Abstract
Background
Adherence to antiretroviral medication for the treatment of HIV is a significant predictor of virologic suppression and is associated with dramatic reductions in mortality and morbidity and other improved clinical outcomes for pediatric patient populations. Effective strategies for addressing adherence problems in youth infected with HIV are needed and require significant attention to the complex interplay of multiple, interacting causal risk factors that lead to poor self-care.
Methods
Within the context of a pilot randomized trial, we evaluated the feasibility and initial efficacy of a multisystemic therapy (MST) intervention adapted to address HIV medication adherence problems against a usual care condition that was bolstered with a single session of motivational interviewing (MI). For 34 participating youth, health outcomes (viral load and CD4 count) were obtained from approximately 10 months pre-baseline through approximately 6 months post-baseline and self-reported medication adherence outcomes were obtained quarterly from baseline through 9 months post-baseline. Using mixed-effects regression models we examined within and between-groups differences in the slopes of these outcomes.
Results
Feasibility was supported, with a 77% recruitment rate and near-maximal treatment and research retention and completion rates. Initial efficacy also was supported, with the MST condition but not the MI condition demonstrating statistically and clinically significant viral load reductions following the start of treatment. There also was some support for improved CD4 count and self-reported medication adherence for the MST but not the MI condition.
Conclusions
MST was successfully adapted to improve the health outcomes of youth poorly adherent to antiretroviral medications. Replication trials and studies designed to identify mechanisms of action are important next steps.
INTRODUCTION
In the United States, most youth perinatally infected with HIV are aging into adolescence (Abrams et al., 2001) where they are joined by an increasing number of behaviorally infected peers (CDC, 1998, 2000). Adherence to antiretroviral (ARV) medication is a significant predictor of virologic suppression and is associated with dramatic reductions in mortality and morbidity and other improved clinical outcomes for pediatric patient populations (Feingold, Rutstein, Meislich, Brown, & Rudy, 2000; Flynn, Rudy, Douglas et al., 2004; Krogstad et al., 2002). However, despite the life-threatening nature of the illness, substantial percentages of youth are poorly adherent to ARV (Steele & Grauer, 2003).
Few interventions targeting adherence in pediatric HIV have been formally evaluated (Reisner et al., 2009; Simoni et al., 2007) and the available studies are characterized by serious limitations, including high treatment non-completion rates, lack of a control group, and few positive findings. Moreover, existing interventions maintain a traditional focus on individual youth-level adherence risk factors (e.g. poor HIV knowledge, low motivation for self-care), a corresponding focus on youth responsibility for improving their own adherence, and a “one-size-fits-all” approach that addresses pre-determined factors without consideration of the specific factors influencing each youth's adherence problems. Social-ecological theory (Bronfenbrenner, 1979) suggests that complex problem behaviors are multiply determined and reflect difficulties within many systems in which youth are embedded. Thus, effective strategies for addressing medication adherence problems in HIV infected youth require significant attention to the complex interplay of multiple, interacting causal risk factors that lead to poor self-care (Pequegnat & Szapocznik, 2000; Simoni et al., 2007).
An innovative approach to adherence problems
Multisystemic Therapy (MST) is a home and community-based treatment originally developed and validated for use with delinquent youth (Henggeler, Schoenwald, Borduin, Rowland & Cunningham, 1998; 2009). MST addresses the multiple determinants of youth problem behaviors, as it intervenes at the level of the individual youth, family, peer and other community systems. In addition, the home-based service delivery approach ensures better retention in treatment (Ellis et al., 2007), and increases access for hard-to-reach populations such as those with limited transportation resources and those in rural settings. An uncontrolled program evaluation of MST adapted to address medication adherence problems in children infected with HIV (Ellis, Naar-King, Cunningham & Secord, 2006) supported intervention feasibility and efficacy.
The purpose of the present study was to conduct a pilot randomized clinical trial (RCT) comparing MST as adapted to address HIV medication adherence problems against a usual care condition that was bolstered with a single session of motivational interviewing (MI). In addition to assessing intervention feasibility, two hypotheses were tested: that, relative to youth in the MI condition, youth in the MST condition would (1) evidence greater improvement in medication adherence and (2) evidence greater improvements in health outcomes (i.e., viral load and CD4 counts).
