Abstract
A 61-year-old man with relapsing-remitting multiple sclerosis developed extranodal large B-cell lymphoma of the stomach following monthly natalizumab infusions for 6 years. Development of lymphoproliferative disorders increases with chronic use of immunosuppression. Cases of primary central nervous system lymphoma as well as one case of peripheral T-cell lymphoma have previously been reported with natalizumab use. Given the absence of a known association between multiple sclerosis and extranodal presentations of diffuse large B-cell lymphoma, a causal association with natalizumab administration cannot be excluded.
Keywords: natalizumab, multiple sclerosis, Non-Hodgkin lymphoma of the stomach
Introduction
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of disorders with varied clinical presentations. Primary extran-odal NHL refers to initial disease involvement in any organ or tissue other than lymph nodes or the spleen. All histologic subtypes of nodal lymphomas can arise extranodally but over 90% of cases are due to diffuse large B-cell lymphoma and mucosa-associated lymphoid tissue (MALT).1 Diffuse large B-cell lymphoma (DLBCL) represents approximately 30% of all lymphomas and is the most common lymphoma subtype worldwide.2 Chronic immunosuppression remains a signifi-cant risk factor underlying the development of both nodal and extranodal DLBCL.3
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS). Monoclonal antibody-based treatment offers new therapeutic options for the management of MS. Natalizumab is a humanized recombinant monoclonal antibody that binds to the α4 subunit of the α4β1 integrin (VLA-4) and interferes with the migration of leukocytes into the CNS.4 Initially approved for the treatment of relapsing forms of MS in 2005, natalizumab was voluntarily withdrawn from the market after two patients were diagnosed with progressive multifocal leukoencephalopathy (PML). In 2006, natalizumab was reapproved with use in the United States restricted to highly active relapsing-remitting MS and patients not responding to or tolerating first-line therapy. Natalizumab-induced impairment of immune surveillance in the CNS is thought to be one possible explanation for the increased incidence of PML and was implicated as a possible causative factor contributing to primary central nervous system lymphoma (PCNSL) after natalizumab use.5 Additional effects of natalizumab on the function and differentiation of immune cells including B lymphocytes may underlie a possible causal relationship in the development of extranodal DLBCL in patients receiving this medication.
Case Report
A 61-year-old man with a long-standing history of MS presented with fevers, unintentional weight loss, and abdominal pain for approximately 5 months. His MS history dated to July 1987 when he developed tingling that originated in his right leg and traveled up to his ear along his right side. Interim attacks included bilateral foot drop, right-sided weakness, left-sided paresthesias, and Lhermitte’s phenomena. Disease-modifying treatment did not start until 1997 at which time he received multiple lines of therapy including interferon β-1b (1997–2002), methotrexate (2001), interferon β-1a with monthly steroids (2002–2007), intravenous immunoglobulin (2005–2006), and riluzone (2006) before starting natalizumab in January 2007. Given disease stability, the patient remained on monthly natalizumab and had received 64 treatments prior to his above presentation.
An upper endoscopy demonstrated a mass lesion in the greater curvature of the stomach. Immunohistochemistry demonstrated a group of CD10(+), CD20(+), BCL-6(+), and MUM-1(+) cells with a Ki-67 of 80%–90%, consistent with a diagnosis of diffuse large B-cell lymphoma. Histologic stains for Helicobacter pylori and Epstein–Barr virus (EBV) were negative. Peripheral blood EBV polymerase chain reaction (PCR) was also negative. A positron emission tomography (PET) scan demonstrated diffuse hyper-metabolic gastric wall thickening measuring up to 3.9 cm along the upper curvature [standard uptake value (SUV) max./avg. 21.7/16.7] with minimally enlarged gastrohepatic lymph nodes (largest measuring 1.2 cm; SUV max./avg. 2.8/2.3) adjacent to the mass (Fig. 1). Natalizumab therapy was stopped.
Figure 1.
