Abstract
Cryostorage is of immense interest in biomedical research, especially for stem cell-based therapies and fertility preservation. Several protocols have been developed for efficient cryopreservation of cells and tissues, and a combination of dimethyl sulfoxide (DMSO) and fetal bovine serum (FBS) is commonly used. However, there is a need for an alternative to FBS because of ethical reasons, high cost, and risk of contamination with blood-borne diseases. The objective of the present study was to examine the possibility of using buffalo (Bubalus bubalis) ocular fluid (BuOF) to replace FBS in cryomedia. Frozen–thawed cells, which were cryopreserved in a cryomedia with BuOF, were assessed for viability, early and late apoptosis, and proliferation. Three cell lines (CHO, HEK, and C18-4), mouse embryonic stem (mES) cells, and primary cells, such as mouse embryonic fibroblast (MEF) cells, human peripheral blood mononuclear cells (hPBMCs), and mouse bone marrow cells (mBMCs), were cryopreserved in cryomedia containing 10% DMSO (D10) with 20% FBS (D10S20) or D10 with 20% BuOF (D10O20). For all three cell lines and mES cells cryopreserved in either D10S20 or D10O20, thawed cells showed no difference in cell viability or cell recovery. Western blot analysis of frozen–thawed-cultured cells revealed that the expression of Annexin V and proliferating cell nuclear antigen (PCNA) proteins, and the ratio of BAX/BCL2 proteins were similar in all three cell lines, mES cells, and hPBMCs cryopreserved in D10S20 and D10O20. However, initial cell viability, cell recovery after culture, and PCNA expression were significantly lower in MEF cells, and the BAX/BCL2 protein ratio was elevated in mBMCs cryopreserved in D10O20. Biochemical and proteomic analysis of BuOF showed the presence of several components that may have roles in imparting the cryoprotective property of BuOF. These results encourage further research to develop an efficient serum-free cryomedia for several cell types using BuOF.
Introduction
Cryopreservation is a technique that enables preservation of structure and function of cells and tissues by preventing or minimizing damage. Cryopreserved cells and tissues can be stored for prolonged periods in limited space at ultra-low temperatures and require less maintenance [1]. Stem cells are used in transplantation and cell-based therapies, which makes these cells a promising tool for regenerative and biomedical research. However, stem cells have a limited lifespan [2], and with increase in the number of passages, their proliferative capacity and differentiation decrease [3, 4]. Embryonic stem (ES) cells are derived from the inner cell mass of blastocyst-stage embryoswith the potential to self-renew and differentiate into different cell lineages [5]. Mouse ES cells are used in various research applications including gene targeting, which helpsgeneratetarget mutant mouse models for functional genomic studies and thereputic applications [6]. Spermatogonial stem cells (SSCs)isolated from donor mice repopulated and produced mature spermatozoa in recipient mouse testes [7]. Maintenance of proliferative and differentiation capacity of ES cells and SSCs when subjected to cryopreservation could extend their applications for regenerative therapy and restoration of fertility in cancer patients who are rendered infertile because of the gonadotoxic effects of chemotherapy and radiotherapy [6].
Several established cell lines of different origins are routinely used for drug assays and gene expression analyses [8]. Various primary cells are also routinely used in biological research. Mouse embryonic fibroblast (MEF) cells are used as feeder cells to maintain ES cells and induced pluripotent stem (iPS) cells in the undifferentiated state [9, 10]. Peripheral blood mononuclear cells (PBMCs) are commonly used for prospective phenotypic and functional analyses in a wide range of infectious disease and clinical vaccine studies [11]. PBMCs are used to develop effective human immunodeficiency virus1 vaccines and infer effector function and cellular or humoral immune responses [12]. Mouse bone marrow cells (mBMCs) are used to study angiogenesis inhibitor drugs in cancer treatment [13, 14]. These cells have potential applications in hematopoietic stem cell transplantation research [15] and regenerative medicine [16]. Each cell type differs in structure and cell membrane composition because they respond differently to cryopreservation. Culture age at the time of freezing and cultivation procedures may also impact freezing success [17]. Current widespread increase in the application of cell culture methods to various areas of biology and medicine emphasizes the need for contamination-free and efficient cell cryopreservation.
During cryopreservation, cellular injury primarily occurs when water, which is an important cell constituent, freezes at ultralow temperatures. Cellular injury can be minimized by adding cryoprotective agents (CPAs). Dimethyl sulfoxide (DMSO) is a widely used permeable CPAs in cell and tissue cryopreservation because of its low toxicity [18]. Fetal bovine serum (FBS) is a commonly used non-permeable CPA that is combined with DMSO for several cell lines and tissues because it supports better cell recovery. However, there is a need to find a replacement for FBS as a cryopreservative because it is expensive. Furthermore, the methods for harvesting blood are inhumane because FBS is harvested from bovine fetuses taken from pregnant cows during slaughter by means of cardiac puncture without any anesthesia [19, 20]. Use of animal serum is also associated with a risk of contamination with viruses [21] and prions as well as possible disease transmission [22], some of which are impossible to remove from serum. Some of these infectious agents, such as bacteria and viruses, are even capable of surviving at low temperatures that are routinely used for cell stock storage (-160°C) [23, 24]. Although FBS is indispensable in biomedical research, FBS-free cryomedia would benefit researchers by conforming to good laboratory practices.
Several alternatives to serum in cryomedia have been considered. Sericin is a protein hydrolysate that is rich in serine and obtained from raw silk during the degumming process. Expression of sericin in Escherichia coli has been shown to prevent freezing stress and promote cell viability [25]. A medium that contained sericin-cryopreserved Chinese-hamster ovary (CHO) and P3UI myeloma cells was as efficient as the conventional FBS-containing medium and superior to several commercial serum-free freezing media[26]. Similarly, replacement of FBS with bovine serum albumin (BSA) in cryomedia showed no significant difference in cell recovery and viability of peripheral blood mononuclear cells [27]. However, the extremely high costs of sericin and BSA limit their use, especially in developing countries.
Bovine ocular fluid (BOF) alone and in combination with sheep plasma and human serum albumin has been evaluated as a serum replacement for different cells [28]. BOF has several active components that promote cell growth, such as vascular endothelial growth factor [29], the 21-KDa acidic-Ca-binding protein [30], insulin like growth factor, hypoxanthine, and fibronectin [31]; however, it does not support cell growth on its own. In addition, ocular fluid has several proteins, including albumin [32], which may act as non-permeable CPAs. Collection of buffalo ocular fluid (BuOF) is feasible in India because there is no religious taboo regarding buffalo slaughter, and buffalo eyes are available in abundance as a slaughter house by-product. The price of BuOF is approximately 7–8-fold lower than that of FBS (in Indian scenario), and aseptic collection of BuOF is possible because eyes are enclosed organs. However, the effect of BuOF on cell cryopreservation has not been evaluated to date.
The aim of the present study was to evaluate if BuOF can replace FBS for cell cryopreservation. We also evaluated the composition of BuOF to identify the component(s) that may play a key role in imparting cryoprotective ability.
Materials and Methods
Collection of BuOF
All animal procedures were approved by the Institute Animal Ethics Committee (IAEC) of Centre for Cellular and Molecular Biology (CCMB), Hyderabad, India. Intact eyeballs from healthy Murrah male calves (n = 12; age, 6–8 months) were collected from the Municipal Slaughter House, Hyderabad, India and transported in phosphate buffered saline (PBS; Invitrogen) on ice. After arrival to the laboratory within 1 h of slaughter, ocular muscles and the optic nerve were trimmed from the eyeballs. After rinsing with ice cold 50% alcohol and PBS several times, the cornea of the eyeball was carefully punctured with a 22-gauge needle, and aqueous humor was collected. The posterior chamber of the eyeball was then cut open using a sterile surgical blade, and the vitreous humor was collected using a 10-ml syringe. Collected aqueous and vitreous humor were pooled from all animals in a given trial (n = 3), centrifuged at 775 × g (15 min at 4°C), and then filtered through 0.45-μm and 0.22-μm filters. The sterile BuOF was aliquoted into cryovials (Nunc) and stored at -30°C until use.