METHOD
Participants
Thirty-four youth and their caregivers were recruited from two pediatric HIV/AIDS clinics. At baseline, youth were on average 15 years old (SD = 2 years); most were girls (65%) and African American (91%). Thirty-three had been perinatally infected. Caregivers were typically the youth's biological or adoptive mother (50%) or grandmother (24%) and most lived in poverty (e.g., 53% reported annual incomes < $10,000).
Procedures
Eligible youth were (a) 9 to 17 years of age, (b) receiving HIV management, (c) residing in stable placement (most often with family) and within a 2-hour drive from either clinic, and (d) met one or more eligibility criteria. These criteria pertained to medication adherence problems and two other HIV risk behaviors targeted by the MST intervention: sexual risk behaviors and substance use. Adherence-related eligibility criteria included (a) during participant screening assessment, youth self-reported medication adherence below 80% in the past 3 months, (b) at least one viral load (VL) reading above 10,000 copies/mL in the past 12 months per clinic chart data, or (c) physician decision to remove youth from medications in past 12 months due to poor medication adherence per clinic chart review. Thirty-three youth (97%) were eligible for the study based on one or more of these adherence-related criteria of whom 25 (74%) had experienced an elevated VL in the past 12 months (see Table 1). The distribution of youth with elevated VL was not significantly different between the MST (80%) and MI (64%) groups (χ2(1) = .39, p n.s.). Most youth (n = 22 or 65%) also reported low adherence with similar distribution of these youth between the MST (65%) and MI (64%) groups. Just one youth was eligible for the study based on non-adherence criteria (i.e., self-reported need to “cut down on drinking” in past 90 days). However, this youth had serious adherence risk indicators (i.e., self-reported 86% adherence; VL reading in excess of 65,000 copies/mL during the study) and consequently was retained for analyses.
Table 1.
Eligibility criteria matrix.
| MST n = 20 | US n = 14 | Total N = 34 | |
|---|---|---|---|
| Elevated viral load only | 4 (20%) | 3 (20%) | 7 (20%) |
| Low adherence only | 3 (15%) | 3 (21%) | 6 (18%) |
| Removed from medications only | 0 | 2 (14%) | 2 (6%) |
| Elevated VL and low adherence | 10 (50%) | 6 (43%) | 16 (47%) |
| Elevated VL and removed from medications | 2 (10%) | 0 | 2 (6%) |
| Non-adherence eligibility | 1 (5%) | 0 | 1 (3%) |
Youth self-report data were collected at baseline and 3-, 6-, and 9-months post-baseline, resulting in a 2 (treatment condition) x 4 (time) factorial design with random assignment of youth to treatment condition. The first two youth recruited from each clinic were assigned to MST (vs. randomized) to facilitate therapist training. Procedures were approved by two university institutional review boards and data were additionally protected by a Federal confidentiality certificate. Informed consent and youth assent were obtained per institutional guidelines. Families were compensated with $30 for each completed research assessment and with $10 for each completed MST fidelity survey.
Treatment conditions
Multisystemic Therapy
MST is a manualized intensive, family-centered, community-based treatment originally designed to target serious antisocial behavior (Henggeler et al., 1998; 2009). Key features include: (a) identification of risk factors across youths’ ecological systems; (b) use of individualized interventions that integrate empirically based clinical treatments into a broader ecological framework; (c) focusing interventions on caregivers to more effectively parent and on adolescents to more effectively cope with problems across systems; (d) home-based treatment delivery; and (e) an intensive quality assurance system supporting therapist fidelity.
MST interventions for youth with HIV targeted problems within the individual child or caregiver, the family system, and the broader community systems within which the family was involved. Therapists drew upon a menu of evidence-based intervention techniques that included cognitive-behavioral therapy, parent training, behavioral family systems therapy and communication skills training. For example, individual interventions included cognitive- behavioral interventions with adolescents to treat depressive symptoms. Family interventions included introducing systematic monitoring and discipline systems in order to increase parental oversight; developing family organizational routines such as set times for taking medication to reduce forgetfulness; helping caregivers to communicate effectively with each other about parenting and developing indigenous social support networks to assist caregivers with medical care. At the community level, interventions within the health care system were considered to be a crucial component. Interventions included helping the family resolve barriers to keeping appointments, and working with the family and the medical treatment team to promote a positive working relationship. Consequently, therapists routinely accompanied families to their medical appointments. Community interventions also included collaboration with other agencies involved with the child or family, such as the Department of Social Services. Finally, HIV-specific interventions such as providing condom use education to sexually active adolescents and their caregivers or pill-swallowing interventions were also conducted.