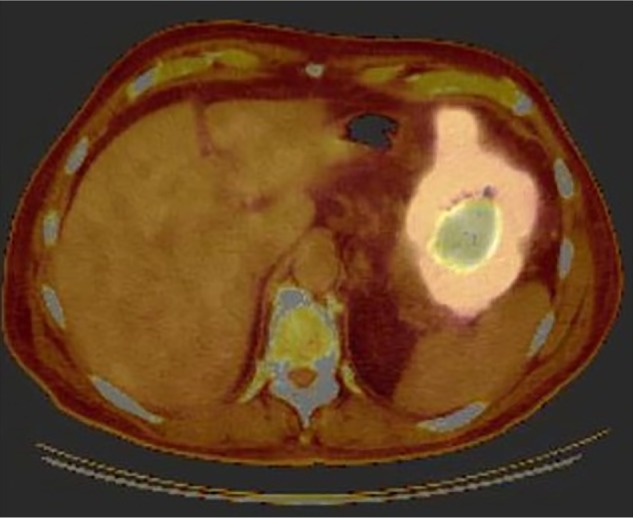
PET at diagnosis. Imaging demonstrates diffuse gastric wall thickening with SUVmax of 21.7 consistent with disease involvement.
Given a borderline performance status, the patient initially received weekly rituximab for four doses without result. Three cycles of bendamustine and rituximab were then given without any improvement in the patient’s extranodal mass. Subsequently, he received four cycles of infusional etoposide, vincristine, doxorubicin, cyclophosphamide with prednisone, and rituximab (R-EPOCH). A PET scan at completion of chemotherapy demonstrated small residual FDG (fluorodeoxyglucose) activity in the body of the stomach measuring 1.9 cm × 0.7 cm (SUV 4.4) consistent with a positive response to therapy (Fig. 2). Repeat upper endoscopy demonstrated a gastric ulcer with no evidence of persistent lymphoma on biopsy. The patient received consoli-dative radiation therapy to the involved site. His treatment course was complicated by multiple episodes of febrile neutropenia attributed to recurrent multidrug-resistant urinary tract infections due to neurogenic bladder requiring self-catheterization. The patient remains in complete remission at 8 months following treatment completion. He is off all immunosuppressive therapy for his MS given the absence of new symptoms. Written consent was obtained from the patient for publication of this study.
Figure 2.
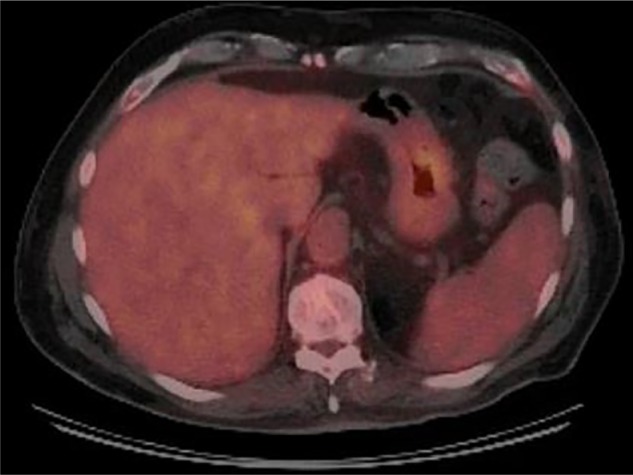
End of treatment PET demonstrating resolution of previously seen FDG uptake and overall positive response to therapy.