Biochemical analysis of BuOF and FBS
Differences in biochemical composition of BuOF and FBS were determined by a medical diagnostic agency (Vijaya Diagnostics; www.vijayadiagnostic.com). A total of three samples each for FBS (Gibco; origin, United States; lot numbers; 494515, 816712, and 835987 respectively) and pooled BuOF were used for analysis. The mean value for each analyzed biochemical and the methods used for analysis are listed in Table 1.
Table 1. Comparative biochemical analysis of BuOF and FBS.
| Parameters (mg/dl) | Method used for analysis | BuOF | FBS | |
|---|---|---|---|---|
| 1 | Total Protein | Biuret | 0.5 ± 0.03 | 3.25 ± 0.23 |
| 2 | Albumin | Bromocresol green (BCG) | 0.2 ± 0.09 | 1.85 ± 0.32 |
| 3 | Globulin | Biuret and BCG | 0.3 ± 0.01 | 1.4 ± 0.2 |
| 4 | Lipoprotein A | Particle enhanced Immunoturbidimetry | 1.8 ± 0.1 | 2.5 ± 0.2 |
| 5 | Triglycerides | Glycerol-3-phosphate oxidase- phenol aminophenazone (GPO-PAP)colorimetry | 13 ± 0.9 | 61 ± 1.4 |
| 6 | Total Cholesterol | (Cholesterol oxidase- peroxidase enzyme) isotope dilution (CHO-POD) IDMS mass spectroscopy | 22.5 ± 1 | 27.5 ± 1.02 |
| 7 | LDL Cholesterol | Enzymatic immunoinhibition | 14 ± 0.9 | 8 ± 0.8 |
| 8 | HDL Cholesterol | (Total cholesterol)–(HDL + VLDL cholesterol) | 6 ± 0.7 | 8 ± 0.6 |
| 9 | VLDL Cholesterol | (TG /5) | 2.6 ± 0.5 | 12.5 ± 0.8 |
| 10 | Glucose | Hexokinase | 56.5 ± 2.3 | 77 ± 4.1 |
| 11 | Ascorbic acid | Spectrophotometry | 7.27 ± 0.7 | 1.13 ± 0.2 |
Values are represent in mean ± SEM.
Cell lines, derivation of primary cells, and cell culture
Derivation of cells from mice was approved by IAEC (permit numbers IAEC 52/2014 and 4/2015). For isolation of human peripheral blood mononuclear cells (hPBMCs), approval was obtained from Institution Ethics Committee (IEC) of CCMB, Hyderabad, India (permit number IEC 35/2015). All reagents were purchased from Invitrogen unless otherwise specified. Cell lines including Chinese hamster ovary cells (CHO-K1ATCC, CCL-61), mouse embryonic stem (mES) cells-R1 (ATCC, SCRC-1011), and human embryonic kidney cells (HEK-293T/17ATCC, CRL-11268) were purchased from American Type Culture Collection (ATCC). The immortalized C18-4 mouse type-A spermatogonia cell line [33] was a gift from Dr. Marie-Claude Hofmann (The University of Texas MD Anderson Cancer Center, Houston, TX, USA). Human PBMCs were isolated as previously described [27] from the blood collected from healthy volunteers of either sex after obtaining written consent (n = 9). Both the MEF cells [34] and mBMCs [35] were derived from C57BL/6 mice (n = 6) as previously described. All adherent cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with high glucose and suspension cells in Roswell Park Memorial Institute (RPMI) 1640 medium. Both media were supplemented with10% heat-inactivated FBS, 1 × non-essential amino acid solution, and 1 × antibiotic–antimycotic solution, and cells were cultured in a 5% CO2 environment at 37°C. Fresh confluent adherent cells after 24 h culture and fresh suspension cells after 72 h culture were considered as the control group.
Cell cryopreservation
The uncontrolled slow freezing protocol was used for cell cryopreservation. Cryomedia for freezing consisted of DMEM/F12-HEPES that contained 10% DMSO (Sigma) alone (D10) and with 20% FBS (v/v) (D10S20) or 20% BuOF (v/v) (D10O20). In each 2-ml cryovial, cell suspension (1×107cells) was added to 1ml of cryomedium and equilibrated for 30 min on ice. The cryovials were placed in an isopropyl alcohol container (Mr. Frosty Freezing Container; Thermo Scientific) and kept in a -80°C freezer (Thermo Scientific). Cells were cooled at an uncontrolled rate of approximately 1°C/min following the manufacturer’s protocols. After 24 h, cryovials were transferred to liquid nitrogen for storage.
Cell thawing
After 1month, the cells were thawed by swirling the cryovials in a 37°C water bath until the contents were completely melted. The thawed content was transferred to a15-ml tube that contained 10-ml DMEM/F12-HEPES supplemented with 10% FBS. The content in the tube was gently mixed, centrifuged at 200 × g for 5 min, and the cell pellet was either suspended in DMEM/high glucose or RPMI 1640 medium with 10% FBS depending on cell type. Cell viability was determined before seeding the cells for culture.
Cell viability and cell recovery assay
Cell viability was assessed immediately after thawing and after 24 h of culture (adherent cells) and 72 h culture (suspension cells). Cell viability was determined by trypan blue dye (Sigma) exclusion analysis. Microscopic evaluation was carried out no later than 10 min after the end of incubation. Approximately 500 cells/group were counted for cell viability analysis.
For cell recovery assay, the cells were seeded according to the post-thaw viability in each group. Adherent cells were cultured in 100-mm culture dishes and suspension cells in 75-mm2 culture flasks (both from TPP) at a density of 2 × 105 live cells/cm2 using the above-mentioned medium and culture conditions. After 24 h, the adhered cells were washed twice with PBS, and the cells were harvested by trypsin (0.25%) digestion. The suspension cells were collected after 72 h of culture by pelleting down at 200 × g for 5 min. The recovered cells were assessed for viability and counted to estimate cell recovery [27] in each cryopreservation group.
Western blot analysis
Frozen–thawed adherent cells cultured for 24 h and suspension cells cultured for 72 h were assessed for the expression of apoptosis and cell proliferation-specific proteins. Total proteins were extracted upon homogenization by sonication in a dissolving buffer (7M urea, 2M thiourea, 4% CHAPS [3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate], 18mM Tris-HCl, 14mM Tris-Base, 0.2% Triton-X, and 50mM dithiothreitol). Single strength ProteCEASE-50, which is an ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor (G-Biosciences) was added to dissolving buffer before protein extraction. Lysed samples (30 μg) were subjected to 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The gels were transferred onto a polyvinylidenedifluoride (PVDF) membrane (Millipore). The membranes were blocked with Starting Block (TBS) Blocking Buffer (Life Technologies) for 1 h at room temperature. All primary and secondary antibodies were purchased from Thermo Scientificunless otherwise specified. The blocked membranes were incubated with the following primary antibodies to evaluate the expression of an early apoptotic protein, Annexin V, 1:1000 (Santa Cruz Biotechnology); a cell proliferation protein, proliferating cell nuclear antigen (PCNA), 1:1000; pro-apoptotic protein, BCL2-associated X protein (BAX), 1:200; and anti-apoptotic protein, B-cell lymphoma 2 (BCL2), 1:1000. To control protein loading on the gels, the membranes were probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 1:1000 antibody. The membranes were then washed with TBS-T and incubated in goat anti-mouse or goat anti-rabbit HRP-conjugated secondary antibody (1:10000) in TBS for 1 h at room temperature. After washing with TBS-T, immune reactivity was revealed by chemiluminescence using a C-DiGit Blot Scanner (Licor) against Super Signal West Femto Chemiluminescent Substrate (Thermo Scientific), and the generated signal was analyzed using a densitometer. Signal from each antibody was normalized to that of GAPDH for each cryopreservation group.
