Abstract
Obesity is associated with breast cancer in post-menopausal women, and breast density is a marker of breast cancer risk. Leptin is produced by the adipose tissue, acts through receptors that are polymorphic in nature, and is considered a cancer growth factor. The relationship between Body Mass Index, leptin, leptin receptors and breast density is not well studied.
A cross-sectional analysis in 392 post-menopausal healthy women was conducted; participants provided permission to obtain copies of their most recent screening mammogram. Nonfasting plasma leptin levels were determined using a commercially available leptin ELISA kit. The Q223R genotypes of the LEPR gene were performed by polymerase chain reaction followed by restriction fragment length polymorphism analysis using DNA extracted from buffy coat samples.
A statistically significant positive relationship was observed between leptin levels and body mass index (P value<0.0001); leptin was significantly positively associated with mammography total breast area and non-dense breast area (P-value<0.0001), while was significantly inversely associated with percent breast density (P-value<0.0001). Leptin levels varied across the LEPR Q223R polymorphism, and were higher in women homozygous for the AA variant. Percent breast density decreased across the LEPR Q223R genotype, with lower percent density in women with the AA genotype. When dense area was considered for quartiles of leptin by LEPR Q223R, a significant inverse trend between leptin levels and dense breast area was observed only among women with the G/G genotype (P-trend<0.001). After adjustment for possible confounders, leptin levels were significantly inversely associated with percent breast density (p=0.01). A significant interaction between body mass index and leptin levels on percent breast density was observed (p=0.03).
These findings suggest that the association between leptin and breast density may vary by LEPR Q223R genotype, and that body mass index and leptin may act in an interactive way in determining breast density.
Keywords: breast cancer, screening, risk factors, prevention, body mass index
INTRODUCTION
Mammographic density is one of the known and strongest risk factors for breast cancer [1]. Women with the highest absolute and/or relative breast density have two to six times the risk of developing breast cancer compared with women with lower density [2–6]. Despite these findings, the underlying biological mechanisms by which breast density increases breast cancer risk remains unclear. Hormonal, genetic, and environmental factors may play a role in determining a woman’s breast density.
Increasing body mass index (BMI) is consistently inversely associated with percent breast density [7–8], and is directly associated with the non-dense area of the breast, which is mostly constituted of fat). However, obesity is an independent risk factor for breast cancer among postmenopausal women [9], a paradox that has yet to be understood. Body mass and body composition are under a multi-factorial endogenous and exogenous control. Thus, it is possible that obesity-related hormones may play a role in explaining this disparate relationship. Although studies have evaluated obesity in relation to percent breast density, limited research has evaluated the role of obesity-related hormones, including adipokines such as leptin, a potential cancer growth factor, on breast density [10].
Leptin is a hormone produced primarily by white adipose tissue that regulates appetite and energy expenditure, as well as reproductive and immune function [11]. It is suggested to promote angiogenesis and pro-inflammatory responses [13–15], to stimulate the proliferation of normal and malignant breast epithelial cells [16] and to be associated with cancer of the breast and of other sites [10, 17–18]. An inverse association between leptin levels and percent breast density has been reported, but it is considered to be mediated by BMI since it disappears after statistical adjustment [19–21]; a direct effect of leptin on percent density has not been demonstrated so far ().
Several genes are involved in leptin function and regulation [12]; polymorphisms in these genes may modify leptin levels and/or activity at the receptor site. Among the others, the LEPR polymorphism Q223R (rs1137101) is a common variant with functional significance, and is thought to be associated with an impaired signaling capacity of the leptin receptor [12]; the homozygous variant AA has been associated with higher mean circulating levels of leptin [22–24]. To date, no prior studies have evaluated leptin receptor polymorphisms in relation to breast density.
Given the complex relationship between BMI, leptin and breast density), we conducted a cross-sectional analysis in post-menopausal healthy women in order to understand the role of leptin on breast density, and to study the potential modifying effect of BMI and LEPR polymorphism on such association.
