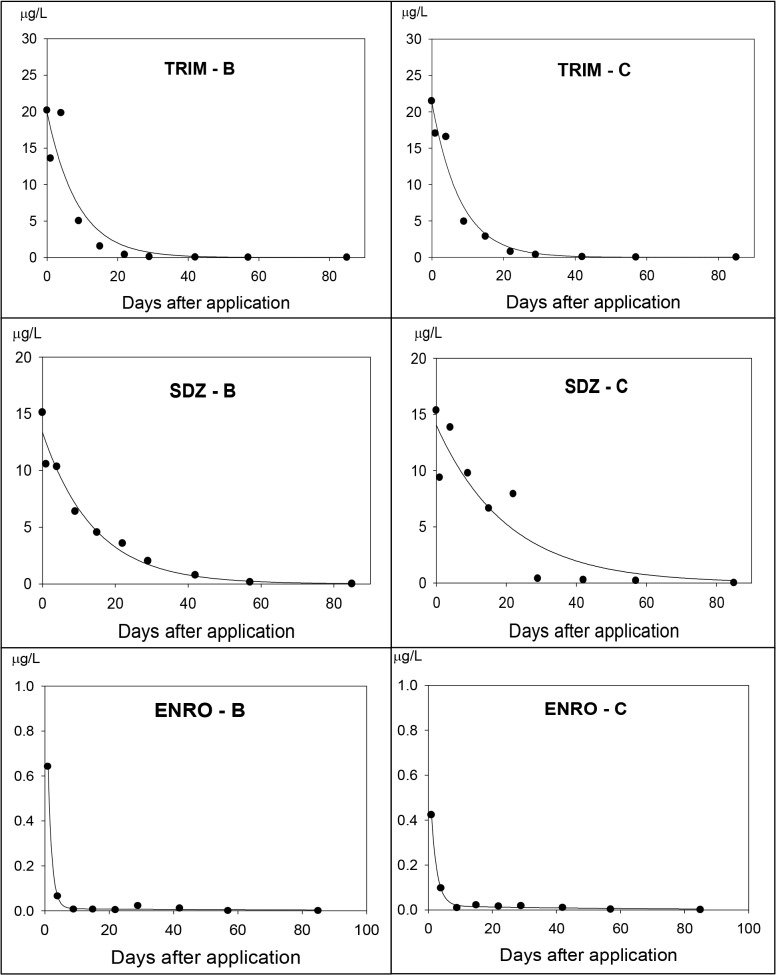
The dissipation of antibiotics was assessed using microcosm systems as described by Laabs et al. ([29], Fig 2). The dissipation profiles of the studied antibiotics followed simple first order kinetics and were fitted to exponential decay equations (see Fig 3 as an exemplary for dissipation curves of the target antibiotics). Exceptions were the dissipation of SDZ in the dark water system (D) and of ENRO in the total water:sediment system under a natural light regime due to a lack of clear temporal trend. For these systems, no dissipation half lives (DT50) were computed. Recovery rates immediately after application (day 0) of SDZ and TRIM in all microcosms varied from 60% to 79% and from 94% to 123%, respectively. In water microcosms, ENRO showed a better recovery rate of 60% (A) and 74% (D) compared with those in water:sediment microcosms where only 32% of the initial ENRO concentration was recovered. This likely reflected the strong sorption of this fluoroquinolone, probably accompanied by the formation of non-extractable residues, as recently reported for difloxacine in soils [14]. Yet, by definition these processes do not hamper the assessment of dissipation rates from the aqueous phase. The respective DT50 times ranged from 0.6 to 44.1 days (both for ENRO; Table 6), i.e, lying either in-between the monthly sampling intervals of the monitoring study or covering only a snapshot of it. In the later case, the monitored background concentrations may be significantly exceeded at selected sampling locations and immediately after application as assumed through the PEC calculation showed in Table 2 (see section Risk assessment). Hence, even if the overall contamination level was low according to commonly accepted risk criteria, this does not exclude the possibility of high local risks for short time intervals.
Fig 3. Dissipation curves of TRIM, SDZ and ENRO in water phase of the total water:sediment systems B (natural light regime) and C (light control).
The concentration scales are different between compounds.
