Abstract
The presence of cleft lip with or without cleft palate (CL/P) in family members of cancer patients was investigated. An epidemiological questionnaire including family history of cancer and congenital oral cleft malformations was administered to 168 cancer survivors and a population-based sample of 170 healthy subjects. In the control group, 1.2% reported a family member with CL/P; among cancer survivors the figure was 4.2% (odds ratio: 3.7; 95% confidence interval: 0.75–17.8; p = .07). Among cancer survivors with a family member with CL/P, there was an apparent excess of testicular cancer and melanoma in comparison with the cancer survivors with no family history of CL/P. These preliminary results suggest a common etiologic background for cancer and CL/P.
Keywords: Cancer susceptibility genes, Congenital defects, Family studies
INTRODUCTION
Cancer is a multifactorial disease in which both genetic and environmental factors play a significant role. Preventive plans for decreasing cancer incidence include both the removal of environmental agents known to be carcinogens and the identification of cancer susceptibility gene polymorphisms or mutations. Subjects carrying specific susceptibility genes can be the target of more aggressive screening programs and chemo-preventive treatments, in addition to being properly advised on their lifestyle behavior.
Several studies have reported an association between pediatric cancers and congenital defects (1–4) and have suggested that these two conditions may have in common variations in genes that regulate growth and tissue development.
One of the most common congenital malformations is cleft lip with or without cleft palate (CL/P), whose prevalence at birth is roughly 1 in 1,000 live births (5). The association between CL/P and pediatric cancer has been controversial (6–9); however, aggregation of cancer in CL/P families as well as the occurrence of adulthood cancers in children born with CL/P have been documented. One study has shown that parents of kids with sporadic CL/P have a higher risk of developing cancer than control families (10); the other reported an increased risk of cancer in adulthood in a population-based cohort of CL/P subjects (11). Genes that have been suspected to be at the basis of this association are the fibroblast signaling pathway (FGF), epithelial cadherin (CDH1), and AXIS inhibition protein 2 (AXIN2) (12). The types of cancer more frequently associated with mutations in the above-mentioned genes are colon-rectal and breast cancers.
The few epidemiological studies conducted so far have addressed the presence of cancer in CL/P subjects and their families; no study, however, has been published on the presence of CL/P in family members of cancer patients. The finding of an increase risk of CL/P in families ascertained through the identification of a cancer case would strengthen the hypothesis that common genetic factors may play a role. In addition, more aggressive cancer preventive measures could be directed toward family members of CL/P cases, if an association is confirmed.
MATERIAL AND METHODS
During 2007 and 2008, a questionnaire on demographics, lifestyle, and family history of cancer and congenital oral cleft malformations was administered to two separate groups of subjects: a cohort of cancer survivors and a population-based sample of healthy subjects. The catching area of the population sample of controls included the area where the hospital chosen for recruitment of cancer survivors was located.
Healthy subjects
Participants from the general population were enrolled in Pittsburgh, Pennsylvania. Subjects 18 years of age and older were asked to join a population-based registry of healthy people from Pittsburgh and surrounding communities. These participants were recruited through flyers posted at churches, community centers, outpatient clinics, health fairs, school events, and libraries and taken out in the local newspapers and the University of Pittsburgh Medical Center newsletters. Institutional Review Board approval was obtained from the University of Pittsburgh. A sample of 176 subjects were administered the supplemental questionnaire on family history for congenital oral cleft malformations, and 170 of them (97%) completed it.
Cancer survivors
Cancer survivors were recruited in one of the hospitals affiliated with the University of Pittsburgh Cancer Center, located in Latrobe (Pennsylvania), starting from January 2007. Patients who were treated for cancer in the past, who were considered in remission for at least 6 months, and who were scheduled for an appointment for a routine checkup were considered eligible for the survey (n = 602).