Proteomic analysis
Total protein from BuOF and FBS samples was extracted using dissolving buffer (7M urea, 2M thiourea, 4% CHAPS, 18mM Trizma base, 2 tablets of EDTA protease inhibitor, 0.2% Triton X, and 50mM DTT). The extracted proteins were then quantified using the amido black assay [36]. One hundred micrograms of each sample in 2 × lamelli buffer (20% glycerol, 4% SDS, 10% 2-mercaptoethanol, 0.004% bromophenol blue, and 0.125M Tris HCl) was subjected to 12% SDS-PAGE in duplicate. The gel was stained in CBB R-250 (Coomassie brilliant blue R-250; Bio Rad) overnight and then de-stained and documented. Each of the electrophoresed sample lanes were divided into five fractions, and each fraction was further processed into finer, 1.5-mm pieces. Each of the fractions was washed in 40mM ammonium bicarbonate in 50% acetonitrile (ACN) for 1 hand twice with water for 1 h, followed by dehydration with 100% ACN. Each fraction was then digested for 18 h with 40μl of sequencing-grade trypsin (10 ng/μl; Promega). The digested peptides (100 μl) were extracted using 0.1% trifluoroacetic acid (TFA) in 50% ACN. The respective samples for each fraction were pooled, desalted, and then vacuum dried. The peptides were then reconstituted in 20 μl of 5% ACN and 0.1% formic acid. The trypsin-digested peptides were subjected to tandem mass spectrometry (MS/MS) using an Orbitrap Nano analyzer (Thermo Scientific). The samples were run in duplicate in collision-induced dissociation mode for 1 h each. The proteins were identified from the obtained MS/MS peak list searched against the Bos taurus database.
Statistical analyses
Data were pooled from each trial for analysis. The results are presented as mean ± SEMof four trials for a cell type in each group. Statistical analysis was conducted using an analysis of variance (ANOVA). Significant differences between the means were determined by analyzing the data with the Fisher’s Least Significance Difference (LSD) test. The level of significance was set at P < 0.05.
Results
Biochemical compositions of BuOF and FBS
The biochemical compositions of BuOF and FBS are shown in Table 1. Most of the components were several-fold higher in FBS except for low-density lipoprotein (LDL) cholesterol and ascorbic acid, which were higher in BuOF.
Post-thaw cell viability, cell recovery, and cell viability after culture
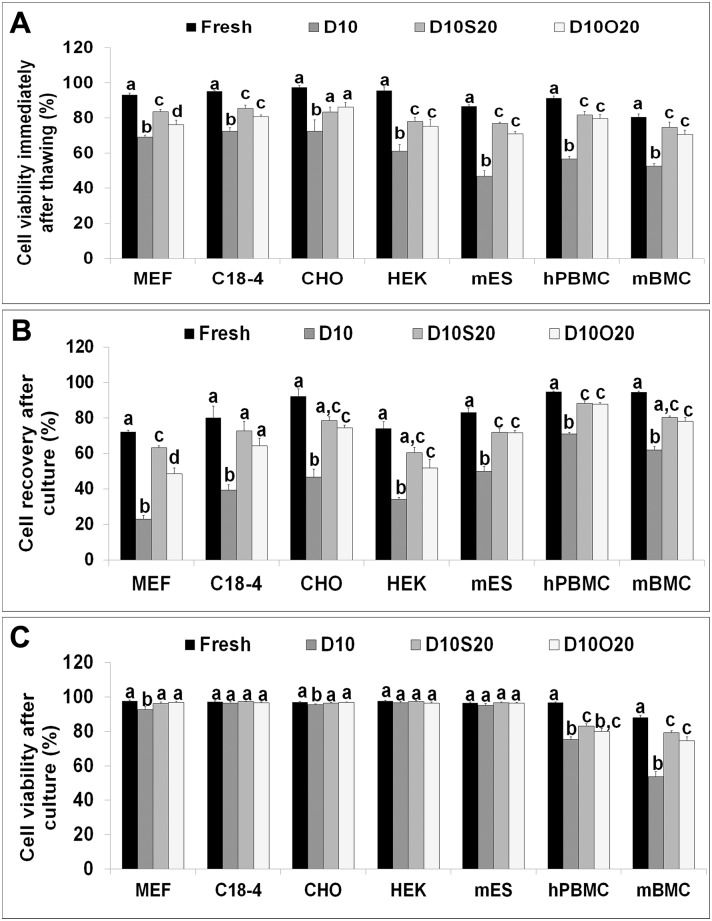
Viability of cells was determined by trypan blue dye exclusion immediately after thawing (Fig 1A). The post-thaw viability of cells in all three adherent cell lines, mES cells, hPBMCs, mBMCs, and MEF cells frozen in D10 was significantly lower than that of fresh cells and cells frozen in D10S20 and D10O20. The viability of C18-4 cells, HEK cells, mES cells, hPBMCs, and mBMCs frozen in D10O20 was significantly lower than that of fresh cells but similar to that of cells frozen in D10S20. However, viability of CHO cells frozen in D10O20 was similar to that of both fresh cells and cells frozen in D10S20. Viability of MEF cells frozen in D10O20 was significantly lower than that of fresh cells and cells frozen in D10S20.
Fig 1. Cell viability and cell recovery in fresh and frozen–thawed cells.
(A) Cell viability of fresh and frozen-thawed cells as determined by trypan blue dye exclusion immediately after thawing. (B) Percentage of cell recovery of frozen–thawed cells after 24 h (MEF, C18-4, CHO, HEK, and mES cells) and 72 h (hPBMCs and mBMCs) of culture. (C) Cell viability as determined by trypan blue dye exclusion after 24 h (MEF, C18-4, CHO, HEK, and mES cells) and 72h (hPBMCs and mBMCs) of culture. Data represent mean ± SEM off our trials for each cell type in each group. Bars with different letters are significantly different at P < 0.05.
Frozen–thawed adherent cells 24 h and suspension cells 72 h after culture were evaluated to determine cell recovery (Fig 1B). There was a significant decline in cell recovery from D10, regardless of cell type; however, the cell recovery was similar in both D10S20 and D10O20 in all three adherent cell lines, mES cells, hPBMCs, and mBMCs. The recovery of C18-4 cells frozen in D10O20 was also similar to that of fresh cells. Fewer MEF cells were recovered in both D10S20 and D10O20 compared with fresh cells, and fewer were recovered in D10O20 compared with D10S20.
Cell viability of all cell types was also determined after culture (Fig 1C). Viability of C18-4, HEK, and mES cells was similar to that of fresh cells in all cryopreserved groups including D10. The viability of MEF cells, CHO cells, hPBMCs, and mBMCs frozen in D10 was significantly lower than that of fresh cells and cells frozen in D10S20 and D10O20. The viability of hPBMCs and mBMCs frozen in D10O20 was significantly lower than that of fresh cells but similar to that of cells frozen in D10S20.