MST was further adapted to support treating youth in rural locations, with Skype-based weekly supervision meetings with a distally-located MST therapist and with the addition of a therapeutic assistant who performed routine tasks (e.g., pill counts) to free up MST therapist time to focus on more complex clinical issues. Interventions were provided in a variety of settings including homes, schools, and medical clinics. Families were seen for a mean of 2.2 (SD = .87) visits per week across a mean of 6 (SD = 1.3) months. A published case study further describes the MST treatment procedures (Letourneau, Ellis, Naar-King, Cunningham, & Fowler, 2010). Two master's level therapists completed a week-long MST training course and a 1.5-day supplemental training specific to HIV care prior to delivering the MST intervention. Therapist fidelity was assessed via monthly caregiver-reported therapist adherence measures (TAM; Henggeler, Borduin, Schoenwald, Huey, & Chapman, 2006). Mean TAM scores for the MST therapists (.76 and .81) were well above the threshold (.61) that indicates minimally adherent treatment. Directors of the two clinics that referred participants reported there was very little impact of the MST intervention on clinic flow. Rather, MST therapists were viewed as providing useful information that benefited patient care in those settings.
Usual Care with Motivational Interviewing
The usual or standard of care provided by both referral clinics to all youth in this study included quarterly clinic visits to address medication and other health needs, with more frequent visits scheduled when clinically indicated. When indicated, clinic teams counseled the youth and caregiver for medication adherence. Severe cases of poor adherence could be addressed by nurse home visits or hospitalization, , although these interventions were rare. Youth randomized to the MI condition also received a single session of motivational interviewing (Miller & Rollnick, 2002). MI seeks to increase motivation and self-efficacy by altering key decisional and self-regulatory balances through eliciting verbalizations that are consistent with change. MI sessions were held with individual youth and targeted medication adherence and either substance use or sexual risk behavior problems as needed. Session attendance was incentivized ($10) and transportation costs covered. Two master's level therapists completed a 2-day MI training course and the 1.5 day supplemental HIV care training. Therapist fidelity to MI was evaluated using the Motivational Interviewing Treatment Integrity coding system (Moyers, Martin, Manuel, Hendrickson, & Miller, 2005). Across sessions the mean percent of adherence to MI was 96.00 (SD = 8.0) indicating strong fidelity.
Measures
Medication adherence
Self-reported medication adherence was collected at each quarterly research assessment. Three one-month recall items assessed percent of days that any medications were taken, that all doses were taken, and that medication was taken according to instructions (Naar-King, Frey, Harris & Arfken, 2005). The mean response to these items provided a single medication adherence score per youth per assessment. For some analyses, scores were dichotomized to reflect <90% vs. ≥ 90% adherence.
Viral load and CD4 outcomes
While youths’ clinic records were reviewed for VL and CD4 counts from 12 months prior to baseline through 9 months post-baseline, on average, the first VL measurement occurred 10.4 (SD = 2.5) months prior to baseline and the last occurred 6.0 (SD = 2.5) months following baseline. When test results were not available within one month of quarterly research assessment dates, labs were scheduled.
Satisfaction with treatment
Patient satisfaction was measured with the 5-item Youth Satisfaction Questionnaire (YSQ) which has adequate internal consistence (coefficient alpha=0.80; Stuntzner-Gibson, Koren, & DeChilo, 1995). Youth were asked to indicate whether they liked the help they got, got the help they wanted, needed more help, got more help than needed, and felt the services helped with their lives. Item scores were summed for a single score, with higher scores indicating greater satisfaction.