MATERIAL AND METHODS
Study Population
This is a cross-sectional analysis using controls from the Mammograms and Masses Study (MAMS). MAMS was initially designed to assess hormones in relation to mammographic density; details have been described elsewhere [25–26]. Women were enrolled through the Magee Women’s Hospital Mammographic Screening and Diagnostic Imaging Program in greater Pittsburgh area, PA. Briefly, eligible women were age 18 years or older visiting Magee-Womens Hospital (Pittsburgh, PA) or a Magee Womancare Center in the greater Pittsburgh area between September 2001 to May 2005 for one of the following reasons: (a) a breast biopsy, (b) an initial surgical consultation after breast cancer diagnosis, or (c) a routine screening mammogram. Women who reported a prior cancer history other than non-melanoma skin cancer, who consumed more than five alcoholic beverages per day, or weighed less than 110 lb (49.9 Kg) or more than 300 lb (136 Kg) were excluded. The MAMS study population consists in total of 1,133 women, 869 of which with a negative screening mammogram, or with benign breast disease; the University of Pittsburgh Institutional Review Board reviewed and approved the study protocol, and all study participants provided written informed consent.
This cross-sectional analysis was restricted to post-menopausal women (n=647). Women were classified as post-menopausal if they did not report periods in the year before enrollment, had ever used menopausal hormone therapy (MHT), had a bilateral oophorectomy, or were 50 years or older at enrollment. Women who reported a hysterectomy without bilateral oophorectomy were considered to be postmenopausal if they had ever used MHT in the past or were 50 years or older at hysterectomy. Subsequently, women who were using MHT at the time of enrollment (n=165), lacking a mammogram (n=35), had their blood drawn more than 4.5 months after the mammogram (n=16), whose questionnaire and/or blood sample was not available (n=23) or who reported a previous history of cancer (n=16) were excluded, leaving a total sample of 392 women eligible for this analysis. Additionally, laboratory data were incomplete for 27 women, thus the final sample consisted of 365 women.
Participants completed a self-administered questionnaire on demographics, medical and reproductive history, lifestyle factors, medication use, and family history of breast cancer. A non-fasting, 40 ml sample of peripheral blood was collected from the study participants at enrollment.
Mammogram
Participants’ provided permission to obtain copies of their most recent screening mammogram. The assessment of mammographic measures has been described in detail elsewhere [25]. One expert reader, masked to the identity status (benign control, well control) and demographic and risk factor characteristics of the subject, read all mammograms. Total breast area and all dense regions were measured using a compensating polar planimeter (LASICO) on the craniocaudal view with the side of breast (right or left) randomly chosen for each participant; non dense breast area was calculated by subtracting dense breast area from total breast area; percent density was calculated by dividing dense breast area by total breast area * 100. The expert reader also provided a subjective measure of film quality (excellent, good, fair, poor, very poor, extremely poor). A reproducibility study (n=28 MAMS participants) showed intraclass correlation coefficients for dense breast area, total breast area and percent density of ρ = 0.86, ρ = 0.99, and ρ = 0.89, respectively [24].
Laboratory measures
Leptin hormone levels were measured using refrozen plasma samples (1 previous freeze-thaw cycle). Nonfasting plasma leptin levels were determined using a commercially available leptin ELISA kit (Biosource International, Camarillo, Ca, USA). Batches were performed using 96 well plates which included 38 samples, 8 leptin standards and quality controls. Forty blinded duplicates (10%) were included as a means of assessing the reliability of the assay. Each sample was run in duplicate within the same plate and a coefficient of variation (CV) was calculated. All CV’s greater than 15% were repeated. The average of the two leptin measurements was used for this analysis.
The genotypes for the sequence variants in the Q223R of the LEPR gene were performed by polymerase chain reaction followed by restriction fragment length polymorphism analysis (PCR-RFLP) using DNA extracted from buffy coat samples. The genotyping was performed using a 7300 RT-PCR system (Applied Biosystem, Foster City, CA). The methods used in the genotyping of LEPR Q223R were based on those described by Gotoda et al. [27] in which a PCR was followed by MspI restriction digest to detect the A to G transition polymorphism at codon 223 of the LEPR gene. Two sequence specific oligonucleotide primers were used: Primer 5’: AAACTCAACGACACTCTCCTT and Primer 3’: TGAACTGACATTAGAGGTGAC. The specific genotypes were identified by gel electrophoresis based on the following molecular weights of the PCR product: wild type (80 bp), homozygote variant (58 bp + 22 bp), and heterozygote (80 bp + 58 bp + 22 bp). As a means of preventing cross-contamination of the PCR product, the DNA extraction and PCR set up were performed on different benches in the laboratory.