Table 6. Dissipation parameters of SDZ, TRIM and ENRO in different test systems.
| Antibiotics | k | DT50 | DT90 | DT99 | R2 |
|---|---|---|---|---|---|
| day-1 | day | Day | day | ||
| Water phase | |||||
| Natural light system (A) | |||||
| SDZ | 0.1369 | 5.1 | 16.8 | 33.6 | 0.9121 |
| TRIM | 0.0238 | 29.1 | 96.7 * | 193.5 * | 0.9030 |
| ENRO | 1.1093 | 0.6 | 2.1 | 4.2 | 0.9337 |
| Light control system (D) | |||||
| SDZ | - | - | - | - | - |
| TRIM | 0.0228 | 30.4 | 101.0 * | 201.0 * | 0.6898 |
| ENRO | 0.5302 | 1.3 | 4.3 | 8.7 | 0.8407 |
| Water:sediment—natural light system (B) | |||||
| SDZ | 0.0798 | 9.8 | 32.5 | 65.0 | 0.9633 |
| TRIM | 0.1151 | 6.0 | 20.0 | 40.0 | 0.8602 |
| ENRO | 0.7649 | 0.9 | 3.0 | 6.0 | 0.9979 |
| Water:sediment—light control system (C) | |||||
| SDZ | 0.0493 | 14.1 | 46.7 | 93.4 * | 0.8364 |
| TRIM | 0.1265 | 5.5 | 18.2 | 36.4 | 0.9639 |
| ENRO | 0.5558 | 1.2 | 4.1 | 8.3 | 0.9945 |
| Total system | |||||
| Water:sediment—natural light system (B) | |||||
| SDZ | 0.029 | 23.9 | 79.4 | 158.8 * | 0.8566 |
| TRIM | 0.0588 | 11.8 | 39.2 | 78.3 | 0.7989 |
| ENRO | - | - | - | - | - |
| Water:sediment—light control system (C) | |||||
| SDZ | 0.0215 | 32.2 | 107.1 * | 214.2 * | 0.7426 |
| TRIM | 0.0862 | 8.0 | 26.7 | 53.4 | 0.9486 |
| ENRO | 0.0157 | 44.1 | 146.7 * | 293.3 * | 0.6951 |
*: outside of incubation period
- Sulfadiazine (SDZ): in the water phase, SDZ degraded faster in the transparent water system (A, DT50 = 5 days) than in the water:sediment systems (B, DT50 = 10 days; C, DT50 = 14 days). In this semi-field experiment, light was partly blocked by the suspended particles in system B and fully inhibited in system C. The results demonstrate the influence of sunlight on the decomposition rate of SDZ in water. Photodegradation of SDZ in water under laboratory incubation has been documented elsewhere (e.g., [51,52,53]). Dissipation rates of SDZ in total water:sediment systems were slower (B, DT50 = 24 days; C, DT50 = 32 days; Table 6). This finding confirmed the photolytic sensitivity of SDZ with a reduction of half-life in the total water:sediment system exposed to sunlight (B), but at the same time points to higher risks of SDZ for benthic microorganisms than for aquatic ones. Yang et al. [54] reported the influence of biodegradation on SDZ dissipation where the half-lives for SDZ in aerobic nonsterile soils ranged from 12 days to 18 days, while under anoxic conditions the half-lives ranged between 57 days and 237 days. The low Koc and Kow values of SDZ (Table 1) indicate weak sorption, high mobility and bioavailability in soil. However, the high organic matter content in the investigated sediment (S1 Table) also created favorable conditions for a stronger sorption of SDZ to the soil [5], which resulted in a longer dissipation time in the water:sediment microcosms (B and C) compared with water microcosm (A). As a result, in the total water:sediment systems, more than 13% of the spiked concentration of SDZ was still quantified on the last day of the incubation period (day 85).
- Trimethoprim (TRIM): Dissipation profiles again followed exponential decay curves (R2 varied from 0.69 to 0.96). Dissipation behavior of TRIM was different among systems compared with SDZ and ENRO. Photodegradation was unlikely to cause dissipation since there were no significant differences of TRIM concentrations between transparent and dark systems (Table 6). Also in the water phase, degradation rate of TRIM was fairly slow, with half-lives of around 30 days. Consequently, TRIM was the most persistent compound of the three studied antibiotics in the water system, and the 90% and 99% dissipation fractions of TRIM in water lay outside the incubation period. In contrast, a rapid dissipation of TRIM was observed in the water phase of the water:sediment systems (DT50 = 5.5 to 11.8 days; Table 6). As the Koc and Kow values of TRIM are low (Table 1), it can be assumed that biodegradation largely controlled the dissipation of TRIM. A study of Liu et al. [55] also showed a fast decomposition of TRIM under non-sterile conditions.