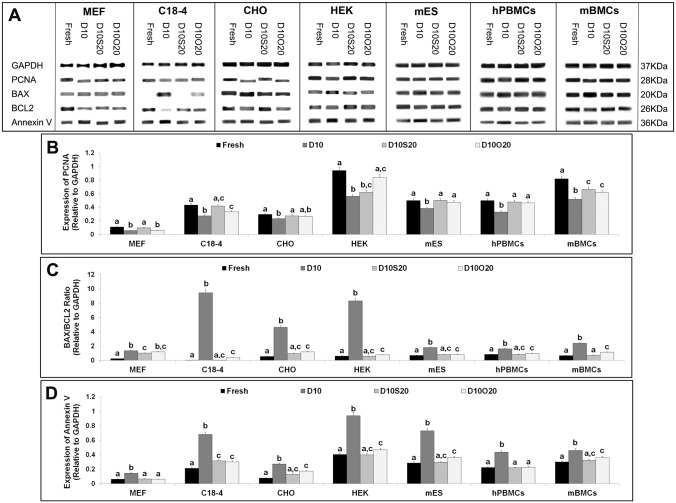
Western blot analysis of frozen–thawed-cultured cells
Expression levels of cell proliferation-, early apoptosis-, pro-apoptosis-, and anti-apoptosis-specific proteins (PCNA, Annexin V, BAX, and BCL2, respectively) were analyzed in frozen–thawed cells after culture (Fig 2A). PCNA expression in all cell types cryopreserved in D10 was lower than that in fresh cells and cells cryopreserved in D10S20 and D10O20 (Fig 2B). PCNA expression in CHO cells, hPBMCs, and mES cells frozen in D10O20 was similar to that of fresh cells and cells frozen in D10S20. PCNA expression in hPBMCs and C18-4 cells frozen in D10O20 was similar to those cells frozen in D10S20 but lower than that in fresh cells. In MEF cells, PCNA expression was lower in cells frozen in D10O20 than in both fresh and cells cryopreserved in D10S20. Interestingly, PCNA expression in HEK cells frozen in D10O20 was similar to that of fresh cells but higher than that of cells frozen in D10S20.
Fig 2. Expression of proliferation-, pro-apoptosis-, anti-apoptosis-, and early apoptosis-specific proteins in frozen-thawed cells.
Expression of cell PCNA, BAX, BCL2, and Annexin V respectively in fresh and frozen–thawed cells after 24 h (MEF, C18-4, CHO, HEK, and mES cells) and 72 h (hPBMCs and mBMCs) of culture. Thirty-microgram aliquots of each cell extract were subjected to SDS-PAGE and western blot analysis. (A) Representative western blotting and densitometry analysis of (B) BAX/BCL2 ratio, (C) PCNA, and (D) Annexin V expression relative to GAPDH. Data represent mean ± SEM off our trials for a cell type in each group. Bars with different letters are significantly different at P < 0.05.
The ratio of BAX/BCL2 proteins was significantly higher in all cell types frozen in D10 than in fresh cells and cells cryopreserved in D10S20 and D10O20 (Fig 2C). Although the BAX/BCL2 ratio was higher in D10O20 compared with fresh cells, it was similar to that of cells frozen in D10S20 in all cell types except mBMCs. mBMCs frozen in D10O20 had higher BAX/BCL2 ratios compared with those frozen in D10S20. Annexin V protein expression in all seven cell types cryopreserved in D10 was higher than in fresh cells and cells cryopreserved in D10S20 and D10O20 (Fig 2D). Annexin V expression in MEF cells, and hPBMCs frozen in D10O20 was similar to that of fresh cells and cells frozen in D10S20. Annexin V expression in C18-4 cells, HEK cells, CHO cells, mES cells, and mBMCs frozen in D10O20 was similar to that of cells frozen in D10S20 but lower than that of fresh cells.
Proteomic analysis of BuOF and FBS
Proteomic analysis of BuOF and FBS was carried out by depleting the samples of abundant proteins and subjecting the samples to MS/MS analysis. Based on MS/MS peak search in the Bos taurus database, a total of 41 proteins in BuOF and 207 proteins in FBS were identified, out of which 16 were common. A list of proteins identified in BuOF and their comparison with FBS is shown in Table 2. Proteins identified in FBS are listed in S1 Table. A protein database search and literature search revealed that BuOF contains 26% glycoproteins, 9% globular proteins, 7% plasma proteins, 9% lipoproteins, and 7% cytoskeletal proteins. Out of the 16 common proteins, albumin was the major protein identified in FBS and BuOF.
Table 2. Proteomic analysis of BuOF and comparison with FBS.
| Name of the protein | Molecular weight (kDa) | Type of protein/role | Whether present or absent in FBS | |
|---|---|---|---|---|
| 1 | Apolipoprotein A-I precursor | 30.3 | High density lipoprotein/promotes proliferation and inhibits apoptosis of cells [77] | Present |
| 2 | Fibronectin variable region | 20.4 | Glycoprotein/induces cell proliferation, inhibits apoptosis [83], and helps improve fertility rate of cryopreserved sperm [84] | Present |
| 3 | Transthyretin precursor | 15.7 | Thyroxine binding prealbumin | Present |
| 4 | Serum albumin | 53.9 | Globular protein/replaces serum in efficient cryopreservation of PBMCcells [27] | Present |
| 5 | Alpha-1-B glycoprotein precursor | 53.5 | Glycoprotein | Present |
| 6 | Gelsolin | 80.7 | Lysophosphatidic acid transport protein/anti-oxidant properties [73] andinvolved in both the control and execution of apoptosis [72] | Present |
| 7 | Complement component 3 | 187.1 | Glycoprotein | Present |
| 8 | Keratin 5, type II | 62.6 | Cytoskeletal protein | Present |
| 9 | Inter-alpha-trypsin inhibitor heavy chain H2 precursor | 106.1 | Carrier protein | Present |
| 10 | Albumin | 69.2 | Globular protein/replace sserum in efficient cryopreservation of PBMC cells [27] | Present |
| 11 | Complement factor B precursor | 85.3 | Circulatory protein | Present |
| 12 | Keratin 7, type II | 50 | Cytoskeletal protein | Present |
| 13 | Transferrin isoform X1 | 77.6 | Glycoprotein/major anti-oxidant protein andprotects spermatozoa against oxidative damage during freeze–thawstress [69] | Present |
| 14 | Keratin 42, type 1 like isoform X3 | 50.3 | Cytoskeletal protein | Present |
| 15 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 isoform X1 | 46.1 | Enzyme/inhibit sapoptosis by inhibitingcaspase-3 [75] | Present |
| 16 | Crystal structure of BSA chain b | 66.4 | Globular protein/replaces serum in efficient cryopreservation of PBMCcells [27] | Present |
| 17 | Alpha-2-HS-glycoprotein precursor | 38.4 | Glycoprotein | Absent |
| 18 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | 22.7 | Globulin glycoprotein/inhibits apoptosis by inhibiting caspase-3 [75] | Absent |
| 19 | Regucalcin | 33.3 | Calcium binding protein/maintains cell homeostasis and playa role as a suppressor protein in cell signaling systems in many cell types [81] | Absent |
| 20 | Crystal structure of bovine factor Vai, chain A | 34.7 | Inactivated form of factor Va | Absent |
| 21 | Endopin 2C | 46.7 | Type of serpin/inhibits cysteine proteases and elastase-like serine proteases therefore eventually inhibit apoptosis [85] | Absent |
| 22 | Fetuin B precursor | 42.6 | Glycoprotein/anti-oxidant and helps maintain sperm morphology by increasing ROS scavenging enzymes [82] | Absent |
| 23 | Fibrinogen alpha chain | 18.1 | Glycoprotein | Absent |
| 24 | Apolipoprotein E | 27.1 | Lipoprotein | Absent |
| 25 | C-type lectin domain family 3, member B precursor | 22.1 | Plasminogen binding protein | Absent |
| 26 | Adiponectin | 26.1 | Hormone(cytokine) | Absent |
| 27 | Transferrin precursor | 77.7 | Glycoprotein/major anti-oxidant protein andprotects spermatozoa against oxidative damage during freeze–thaw stress [69] | Absent |
| 28 | Inter-alpha-trypsin inhibitor heavy chain 1 precursor | 101.2 | Serine protease inhibitor | Absent |
| 29 | keratin 25, type I like | 49.3 | Intermediate filament | Absent |
| 30 | Bovine hemoglobin chain C | 15 | Metalloprotein | Absent |
| 31 | Hemoglobin, beta | 16 | Globin protein | Absent |
| 32 | Apolipoprotein N | 28.5 | Lipoprotein | Absent |
| 34 | Collagen, type III, alpha 1 | 137.1 | Fibrous scleroprotein | Absent |
| 35 | Immunoglobulin light chain | 10.4 | Polypeptide | Absent |
| 36 | Alpha-2-macroglobulin variant 1 | 115.1 | Plasma protein/proteinase inhibitor and anti-apoptotic protein [86] | Absent |
| 37 | Alpha-2-macroglobulin variant 4 | 42.3 | Plasma protein/proteinase inhibitor and anti-apoptotic protein [86] | Absent |
| 38 | Kininogen isoform X2 | 44.4 | Polypeptide | Absent |
| 39 | Protein dimmed | 39.4 | Helix-loop-helix protein | Absent |
| 40 | Primary amine oxidase | 81.7 | Copper containing enzymes/biological regulator of cell growth and differentiationand is also involved in apoptosis regulation by altering membranes [78] | Absent |
| 41 | Actin, alpha 2, smooth muscle, aorta | 39.3 | Globular protein | Absent |
| 42 | Methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1-like | 81.6 | Ligase | Absent |
Discussion
Because FBS is enriched with several growth factors and proteins, it is routinely used as a media supplement for cell and tissue cultures. FBS is also used as a non-penetrating cryoprotectant to cryopreserve several cell types. High cost, ethical concerns, and the possibility of transmission of blood-borne diseases has led to a need to find a substitute for cryopreservation [22]. To date, many serum replacements have been introduced for cell cryopreservation [25–27]; however, their use is limited, primarily because of their high cost and difficulty in procurement. In the present study, we investigated if BuOF can replace FBS for cryopreservation of several different cell types.