Statistical analysis
Continuous variables are presented as mean ± Standard Deviation or median (25th, 75th percentiles) and discrete variables as percentages. Wilcoxon rank sum test was performed for comparison of medians. Pearson correlation coefficients were estimated for the associations between leptin, BMI, and mammographic density outcomes (total breast area, dense area, non-dense area, percent density). The analysis of variance allowed for comparison of leptin levels and breast density across categories of the LEPR Q223R genetic polymorphism and of BMI; multiple linear regression was performed to assess the association between plasma leptin levels and percent breast density; covariates considered in the model were: age (years), time since fasting, BMI (kg/m2), batch, education (≤ high school/post-secondary education), race (white/other), smoking status (never/ever), family history of cancer (yes/no) and previous breast biopsy (yes/no). All multivariate analyses were adjusted for mammography film quality and time of blood draw. Breast density outcomes were square root transformed; leptin levels were transformed using the natural logarithm in order to reach normality of the distribution. Tests for linear trend were assessed by modeling the leptin exposure as an ordinal variable. Analyses were performed using SAS version 9.2; all P-values are two-sided.
RESULTS
A general description of the population under study (Table 1) shows that this is a sample of prevalently white, educated post-menopausal women, two thirds of which had a BMI greater that 25 kg/m2. More than eighty percent of the women had at least one pregnancy and 78% of women had at least one live birth. Roughly 18% of the sample reported a previous breast biopsy. Median leptin levels were 34.3 ng/ml. The average percent breast density was 27%.
Table 1.
Descriptive characteristics of study population (n=365)
| Characteristic | Mean ± SD Median (25th, 75th) |
N (%) | |
|---|---|---|---|
| Age (years) | 61.9 ± 8.4 | ||
| < 60 | 172 (47.1) | ||
| 60–69 | 119 (32.6) | ||
| ≥ 70 | 74 (20.3) | ||
| Race | |||
| White | 340 (93.2) | ||
| Other | 25 (6.8) | ||
| Education | |||
| ≤ High school | 93 (25.5) | ||
| Post-secondary education | 264 (72.3) | ||
| BMI (kg/m2) | 28.7 ± 6.1 | ||
| < 25 | 109 (29.9) | ||
| 25 to < 30 | 127 (34.8) | ||
| ≥ 30 | 129 (35.3) | ||
| Age at menopause (years) | 48.3 ± 5.3 | ||
| Age at menarche (years) | |||
| < 12 | 71 (19.5) | ||
| 12 | 103 (28.2) | ||
| 13 | 110 (30.1) | ||
| ≥ 14 | 80 (21.9) | ||
| Pregnancy | |||
| No | 65 (17.8) | ||
| Yes | 300 (82.2) | ||
| Number of live births | |||
| None | 82 (22.4) | ||
| 1 | 41 (11.2) | ||
| 2 | 102 (28.0) | ||
| 3 | 81 (22.2) | ||
| ≥ 4 | 59 (16.2) | ||
| History of Breast Biopsy | |||
| No | 298 (81.6) | ||
| Yes | 66 (18.1) | ||
| Oopherectomy | |||
| No | 289 (79.2) | ||
| Yes | 74 (20.3) | ||
| Family history of cancer | |||
| No | 131 (35.9) | ||
| Yes | 232 (63.6) | ||
| Smoking status | |||
| Never | 215 (58.9) | ||
| Ever | 150 (41.1) | ||
| Leptin Q223R* | |||
| Wild type | 110 (30.1) | ||
| Heterozygous | 190 (52.1) | ||
| Homozygous variant | 65 (17.8) | ||
| Leptin levels (ng/ml) | 43.0 ± 34.5 | ||
| 34.3 (18.9, 57.9) | |||
| Mammographic Density Measures | |||
| Dense area (cm2) | 35.6 (22.3, 55.8) | ||
| Non-dense area (cm2) | 104.6 (63.2, 168.0) | ||
| Total area (cm2) | 149.6 (106.2, 214.7) | ||
| Percent Breast Density | 27.0 (13.8, 40.6) | ||
Note: Percentages may not sum to 100% due to missing data;
p-value for HW equilibrium=0.27
A statistically significant positive relationship was observed between leptin levels and BMI (P-value<0.0001; Table 2); leptin was significantly positively associated with total breast area and non-dense breast area as measured by mammography (P-value<0.0001; Table 2). The relationship between leptin and percent breast density was inverse and statistically significant (P-value<0.0001). Similar results were observed when BMI was considered in relation to breast density (Table 2).