Statistical Analysis
Analyses were performed using mixed-effects regression models (MRMs) in HLM software (version 6.08; Raudenbush, Bryk, & Congdon, 2004; Raudenbush & Bryk, 2002). The VL and CD4 models tested the outcome slopes prior to the start of MST or MI, the change in outcome slopes following the start of the interventions, and between-group differences in these slopes. The level-1 model included a term for the number of months from each youth's first VL and CD4 measurement and a term for the number of months between measurements following the youth's MST or MI start date. The level-2 model included an indicator for treatment condition and cross-level interaction terms were added for the level-1 and level-2 terms. Planned comparisons, reported as χ2 statistics, were specified for terms not directly tested by this model. Medication adherence was modeled as a dichotomous outcome indicating less than 90% adherence or more. The model tested the rate of change in medication adherence following the start of MST or MI. The level-1 linear term was the number of months from the MST or MI start date; measurements occurring before the start of MST or MI were coded as 0.
RESULTS
Feasibility
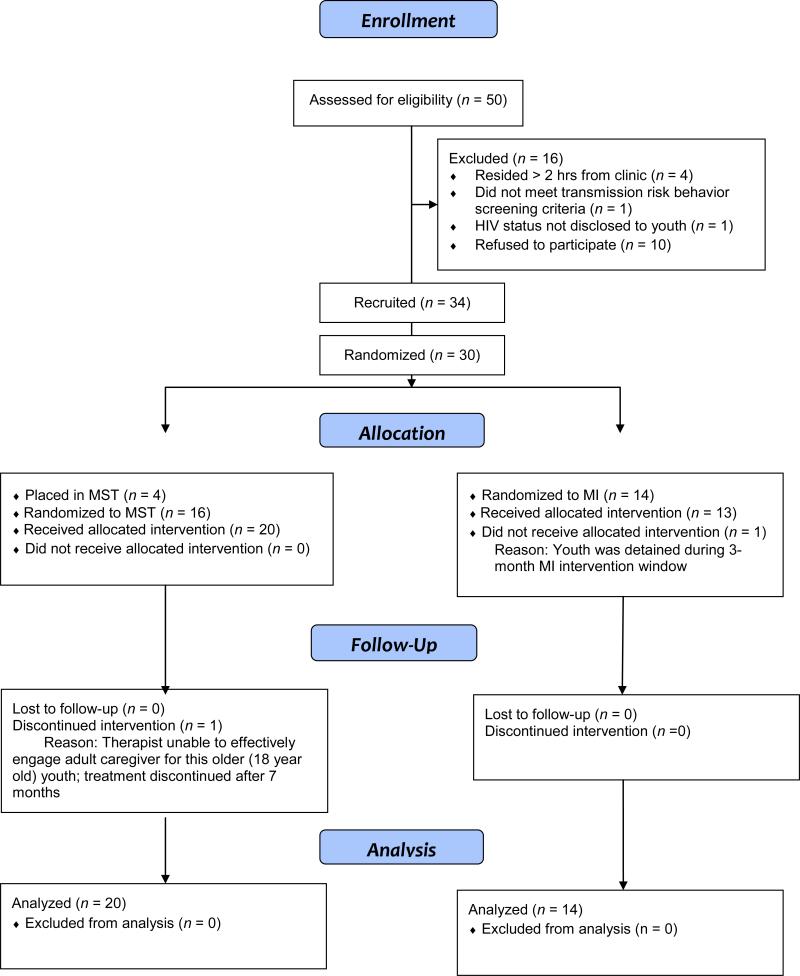
Most (77%) of youth and caregivers approached to participate in this study did so and all but one youth completed their interventions (see Figure 1). There was 100% retention through the third research assessment and a 97% overall study completion rate. In general, youth reported high satisfaction with MST and MI, with both groups achieving mean scores above 4.0 on this 5-point scale.
Figure 1.
Consort figure.
Viral Load
Viral load results are presented in Table 2 and depicted in Figure 2. Groups did not differ significantly on initial VL, which, on average, occurred 10 months prior to study participation. The average initial log VL was 4.01 (MST) and 3.46 (MI; β = −0.55, p = .274). Between the initial measurement and the start of MST or the MI session, VL for MST youth decreased at a rate of −0.06 logs per month (p = .054), and VL for MI youth decreased at a rate of −0.08 logs per month (χ2 = 5.78, p = .015). These pre-MST/MI slopes were not significantly different (β = − 0.02, p = .614). Moreover, the average VL did not differ significantly between MI and MST at the start of treatment, β = −0.72, SE = 0.71, t (31) = −1.01, p = .32, 95% CIβ = −2.11, 0.67.