The biochemical composition of BuOF was found to be different from that of FBS. All constituents except for LDL cholesterol and ascorbic acid, were found to be present in a higher concentrations in FBS (approximately 1.8- and 6.4-fold higher in BuOF, respectively; Table 1). LDL cholesterol contains 85–90% lipids as well as 10–15% proteins [37] and is responsible for the gelation process in freeze–thawing [37–40]. Presence of higher LDL cholesterol content in BuOF could be beneficial for cell cryopreservation by providing fluid stability. LDL cholesterol protected sperm cells against cold shock by preventing the efflux of phospholipids and cholesterol from the sperm cell membrane [41]. Egg yolk, which is rich in LDL cholesterol, has been proven to be an effective cryopreservative for spermatozoa of bulls [42, 43], stallions [44], rams [45], and dogs [46].
Ascorbic acid is a micronutrient that prevents both membrane depolarization and cytochrome C release events that occur during apoptosis [47]. Lane et al. observed a significant decrease in the levels of lactate dehydrogenase in mouse embryos cryopreserved with ascorbic acid [48]. Presence of a high concentration of ascorbic acid in BuOF could have a role in preventing damage by free radicals, which is one of the reasons implicated in loss of viability during or immediately after cell freezing [49]. Thus, viability of different cell lines was maintained in this study. Furthermore, a high concentration of ascorbic acid in BuOF could be responsible for the similarity in Annexin V expression and BAX/BCL2 ratio in cell lines and hPBMCs cryopreserved in BuOF and FBS. However, it remains unclear if the effect was exclusively because of ascorbic acid or some other components in BuOF.
Annexin V expression has been used to evaluate early apoptotic cells [50], whereas the ratio of BAX (pro-apoptotic) to BCL2 (anti-apoptotic) gene expression indicates susceptibility of cryopreserved cells to apoptosis [51]. Previous study revealed that Annexin V assay can be used to detect membrane integrity of frozen–thawed human spermatozoa [52]. Moreover, the BAX/BCL2 ratio is used to determine the quality of embryos and oocytes in different stages of in vitro culture [53]. The relative Annexin V expression and BAX/BCL2 ratio were similar in all cell types cryopreserved in either D10S20 or D10O20except mBMCs and in cells frozen in D10. These findings indicate that both FBS and BuOF have similar ability to inhibit apoptosis in most cell types cryopreserved in this study. Although in mouse primary suspension cells such as mBMCs frozen in D10O20, the BAX/BCL2 ratio was similar to that of cells frozen in D10. Nevertheless, Annexin V expression in mBMCs cryopreserved in either group was not different. These findings indicate that unlike BuOF, FBS was beneficial for preventing late apoptosis in mBMCs. Interestingly, Annexin V expression and BAX/BCL2 ratio in human primary cells (hPBMCs) was similar in both groups. These results suggest that mBMCs are rather susceptible to apoptosis during cryopreservation, unlike hPBMCs. The components of the p53 pathway that control late cellular apoptosis in response to DNA damage are reported to be more active in mouse cells than in human cells [54, 55]. This could potentially explain the elevated BAX/BCL2 ratio in mouse primary cells.
Recovery of viable cells from cryogenic storage is a major concern [56]. Initially, viability of all cell types was estimated immediately after thawing to compare BuOF and FBS cryopreservation abilities, as reported in earlier studies [26, 27, 57]. Although viability of MEF cells frozen in D10O20 immediately after thawing was low, expression of Annexin V and BAX/BCL2 ratio in cultured cells were not different from cells frozen in D10S20. These findings suggest that though BuOF could not preserve the initial viability nevertheless, it could prevent both early and late apoptosis of frozen-thawed MEF cells. On the contrary, all cells types frozen in D10 not only had lower cell viability immediately after thawing but also had elevated Annexin V and BAX/BCL2 ratio in cultured cells. These findings indicate that presence of FBS or BuOF in cryomedia significantly enhances cell survival and prevented apoptosis. Interestingly, the cell viability of few cultured adherent cells such as C18-4, HEK and mES cells that were frozen in D10 did not differ from cells frozen in other groups, but the Annexin V and BAX/BCL2 ratio in these cells were significantly higher. These results indicate that mere recovery of cell viability of frozen-thawed cells cannot be a reliable indicator for the cryoprotection provided by a cryopreservation media [58]. Evaluation of apoptosis-specific proteins expression in frozen-thawed cells is also essential [59]. Because different cells grow at different rates, cell recovery post-culture is an ideal method to assess their growth rate after cryopreservation. Recovery of cells can be assessed by percentage of cell recovery [60] and expression of proliferation cell-specific proteins, such as PCNA [61]. Frozen–thawed murine neural precursor cells retained their proliferative capacity, as shown by the PCNA expression [62]. Percentage of cells recovered and PCNA expression in cells frozen in DMSO alone (D10) was significantly lower than that of cells frozen with DMSO with FBS or BuOF in all cell types used in this study. These findings further support the use of FBS or BuOF in cryomedia for cell cryopreservation.
However, for the MEF cells cryopreserved in D10O20, cell recovery and PCNA expression were significantly lower than that in the cells cryopreserved in D10S20, and the viability of cultured cells recovered after 24h was not different from fresh cells and cells frozen in D10S20. This difference could be because MEF cells are primary cells and require more than 24 h for restoration of cell growth and proliferation after cryopreservation. Another plausible explanation could be presence of higher protein, lipoprotein, and triglyceride contents in FBS led to better cryopreservation of MEF cells. Bradykinin [63], tubulin [64], heat shock protein 90 [65], and superoxide dismutase [66] are reported to play roles in bull sperm cryopreservation. Enolase, a metalloenzyme, could replace DMSO in cryopreservation of rat hepatocytes by reducing oxidative stress and cellular toxicity [67]. Presence of these proteins exclusively in FBS could potentially provide superior cryoprotection.