Table 2.
Correlation coefficients between leptin levels, body mass index and mammographic patterns
| Leptin (log, ng/ml) | BMI (kg/m2) | |||
|---|---|---|---|---|
| Characteristic | r | P-value | r | P-value |
| Age (years) | 0.01 | 0.8 | −0.03 | 0.6 |
| BMI (kg/m2) | 0.72 | <0.0001 | -- | -- |
| Breast Density^ | ||||
| Dense Area (cm2) | −0.06 | 0.2 | −0.03 | 0.6 |
| Non-Dense Area (cm2) | 0.55 | <0.0001 | 0.70 | <0.0001 |
| Total Area (cm2) | 0.53 | <0.0001 | 0.71 | <0.0001 |
| Percent Density | −0.41 | <0.0001 | −0.45 | <0.0001 |
square root transformed values
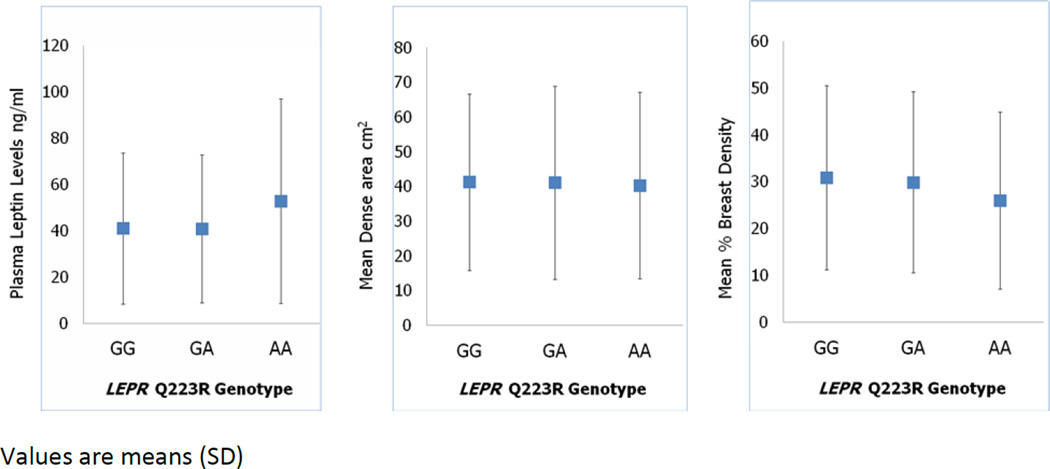
Leptin levels varied across the LEPR Q223R polymorphism (Figure 1), and were higher in women homozygous for the AA variant. Percent breast density decreased across the LEPR Q223R genotype, with lower percent density in women with the AA genotype. These differences, however, were not statistically significant (tests of trend according to LEPR Q223R genotype: leptin p=0.17; percent density p=0.15). No variation of dense area across LEPR Q223R genotype was observed (test of trend, dense area p=0.23).
Figure 1.
Relationship between LEPR Q223R polymorphism, leptin levels and breast density.