Table 2.
Results from mixed-effects regression models evaluating within and between-groups slope differences for log viral load and CD4 count outcomes.
| β | SE | df | p | 95% CIβ | |
|---|---|---|---|---|---|
| Viral Load | |||||
| Intercept (β00) | 4.01 | 0.32 | 32 | <.001 | 3.38, 4.64 |
| MI (β01) | −0.55 | 0.50 | 32 | .274 | −1.53, 0.43 |
| Linear (β10) | −0.06 | 0.03 | 32 | .054 | −0.12, −0.01 |
| MI × Linear (β11) | −0.02 | 0.04 | 32 | .614 | −0.10, 0.06 |
| Post-Linear (β20) | −0.14 | 0.08 | 32 | .088 | −0.30, 0.02 |
| MI × Post-Linear (β21) | 0.33 | 0.14 | 32 | .027 | 0.05, 0.59 |
| CD4 | |||||
| Intercept (β00) | 378.13 | 71.94 | 32 | <.001 | 237.13, 519.13 |
| MI (β01) | 181.38 | 112.22 | 32 | .116 | −38.57, 401.33 |
| Linear (β10) | −1.35 | 3.03 | 32 | .659 | −7.29, 4.59 |
| MI × Linear (β11) | 0.28 | 4.66 | 32 | .953 | −8.85, 9.41 |
| Post-Linear (β20) | 12.93 | 7.08 | 32 | .076 | −0.95, 26.81 |
| MI × Post-Linear (β21) | −16.05 | 12.52 | 32 | .209 | −40.59, 8.49 |
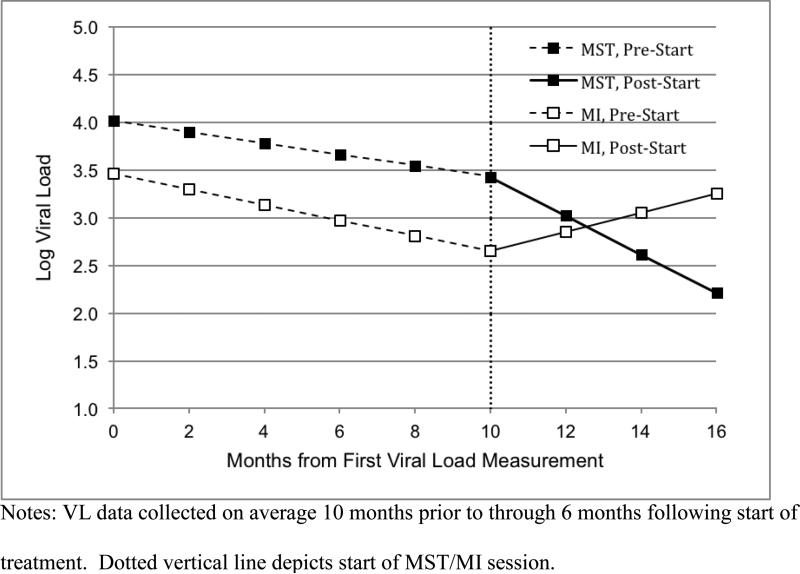
Figure 2.
Graph depicting MST and MI group mean viral loads from 12 months pre-baseline through 9 months post-baseline.
Following the start of the study interventions (depicted on Figure 2 by the dotted vertical line), the pre-MST rate of change in viral load increased from −0.06 to −0.20 logs per month. This was not a significant change in slopes (β = −0.14, p = .088). However, the −0.20 monthly rate of change was significant (χ2 = 9.91, p = .002). The pre-MI session slope of −0.08 changed to 0.10 following the MI session. This was not a significant change in slopes (χ2 = 2.56, p = .106), and the rate of change following the MI session was not significant (χ2 = 1.13, p = .288). Most importantly, the change in slopes from pre-MST/MI to the phase following the start of MST/MI was significantly different for the two groups (i.e., −0.14 change for MST and .18 change for MI; β = 0.33, p = .027). Further, the actual slopes following the start of MST/MI were significantly different for the two groups (i.e., −0.20 versus 0.10; χ2 = 7.02, p = .008). The average log viral load at the start of MST and MI was 3.31 and 2.48. These levels changed to 2.09 and 3.09, respectively across an average 6-month follow-up for these VL data.