- Enrofloxacin (ENRO): of the three studied antibiotics, ENRO was the least persistent in water systems (A and D) (Table 6). In transparent water system A, ENRO rapidly decomposed immediately after application (DT50 < 1), and after 4 days of incubation, 99% of the initial spiked concentration had dissipated. Meanwhile in the dark water system D, 50% and 99% of ENRO dissipation fractions were recorded at day 1 and day 9, respectively. Fluoroquinolones have frequently been reported to show low biodegradation in the environment [56], but fast degradation with, e.g., pure fungal cultures (e.g., [16,17]). It is therefore a question of to what degree microorganisms affected the ENRO dissipation process.
The fast degradation of ENRO in system A could be subjected to the influence of light. Particularly under mildly alkaline conditions as prevailing in the test systems (pH value ca. 8, see S1 Fig), photodegradation of ENRO has been shown to be enhanced ([57]). However, photolysis sensitivity of ENRO in the water phase of water:sediment systems was unclear (DT50 recorded at about one day for both system B and C). As described above, it is likely that this did not reflect fast biodegradation but rather rapid sorption of the compound to the sediment phase. In the totally dark water:sediment system (C), ENRO performed as the most persistent antibiotic with a DT50 value of 44 days and the dissipation fractions of 90% and 99% exceeded the incubation time. The work of Boxall et al. [58] has also confirmed the high persistence of ENRO in soils and sediments (DT50 = 152 days). Once bound to sediment, however, the fluorquinolones may not necessarily be easily re-released to affect microbial communities [14]. Andrieu et al. [59] reported that ENRO residue concentrations after application probably did not cause severe toxic effects to exposed aquatic ecosystems.
Application of dissipation equations to natural water systems
At a given sampling point, based on the year-round fluctuation of the measured concentrations, any peak of concentration of an individual antibiotic recorded at a given sampling date would be assigned to the initial concentration (C0) of the dissipation equation. Accordingly, the antibiotic concentration measured in the next sampling month would be equal to the Ct of the respective equation. Predicted antibiotic concentrations based on the computed exponential decay equations were then compared with the measured concentrations in canal water. The findings show that for the site Pangasius 2, the dissipation equations of TRIM in water systems both with and without light control (A and D) predicted almost exactly (r = 0.96, Table 7) the measured concentrations of TRIM in canal water samples collected at points P2a and P2b. Only at sampling point P2c, there were differences between the predicted and measured antibiotic concentrations, which most likely reflected the influence of a more complex water network at that point. Also the low monitored concentrations of ENRO and SDZ at the points P2a and P2b of the site Pangasius 2 were well predicted from the dissipation behavior in the water systems at the sampling dates (Table 7). Predicted antibiotic concentrations in canal water at the Hatchery sites did not correlate with measured concentrations. At these sites, various fingerling species (e.g carp, tench, tilapia, catfish) were cultivated, leading to mixing of the wastewater every 2 or 3 months. There was also no correlation at the Pangasius 1 site, where the fish ponds were mainly discharged to the rice fields before reaching the canal water. Apparently, deviations from the simple prediction of concentrations using the dissipation functions can be seen as an indicator of mixed wastewater sources or indirect discharge in the canal.
Table 7. Comparison between measured concentrations (ng L-1) in canals (P2a and P2b) and predicted concentrations (ng L-1) based on dissipation equations.