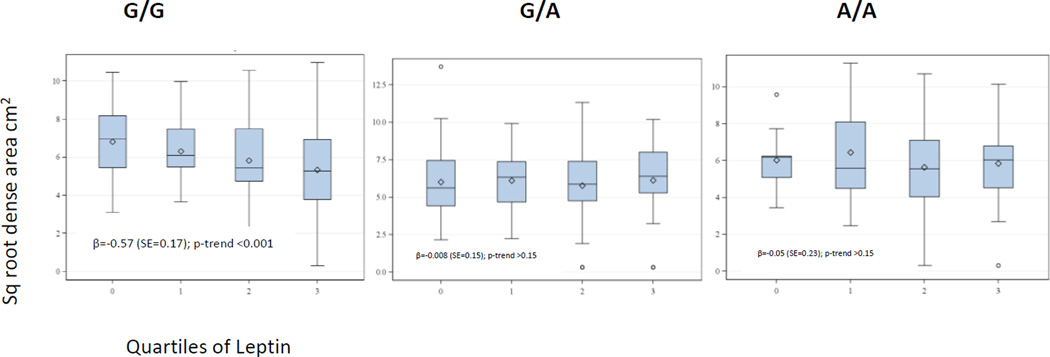
When the dense area was considered according to quartiles of leptin and stratified by LEPR Q223R (Figure 2), a significant inverse trend between leptin levels and dense breast area was observed only among women with the G/G genotype (P-trend<0.001).
Figure 2.
Dense Breast Area for Quartiles of Leptin by LEPR Q223R
After adjustment for possible confounders, leptin levels were significantly inversely associated with percent breast density (p=0.01 - Table 3). Similar results were obtained when BMI was considered, with a significant inverse trend in the association of percent breast density with increasing tertiles of BMI (p=0.01). When an interaction term was included in the model, a significant interaction between BMI and leptin levels on percent breast density was observed (p=0.03). Further analysis of such interaction suggests that the association between BMI and breast density/percent breast density is present in women in the lower leptin quartile; on the other hand, leptin levels are inversely associated with breast density and percent breast density in women in the higher BMI category (data not shown).
Table 3.
Multivariable associations between Percent Breast Density and: Leptin, LEPR Q223R, BMI (kg/m2)
| Percent breast density (square root transformed) | |||||
|---|---|---|---|---|---|
| Age and BMI Adjusted |
Multivariable |
||||
| β | P | ||||
| Leptin (log, ng/ml) |
−0.005 | 0.006 | −0.004* | 0.01 | |
| LEPR | |||||
| GG | −0.004 | 0.19 | −0.005^ | 0.13 | |
| GA | −0.002 | 0.42 | −0.001^ | 0.66 | |
| AA | −0.011 | 0.003 | −0.011^ | 0.004 | |
| BMI (kg/m2) | |||||
| <25 | −0.004# | 0.32 | |||
| 25–29.9 | −0.002# | 0.41 | |||
| ≥30 | −0.007#; ** | 0.03 | |||
| Leptin * BMI | 0.39# | 0.03 | |||
Adjusted for race (white/others), education (≤ high school/>high school), BMI (<25; 25–29.9; ≥30), smoking status (ever/never), family history of cancer (y/n), previous breast biopsy (y/n), LEPR Q223R, and batch
Adjusted for race (white/others), education (≤ high school/>high school), BMI (<25; 25–29.9; ≥30), smoking status (ever/never), family history of cancer (y/n), previous breast biopsy (y/n), leptin, and batch
Adjusted for race (white/others), education (≤ high school/>high school), smoking status (ever/never), family history of cancer (y/n), previous breast biopsy (y/n), LEPR Q223R, and batch;
p for trend: 0.01
The LEPR Q223R polymorphism was inversely associated with percent breast density, with similar associations observed across levels of the gene polymorphism (Table 3). No significant associations were observed in the multivariable analyses when breast density and total breast area were considered (data not shown).
DISCUSSION
Findings from this cross-sectional study of post-menopausal women suggest an inverse association between plasma leptin levels and mammographic percent density, but not with breast dense area; the results held after adjustment for several possible confounding factors including age and BMI. Furthermore, we observed for the first time an interaction between leptin and BMI in their association with percent breast density. This novel finding suggests that in addition to both leptin levels and BMI having independent associations with breast density, their concurrent presence may increase the chance of density breast patterns that result in higher breast cancer risk.
