Abstract
Acanthamoeba- related infections, such as amoebic keratitis and granulomatous amoebic encephalitis, can develop in high-risk population through contaminated water sources. Thus, surveying water resources, particularly those available for human use, is of the utmost importance. In the present study, 67 water samples were collected from water resources in East Azerbaijan, a province in northwestern Iran. Samples were cultured on enriched non-nutrient agar plates, and sequencing-based approaches were used for genotyping. The pathogenic potential of the isolates was determined using thermo- and osmo-tolerance tests. Acanthamoeba were detected in 17 (25.4%) of the 67 collected samples. Sequencing analysis revealed that the isolates belonged to the T3 (23.52%), mixed T3/T4 (5.88%), T4 (58.82%), T5 (5.88%), and T13 (5.88%) genotypes. Through thermo- and osmo-tolerance tests, 88.23% of isolates were resistant to 37 °C, 40 °C temperature, and 0.5 M and 1 M osmolarity; thus, these isolates had the potential for pathogenicity. These findings point toa serious public health concern in the studied region. This study is the first to report Acanthamoeba isolated from drinking and recreational water sources in East Azerbaijan and Acanthamoeba T13 isolated from tap water in Iran.
Keywords: Acanthamoeba, East Azerbaijan, water resources, PCR, thermo-tolerance, osmo-tolerance
Introduction
Free-living amoebae (FLA) belonging to the Acanthamoeba genus are widely distributed in the environment and can be pathogenic to humans.1 The cyst stage of these amoebae is highly resistant to desiccation, wide ranges of pH, temperature changes, and chemicals.2–4 The Acanthamoebae genus can be simply distinguished from other genera of FLA by microscopic investigation and the morphological differences between trophozoites and cysts (particularly two-wall cysts) among genera. Molecular methods based on 18S rRNA gene sequencing have introduced 19 genotypes of this protozoa so far,5 most of which are clinically important because they can potentially cause amoebic keratitis (AK) and, albeit rarely, granulomatous amoebic encephalitis (GAE).3,6–8 AK is a sight-threatening corneal disease that manifests itself as severe eye pain, photophobia, blurred vision, and neuritis. Generally, only a single eye is affected by Acanthamoeba, but the prognosis of this devastating eye disease is poor.9 Acanthamoeba genotypes related to keratitis are mainly T3 and T4; however, various reports have mentioned other genotypes such as T2, T5, T6, T11, T12, and, recently, T13 and T15.10,11 GAE is a rare and fatal disease occurring mainly in immunosuppressed patients including patients with HIV, leukemia, and diabetes. Genotypes related to GAE mainly belong to T1, T2, T4, T10, and T12.8
Among the genotypes, T4 is the most abundant in the environment and the most common to be isolated from patients with GAE and AK.12 The thermo-tolerant amoebae seem to have an increased ability to cause human infections; however, further testing, such as their osmo-tolerance ability, should be performed. Osmo- and thermo-tolerance tests are two plating assay tests used to evaluate the potential pathogenicity of Acanthamoeba isolates.
Since 1990, because of the increasing number of contact lens wearers, reports of AK have also been increasing. The incidence of AK is more in contact lens wearers than in other groups and is estimated to be 0.27–0.33 per 10,000 people.13 According to a 2013 review by Lorenzo Morales et al, the number of reported AK cases has been rising significantly since 2004. Recent studies have revealed that the AK incidence rate varies between 17 and 70 cases per million contact lens wearers.9 However, the exact incidence of AK worldwide is very difficult to establish because of the large number of unreported cases.