| TRIM | Natural light: Ct = C0 e-0.0238t | Light control: Ct = C0 e-0.0228t | ||||||
|---|---|---|---|---|---|---|---|---|
| P2a | P2b | P2a | P2b | |||||
| measured | predicted | measured | predicted | measured | predicted | measured | predicted | |
| C0 | 41 | 41 | 42 | 42 | 41 | 41 | 42 | 42 |
| C24 | 32 | 23 | 29 | 24 | 32 | 24 | 29 | 24 |
| C57 | 11 | 11 | 12 | 11 | 11 | 11 | 12 | 12 |
| C0 | 37 | 37 | 25 | 25 | 37 | 37 | 25 | 25 |
| C34 | 14 | 16 | 16 | 11 | 14 | 17 | 16 | 12 |
| C66 | 1 | 8 | 1 | 5 | 1 | 8 | 1 | 6 |
| Correlation R | 0.96 | 0.96 | ||||||
| Estimated max. conc. | 84 | 86 | 82 | 83 | ||||
| ENRO | Natural light: Ct = C0 e-1.1093t | Light control: Ct = C0 e-0.5302t | ||||||
| P2a | P2b | P2a | P2b | |||||
| measured | predicted | measured | predicted | measured | predicted | measured | predicted | |
| C0 | 59 | 59 | 43 | 43 | 59 | 59 | 43 | 0.043 |
| C24 | 20 | 0 | 0 | 0 | 20 | 0 | 0 | 0 |
| C57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C0 | 19 | 19 | 19 | 0.019 | ||||
| C34 | 0 | 0 | 0 | 0 | ||||
| C66 | 0 | 0 | 0 | 0 | ||||
| C88 | 0 | 0 | 0 | 0 | ||||
| SDZ | Natural light: Ct = C0 e-0.1369t | |||||||
| P2a | P2b | |||||||
| measured | predicted | measured | predicted | |||||
| C0 | 10 | 10 | ||||||
| C24 | 0 | 0 | ||||||
| C0 | 25 | 25 | 9 | |||||
| C40 | 2 | 0 | 0 | |||||
| C0 | 10 | 10 | ||||||
| C34 | 0 | 0 | ||||||
| C66 | 0 | 0 | ||||||
| C88 | 0 | 0 | ||||||
| Estimated max. conc. | 1515 | 580 | ||||||
To estimate the potential maximum antibiotic concentrations that could occur under given environmental conditions, the derived dissipation equations were used to back-calculate the initial antibiotic concentration at application (C0) from the highest concentration of the antibiotic detected at the site during the year (Ct). A maximum of 30 days was assumed between the application of the antibiotic and the sample measurement (t = 30), to give a maximum initial application concentration.
Accordingly, the predicted maximum concentrations in canal water at the day of application of TRIM at the two Pangasius sampling points P2a and P2b was approximately 80 ng L-1; of SDZ was about 1515 ng L-1 at point P2a and 580 ng L-1 at point P2b (Table 7). For ENRO, its fast decomposition under natural water conditions (DT50 < 1) did not allow feasible maximum concentrations within 30 days of the sampling day to be provided. Nevertheless, the high detected concentrations of ENRO in canal water implied that a large amount of ENRO had been used and discharged from the surrounding fish farms close to the sampling date. Those estimated concentrations were lower than the PEC, PNEC and MIC values shown in Table 2 and Table 5. The difference between these extrapolated concentrations and the PECs (derived from worst-case assumption) could be caused by the following reasons: 1) only 30% of the water in the fish ponds was discharged to the environment at the Pangasius site; 2) a portion of the applied antibiotics was taken up by the fish; 3) a fish farm contained several fish ponds, and antibiotics would only be applied to a pond where sick fish might predominate, while the worst-case scenario considered a total amount of antibiotics applied for the entire farm.
In summary, these results confirm the low potential for antibiotic pollution in water sources in the Mekong Delta, which is unlikely to cause an overall risk to aquatic organisms. On the other hand, it is still an open discussion to what degree a long-term and frequent occurrence of lower antibiotic concentration levels may still facilitate the development of bacterial resistance (see [60], for a recent review).
Conclusions
Among the main aquaculture producers in Asia, Vietnam had the highest reported number of different types of antibiotics in use [6]. This study also recorded widespread use of antibiotics in both hatcheries and mature pangasius farms. Local fish farmers’ knowledge of the properties or dosage of used antibiotics was not optimal; antibiotics have also been used for prophylaxes, without worker protection and without exact knowledge about the required dosage. The questionnaires revealed that applications mainly consisted of sulfonamides (like SMX, SDZ), jointly with TRIM, tetracyclines (not studied here), fluorquinolones (ENRO), and penicillins (also not studied here). This study also provides the first large scale background database on the presence of the four different currently used antibiotics SMX, SDZ, TRIM and ENRO in different drinking water sources in the Mekong Delta. The monitoring of surface waters, however, revealed that SMX, SDZ, TRIM and ENRO commonly occurred in the aquatic environment, but their concentrations were generally low in surface water. Concentrations of SMX and TRIM in the areas dominated by fish hatcheries were higher than in the area dominated by mature pangasius culture where wastewater was drained to surrounding rice fields both before being discharged to surrounding water systems (Pangasius 1). Nevertheless, since neither the measured nor the predicted concentrations reached critical PNEC or MIC values in any of the samples, it is suggested that, at least according to these toxicity standards, dilution by rain, river and canal water is sufficient to prevent high concentrations in surface waters.