Although the protein content of BuOF was much lower than that of FBS, a comparative proteomic analysis of BuOF and FBS was carried out to identify proteins/peptides that may be critical for cryopreservation. A total of 41 proteins were identified in BuOF through MS/MS analysis. The majority of proteins were glycoproteins, globular proteins, and lipoproteins. Out of the 41 identified proteins, 16 were also present in FBS (Table 2). Transport proteins such as albumin, serotransferrin, transthyretin, and apolipoprotein AI that were present in both FBS and BuOF were previously reported in human vitreous humor [68], which validates the proteomic approach that was followed in this study.
These proteins are biologically essential, have important roles in cell homeostasis, and act as hormone carriers. Transferrin protects spermatozoa against oxidative damage during freeze–thaw stress [69]. Fibronectin, which is another important glycoprotein that was identified in BuOF, plays an important role in cell–cell aggregation, cell–substratum adhesion, attachment of cells to extracellular matrix components, and cellular motility [70]. Gelsolin, which is an actin filament that caps and severs proteins that enhance the rate of cell migration [71], is involved in both the control and execution of apoptosis [72]. This lysophosphatidic acid transport protein is thought to have anti-oxidant properties [73]. The presence of these proteins in BuOF could be a reason for lower expression of pro-apoptotic proteins in cryopreserved cell lines.
Several proteolytic enzymes have been implicated in apoptosis and associated processes. α-1 Antiproteinase (A1P1) in BuOF is a serine protease inhibitor (serpin) [74] that regulates apoptosis by inhibiting caspase-3 activity [75]. Many proteins identified in BuOF and FBS were found to be involved in lipid transport and binding as well as cell migration. Transthyretin, which is a transport protein in BuOF, is involved in lipid metabolism [76]. Apolipoprotein A-I, which is a major component of high-density lipoprotein (HDL), has been reported to promote proliferation and inhibit apoptosis of endothelial and vascular smooth muscle cells [77]. Presence of these proteins in BuOF may be critical for preventing cell apoptosis during freezing and thawing.
A few proteins were exclusively identified in BuOF. Amine oxidase and kininogen are reported to play crucial role in prevention of cellular apoptosis [78, 79]. Actin plays a crucial role in cell structure, motility, and cell division [77]. Damage to actin modifies microtubule organization in the presence of cryoprotectants such as propanediol and DMSO [80]. The globular and glycoproteins present in BuOF contribute to important cellular functions, such as mobility and contraction of cells during cell division. Regucalcin has a role as a suppressor protein in cell signaling systems of many cell types [81]. Fetuin, a glycoprotein and protease inhibitor, increases superoxide dismutase and glutathione peroxidase enzymatic activities to minimize membrane and DNA damage in cryopreserved semen [82]. Whether these proteins have a critical role in cell cryopreservation needs to be investigated further.
This is a preliminary study that identified BuOF as a replacement for FBS in cryopreservation media. The composition of ocular fluid may vary with age, sex, breed, origin, and physiological and health status of the animal. Variation in the composition of ocular fluid may affect cell survival and gene expression of cryopreserved cells. Therefore, further investigations and standardizations are needed before BuOF can find its use for commercial applications.
In conclusion, this study demonstrates efficient cryopreservation of adherent cell lines, such as CHO, HEK, C18-4, and mES cells and primary suspension cells such as hPBMC and mBMC in 20% BuOF, and its potential to replace FBS in cryomedia. Because mouse primary cells (mBMCs and MEF cells) are susceptible to cryodamage, cryopreservation using BuOF needs further investigation. Proteomic and biochemical analyses of BuOF identified several components that may have crucial roles in cell cryopreservation. Further studies that elucidated the effect of BuOF in several different cell types could help establish BuOF as a vital component for serum-free cryopreservation protocols.
Supporting Information
(DOCX)
Acknowledgments
We would like to thank Mr. Prabhu for his assistance in collection of buffalo eyes from the slaughter house.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Council for Scientific and Industrial Research (CSIR), Government of India (GOI) (grant number BSC0207) and Central Zoo Authority (CZA), GOI (grant number GAP0389). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stephenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: is it time to change the strategy? World journal of gastroenterology: WJG. 2010;16(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem cells. 2004;22(4):487–500. 10.1634/stemcells.22-4-487 . [DOI] [PubMed] [Google Scholar]
- 3. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing research reviews. 2006;5(1):91–116. 10.1016/j.arr.2005.10.001 . [DOI] [PubMed] [Google Scholar]
- 4. Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging cell. 2006;5(3):213–24. 10.1111/j.1474-9726.2006.00213.x . [DOI] [PubMed] [Google Scholar]
- 5. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6. . [DOI] [PubMed] [Google Scholar]
- 6. Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, et al. Efficient transfection of embryonic and adult stem cells. Stem cells. 2004;22(4):531–43. 10.1634/stemcells.22-4-531 . [DOI] [PubMed] [Google Scholar]
- 7. de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. Journal of andrology. 2000;21(6):776–98. . [PubMed] [Google Scholar]
- 8. Khan KH. Gene expression in Mammalian cells and its applications. Advanced pharmaceutical bulletin. 2013;3(2):257–63. 10.5681/apb.2013.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. 10.1126/science.1151526 . [DOI] [PubMed] [Google Scholar]
- 10. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. . [DOI] [PubMed] [Google Scholar]
- 11. Sambor A, Garcia A, Berrong M, Pickeral J, Brown S, Rountree W, et al. Establishment and maintenance of a PBMC repository for functional cellular studies in support of clinical vaccine trials. Journal of immunological methods. 2014;409:107–16. 10.1016/j.jim.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makedonas G, Betts MR. Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV. Immunological reviews. 2011;239(1):109–24. 10.1111/j.1600-065X.2010.00968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spencer HT, Sleep SE, Rehg JE, Blakley RL, Sorrentino BP. A gene transfer strategy for making bone marrow cells resistant to trimetrexate. Blood. 1996;87(6):2579–87. . [PubMed] [Google Scholar]
- 14. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation research. 1999;85(3):221–8. . [DOI] [PubMed] [Google Scholar]
- 15. Yair Reisner L, Asher Meshorer, Nathan Sharon. Hemopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Nati Acad Sci USA. 1978;75(6):2933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatric transplantation. 2003;7 Suppl 3:86–8. . [DOI] [PubMed] [Google Scholar]
- 17. Schmale K, Rademacher T, Fischer R, Hellwig S. Towards industrial usefulness—cryo-cell-banking of transgenic BY-2 cell cultures. Journal of biotechnology. 2006;124(1):302–11. 10.1016/j.jbiotec.2006.01.012 . [DOI] [PubMed] [Google Scholar]
- 18. Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo letters. 2004;25(6):375–88. . [PubMed] [Google Scholar]