Previous studies have reported wearing lenses (particularly soft ones) and contact with contaminated water as great risks for developing AK; for example, Kilvington et al published the first report of tap water as a source of AK in 1990.14,15 Indeed, most patient cases of AK develop after exposure to contaminated water or a history of swimming or bathing in suspected water sources. Winck et al determined a 9.5% contamination with Acanthamoeba spp. in 136 tap water samples of Rio Grande do sul, Brazil. In their study, T2, T2/T6, T4, and T6 genotypes were detected. By thermo- and osmo-tolerance tests, 50% of these isolates were found to have low pathogenicity.16 Lorenzo-Morales et al reported T1, T2, T3, T4, and T7 in 16 of 37 freshwater samples from the Nile delta region of Egypt.17
Notably, the occurrence of Acanthamoeba in water sources could be a dual danger, because these amoebae could also harbor pathogenic microorganisms such as Legionella, Pseudomonas, and Helicobacter.18
In Iran, the prevalence of AK continues to rise. Rezaeian et al reported an increasing trend during a 10-year study of AK patients.19 Niyyati et al reported different genotypes of Acanthamoeba from clinical cases (AK), and most strains belonged to the genotype T4. Furthermore, genotype T3 was isolated for the first time from AK patients in Iran.20 In recent years, reports of AK have increased in East Azerbaijan, a province in northwestern Iran. This region has a mountain climate with regular seasons, and is located approximately 2910 m above sea level.
In the current study, morphological and molecular methods were used to investigate Acanthamoebae in water sources of various regions of East Azerbaijan. The pathogenic potential of the isolates was also determined using thermo- and osmo-tolerance assays.
Material and Methods
Sampling geographical area and sample resources
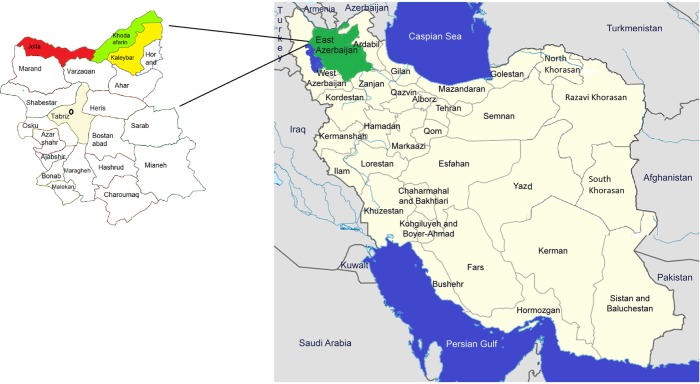
In total, 67 water samples were collected from different water resources of Kaleybar, Khodaafarin, and Jolfa counties of East Azerbaijan in northwestern Iran (Fig. 1). Khodaafarin and Jolfa counties are limited by the ArasRiver in the north. The three named counties have a total area of 5,267 km2 in the north of the province and a population of ∼138,980 reported in the 2012 census (Kaleybar, Khodaafarin, and Jolfa had populations of 48,837, 34,977, and 55,166, respectively).
Figure 1.
Map of the East Azerbaijan Province and Kaleybar (yellow), Khodaafarin (green), and Jolfa (Red) counties, Iran.
Water samples (∼1000 mL) were obtained from cold springs (29), tap water (24), rivers (5), hot springs (6), and household wells (3). Twenty-five samples were collected from urban regions and 42 samples from rural regions. The samples were transferred to the Protozoology Laboratory of Shahid Beheshti University of Medical Science, Tehran, Iran, within 24 hours and stored at room temperature.
Filtration and cultivation
Immediately after being transferred to the laboratory, approximately 250 mL of each sample was filtered through a cellulose nitrate membrane with a pore size of 1.6 μm.21 After that, the center of each membrane was cut out and placed on plates containing 1.5% non-nutrient agar (NNA) medium and heat-inactivated Escherichia coli for culturing.8 Non-nutrient agar 1.5% (NNA) was prepared using Bacto-agar (Difco) and distilled water. Bacteria were used as a food source for amoebae outgrowth. This medium is not rich in nutrients; thus, unwanted organisms do not grow in it. The samples were incubated at room temperature for up to 2 months.
Microscopic examination and cloning
After 1 week, investigation of the plates began and continued daily for up to 2 months. A magnification of 100 × was used to identify positive samples. Amoebae were morphologically identified on the positive plates by their flat-shaped trophozoites with acanthopodia and double-wall cysts. Cloning was then performed to eliminate any microorganism contamination (bacteria or fungi). To this end, a few amoebae were transferred to fresh plates, and replicates were made to achieve a plate without bacterial and fungal contamination.