After application, SDZ, TRIM and ENRO exhibited different dissipation patterns in water and water:sediment systems under tropical climate. The dissipation half-lives ranged from 1 to 44 days, depending on the availability of sunlight and sediment. TRIM was the most persistent antibiotic in water systems and not susceptible to photodegradation. Photolysis, however, affected the dissipation of ENRO and SDZ. While SDZ prevailed to some degree in water:sediment systems, thus being prone to long-distance transport, ENRO showed strong sorption to sediment after application, suggesting that benthic organisms may be more at risk than aquatic ones by these compounds. The good match between monitored and predicted antibiotic concentrations supports the assumption that the semi-field experimental design was suitable for assessing realistic dissipation rates. It helped to validate the models that could be considered as conceptual models for further risk assessment
Despite low concentrations of antibiotics in the canals as well as in the rivers, the large amount of water transported by the Mekong River (457 km3 per year, [61]) results in large overall loads of antibiotics (ENRO ca. 5800 tons, SDZ ca. 1800 tons, SMX 12300 tons, and TRIM ca. 6400 tons per year as a first rough estimate, based on the discharge and concentration detected in the Hau River (CTPS)). Hence, reducing overall loads of antibiotics is still an important policy requirement. Management measures to be considered should focus on 1) reducing fish stocking densities, 2) constructing sedimentation reservoirs for wastewater sedimentation and decomposition, and 3) education programs for local farmers, emphasizing the role of individuals in the context of sustainable aquaculture development, to raise awareness of proper antibiotic use and management, reverse effects from bacterial resistance, and legality of use.
Supporting Information
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors acknowledge the financial support from the Federal Ministry of Education and Research (BMBF), Germany through the Water-related Information System for the Sustainable Development of the Mekong Delta (WISDOM) project in Vietnam. We also would like to thank the Advanced Laboratory, Can Tho University for the use of their facility and their support during sample preparation. We are also grateful to the DONRE of Can Tho and the DONRE of An Giang for their cooperation. We especially would like to thank Ms. Gail Renaud for her English editing support to this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants (0330777 A) from the Federal Ministry of Education and Research, Germany (BMBF, http://www.bmbf.de). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Food and agriculture organization of the United Nations (2012) The state of world fisheries and aquaculture 2012. Available: http://www.fao.org/docrep/016/i2727e/i2727e.pdf
- 2. General Statistics Office (2012) Statistical yearbook of Vietnam 2012 Statistical Publishing House, Vietnam. [Google Scholar]
- 3. Bosma RH, Hanh CTT, Potting J (2009) Environmental impact assessment of the pangasius sector in the Mekong Delta Wageningen University, Wageningen, The Netherlands. [Google Scholar]
- 4. Lam PT, Tam BM, Thuy TTN, Gooley GJ, Ingram BA, Hao NV, et al. (2009) Current status of farming practices of striped catfish, Pangasianodon hypophthalmus in the Mekong Delta, Vietnam. Aquaculture 296: 227–236. [Google Scholar]
- 5. Thiele-Bruhn S (2003) Pharmaceutical antibiotic compounds in soils—a review. Journal of Plant Nutrition and Soil Science 166: 145–167. [Google Scholar]
- 6. Rico A, Satapornvanit K, Haque MM, Min J, Phuong NT, Telfer TC, et al. (2012) Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Reviews in Aquaculture 4: 1–19. [Google Scholar]
- 7. Tuan XL and Munekage Y (2004) Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Vietnam. Marine Pollution Bulletin 49: 922–929. [DOI] [PubMed] [Google Scholar]