CD4 Count
CD4 results are presented in Table 2. Groups did not differ significantly on initial CD4 count. The average initial CD4 was 378 (MST) and 560 (MI; β = 181.38, p = .116). Between the initial measurement and the start of the study interventions, CD4 did not change significantly for youth in the MST group (β = 1.35, p = .659) or youth in the MI group (χ2 = 0.09, p > .500). Likewise, the slopes for the two groups were not significantly different (β = 0.28, p = .953). Following the start of the study interventions, for MST youth, the rate of change in CD4 did not differ significantly from the rate of change prior to the start of MST (β = 12.93, p = .076). However, CD4 did increase significantly following the start of MST (χ2 = 4.96, p = .024). For MI youth the rate of change in CD4 did not differ significantly before and after the start of MI (χ2 = 0.09, p > .500) and CD4 did not change significantly following the start of MI (χ2 = 0.25, p > .500). The change in slope before and after the start of MST was not significantly different from the change in slope before and after the MI session (β = −16.05, p = .209). Likewise, the MST and MI slopes following the start of the study interventions did not differ significantly (χ2 = 2.53, p = .107).
Medication Adherence
Youth randomized to MST and MI did not differ significantly on the average pre-MST or pre-MI level of medication adherence (β = 0.19, SE = 1.21, df = 30, OR = 1.21, p = .877). For MST youth, the level of medication adherence increased significantly following the start of the intervention (β = 0.22, SE = 0.09, df = 120, OR = 1.24, p = .022), and for MI youth, the rate of change was not significant (χ2 = 1.03, p = .311). However, the rates of change in medication adherence did not differ significantly for MST youth and MI youth (β = −0.07, SE = 0.17, df = 120, OR = 0.93, p = .693).
DISCUSSION
The present study provided an examination of the feasibility and initial efficacy of an innovative and intensive HIV medication adherence intervention. As with an earlier uncontrolled assessment (Ellis et al., 2006), the present study supported the feasibility of MST for youth with medication adherence problems. Specifically, recruitment, retention and clinical and research completion rates were high as was client satisfaction. Results also supported the efficacy of the MST intervention. Youth in the MST condition experienced significantly greater VL reductions in comparison to their MI counterparts. There was some evidence that CD4 and self-reported adherence improved significantly for the MST condition, although between-groups differences were not significant.
Limitations
Generalizability of study results might be limited due to the small sample size and overrepresentation of African American and perinatally infected participants. Also, the significant between-groups differences in VL were not accompanied by significant differences in self-reported adherence ratings, suggesting problems with the self-report instrument. A true “untreated” standard of care condition might have provided a cleaner test of the MST intervention effects.
Conclusions
Results from this trial provide preliminary support for MST and are especially encouraging considering the small sample size of this pilot. To our knowledge, MST is the first adolescent adherence intervention to demonstrate reduced VL in the context of a randomized trial. However, replication is a necessary next step before broader intervention dissemination is warranted.
Contributor Information
Elizabeth J. Letourneau, Department of Mental Health, John Hopkins University, Baltimore, MD,USA
Deborah A. Ellis, Department of Pediatrics, Wayne State University, Detroit, MI, USA
Sylvie Naar-King, Department of Pediatrics, Wayne State University, Detroit, MI, USA.
Jason E. Chapman, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
Phillippe B. Cunningham, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
Sandra Fowler, Department of Pediatrics, Medical University of South Carolina, Charleston, SC, USA.