Thermo- and osmo-tolerance tests
These two tests were conducted to assay the potential pathogenicity of the amoebae. For the thermo-tolerance test, two sets of culture plates were prepared using an isolated cyst-saturated block of NNA medium. One set was incubated at 37 °C, and the other was incubated at 40 °C for up to 7 days after cultivation. Plates were investigated daily by a light microscope (400 × magnification).
To accomplish the osmo-tolerance test, first, two sets of NNA medium with 0.5 and 1 M d-mannitol were prepared. Next, one amoeba-saturated block was placed in the center of the prepared plates. The plates were then incubated at room temperature and were examined like the thermo- tolerance test.
DNA extraction
Total genomic DNA was extracted by the modified phenol/chloroform method.22 Trophozoites and cysts were harvested by sterile PBS, pH 7.2, from the surface of NNA plates. After centrifugation at 500 g for five minutes, the pellet was resuspended in lysis buffer containing 50 mmol/L NaCl, 10 mmol/L EDTA, 50 mmol/L Tris-HCl, and with pH 8.0 and SDS 1%; incubation was performed at 60 °C overnight with 0.25 mg/mL proteinase K. The solution was then incubated at 100 °C to inactivate proteinase K. This process continued using phenol/chloroform/isoamyl alcohol (25:24:1) and chloroform/isoamyl alcohol (24:1). Finally, DNA was recovered by cold absolute ethanol and sodium acetate (3 M).
PCR analysis
DNA was amplified by using JDP primers (genus-specific primers),23,24 including the primer pair JDP1 (5′-GGCCCAGATCGTTTACCGTGAA) as forward primer and JDP2 (5′-TCTCACAAGCTGCTAGGGAGTCA) as reverse primer.25 These primers can amplify an approximately 500 bp fragment. PCR was performed in a 30-amplicon (Taq DNA Polymerase Master Mix) mix ready-made mixture. The final mixture of reaction contained 25 μL Taq Master Mix, 5 ng DNA, 0.1 μm of each primer, and distilled water. PCR was carried out under the following conditions: an initial denaturation step at 94 °C for 1 minute and 35 repetitions at 94 °C for 35 seconds, and an annealing step at 56 °C for 45 seconds and at 72 °C for 1 minute. To confirm the PCR results, its products were separated by using 1.5% gel agarose, and gels were stained with an ethidium bromide solution and examined under UV light.
Sequencing of PCR products
The PCR products of 17 isolates were submitted for sequencing using an ABI 3130X automatic sequencer at the Takapouzist Company, Tehran. In order to classify the 17 isolated Acanthamoeba, homology analyses of the obtained sequences with genes of gene bank were carried out using the BLAST (Basic Local Alignment Search Tool) program of the US National Center for Biotechnology Information (NCBI) site.
Results
Overall, 17 (25.4%) of the 67 water samples were found to be positive for Acanthamoeba spp. by both microscopic and molecular methods (Table 1, Fig. 2).
Table 1.
Number and percentages of positive samples collected from various water resources in East Azerbaijan, Northwest Iran.