- 8. Managaki S, Murata A, Takada H, Tuyen BC, Chiem NH (2007) Distribution of macrolides, sulfonamides and trimethoprim in tropical waters: ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environmental Sciences & Technology 41: 8004–8010. [DOI] [PubMed] [Google Scholar]
- 9. General Statistics Office (2008) Results of the survey on household living standards 2008 Statistical publishing house, Vietnam. [Google Scholar]
- 10. Reis N and Mollinga PP (2012) Water supply or ‘Beautiful latrines’? Microcredit for rural water supply and sanitation in the Mekong Delta, Vietnam. ASEAS–Austrian Journal of South-East Asian Studies 5(1): 10–29. [Google Scholar]
- 11. Toan PV, Sebesvari Z, Bläsing M, Rosendahl I, Renaud FG (2013) Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Science of the Total Environment 452–453: 28–39. 10.1016/j.scitotenv.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 12. Wilbers GJ, Becker M, Nga LT, Sebesvari Z, Renaud FG (2014) Spatial and temporal variability of surface water pollution in the Mekong Delta, Vietnam. Science of the Total Environment 485–486: 653–665. 10.1016/j.scitotenv.2014.03.049 [DOI] [PubMed] [Google Scholar]
- 13. Forster M, Laabs V, Lamshoft M, Groeneweg J, Zuhlke S, Spiteller M, et al. (2009) Sequestration of manure-applied sulfadiazine residues in soils. Environmental Science & Technology 43: 1824–1830. [DOI] [PubMed] [Google Scholar]
- 14. Rosendahl I, Siemens J, Kindler R, Groeneweg J, Zimmermann J, Czerwinski S, et al. (2012) Persistence of the fluoroquinolone antibiotic difloxacin in soil and lacking effects on N-turnover. Journal of Environmental Quality 41: 1275–1283. 10.2134/jeq2011.0459 [DOI] [PubMed] [Google Scholar]
- 15. Jessick AM, Moorman TB, Coats JR (2013) Fate of erythromycin in sediment-containing surface water microcosms: how does aged erythromycin in sediment influence bioavailability? In: Cobb GB and Smith PN, editors. Evaluating veterinary pharmaceutical behavior in the environment. ACS Symposium Series 1126: 161–178. [Google Scholar]
- 16. Wetzstein H- G, Schmeer N, Karl W (1997) Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus gloeophyllum striatum: Identification of metabolites. Applied and Environmental Microbiology 63: 4272–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wetzstein H- G, Stadler M, Tichy H- V, Dalhoff A, Karl W (1999) Degradation of ciprofloxacin by basidiomycetes and identification of metabolites generated by the brown rot fungus gloeophyllum striatum. Applied and Environmental Microbiology 65 (4): 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sukul P and Spiteller M (2007) Fluoroquinolone antibiotics in the environment. In: Ware GW, Nigg HN, Doerge DR, editors. Reviews of Environmental Contamination and Toxicology 191: 131–162. [DOI] [PubMed] [Google Scholar]
- 19. Bjorklund NV, Rabergh CMI, Bylund G (1991) Residues of oxolinic acid and oxytetracycline in fish and sediments from fish farms. Aquaculture 97: 85–96. [Google Scholar]
- 20. Coyne R, Hiney M, O’Connorb B, Kerry J, Cazabona D, Smith P (1994) Concentration and persistence of oxytetracycline in sediments under a marine salmon farm. Aquaculture 123: 31–42. [Google Scholar]
- 21. Nepejchalova L, Svobodova Z, Kolarova J, Frgalova K, Valova J, Nemethova D (2008) Oxytetracycline assay in pond sediment. Acta Veterinaria Brno 77: 461–466. [Google Scholar]
- 22. Phong TK, Nhung DTT, Hiramatsu K, Watanabe H (2009) Prediction of the Fate of oxytetracycline and oxolinic acid in a fish pond using simulation model—A preliminary study. Journal of the Faculty of Agriculture, Kyushu University 54 (2): 513–521. [Google Scholar]
- 23.Chau NDG, Sebesvari Z, Amelung W, Renaud F (2015) Pesticide pollution of multiple drinking water sources in the Mekong Delta, Vietnam: evidence from two provinces. Environmental Science and Pollution Research. 10.1007/s11356-014-4034-x [DOI] [PubMed]
- 24. Ciarlone AE, Fry BW, Ziemer DM (1990) Some observations on the adsorption of tetracyclines to glass and plastic labware. Microchemical Journal 42(2): 250–255. [Google Scholar]