A trained nurse would approach the patient after the routine checkup and propose participation in the study. Patients who agreed would sign an informed consent and fill the same self-administered questionnaire completed by the healthy population. Starting from December 1, 2007, through June 1, 2008, the supplemental questionnaire on family history for oral cleft congenital malformations was added to the main questionnaire. Out of 206 cancer survivors contacted during that period, 168 (82%) agreed to participate in the study.
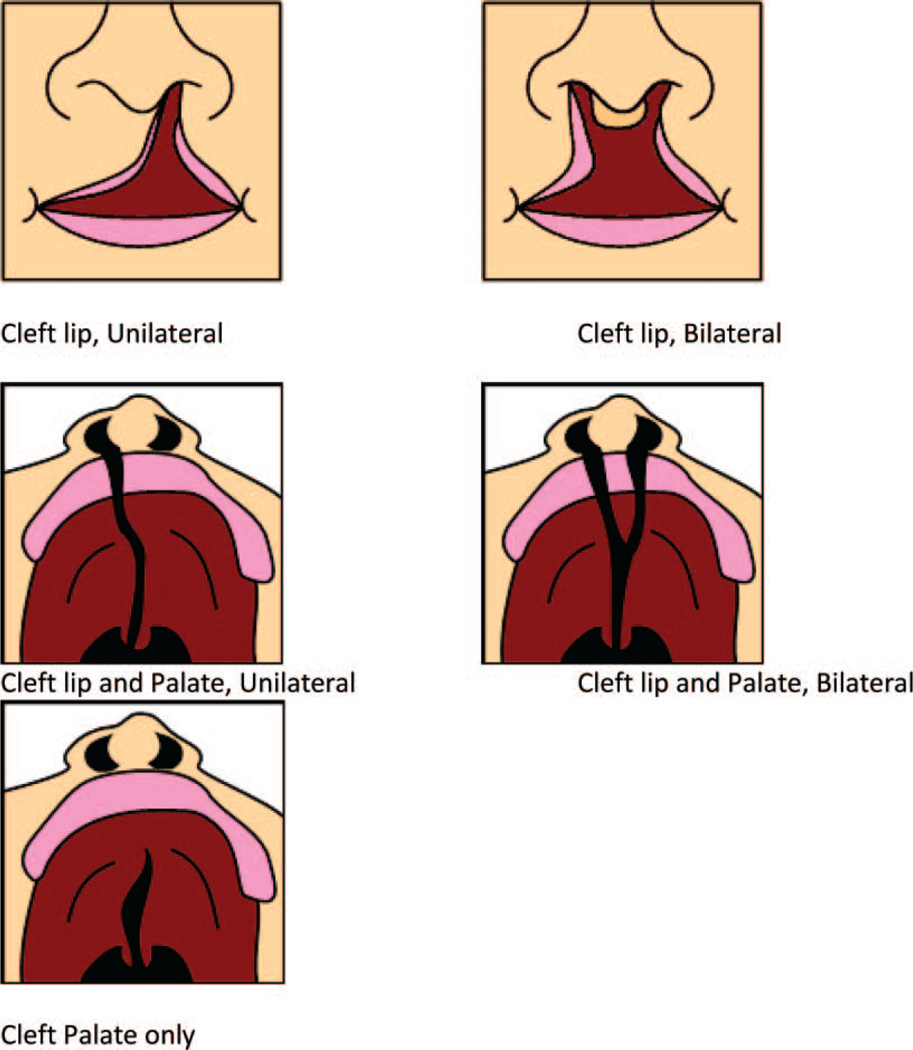
The questionnaire included a general section on demographics, some behavioral risk factors such as smoking and drinking habits, a detailed family history for cancer in first-degree relatives, and a section investigating the presence of CL/P in first and second-degree family members. Information collected included the type of CL/P (unilateral, bilateral, cleft palate only, lip only, or cleft lip plus palate) and the relationship between the index case and the family member. A schematic drawing of the various cleft palate subtypes was included in the questionnaire in order to facilitate and standardize the answers (Figure 1).