Only two prior studies have assessed leptin in relation to mammographic density among postmenopausal women [19–20]. Stuedal et al. [19] observed an inverse association between leptin and both measures of breast density (dense area and percent density) which attenuated with adjustment for BMI and mammographic nondense area. Additionally, their findings suggest that the association between leptin and breast density may vary by the degree of breast fat.A difference between the present study and the previously published report by Steudal et al. is the source population, consisting of Northern European women, with a lower average BMI (26.7 versus 28.7 kg/m2 in the present study) and lower leptin levels. These factors may in part explain the different results observed.
A recent study by McCormack et al. [20] also examined leptin levels among a population of postmenopausal White and Afro-Caribbean women. Although leptin was inversely related to percent density in ethnic-age-adjusted models, this association was attenuated with further adjustment by BMI. The study was conducted in UK, included a smaller sample size than the current analysis, was diverse in ethnic composition, and had an average gap between mammography and blood draw of 1.7 years. Therefore the comparability with the present study is low.
In the current study, leptin levels varied with the presence of a functional polymorphism, LEPR Q223R in the direction expected from the functional activity of the polymorphism, but the association was not statistically significant. The association between leptin and percent density did not differ when the data were stratified by the genetic polymorphism. When analyzing the dense area, leptin was inversely associated with density area among women with G/G genotype; however, no associations between leptin and dense area with the G/A or A/A genotypes were observed. Despite the overall null findings, the results suggest that the association between leptin and dense area may vary by LEPR Q223R genotype. This is the first report to examine this relationship and our findings suggest that leptin local activity in the breast tissue may be mediated by the presence of leptin receptors, and that polymorphisms in the receptor genes may have functional effects in the target tissue.
This study has several strengths: the inclusion of a large number of women who fully participated in this effort by answering the questionnaire and furnishing a blood sample in addition to providing access to their mammogram. This is also the first study to address the complex relationship between BMI, leptin levels and leptin receptor polymorphisms on mammographic patterns. In fact, although some studies have addressed some of these factors individually, none have provided a more global picture by including all of these relevant factors within the same analysis.
The original study design had some limitations, since it was based on voluntary participation of women; the sample resulting from this selection was composed mainly of white, educated women and this may limit the generalizability of our results. In addition, leptin measures were performed at one time point, since there was only one blood draw per subject, thus we were unable to account for any potential within-subject leptin variability. Samples included in this study were re-frozen once, and the effect of thawing/freezing on leptin levels is unknown. Despite this, prior research suggests that leptin levels are relatively stable over a 3-year period (ICC=0.58)[28]. Leptin levels can vary by time of day and time since last food consumption; the blood samples collected in the parent study were non-fasting. While the analyses were adjusted for time of blood draw, the leptin values presented here do not represent fasting levels. The relation between leptin levels and food consumption and with time of the day when the sample is drawn may be some of the reasons contributing to the diverging results observed across published studies.
In conclusion, this study suggests a possible interaction between leptin and BMI in determining breast density in post-menopausal women. In addition, the results indicate that genetic variants regulating the local breast tissue metabolism and growth through the control of hormonal levels, such as leptin may be relevant in breast cancer risk when breast density is considered.