REFERENCES
- Abrams EJ, Weedon TP, Bertolli J, Bornschlegel K, Cervia J, m Mendez H, Thomas P. For the New York City Pediatric Spectrum of Disease Consortium: Aging cohort of HIV-infected children in New York City. Pediatric Infectious Disease Journal. 2001;20:511–517. doi: 10.1097/00006454-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Harvard University Press; Cambridge, MA: 1979. [Google Scholar]
- Centers for Disease Control and Prevention . Trends in the HIV and AIDS Epidemic. U.S. Department of Health and Human Services; Atlanta, GA.: 1998. [Google Scholar]
- Centers for Disease Control and Prevention Cumulative AIDS cases; exposure categories; ten states/territories and cities reporting the highest number of AIDS cases international statistics. 2000 Available: http://www.cdc.gov/hiv/stats.htm [2001]
- Ellis DA, Podolski C, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Cunningham PB, Secord E. Use of Multisystemic Therapy to improve antiretroviral adherence and health outcomes in HIV-infected pediatric patients: Evaluation of a pilot program. AIDS Patient Care and STDs. 2006;20:112–121. doi: 10.1089/apc.2006.20.112. [DOI] [PubMed] [Google Scholar]
- Feingold AR, Rutstein RM, Meislich D, Brown T, Rudy BJ. Protease Inhibitor Therapy in HIV Infected Children. AIDS Patient Care STDS. 2000;14:589–593. doi: 10.1089/10872910050193761. [DOI] [PubMed] [Google Scholar]
- Flynn PM, Rudy BJ, Douglas SD, Lathye J, Spector SA, Martinez J, Lindsey JC. Virologic and immunologic outcomes after 24 weeks in HIV Type 1-infected adolescents receiving Highly Active Antiretroviral Therapy. Journal of Infectious Diseases. 2004;190:271–279. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Borduin CM, Schoenwald SK, Huey SJ, Chapman JE. Multisystemic Therapy Adherence Scale - Revised (TAM–R) Medical University of South Carolina, Department of Psychiatry and Behavioral Sciences; Charleston, SC: 2006. [Google Scholar]
- Henggeler SW, Schoenwald SK, Borduin CM, Rowland MD, Cunningham PB. Multisystemic treatment of antisocial behavior in children and adolescents. Guilford Press; New York: 1998. [Google Scholar]
- Henggeler SW, Schoenwald SK, Borduin CM, Rowland MD, Cunningham PB. Multisystemic treatment of antisocial behavior in children and adolescents (Second Ed.) Guilford Press; New York: 2009. [Google Scholar]
- Krogstad P, Lee S, Johnson G, et al. the Pediatric AIDS Clinical Trials Group 377 Study Team Nucleoside-analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir for pretreated children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:991–1001. doi: 10.1086/338814. [DOI] [PubMed] [Google Scholar]
- Letourneau EJ, Ellis DA, Naar-King S, Cunningham PB, Fowler SL. Case study: Multisystemic Therapy as an intervention for adolescents who engage in HIV transmission risk behaviors. Journal of Pediatric Psychology. 2010;35:120–127. doi: 10.1093/jpepsy/jsp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change (vol. 2) Guildford; New York: 2002. [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Hendrickson SML, Miller WR. Assessing competence in the use of motivational interviewing. Journal of Substance Abuse Treatment. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Frey M, Harris M, Arfken C. Measuring adherence to treatment of pediatric HIV/ AIDS. AIDS Care. 2005;17:345–349. doi: 10.1080/09540120412331299753. [DOI] [PubMed] [Google Scholar]
- Pequegnat W, Szapocznik J, editors. Working with families in the era of HIV/AIDS. Sage; Thousand Oaks, CA: 2000. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Scientific Software International, Inc.; Lincolnwood, IL: 2004. [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Topics in HIV Medicine. 2009;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Montgomery A, Martin E, New M, Demas PA, Rana S. Adherence to antiretroviral therapy for pediatric HIV infection: A qualitative systematic review with recommendations for research and clinical management. Pediatrics. 2007;119:e1371–e1383. doi: 10.1542/peds.2006-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele RG, Grauer D. Adherence to antiretroviral therapy for pediatric HIV infection: review of the literature and recommendations for research. Clinical Child & Family Psychology Review. 2003;6:17–30. doi: 10.1023/a:1022261905640. [DOI] [PubMed] [Google Scholar]
- Stuntzner-Gibson D, Koren PE, DeChilo N. The Youth Satisfaction Questionnaire: What kids think of services. Families in Society. 1995;76:616–624. [Google Scholar]