Figure 1.
Schematic graphs utilized for the questionnaire to identify family members with CL/P.
Statistical analysis
Comparison between cancer survivors and healthy subjects was performed using chi square for categorical variables and t-test for continuous variables. Statistical significance was set at .05. Conditional logistic regression was used to assess the association between cancer and CL/P. The ratio of the number of cases observed for each cancer type in survivors with family history of CL/P was divided by the number of cases expected in survivors without family history of CL/P (O/E). All analyses were conducted with SAS statistical package (Cary, North Carolina).
RESULTS
The questionnaire was answered by 338 subjects: 168 cancer survivors and 170 healthy subjects. The description of the population is reported in Table 1. Healthy subjects were significantly younger and more educated than cancer survivors. In addition, healthy subjects included a significantly larger proportion of ethnicities other than Whites.
Table 1.
Description of the Study Population
| Variable | Cancer Survivors (n = 168) Mean ± SD |
Healthy Population (n = 170) Mean ± SD |
p value |
|---|---|---|---|
| Age (years) | 62.61 ± 11.15 | 35.84 ± 15.16 | .0001 |
| Number of 1st degree relatives | 7 ± 3 | 5 ± 2 | |
| N (%) | N (%) | ||
| Gender | |||
| Males | 43 (26) | 51 (30) | |
| Females | 124 (74) | 119 (70) | n.s. |
| Education | |||
| ≤ High school | 73 (43.7) | 10 (5.9) | |
| Some college | 48 (28.7) | 52 (30.6) | |
| College graduate | 29 (17.4) | 66 (38.8) | |
| Postgraduate | 17 (10.2) | 42 (24.7) | .0001 |
| Ethnicity | |||
| White | 166 (99.4) | 146 (85.9) | |
| Other | 1 (0.6) | 24 (14.1) | .0001 |
| Family history of cancer (first degree) | 107 (64) | 58 (34) | .001 |
| Cancer type | n/a | ||
| Breast | 81(48.5) | ||
| Colon-rectal | 20 (12) | ||
| Lymphoma | 12 (7.2) | ||
| Lung | 9 (5.4) | ||
| Skin | 7 (4.1) | ||
| Testicular | 4 (2.4) | ||
| Gastro-esophageal | 2 (1.2) | ||
| Sarcoma | 2 (1.2) | ||
| Othersa | 8(4.8) | ||
| Two primaries | 20 (12) | ||
| Three primaries | 1 (0.6) | ||
| Missing | 1 (0.6) | ||
| Cleft in family | 7 (4.2) | 2 (1.2) | .07 |
Note: Totals may vary because of the missing values.
Includes one of each of the following: cervix, bladder, endometrial, kidney, oral, ovary, prostate, or eye.
As expected, among cancer survivors the most represented cancers were those with higher survival, i.e., breast, colon-rectal, lymphoma/leukemia. A large proportion of survivors reported a history of two or more primary cancers (20/167, 12%).
The presence of a family member (including self) with CL/P was 1.2% in the sample of the general population and 4.2% among cancer survivors (p = .07; Table 1). The odds ratio of CL/P with cancer was 3.7 (95% confidence interval: 0.75–17.8). Because a difference in ethnicity was observed in the study populations, the analysis was also restricted to Whites; the prevalence of CL/P was 4.3% in cancer survivors and 1.4% in healthy controls. In order to further address differences in demographic characteristics, a stratified analysis according to quartiles of age (≤30, 31–52, 53–64, and >64 years) was performed; the prevalence of CL/P was 0%, 3.1%, 6.8%, and 2.6% among cancer survivors and 0%, 2.0%, 3.9%, and 0% among healthy subjects respectively. When the data were stratified according to education, all the cases were among people with high school or some college education; no case was reported in families of people who had completed college or more.