Acknowledgments
Funding resource: Research supported in part by the R25 award CA57703, the P20 CA 132385-01, the Department of Defense grant DAMD 17-02-1-0553, the Pennsylvania Department of Health grant P2777693, and the NIH/NCRR General Clinical Research Center grant MOI-RR000056.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Ethical standards: the study was approved by the IRB of the University of Pittsburgh
References
- 1.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, Tritchler DL. Mammographic densities and breast cancer risk. Breast Dis. 1998;10(3–4):113–126. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 3.Byrne C, Schairer C, Brinton LA, Wolfe J, Parekh N, Salane M, Carter C. Hoover : Effects of mammographic density and benign breast disease on breast cancer risk (United States) Cancer Causes Control. 2001;12(2):103–110. doi: 10.1023/a:1008935821885. [DOI] [PubMed] [Google Scholar]
- 4.Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, Astrahan M, Siozon CC, Pike MC. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev. 2003;12(4):332–338. [PubMed] [Google Scholar]
- 5.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, menopause status. J Natl Cancer Inst. 1995;87(21):1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 6.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 7.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- 8.Samimi G, Colditz GA, Baer HJ, Tamimi RM. Measures of energy balance and mammographic density in the Nurses' Health Study. Breast Cancer Res Treat. 2008;109(1):113–122. doi: 10.1007/s10549-007-9631-7. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;20(16):1218–1226. doi: 10.1093/jnci/djg022. 95. [DOI] [PubMed] [Google Scholar]
- 10.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207(1):12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 12.Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol. 2005;162(2):101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- 13.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 14.Iversen PO, Drevon CA, Reseland JE. Prevention of leptin binding to its receptor suppresses rat leukemic cell growth by inhibiting angiogenesis. Blood. 2002;100(12):4123–4128. doi: 10.1182/blood-2001-11-0134. [DOI] [PubMed] [Google Scholar]
- 15.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83(10):1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94(22):1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 18.Han CZ, Du LL, Jing JX, Zhao XW, Tian FG, Shi J, Tian BG, Liu XY, Zhang LJ. Associations among lipids, leptin, and leptin receptor gene Gin223Arg polymorphisms and breast cancer in China. Biol Trace Elem Res. 2008;126(1–3):38–48. doi: 10.1007/s12011-008-8182-z. [DOI] [PubMed] [Google Scholar]
- 19.Stuedal A, Ursin G, Veierod MB, Bremnes Y, Reseland JE, Drevon CA, Gram IT. Plasma levels of leptin and mammographic density among postmenopausal women: a cross-sectional study. Breast Cancer Res. 2006;8(5):R55. doi: 10.1186/bcr1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormack VA, Dowsett M, Folkerd E, Johnson N, Palles C, Coupland B, Holly JM, Vinnicombe SJ, Perry NM, dos Santos Silva I. Sex steroids, growth factors and mammographic density: a cross-sectional study of UK postmenopausal Caucasian and Afro-Caribbean women. Breast Cancer Res. 2009;11(3):R38. doi: 10.1186/bcr2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maskarinec G, Woolcott C, Steude JS, Franke AA, Cooney RV. The relation of leptin and adiponectin with breast density among premenopausal women. Eur J Cancer Prev. 2010;19(1):55–60. doi: 10.1097/CEJ.0b013e328333fb0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinton ND, Lee AJ, Ross RJ, Eastell R, Blakemore AI. A single nucleotide polymorphism (SNP) in the leptin receptor is associated with BMI, fat mass and leptin levels in postmenopausal Caucasian women. Hum Genet. 2001;108(3):233–236. doi: 10.1007/s004390100468. [DOI] [PubMed] [Google Scholar]
- 23.Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–4439. doi: 10.1210/jcem.86.9.7842. [DOI] [PubMed] [Google Scholar]
- 24.Ragin CC, Dallal C, Okobia M, Modugno F, Chen J, Garte S, Taioli E. Leptin levels and leptin receptor polymorphism frequency in healthy populations. Infect Agent Cancer. 2009;(4 Suppl 1):S13. doi: 10.1186/1750-9378-4-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeves KW, Gierach GL, Modugno F. Recreational physical activity and mammographic breast density characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16(5):934–942. doi: 10.1158/1055-9965.EPI-06-0732. [DOI] [PubMed] [Google Scholar]
- 26.Hudson AG, Gierach GL, Modugno F, Simpson J, Wilson JW, Evans RW, Vogel VG, Weissfeld JL. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(3):680–687. doi: 10.1158/1055-9965.EPI-07-2739. [DOI] [PubMed] [Google Scholar]
- 27.Gotoda T, Manning BS, Goldstone AP, Imrie H, Evans AL, Strosberg AD, McKeigue PM, Scott J, Aitman TJ. Leptin receptor gene variation and obesity: lack of association in a white British male population. Hum Mol Genet. 1997;6(6):869–876. doi: 10.1093/hmg/6.6.869. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan RC, Ho GY, Xue X, Rajpathak S, Cushman M, Rohan TE, Strickler HD, Scherer PE, Anastos K. Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1291–1293. doi: 10.1158/1055-9965.EPI-06-1089. [DOI] [PubMed] [Google Scholar]