- 25. Dalkmann P, Broszat M, Siebe C, Willaschek E, Sakinc T, Huebner J (2012) Accumulation of pharmaceuticals, enterococcus, and resistance genes in soils irrigated with wastewater for zero to 100 years in central Mexico. PLoS ONE 7(9): e45397 10.1371/journal.pone.0045397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drugbank (2013). Available: http://www.drugbank.ca/drugs/DB00359
- 27. Li ZH, Randak T (2009) Residual pharmaceutically active compounds (PhACs) in aquatic environment—status, toxicity and kinetics: a review. Veterinarni Medicina 52(7): 295–314. [Google Scholar]
- 28.University of Hertfordshire (2011) VSDB: Veterinary substances database. Last updated 2011. Available: http://sitem.herts.ac.uk/aeru/vsdb/1745.htm
- 29. Laabs V, Wehrhan A, Pinto A, Dores E, Amelung W (2007) Pesticide fate in tropical wetlands of Brazil: an aquatic microcosm study under semi-field conditions. Chemosphere 67: 975–989. [DOI] [PubMed] [Google Scholar]
- 30. Gobel A, Thomsen A, McArdell CS, Alder AC, Giger W, Theiß N, et al. (2005) Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. Journal of Chromatography A 1085 (2): 179–189. [DOI] [PubMed] [Google Scholar]
- 31. Golet EM, Strehler A, Alder AC, Giger W (2002) Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Analytical Chemistry 74: 5455–5462. [DOI] [PubMed] [Google Scholar]
- 32.Dung TT, Ngoc NTN, Thinh NQ, Thy DTM, Tuan NA, Shinn A, et al. (2008) Common diseases of pangasius catfish farmed in Vietnam. Global Aquaculture Advocate: 77–78. Available: http://cenres.ctu.edu.vn/Publication/Tai%20lieu%20xuat%20ban%20quoc%20te%20nam%202008/07_tapchiquocte_2008_Nhu%20Ngoc.pdf
- 33.Serrano PH (2005) Responsible use of antibiotics in aquaculture. FAO Fisheries Technical Paper 469. Rome, 97p.
- 34. TT-BNN (2009) Circular to promulgate the list of drugs, chemicals, antibiotics banned for uses, limited to use Ministry of Agriculture and rural development, Vietnam: Available: http://www.spsvietnam.gov.vn/Lists/VBPQ_EN/Attachments/480/15-2009-TT-BNN_Eng.pdf [Google Scholar]
- 35. Phuong NT, Oanh DTH (2009) Striped catfish (Pangasianodon hypophthalmus) aquaculture in Viet Nam: an unprecedented development within a decade In: De Silva SS, Davy FB, editors. Success stories in Asian aquaculture. Springer, NACA and IDRC, Dordrecht, Bangkok and Ottawa, pp. 133–149. [Google Scholar]
- 36. Carballo E, van Eer A, van Schie T, Hilbrands A (2008) Small-scale freshwater fish farming Agrodok 15. Agromisa Foundation and CTA, Wageningen, 84p. [Google Scholar]
- 37. Riviere JE, Papich MG (2009) Sulfonamides and potentiated sulfonamides In: Riviere JE and Papich, editors. Veterinary Pharmacology and Therapeutics 9th edition, pp. 835–860. [Google Scholar]
- 38. Lin AY, Yu TH, Lin CF (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan. Chemosphere 74: 131–141. 10.1016/j.chemosphere.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 39. Wang X, He X, Chen B, Xie C (2011) Rice field for the treatment of pond aquaculture effluents. African Journal of Biotechnology 10(34): 6456–6465. [Google Scholar]
- 40. Trieu TTN, Lu M (2014) Estimates of nutrient discharge from striped catfish farming in the Mekong River, Vietnam, by using a 3D numerical model. Aquaculture International 22: 469–483. [Google Scholar]
- 41. GSO (General Statistics Office) (2014) Statistical yearbook of Vietnam 2013 Statistical Publishing House, Vietnam. [Google Scholar]
- 42.U. S. Environmental Protection Agency (2011) Finalization of guidance on incorporation of water treatment effects on pesticide removal and transformations in drinking water exposure assessments. Available: http://www.epa.gov/pesticides/science/efed/policy_guidance/team_authors/water_quality_tech_team/wqtt_dw_treatment_effects_removal_transformation.htm
- 43. Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, et al. (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Science of the Total Environment 473–474: 619–641. 10.1016/j.scitotenv.2013.12.065 [DOI] [PubMed] [Google Scholar]
- 44. Koschorreck J, Koch C, Ronnefahrt I (2002) Environmental risk assessment of veterinary medicinal products in the EU—a regulatory perspective. Toxicology Letters 131: 117–124. [DOI] [PubMed] [Google Scholar]