Discussion
This is the first report of extranodal DLBCL of the stomach in a patient receiving nataliziumab therapy for MS. No other nodal or extranodal presentations of DLBCL have been reported in the population of MS patients treated with natalizumb. In other treatment groups, an individual with Crohn’s disease was diagnosed with DLBCL over 6 months after completing natalizumab therapy.6 PCNSL and one case of peripheral T-cell lymphoma have both been described in patients with MS receiving natalizumab.7,8 Interestingly, the majority of reported cases had no identified EBV-driven etiology. Studies have shown that patients with MS do not have a predilection for the development of NHL.9
Histologically, DLBCL reflects moderate to higher grade disease activity and is often associated with a more aggressive initial presentation. In a recent study of extranodal NHL, approximately 50% of cases were DLBCL.10 The most common extranodal site is the gastrointestinal tract with involvement of the stomach composing the majority of cases.11 The overall incidence of primary extranodal NHL of the gastrointestinal tract is approximately 1 per 100,000 individuals.1 A single-center review identified 371 primary gastrointestinal NHL cases over a 4-year period of which 74.8% of cases were confined to the stomach.11 The incidence of extranodal gastrointestinal NHL appears to be increasing with H. pylori infection implicated in the majority of gastric MALT cases.10 Frequent symptoms prompting clinical evaluation include abdominal pain, nausea, and weight loss although clinical presentations of extranodal NHL vary. Genetic studies have revealed differences in lymphomagenesis between nodal and extranodal DLBCL that suggest the two diseases merit consideration as separate entities.12
The use of concurrent natalizumb in the presented case raises questions regarding a possible relationship between VLA-4 inhibition and the development of extran-odal DLBCL. Outside of its inhibitory effects on lymphocyte transportation into the CNS, natalizumab appears to decrease the migratory capacity of circulating immune cells and alter expression of genes involved in B-cell activation and differentiation.13 Although impaired immune surveillance with natalizumab appears possible mechanistically, further confirmatory studies are needed to better understand the clinical implications of this phenomenon.
It is difficult to establish a direct causal association between natalizumab use and the development of extranodal DLBCL of the stomach on the basis of one reported case. However, it is important to document this event in the available literature given prior questions regarding natalizumab use and the development of other rare lymphoproliferative disorders such as PCNSL and peripheral T-cell lymphoma. As long-term use of natalizumab increases, heightened clinical suspicion remains imperative given the potential unanticipated complications from this potent method of immunosuppression.
Footnotes
ACADEMIC EDITOR: William C. S. Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: JYL. Contributed to the writing of the manuscript: JYL. Jointly developed the structure and arguments for this paper: JYL, HVN. Made critical revisions and approved final version: JYL, DWK, AS, HVN. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19:1992–9. doi: 10.1093/annonc/mdn525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110:29–36. doi: 10.1182/blood-2007-01-041871. [DOI] [PubMed] [Google Scholar]
- 3.Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109:208–88. doi: 10.1038/bjc.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnke C, Menge T, Hartung HP, et al. Natalizumab and progressive multi-focal leukoencephalopathy: what are the causal factors and can it be avoided? JAMA Neurol. 2013;70:398–402. [Google Scholar]
- 5.Stüve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–7. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 6.Targan SR, Feagan BG, Fedorak RN, et al. International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE trial. Gastroenterology. 2007;132:1672–83. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Schweikert A, Kremer M, Ringel F, et al. Primary central nervous system lymphoma in a patient treated with natalizumab. Ann Neurol. 2009;66:403–6. doi: 10.1002/ana.21782. [DOI] [PubMed] [Google Scholar]
- 8.Schowinsky J, Corboy J, Vollmer T, Kleinschmidt-DeMasters BK. Natalizumab- associated complication? First case of peripheral T cell lymphoma. Acta Neuropathol. 2012;123:751–2. doi: 10.1007/s00401-012-0967-7. [DOI] [PubMed] [Google Scholar]
- 9.Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the inter-lymph consortium. Blood. 2008;111:4029–38. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell JM, Auer-Grzesiak I, Zhang J, Andrews CN, Stewart D, Urbanski SJ. Increase incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol. 2012;26:452–6. doi: 10.1155/2012/480160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch P, del Valle F, Berdel WE, et al. German Multicenter Study Group Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3861–73. doi: 10.1200/JCO.2001.19.18.3861. [DOI] [PubMed] [Google Scholar]
- 12.Kuper-Hommel MJ, van de Schans SA, Vreugdenhil G, van Krieken JH, Coebergh JW. Undertreatment of patients with localized extranodal compared with nodal diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:1698–705. doi: 10.3109/10428194.2012.753447. [DOI] [PubMed] [Google Scholar]
- 13.Lindberg RL, Achtnichts L, Hoffmann F, Kuhle J, Kappos L. Natalizumab alters transcriptional expression profiles of blood cell subpopulations of multiple sclerosis patients. J Neuroimmunol. 2008;194:153–64. doi: 10.1016/j.jneuroim.2007.11.007. [DOI] [PubMed] [Google Scholar]