Table 2 shows the distribution in cancer type in cancer survivors who reported a family member with CL/P and cancer survivors who had no family members with CL/P. There was no overall difference in cancer type in the two groups; breast and colon-rectal cancers were the two most prevalent cancers in the two groups. In the group of cancer survivors who reported a family member with CL/P there was an excess of testicular cancer and melanoma in comparison with the cancer survivors who had no family history of CL/P.
Table 2.
Association Between Cancer Type in Cancer Survivors and CL/P in Family Members
| Family History for CL/P | Observed/ Expected |
||
|---|---|---|---|
| Yes | No | ||
| Cancer Survivors | 7 | 161 | |
| Breast | 3 (42.8%) | 78 (49%) | 0.9 |
| Colon-rectal | 1 (14.3%) | 19 (12%) | 1.2 |
| Melanoma | 1 (14.3%) | 4 (2.5%) | 5.7 |
| Testicular | 1 (14.3%) | 3 (1.9%) | 7.6 |
| Two primaries | 1 (14.3%)a | 19 (12%) | 1.2 |
| Three primaries | 0 | 1 (0.6%) | – |
| Others/missing | 0 | 35 (22%) | – |
Note: Overall chi square (Fisher exact test) = 0.54.
Breast, lung.
There was no association between the type of CL/P in the family member and the type of cancer observed in the cancer survivors, nor was a pattern observed between cleft type and family relationship between the cleft cases and the cancer survivors (Table 3). There was an excess of female CL/P reported by the population under study, but this did not correlate with the gender of the index case.
Table 3.
Description of the Family Member With CL/P and of the Corresponding Index Case in the Same Family
| Type of CL/P in Family Member |
N (%) | Description of Index Case | CL/P Family Relationship to the Index Case |
Average Age of Index Case (Mean ± SD) |
|---|---|---|---|---|
| Lip only, unilateral | 3 (33) | Breast + lung | Niece | 58.3 ± 20.6 |
| Colon-rectal | Nephew | |||
| TESTICLES | Nephew + niece | |||
| Lip only, bilateral | 1 (11) | Healthy subject | Niece | 44 |
| Palate only | 1 (11) | Breast | Niece | 58 |
| Lip and palate, unilateral | 2 (23) | Healthy subject | Granddaughter | 66 ± 4.2 |
| Melanoma | Self | |||
| Lip and palate, bilateral | 1 (11) | Breast | Niece | 64 |
| Location unknown | 1 (11) | Breast | Paternal uncle | 61 |
| Total | 9 (100) | |||
| Average age index cases | 9 | 59.3 ± 12.3 | ||
| Average age remaining population | 327 | 48.8 ± 18.9a |
p values for the t-test for unequal variances = .034.
DISCUSSION
This study assesses the prevalence of CL/P in family members of cancer patients and suggests that CL/P is much more frequent (up to four times) in families of cancer survivors in comparison with families of a population-based sample of controls. The results are not statistically significant, possibly because of the small sample size of this study relative to the rarity of CL/P occurrence. The power to detect a statistically significant difference in the present study was in fact only 60%.
The interpretation of these preliminary results should be done with caution. Although it cannot be dismissed that this could be a chance finding, it is possible that the result is another piece of evidence in favor of the hypothesis of common genetic factors at the basis of the etiology of both CL/P and cancer. Similar to the excess of CL/P in relatives of cancer survivors, this study shows that cancer survivors have a significantly higher proportion of relatives with cancer, as expected from previous literature; however, there was no overlap between relatives with cancer and relatives with CL/P. Probably a larger study could address a possible link between cancer and CL/P in relatives of cancer survivors.
The strengths of this study are the use of a standardized questionnaire surveying family history for CL/P, the inclusion of population-based controls drawn from a healthy registry assembled for research purposes, the large number of well-characterized cancer survivors, and the more efficient study design, which starts from a more common disease (cancer) and aims at detecting a rarer condition (CL/P) in family members.