| SOURCE | NUMBER OF SAMPLES | NUMBER OF POSITIVE SAMPLES | PERCENTAGE |
|---|---|---|---|
| Coldspring | 29 | 5 | 17.2 |
| Tap water | 24 | 9 | 37.5 |
| River | 5 | 1 | 20 |
| Hotspring | 6 | 1 | 16.7 |
| Householdwell | 3 | 1 | 33.3 |
| Total | 67 | 17 | 25.4 |
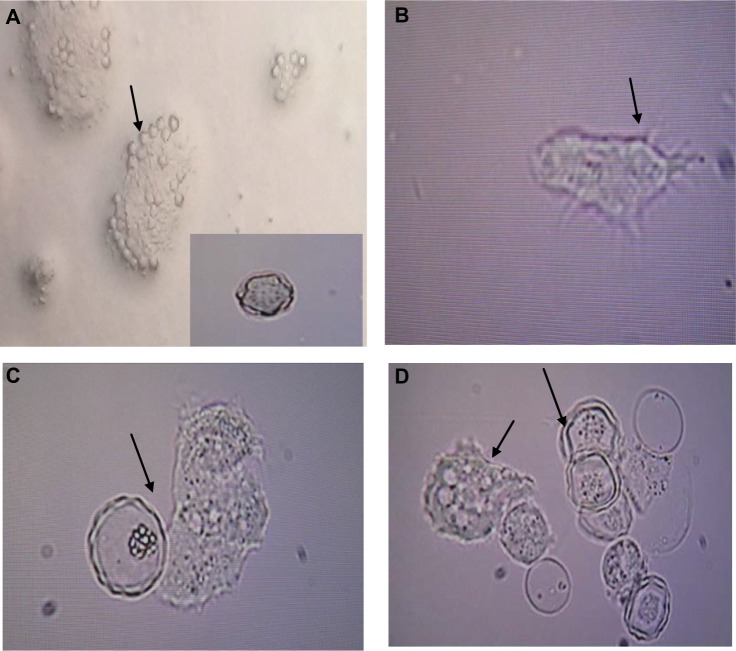
Figure 2.
Light micrographs of (A) Acanthamoeba cysts with star-shaped endocysts (arrows); (B) Acanthamoeba trophozoite with spine like structures called acantopodia, ×400; (C, D)trophozoites and double-walled cysts, ×400.
More tap water than cold spring water was contaminated based on the number of samples collected from each source (Table 1). Indeed, tap water was found to be a contaminated source (with 37.5%). The occurrence of Acanthamoeba spp. was 36% (9 out of 25 samples) and 19.05% (8 out of 42 samples) in rural and urban regions, respectively.
Morphological detection revealed flat-shaped trophozoites with a single nucleus, various vacuoles and spine-like structures called acanthopodia (Fig. 2). Trophozoites were between 20 and 30 μm long. Cysts were detected by their star, triangular, or square endocysts and measured 10 μm.
The 17 Acanthamoeba isolates amplified a 500-bp product using the JDP primer pairs. Alignment analysis of the isolated Acanthamoeba spp. using BLAST revealed that these isolates belonged to T3 (BN39, 43, 59, and 67, 23.5%), mixed T3–T4 (BN2, 5.9%), T4 (58.8%), T5 (BN63, 5.9%), and T13 (BN38, 5.9%) genotypes (Table 2). Two T4 genotype isolates (BN3 and BN10) corresponded to A. polyphaga, four T3 genotype isolates (BN39, BN43, BN59, and BN67) corresponded to A. grifinii, and the T5 genotype isolate (BN63) corresponded to A. lenticulata. It is noteworthy that mixed contamination of T4 and T3 was found in one sample (BN2) that belonged to a household well (Table 2). The BLAST algorithm calculates similarity scores for local alignments including the query coverage (percent of the query sequence that overlaps the subject sequence) and max identity (percent of similarity between the query and the subject sequences over the length of the coverage area). The present BLAST analysis of the obtained sequences reflected a high percentage of query coverage and identity with the gene deposited in the gene bank (Table 2).
Table 2.
Locality, sources, and isolated genotypes from drinking and recreational water sources of East Azerbaijan, northwest Iran, and their thermo-tolerance and osmo-tolerance ability.