- 19. Mellor DJ, Gregory NG. Responsiveness, behavioural arousal and awareness in fetal and newborn lambs: experimental, practical and therapeutic implications. New Zealand veterinary journal. 2003;51(1):2–13. 10.1080/00480169.2003.36323 . [DOI] [PubMed] [Google Scholar]
- 20. Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Alternatives to laboratory animals: ATLA. 2002;30(2):219–27. Epub 2002/04/25. . [DOI] [PubMed] [Google Scholar]
- 21. Erickson GA, Bolin SR, Landgraf JG. Viral contamination of fetal bovine serum used for tissue culture: risks and concerns. Developments in biological standardization. 1991;75:173–5. . [PubMed] [Google Scholar]
- 22. Tekkatte C, Gunasingh GP, Cherian KM, Sankaranarayanan K. "Humanized" stem cell culture techniques: the animal serum controversy. Stem cells international. 2011;2011:504723 10.4061/2011/504723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;46(2):146–52. . [DOI] [PubMed] [Google Scholar]
- 24. Hubalek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46(3):205–29. . [DOI] [PubMed] [Google Scholar]
- 25. Tsujimoto K, Takagi H, Takahashi M, Yamada H, Nakamori S. Cryoprotective effect of the serine-rich repetitive sequence in silk protein sericin. Journal of biochemistry. 2001;129(6):979–86. . [DOI] [PubMed] [Google Scholar]
- 26. Sasaki M, Kato Y, Yamada H, Terada S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnology and applied biochemistry. 2005;42(Pt 2):183–8. 10.1042/BA20050019 [DOI] [PubMed] [Google Scholar]
- 27. Germann A, Schulz JC, Kemp-Kamke B, Zimmermann H, von Briesen H. Standardized Serum-Free Cryomedia Maintain Peripheral Blood Mononuclear Cell Viability, Recovery, and Antigen-Specific T-Cell Response Compared to Fetal Calf Serum-Based Medium. Biopreservation and biobanking. 2011;9(3):229–36. 10.1089/bio.2010.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filipic B, Shehata M, Toth S, Schwarzmeier J, Koren S. Novel serum replacement based on bovine ocular fluid: a useful tool for cultivation of different animal cells in vitro. Altex. 2002;19(1):15–20. . [PubMed] [Google Scholar]
- 29. Miller JW, Adamis AP, Shima DT, D'Amore PA, Moulton RS, O'Reilly MS, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. The American journal of pathology. 1994;145(3):574–84. Epub 1994/09/01. [PMC free article] [PubMed] [Google Scholar]
- 30. Camina JP, Casabiell XA, Perez FR, Lage M, Casanueva FF. Isolation of a bioactive Ca(2+)-mobilizing complex lipid from bovine vitreous body. Biochemical and biophysical research communications. 1998;244(3):696–700. Epub 1998/04/16. 10.1006/bbrc.1998.8320 . [DOI] [PubMed] [Google Scholar]
- 31. Webster L, Stanbury RM, Chignell AH, Limb GA. Vitreous intercellular adhesion molecule 1 in uveitis complicated by retinal detachment. The British journal of ophthalmology. 1998;82(4):438–43. Epub 1998/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grus FH, Joachim SC, Pfeiffer N. Proteomics in ocular fluids. Proteomics Clinical applications. 2007;1(8):876–88. 10.1002/prca.200700105 . [DOI] [PubMed] [Google Scholar]
- 33. Hofmann MC, Braydich-Stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem cells. 2005;23(2):200–10. 10.1634/stemcells.2003-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samuelson LC, Metzger JM. Isolation and Freezing of Primary Mouse Embryonic Fibroblasts (MEF) For Feeder Plates. CSH protocols. 2006;2006(2). 10.1101/pdb.prot4482 . [DOI] [PubMed] [Google Scholar]
- 35. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–77. Epub 2001/05/12. . [DOI] [PubMed] [Google Scholar]
- 36. Schaffner W, Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Analytical biochemistry. 1973;56(2):502–14. Epub 1973/12/01. . [DOI] [PubMed] [Google Scholar]
- 37. Hu JH, Li QW, Li G, Chen XY, Yang H, Zhang SS, et al. The cryoprotective effect on frozen–thawed boar semen of egg yolk low density lipoproteins. Asian–Aust J Anim Sci 2006;(19):486–90.17040943 [Google Scholar]
- 38. Lamia Tainturiera, Lae T.J., Chantal, Olivier G., Jean L.C., et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl1, a commercial egg yolk extender. Theriogenology 2004;61 895–907. [DOI] [PubMed] [Google Scholar]
- 39. Boe-Hansen GB, Morris ID, Ersboll AK, Greve T, Christensen P. DNA integrity in sexed bull sperm assessed by neutral Comet assay and sperm chromatin structure assay. Theriogenology. 2005;63(6):1789–802. Epub 2005/03/15. 10.1016/j.theriogenology.2004.08.004 . [DOI] [PubMed] [Google Scholar]
- 40. Moussa M, Marinet V, Trimeche A, Tainturier D, Anton M. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology. 2002;57(6):1695–706. Epub 2002/05/31. . [DOI] [PubMed] [Google Scholar]
- 41. Amirat L, Tainturier D, Jeanneau L, Thorin C, Gerard O, Courtens JL, et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl, a commercial egg yolk extender. Theriogenology. 2004;61(5):895–907. . [DOI] [PubMed] [Google Scholar]
- 42. Hu JH, Li QW, Jiang ZL, Li WY. Effects of different extenders on DNA integrity of boar spermatozoa following freezing-thawing. Cryobiology. 2008;57(3):257–62. Epub 2008/10/07. 10.1016/j.cryobiol.2008.09.004 . [DOI] [PubMed] [Google Scholar]
- 43. Vera-Munoz O, Amirat-Briand L, Diaz T, Vasquez L, Schmidt E, Desherces S, et al. Effect of semen dilution to low-sperm number per dose on motility and functionality of cryopreserved bovine spermatozoa using low-density lipoproteins (LDL) extender: comparison to Triladyl and Bioxcell. Theriogenology. 2009;71(6):895–900. Epub 2009/01/02. 10.1016/j.theriogenology.2008.10.010 . [DOI] [PubMed] [Google Scholar]
- 44. Pillet E, Duchamp G, Batellier F, Beaumal V, Anton M, Desherces S, et al. Egg yolk plasma can replace egg yolk in stallion freezing extenders. Theriogenology. 2011;75(1):105–14. Epub 2010/09/14. 10.1016/j.theriogenology.2010.07.015 . [DOI] [PubMed] [Google Scholar]
- 45. Moustacas VS, Zaffalon FG, Lagares MA, Loaiza-Eccheverri AM, Varago FC, Neves MM, et al. Natural, but not lyophilized, low density lypoproteins were an acceptable alternative to egg yolk for cryopreservation of ram semen. Theriogenology. 2011;75(2):300–7. Epub 2010/10/12. 10.1016/j.theriogenology.2010.08.016 . [DOI] [PubMed] [Google Scholar]
- 46. Varela Junior AS, Corcini CD, Ulguim RR, Alvarenga MV, Bianchi I, Correa MN, et al. Effect of low density lipoprotein on the quality of cryopreserved dog semen. Animal reproduction science. 2009;115(1–4):323–7. Epub 2008/12/23. 10.1016/j.anireprosci.2008.11.002 . [DOI] [PubMed] [Google Scholar]
- 47. Kc S, Carcamo JM, Golde DW . Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(12):1657–67. 10.1096/fj.05-4107com . [DOI] [PubMed] [Google Scholar]
- 48. Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Human reproduction. 2002;17(10):2686–93. . [DOI] [PubMed] [Google Scholar]
- 49. Tariq SA. Role of ascorbic acid in scavenging free radicals and lead toxicity from biosystems. Molecular biotechnology. 2007;37(1):62–5. . [DOI] [PubMed] [Google Scholar]
- 50. Zhang G, Gurtu V, Kain SR, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. BioTechniques. 1997;23(3):525–31. Epub 1997/09/23. . [DOI] [PubMed] [Google Scholar]
- 51. Rao BS, Mahesh YU, Charan KV, Suman K, Sekhar N, Shivaji S. Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology. 2012;64(3):176–84. Epub 2012/01/28. 10.1016/j.cryobiol.2012.01.005 . [DOI] [PubMed] [Google Scholar]
- 52. Glander HJ, Schaller J. Binding of annexin V to plasma membranes of human spermatozoa: a rapid assay for detection of membrane changes after cryostorage. Molecular human reproduction. 1999;5(2):109–15. . [DOI] [PubMed] [Google Scholar]