- 45.European Commission (2003) Technical guidance document on risk assessment part II, pp:99–102. Available: https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf
- 46. Salmon SA, Watts JL (2000) Minimum inhibitory concentration determinations for various antimicrobial agents against 1570 bacterial isolates from Turkey poults. Avian diseases 44: 85–98. [PubMed] [Google Scholar]
- 47. Wust J, Wilkins TD (1978) Susceptibility of anaerobic bacteria to sulfamethoxazole/trimethoprim and routine susceptibility testing. Antimicrobial agents and chemotherapy 14(3): 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiggins GL, McLaughlin JV, Bickham ST, Jones WL, Balows A (1970) Susceptibility of Neisseria meningitidis strains from the civilian population to sulfadiazine, penicillin, and rifampin. Applied microbiology 20(6): 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ciglasch H, Busche J, Amelung W, Totrakool S, Kaupenjohann M (2006) Insecticide dissipation after repeated field application to a northern Thailand. Journal of Agricultural and Food Chemistry 54: 8551–8559. [DOI] [PubMed] [Google Scholar]
- 50. Rosendahl I, Laabs V, Ahowe CA, James B, Amelung W (2009) Insecticide dissipation from soil and plant surfaces in tropical horticulture of southern Benin, West Africa. Journal of Environmental Monitoring 11:1157–1164. 10.1039/b903470f [DOI] [PubMed] [Google Scholar]
- 51. Boreen AL, Arnold WA, McNeill K (2004) Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environmental Science and Technology 38: 3933–3940. [DOI] [PubMed] [Google Scholar]
- 52. Perisa M, Babic S, Skoric I, Fromel T, Knepper TP (2013) Photodegradation of sulfonamides and their N4-acetylated metabolites in water by simulated sunlight irradiation: kinetics and identification of photoproducts. Environmental Science and Pollution Research 20: 8934–8946. 10.1007/s11356-013-1836-1 [DOI] [PubMed] [Google Scholar]
- 53. Lemanska-Malinowska N, Felis E, Surmacz-Gorska J (2013) Photochemical degradation of sulfadiazine. Archives of environmental protection 39(3): 79–91. [Google Scholar]
- 54. Yang JF, Ying GG, Yang LH, Zhao JL, Liu F, Tao R, et al. (2009) Degradation behavior of sulfadiazine in soils under different conditions. Journal of Environmental Science and Health Part B 44: 241–248. [DOI] [PubMed] [Google Scholar]
- 55. Liu F, Ying GG, Yang JF, Zhou LJ, Tao R (2010) Dissipation of sulfamethoxazole, trimethoprim and tylosinin a soil under aerobic and anoxic conditions. Environmental Chemistry 7: 370–376. [Google Scholar]
- 56. Alexy R, Kumpel T, and Kummerer K (2004) Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere 57: 505–512. [DOI] [PubMed] [Google Scholar]
- 57. Dolar D, Kosutic K, Perisa M, Babic S (2013) Photolysis of enrofloxacine and removal of its photodegradation products from water by reverse osmosis and nanofiltration membranes. Separation and Purification Technology 115: 1–8. [Google Scholar]
- 58. Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, Levy LS (2006) Uptake of veterinary medicines from soils into plants. Journal of Agricultural and Food Chemistry 54(6): 2288–2297. [DOI] [PubMed] [Google Scholar]
- 59. Andrieu M, Rico A, Phu TM, Huong DTT, Phuong NT, van den Brink PJ (2015) Ecological risk assessment of the antibiotic enrofloxacin applied to Pangasius catfish farms in the Mekong Delta, Vietnam. Chemosphere 119: 407–414. 10.1016/j.chemosphere.2014.06.062 [DOI] [PubMed] [Google Scholar]
- 60. Jechalke S, Heuer H, Siemens J, Amelung W, Smalla K (2014) Fate and effects of veterinary antibiotics in soil. Trends in Microbiology 22(9): 536–545. 10.1016/j.tim.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 61.Mekong River Commission (2009) The flow of the Mekong. MRC Management Information booklet series 2009. Available: http://www.mrcmekong.org/assets/Publications/report-management-develop/MRC-IM-No2-the-flow-of-the-mekong.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.