| CODE | LOCALITY | SOURCE | GENOTYPE | GROWTH AT 37 °C | GROWTH AT 40 °C | GROWTH AT MANNITOL 0.5 M/1 M | QUERY COVERAGE/MAX. IDENTITY (%) | ACCESSION NO. |
|---|---|---|---|---|---|---|---|---|
| BN-1 | Kaleybar | Coldspring | T4 | + | + | +/+ | 96/99 | KR07213 |
| BN-2 | Kaleybar | Well | T3–T4 | + | + | +/+ | 89/99 100/100 |
KR07214 KR07215 |
| BN-3 | Kaleybar | River | T4 | + | + | +/+ | 97/99 | KR07216 |
| BN-10 | Kaleybar | Coldspring | T4 | + | + | +/+ | 96/99 | KR07217 |
| BN-13 | Kaleybar | Coldspring | T4 | + | + | +/+ | 98/99 | KR07218 |
| BN-25 | Khodaafarin | Coldspring | T4 | − | − | −/− | 97/99 | KR07219 |
| BN-30 | Khodaafarin | Coldspring | T4 | + | + | +/+ | 96/99 | KR07220 |
| BN-38 | Jolfa | Tap water | T13 | + | + | +/+ | 100/100 | KR07221 |
| BN-39 | Khodaafarin | Tap water | T3 | + | + | +/+ | 100/100 | KR07222 |
| BN-41 | Jolfa | Tap water | T4 | + | + | +/+ | 96/98 | KR07223 |
| BN-43 | Jolfa | Tap water | T3 | + | + | +/+ | 100/100 | KR07224 |
| BN-51 | Jolfa | Hotspring | T4 | + | + | +/+ | 94/97 | KR07225 |
| BN-55 | Jolfa | Tap water | T4 | + | + | +/+ | 97/99 | KR07226 |
| BN-59 | Jolfa | Tap water | T3 | + | + | +/+ | 100/100 | KR07227 |
| BN-60 | Jolfa | Tap water | T4 | − | − | +/+ | 99/98 | KR07228 |
| BN-63 | Jolfa | Tap water | T5 | − | − | −/− | 100/100 | KR07229 |
| BN-67 | Jolfa | Tap water | T3 | − | − | −/− | 100/100 | KR07230 |
Through thermo- and osmo-tolerance tests, it was found that 88.23% of isolates were resistant to 37 °C, 40 °C temperature and 0.5 M and 1 M osmolarity (Table 2). Only three isolates belonging to T4, T5, and T3 (BN25, BN63, and BN67, respectively) were not resistant (Table 2). One of the resistant isolates belonging to T4 (BN51) was isolated from a hot spring with a temperature of 68 °C, and another one belonging to T4 (BN13) was isolated from a cold spring with a temperature of 4 °C.
Discussion
The present study indicates the presence of Acanthamoeba spp. in 25.4% of water resources in the studied region. This relatively high occurrence of Acanthamoeba spp. in water resources is in accordance with a study by Rezaeian et al, which revealed a significant increase of AK in Iran.26 This region is one of the most famous tourist attractions in East Azerbaijan Province. With respect to the frequent human activity in the studied sampling sites, the presence of the Acanthamoeba is a potential hazard to the public health of native people and tourists.
In the present study, more tap water sources tested positive for Acanthamoeba than the other sources, as shown in Table 1. This result confirms the resistance of the cyst stage of Acanthamoeba to chlorine (which is the main and sometimes only material used for cleaning the water). This relatively high occurrence can be caused by cyst formation and may highlight that, despite filtration, chlorination, and treatment processes, amoebae are able to colonize the water distribution systems in the area, probably by biofilm formation, as previously suggested in other regions by Cabral et al.27
Also, isolation of a pathogenic genotype of Acanthamoeba (thermo- and osmo-resistant T4) from one hot-spring located in Jolfa County is important, because this spring is used for therapeutic purposes. Another important finding is the high occurrence of Acanthamoeba in cold springs, because these springs are important recreational centers. The present study reveals that T4 is the predominant environmental genotype; because of its higher virulence and increased ability to transmit between hosts, the predominance of T4 increases the risk of infection in the studied region.8 The predominance of T4 is in accordance with a study by Niyyati et al conducted on river water in Tehran, but contradicts the findings of Huang and Hsu, who found T15 to be the predominant type in springs of recreational areas in Taiwan.28,29 T4 was the most frequently isolated genotype from clinical cases; however, other detected genotypes in the current study (T3, T5, and T13) are pathogenic to humans, too.10,11 The T5 genotype (A. lenticulata), the genotype that was isolated in this study, was first reported as an agent of AK by Spanakos et al in Greece and the world in 2006.30 Furthermore, there are various reports of keratitis that resulted from A. polyphaga, such as a study by Jones et al.31 As far as we know, this is the first study to report the genotype T13 in water sources in Iran.