The fact that more CL/P cases in the family were reported by cancer survivors than by controls cannot be ascribed to recall bias among cancer survivors or to differential reporting according to case/control status, since CL/P is not known by the general population or by health care providers to be a risk factor for cancer. Underreporting is also unlikely, since this is a very obvious congenital defect, and a schematic graph of the possible phenotypic presentations of CL/P was included in the questionnaire.
We have observed in this study more female than male CL/P in relatives; the epidemiology of sporadic CL/P indicates a higher prevalence of this abnormality in males than in females. It is possible that our result is due to the small number of subjects ascertained, by referral bias from the family members or by a specific common etiologic factor between cancer and CL/P that is associated with gender.
The two populations under study, cancer survivors and healthy controls, differ significantly in age, education, and race. The excess of White patients among cancer survivors cannot be linked to the excess of family members with CL/P reported by this group, since ethnicity plays a small role in CL/P prevalence, and if any difference exists, Asians and Native Indians seem to be more prone to show CL/P than Whites (13). In addition, a post hoc analysis restricted to Whites did not change the main result.
The confounding role of education cannot be excluded: cancer survivors are less educated and perhaps less informed of what a congenital defect is, although the graph included in the questionnaire should have helped the respondents. If less reporting was done by cancer survivors, it can be hypothesized that the real difference in CL/P prevalence in families of cancer survivors versus families of healthy controls is even greater than what was observed in the current study, thus corroborating the results. Cancer survivors are also significantly older than the control sample, and this is a limitation of this exploratory study. A stratified analysis according to age was conducted in an attempt to address this aspect, and the percentage of relatives with CL/P was higher among cancer survivors than among controls across strata of age. However, a further study with properly age-matched controls would be necessary to fully address this limitation.
Possible mechanisms at the basis of an association between CL/P and cancer are shared genetic factors. It has been reported (12, 13) that polymorphisms in genes involved in cell–cell adhesion and cell motility are associated with CL/P, and the same genes may be involved in carcinogenesis.
Polymorphisms in fibroblast signaling pathway genes, epithelial cadherin, and AXIS inhibition protein 2 have been associated with both CL/P and colon-rectal, gastric, or breast cancers, although studies in this direction are very scarce (12).
A limitation of our study is the inclusion of cancer survivors instead of newly diagnosed cancer patients. A question that cannot be addressed by the current study design is the association between CL/P and cancers that are highly fatal, such as lung cancer. Another consequence of studying cancer survivors is that the association between cancer type and CL/P in family members cannot be assessed, since survivorship is a characteristic that varies according to cancer type. In fact, the sample of cancer survivors studied here is largely composed of breast cancer, colon-rectal cancer, and lymphoma patients, three cancer types known to have good prognosis and longer survival. Future studies should propose similar design but the inclusion of incident cancer cases.
Another aspect to be addressed in future studies is the size of the families identified through the index case. Although the number of first-degree relatives was collected through the questionnaire, the information on number of second-degree relatives is lacking for both cancer survivors and healthy controls.
When we compared cancer types among cancer survivors who reported a CL/P case in their family versus cancer survivors who did not, we found in the first group (survivors with a positive family history for CL/P) an apparent excess of melanoma and testicular cancer cases. This result should be by no mean considered conclusive and has to be interpreted with caution because of the small sample size, but it deserves further investigation, since it could indicate common etiologic factors for these rare cancer types and CL/P.
This preliminary finding should be confirmed in further epidemiological studies on large cohorts of incident cancer cases, in which a detailed family history for CL/P is collected with a standardized questionnaire, along with testing for polymorphisms in candidate genes shown to be associated with both CL/P and cancer. However, if confirmed, these results could open potential new venues in the understanding of cancer etiology as well as cancer prevention in families with a history of congenital defects.