- 53. Yang MY, Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Animal reproduction science. 2002;70(3–4):159–69. . [DOI] [PubMed] [Google Scholar]
- 54. Gottifredi V SSY, Prives C. Regulation of p53 after different forms of stress and at different cell cycle stages. Cold Spring Harbor Symp Quant Biol. 2000;65:483–8. [DOI] [PubMed] [Google Scholar]
- 55. Schultz L.B NHC, Malikzay A, DiTullio R.A Jr., Stavridi E.S, Halazonetis T.D. The DNA Damage Checkpoint and Human Cancer. Cold Spring Harbor Symp Quant Biol. 2000;65:489–98. [DOI] [PubMed] [Google Scholar]
- 56. Osterling M, Earley E. A reliable method for recovering cryopreserved hybridoma cells for cloning. Journal of Tissue Culture Methods. 1985;9(3):171–3. 10.1007/BF01665926 [DOI] [Google Scholar]
- 57. Muller P, Aurich H, Wenkel R, Schaffner I, Wolff I, Walldorf J, et al. Serum-free cryopreservation of porcine hepatocytes. Cell and tissue research. 2004;317(1):45–56. Epub 2004/06/09. 10.1007/s00441-004-0894-6 . [DOI] [PubMed] [Google Scholar]
- 58. Xiao M, Dooley DC. Assessment of cell viability and apoptosis in human umbilical cord blood following storage. Journal of hematotherapy & stem cell research. 2003;12(1):115–22. Epub 2003/03/29. 10.1089/152581603321210190 . [DOI] [PubMed] [Google Scholar]
- 59. Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, et al. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. Journal of biomedical science. 2006;13(3):433–45. Epub 2005/12/24. 10.1007/s11373-005-9051-9 . [DOI] [PubMed] [Google Scholar]
- 60. Zhao J, Hao HN, Thomas RL, Lyman WD. An efficient method for the cryopreservation of fetal human liver hematopoeitic progenitor cells. Stem cells. 2001;19(3):212–8. 10.1634/stemcells.19-3-212 . [DOI] [PubMed] [Google Scholar]
- 61. Kubben FJ, Peeters-Haesevoets A, Engels LG, Baeten CG, Schutte B, Arends JW, et al. Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut. 1994;35(4):530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Milosevic J, Storch A, Schwarz J. Cryopreservation does not affect proliferation and multipotency of murine neural precursor cells. Stem cells. 2005;23(5):681–8. 10.1634/stemcells.2004-0135 . [DOI] [PubMed] [Google Scholar]
- 63. Shukla MK, Misra AK. Effect of Bradykinin on Murrah buffalo (Bubalus bubalis) semen cryopreservation. Animal reproduction science. 2007;97(1–2):175–9. Epub 2006/04/08. 10.1016/j.anireprosci.2006.02.015 . [DOI] [PubMed] [Google Scholar]
- 64. Eyer J, White D, Gagnon C. Presence of a new microtubule cold-stabilizing factor in bull sperm dynein preparations. The Biochemical journal. 1990;270(3):821–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fukuda A, Osawa T, Oda H, Tanaka T, Toyokuni S, Uchida K. Oxidative stress response in iron-induced acute nephrotoxicity: enhanced expression of heat shock protein 90. Biochemical and biophysical research communications. 1996;219(1):76–81. 10.1006/bbrc.1996.0184 . [DOI] [PubMed] [Google Scholar]
- 66. Wang P, Wang YF, Wang H, Wang CW, Zan LS, Hu JH, et al. HSP90 expression correlation with the freezing resistance of bull sperm. Zygote. 2014;22(2):239–45. 10.1017/S096719941300004X . [DOI] [PubMed] [Google Scholar]
- 67. Averill-Bates DA, Yée MC-S, Grondin M, Sarhan F, Ouellet F. C-1003: Cryopreservation of rat hepatocytes with wheat proteins: Role in oxidative stress protection. Cryobiology. 2014;69(3):512–3. 10.1016/j.cryobiol.2014.09.332 [DOI] [Google Scholar]
- 68. Koss MJ, Hoffmann J, Nguyen N, Pfister M, Mischak H, Mullen W, et al. Proteomics of vitreous humor of patients with exudative age-related macular degeneration. PloS one. 2014;9(5):e96895 Epub 2014/05/16. 10.1371/journal.pone.0096895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li P, Hulak M, Koubek P, Sulc M, Dzyuba B, Boryshpolets S, et al. Ice-age endurance: the effects of cryopreservation on proteins of sperm of common carp, Cyprinus carpio L. Theriogenology. 2010;74(3):413–23. 10.1016/j.theriogenology.2010.02.024 . [DOI] [PubMed] [Google Scholar]
- 70. Reid T, Kenney C, Waring GO. Isolation and characterization of fibronectin from bovine aqueous humor. Investigative ophthalmology & visual science. 1982;22(1):57–61. Epub 1982/01/01. . [PubMed] [Google Scholar]
- 71. Cunningham CC, Stossel TP, Kwiatkowski DJ. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991;251(4998):1233–6. Epub 1991/03/08. . [DOI] [PubMed] [Google Scholar]
- 72. Burtnick LD, Urosev D, Irobi E, Narayan K, Robinson RC. Structure of the N-terminal half of gelsolin bound to actin: roles in severing, apoptosis and FAF. The EMBO journal. 2004;23(14):2713–22. 10.1038/sj.emboj.7600280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pages C, Simon MF, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release. Prostaglandins & other lipid mediators. 2001;64(1–4):1–10. . [DOI] [PubMed] [Google Scholar]
- 74. Gettins PG. Serpin structure, mechanism, and function. Chemical reviews. 2002;102(12):4751–804. . [DOI] [PubMed] [Google Scholar]
- 75. Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, et al. alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. The American journal of pathology. 2006;169(4):1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ando Y. [Transthyretin: it's miracle function and pathogenesis]. Rinsho byori The Japanese journal of clinical pathology. 2009;57(3):228–35. Epub 2009/04/15. . [PubMed] [Google Scholar]
- 77. Dominguez R, Holmes KC. Actin structure and function. Annual review of biophysics. 2011;40:169–86. 10.1146/annurev-biophys-042910-155359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Toninello A, Pietrangeli P, De Marchi U, Salvi M, Mondovi B. Amine oxidases in apoptosis and cancer. Biochimica et biophysica acta. 2006;1765(1):1–13. 10.1016/j.bbcan.2005.09.001 . [DOI] [PubMed] [Google Scholar]
- 79. Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(19):8485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vincent C, Pruliere G, Pajot-Augy E, Campion E, Garnier V, Renard JP. Effects of cryoprotectants on actin filaments during the cryopreservation of one-cell rabbit embryos. Cryobiology. 1990;27(1):9–23. . [DOI] [PubMed] [Google Scholar]
- 81. Yamaguchi M. Role of regucalcin in maintaining cell homeostasis and function (review). International journal of molecular medicine. 2005;15(3):371–89. . [PubMed] [Google Scholar]
- 82. Sariozkan S, Tuncer PB, Buyukleblebici S, Bucak MN, Canturk F, Eken A. Antioxidative effects of cysteamine, hyaluronan and fetuin on post-thaw semen quality, DNA integrity and oxidative stress parameters in the Brown Swiss bull. Andrologia. 2015;47(2):138–47. 10.1111/and.12236 . [DOI] [PubMed] [Google Scholar]
- 83. Han SW, Roman J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene. 2006;25(31):4341–9. 10.1038/sj.onc.1209460 . [DOI] [PubMed] [Google Scholar]
- 84. Glander HJ, Herrmann K, Haustein UF. The equatorial fibronectin band (EFB) on human spermatozoa--a diagnostic help for male fertility? Andrologia. 1987;19(4):456–9. . [DOI] [PubMed] [Google Scholar]
- 85. Hook VY, Hwang SR. Novel secretory vesicle serpins, endopin 1 and endopin 2: endogenous protease inhibitors with distinct target protease specificities. Biological chemistry. 2002;383(7–8):1067–74. 10.1515/BC.2002.115 . [DOI] [PubMed] [Google Scholar]
- 86. De Souza EM, Meuser-Batista M, Batista DG, Duarte BB, Araujo-Jorge TC, Soeiro MN. Trypanosoma cruzi: alpha-2-macroglobulin regulates host cell apoptosis induced by the parasite infection in vitro. Experimental parasitology. 2008;118(3):331–7. 10.1016/j.exppara.2007.09.004 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.