The isolation of Acanthamoeba from both cold and hot springs (4 °C and 68 °C, respectively) suggests the resistance of Acanthamoeba spp. in wide temperature ranges (a range of 64 °C in the present study). Notably, isolates BN25, BN30) belonging to the T4 genotype and isolates (BN60, BN63) belonging to the T5 genotype revealed different tolerance levels to high temperature and osmolarity due to the level of heat shock proteins (HSP60 and HSP70) secreted by amoebae strains. This is consistent with previous studies, which showed that the same genotypes could show different tolerance levels to high temperature and osmolarity.8,32 Mirjalili et al suggested that some T4 types have less pathogenic effects in vivo and in vitro.33 Indeed, the ability of pathogenic Acanthamoeba to secrete high levels of heat shock proteins (HSP60 and HSP70) have led researchers to set up a simple plating assay for detecting pathogenic Acanthamoeba from nonpathogenic strains. Interestingly, the nonpathogenic strains were within T4, T3, and T5 genotypes. However, more tests, including cell culture assay and in vivo studies, are required to pathogenically evaluate the isolated amoebae.
In Iran, several studies have reviewed the occurrence of Acanthamoeba spp. in water resources of different parts of the country.34,35 Some previousstudies have reported the presence of FLA in hot springs, particularly two studies that were carried out in Ardebil Province.36,37 Acanthamoeba spp. were reported from other water resources of Iran as well; for example, one study reported their occurrence of 30% in surface waters of Gilan Province.38 Nazar et al isolated T4 and T5 genotypes with an occurrence of 32% in recreational areas of Tehran.39 Niyyati et al in 2015 also reported pathogenic genotypes belonging to T3, T4, T5, and T11 isolated from tap waters of tourist attractions on Kish Island in southern Iran.40 Tania Tanveer et al found seven pathogenic and nonpathogenic genotypes of Acanthamoeba spp., including T2–T10, T4, T5, T7, T15, T16, and T17, in water resources of Khyber, Pakhtunkhwa, Pakistan. In their study, 32 out of 35 (92%) samples were positive for Acanthamoeba spp.; which is higher than that in the current study, which may be caused by climate differences between the two regions.41 In all of these studies (as in the current study), T4 was detected; this result demonstrates that T4 is the most predominant genotype in environmental sources.
Conclusion
In conclusion, the present research showed the high occurrence of Acanthamoeba in water sources including drinking water in East Azerbaijan. Based on the results, it is clear that the pore size of the filtration membranes and the filtration process are not able to eliminate amoebae. To decrease infections of Acanthamoeba spp., tap water and hot springs must be monitored and disinfected with appropriate disinfectants by health authorities. Indeed, the disinfectant type and dose, as well as improved purification processes such as filter pore sizes, are crucial factors in eliminating Acanthamoeba from drinking and hot spring water sources. Furthermore, posting warning signs in recreational areas, such as cold springs and rivers, may be useful for decreasing Acanthamoeba spp. infections.
Acknowledgments
We are grateful to Dr. Niloofar Taghipoor and Miss Samira Dodangeh for their kind assistance.