ACKNOWLEDGMENT
We are very grateful to Diane Bartels, who has completed the questionnaires collection in Latrobe. Renato Menezes helped in designing the part of the questionnaire on oral cleft malformations. The study was partially supported by the National Istitutes of Health grant CA133922-01A1 to ET.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.Windham GC, Bjerkedal T, Langmark F. A population-based study of cancer incidence in twins and in children with congenital malformations or low birth weight, Norway, 1967–1980. J. Am Epidemiol. 1985;121:49–56. doi: 10.1093/oxfordjournals.aje.a113982. [DOI] [PubMed] [Google Scholar]
- 2.Mili F, Khoury MJ, Flanders WD, Greenberg RS. Risk of childhood cancer for infants with birth defects. I. A record-linkage study, Atlanta, Georgia, 1968–1988. J. Am Epidemiol. 1993;137:629–638. doi: 10.1093/oxfordjournals.aje.a116720. [DOI] [PubMed] [Google Scholar]
- 3.Mili F, Lynch CF, Khoury MJ, Flanders WD, Edmonds LD. Risk of childhood cancer for infants with birth defects. II. A record-linkage study, Iowa, 1983–1989. J. Am Epidemiol. 1993;137:639–644. doi: 10.1093/oxfordjournals.aje.a116721. [DOI] [PubMed] [Google Scholar]
- 4.Narod SA, Hawkins MM, Robertson CM, Stiller CA. Congenital anomalies and childhood cancer in B. Greatritain. J. Am G. Humenet. 1997;60:474–485. [PMC free article] [PubMed] [Google Scholar]
- 5.Mossey PA, Little J. Epidemiology of oral clefts: an international perspective. C. In: Wyszynski DF, editor. Inleft Lip and Palate: O. Fromrigin to Treatment. New York, NY: Oxford University Press; 2002. pp. 127–158. [Google Scholar]
- 6.Steinwachs EF, Amos C, Johnston D, Mulliken J, Stal S, Hecht JT. Nonsyndromic cleft lip and palate is not associated with cancer or other birth defects. J. Am G. Medenet. 2000;90:17–24. doi: 10.1002/(sici)1096-8628(20000103)90:1<17::aid-ajmg4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Nishi M, Miyake H, Takeda T, Hatae Y. Congenital malformations and childhood cancer. P. Medediatr Oncol. 2000;34:250–254. doi: 10.1002/(sici)1096-911x(200004)34:4<250::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Mertens AC, Wen W, Davies SM, Steinbuch M, Buckley JD, Potter JD, Robison LL. Congenital abnormalities in children with acute leukemia: a report from the Children’s G. Cancerroup. J Pediatr. 1998;133:617–623. doi: 10.1016/s0022-3476(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 9.Yu CC, Wong FH, Lo LJ, Chen YR. Hereditary cleft lip/palate and Wilms’ tumor: a rare association. P. Cleftalate. J. Craniofac. 2002;39:376–379. doi: 10.1597/1545-1569_2002_039_0376_hclpaw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 10.Zhu JL, Basso O, Hasle H, Winther JF, Olsen JH, Olsen J. Do parents of children with congenital malformations have a higher cancer risk? A nationwide study in Denmark. J. Br Cancer. 2002;87:524–528. doi: 10.1038/sj.bjc.6600488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bille C, Winther JF, Bautz A, Murray JC, Olsen J, Christensen K. Cancer risk in persons with oral cleft—a population-based study of 8,093 cases. J. Am Epidemiol. 2005;161:1047–1055. doi: 10.1093/aje/kwi132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menezes R, Marazita ML, Goldstein McHenry T, Cooper ME, Bardi K, Brandon C, Letra A, Martin RA, Vieira AR. AXIS inhibition protein 2, orofacial clefts and a family history of cancer. J D. Ament Assoc. 2009;140:80–84. doi: 10.14219/jada.archive.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira AR. Association between the transforming growth factor alpha gene and nonsyndromic oral clefts: A HuGE review. J. Am Epidemiol. 2006;163:790–810. doi: 10.1093/aje/kwj103. [DOI] [PubMed] [Google Scholar]