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
FUNDING: This work was funded by the Shahid Beheshti University of Medical Sciences. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments and made critical revisions and approved final version: MN. Performed the experiments: HB, ZL. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Marciano-Cabral F, Puffenbarger R, Cabral GA. The increasing importance of Acanthamoeba infections. J Eukaryot Microbiol. 2000;47(1):29–36. doi: 10.1111/j.1550-7408.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier JS, Miller D, Liang B, Capriotti JA. In vitro efficacy of a povidone-iodine 0.4% and dexamethasone 0.1% suspension against ocular pathogens. J Cataract Refract Surg. 2011;37(4):763–6. doi: 10.1016/j.jcrs.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non- opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34(9):1001–27. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Thomas V, Loret JF, Jousset M, Greub G. Biodiversity of amoebae and amoebae- resisting bacteria in a drinking water treatment plant. Environ Microbiol. 2008;10(10):2728–45. doi: 10.1111/j.1462-2920.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 5.Magnet A, Henriques-Gil N, Galván-Diaz AL, Izquiedo F, Fenoy S, del Aguila C. Novel Acanthamoeba 18S rRNA gene sequence type from an environmental isolate. Parasitol Res. 2014;113(8):2845–50. doi: 10.1007/s00436-014-3945-2. [DOI] [PubMed] [Google Scholar]
- 6.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free- living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–95. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013;29(4):181–7. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012;5(6):262. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grün A-L, Stemplewitz B, Scheid P. First report of an Acanthamoeba genotype T13 isolate as etiological agent of a keratitis in humans. Parasitol Res. 2014;113(6):2395–400. doi: 10.1007/s00436-014-3918-5. [DOI] [PubMed] [Google Scholar]
- 12.Stothard DR, Schroeder-Diedrich JM, Awwad MH, et al. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J Eukaryot Microbiol. 1998;45(1):45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seal D. Acanthamoeba keratitis update – incidence, molecular epidemiology and new drugs for treatment. Eye. 2003;17(8):893–905. doi: 10.1038/sj.eye.6700563. [DOI] [PubMed] [Google Scholar]
- 14.Moore MB, McCulley JP, Luckenbach M, et al. Acanthamoeba keratitis associated with soft contact lenses. Am J Ophthalmol. 1985;100(3):396–403. doi: 10.1016/0002-9394(85)90500-8. [DOI] [PubMed] [Google Scholar]
- 15.Kilvington S, Larkin DF, White DG, Beeching JR. Laboratory investigation of Acanthamoeba keratitis. J Clin Microbiol. 1990;28(12):2722–5. doi: 10.1128/jcm.28.12.2722-2725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winck MAT, Caumo K, Rott MB. Prevalence of Acanthamoeba from tap water in Rio Grande do Sul, Brazil. Curr Microbiol. 2011;63(5):464–9. doi: 10.1007/s00284-011-0003-5. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo-Morales J, Ortega-Rivas A, Martínez E, et al. Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile Delta region, Egypt. Acta Trop. 2006;100(1):63–9. doi: 10.1016/j.actatropica.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Winiecka-Krusnell J, Linder E. Bacterial infections of free-living amoebae. Res Microbiol. 2001;152(7):613–9. doi: 10.1016/s0923-2508(01)01240-2. [DOI] [PubMed] [Google Scholar]
- 19.Rezeaian M, Farnia S, Niyyat iM, Rahimi F. Amoebic keratitis in Iran (1997–2007) Iran J Parasitol. 2007;2(3):1–6. [Google Scholar]
- 20.Niyyati M, Lorenzo-Morales J, Rezaie S, et al. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009;121(3):242–5. doi: 10.1016/j.exppara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Rezaeian M, Niyyati M, Salehi M, et al. Isolation of Acanthamoeba spp. from different environmental sources. Iran J Parasitol. 2008;3(1):44–7. [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. Vol. 1. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Matin A, Jeong SR, Faull J, Rivas AO, Khan NA. Evaluation of prokaryotic and eukaryotic cells as food source for Balamuthia mandrillaris. Arch Microbiol. 2006;186(4):261–71. doi: 10.1007/s00203-006-0142-4. [DOI] [PubMed] [Google Scholar]
- 24.Booton GC, Kelly DJ, Chu YW, et al. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J Clin Microbiol. 2002;40(5):1621–5. doi: 10.1128/JCM.40.5.1621-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder JM, Booton GC, Hay J, et al. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39(5):1903–11. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niyyati M, Rahimi F, Lasejerdi Z, Rezaeian M. Potentially pathogenic free-living amoebae in contact lenses of the asymptomatic contact lens wearers. Iran J Parasitol. 2014;9(1):14–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Marciano-Cabral F, Jamerson M, Kaneshiro E. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J Water Health. 2010;8(1):71–82. doi: 10.2166/wh.2009.129. [DOI] [PubMed] [Google Scholar]
- 28.Niyyati M, Lasjerdi Z, Nazar M, Haghighi A, Nazemalhosseini Mojarad E. Screening of recreational areas of rivers for potentially pathogenic free-living amoebae in the suburbs of Tehran, Iran. J Water Health. 2012;10(1):140–6. doi: 10.2166/wh.2011.068. [DOI] [PubMed] [Google Scholar]
- 29.Huang S-W, Hsu BM. Isolation and identification of Acanthamoeba from Taiwan spring recreation areas using culture enrichment combined with PCR. Acta Trop. 2010;115(3):282–7. doi: 10.1016/j.actatropica.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Spanakos G, Tzanetou K, Miltsakakis D, Patsoula E, Malamou-Lada E, Vakalis NC. Genotyping of pathogenic acanthamoebae isolated from clinical samples in Greece – report of a clinical isolate presenting T5 genotype. Parasitol Int. 2006;55(2):147–9. doi: 10.1016/j.parint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Jones D. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningio-encephalitis. Trans Ophthalmol Soc U K. 1975;95:221–32. [PubMed] [Google Scholar]
- 32.Memari F, Niyyati M, Haghighi A, Seyyed Tabaei SJ, Lasjerdi Z. Occurrence of pathogenic Acanthamoeba genotypes in nasal swabs of cancer patients in Iran. Parasitol Res. 2015;114(5):1907–12. doi: 10.1007/s00436-015-4378-2. [DOI] [PubMed] [Google Scholar]
- 33.Mirjalali H, Niyyati M, Abedkhojasteh H, Babaei Z, Sharifdini M, Rezaeian M. Pathogenic assays of Acanthamoeba belonging to the T4 genotype. Iran J Parasitol. 2013;8(4):530. [PMC free article] [PubMed] [Google Scholar]
- 34.NIYYATI M, REZAEIAN M. Current Status of Acanthamoeba in Iran: A Narrative Review Article. Iranian Journal of Parasitology. 2015;10(2):157–63. [PMC free article] [PubMed] [Google Scholar]
- 35.Behniafar H, Niyyati M, Lasjerdi Z, Dodangeh S. High Occurrence of Potentially Pathogenic Free Living Amoebae in Water Bodies of Kaleybar and Khodaafarin, East Azerbaijan Province. Current World Environment. 10(Special Issue):1–5. [Google Scholar]
- 36.Badirzadeh A, Niyyati M, Babaei Z, Amini H, Badirzadeh H, Rezaeian M. Isolation of free-living amoebae from sarein hot springs in Ardebil Province, Iran. Iran J Parasitol. 2011;6(2):1. [PMC free article] [PubMed] [Google Scholar]
- 37.Solgi R, Niyyati M, Haghighi A, et al. Thermotolerant Acanthamoeba spp. isolated from therapeutic hot springs in Northwestern Iran. J Water Health. 2012;10(4):650–6. doi: 10.2166/wh.2012.032. [DOI] [PubMed] [Google Scholar]
- 38.Niyyati M, Nazar M, Lasjerdi Z, Haghighi A, Nazemalhosseini E. Reporting of T4 genotype of Acanthamoeba isolates in recreational water sources of Gilan Province, Northern Iran. Novelty Biomed. 2015;3(1):20–4. [Google Scholar]
- 39.Nazar M, Haghighi A, Niyyati M, et al. Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. J Water Health. 2011;9(3):603–8. doi: 10.2166/wh.2011.152. [DOI] [PubMed] [Google Scholar]
- 40.Niyyati M, Lasgerdi Z, Lorenzo-Morales J. Detection and molecular characterization of potentially pathogenic free-living amoebae from water sources in KishIsland, Southern Iran. Microbiol Insights. 2015;8(suppl 1):1. doi: 10.4137/MBI.S24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanveer T, Hameed A, Muazzam AG, Jung SY, Gul A, Matin A. Isolation and molecular characterization of potentially pathogenic Acanthamoeba genotypes from diverse water resources including household drinking water from Khyber Pakhtunkhwa, Pakistan. Parasitol Res. 2013;112(8):2925–32. doi: 10.1007/s00436-013-3465-5. [DOI] [PubMed] [Google Scholar]