Summary
Background
Anti-TNFα biologics induce and maintain remission in inflammatory bowel disease (IBD). Also, they have been reported to induce or unmask idiopathic inflammatory demyelinating disease of the central nervous system (IIDD).
Aim
To determine if anti-TNFα biologics increased the risk of IIDD in a large cohort of patients with IBD.
Methods
We retrospectively identified adult patients referred to the Mayo Clinic, Rochester, MN for management of IBD from a five state capture area (Minnesota, Wisconsin, North Dakota, South Dakota, and Iowa) between 1996 and 2010. IIDDs were identified in both Crohn’s disease (CD) and ulcerative colitis (UC) patients with and without anti-TNFα exposure using the 2010 McDonald MRI criteria. The risk of IIDDs in patients with and without anti-TNFα exposure was estimated for IBD; CD and UC groups separately.
Results
A total of 9095 patients with IBD were identified (4342 CD and 4753 UC). Four patients with CD with exposure to anti-TNFα agents (4/2054) and five patients with CD without anti-TNFα exposure (5/2288) developed a confirmed IIDD. One patient with UC with exposure to anti-TNFα agents (1/1371) and five patients with UC without anti-TNFα agents developed a confirmed IIDD (5/3382). The percent of IIDDs in patients with and without anti-TNFα exposure was; IBD: 0.15% and 0.18% (RR=0.83, 95%CI: 0.28-2.42; p=0.729); CD: 0.19% and 0.22% (RR=0.89, 95%CI: 0.24-3.31; p=0.863); UC: 0.07% and 0.15% (RR=0.49, 95%CI: 0.06-4.22; p=0.510).
Conclusions
Anti-TNFα biologics do not appear to impact the risk of developing clinical IIDD in patients with IBD.
Keywords: multiple sclerosis, demyelinating diseases, anti-TNFα inhibitor
Introduction
Anti-TNFα biologics are effective treatments for inflammatory bowel diseases (IBD).(1-6) Among other potential adverse effects, anti-TNFα biologics have been implicated in the worsening of multiple sclerosis (MS) and in the development of central nervous system idiopathic inflammatory demyelinating diseases (IIDDs).(7-11)
Epidemiological studies have suggested an association between IBD and MS.(12, 13) Possible explanations for this association include a brain-gut interaction, shared genetic susceptibility, and similar environmental risk factors of disease (smoking, higher socioeconomic status, vitamin D deficiency, and colder climates). MS has also been suggested to be an extraintestinal manifestation of IBD.(14-20) While large population-based studies have shown an increased incidence of MS among IBD patients, most studies have not accounted for anti-TNFα exposure.(12, 13, 21)
Individuals with IIDD may have one or more non-specific neurological symptoms including weakness, tingling, numbness, and vision changes depending on the location of the demyelination in the central nervous system. It is unclear whether anti-TNFα biologics cause IIDD. There are published reports of IIDDs in patients exposed to anti-TNFα agents; however these have mostly been non-comparative studies and case reports.(7, 11, 22-28). Current guidelines recommend discontinuing anti-TNFα agents in patients with neurological symptoms and white matter lesions. Despite the paucity of data, anti-TNFα agents are relatively contraindicated in patients with a personal or family history of IIDDSs.(29)
We hypothesized that anti-TNFα agents do not substantially increase the risk of IIDDs in IBD patients. We performed a retrospective study using a large 5-state cohort to determine the risk of IIDDs in IBD patients with and without exposure to anti-TNFα agents.
Materials and Methods
Study Design
We retrospectively studied a cohort of adult patients with UC and CD evaluated at the Mayo Clinic, Rochester, MN between 01/01/1996 to 12/31/2010. We included patients from a five state capture area within latitudes 40-50°N from and surrounding Olmsted County (Minnesota, Wisconsin, North Dakota, South Dakota, and Iowa). The study was approved by the Institutional Review Board of the Mayo Clinic.
Inflammatory bowel disease case ascertainment
Patients were identified using ICD-9 codes for UC, CD, and their state of residence. The UC and CD cohorts were divided according to anti-TNFα exposure status (search terms; infliximab, adalimumab, golimumab, and certolizumab) (Figure 1). IBD diagnosis was established using endoscopic, histologic, and radiographic criteria.
Figure 1.
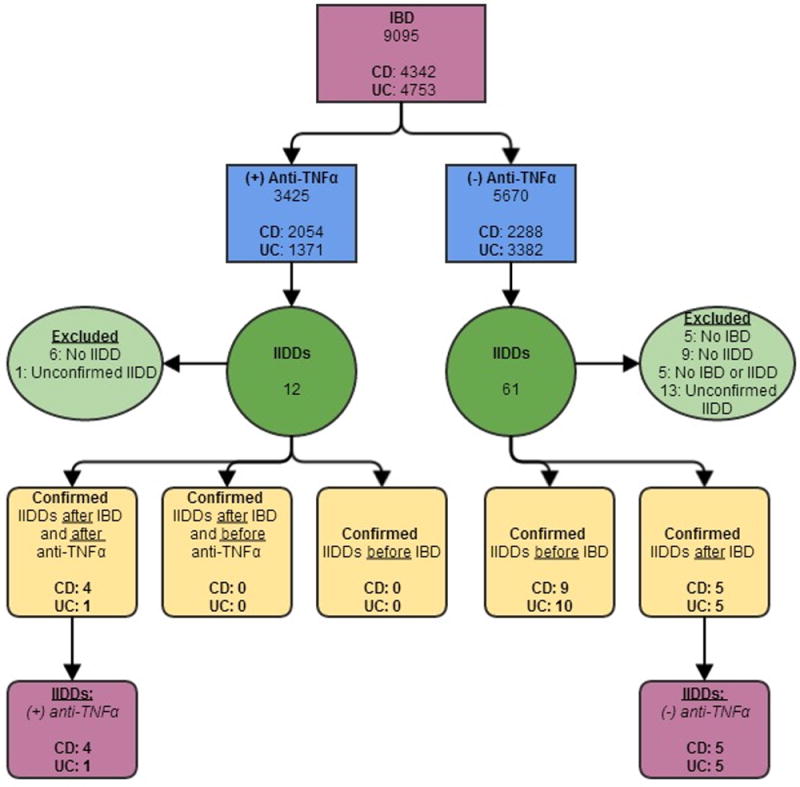
Case ascertainment of IIDD in IBD patients with and without anti-TNFα exposure.
Inflammatory bowel disease (IBD), Crohn’s disease (CD), Ulcerative colitis (UC), anti-tumor necrosis factor alpha (anti-TNFα), idiopathic inflammatory-demyelinating disease of the central nervous system (IIDD)
Central nervous system idiopathic inflammatory-demyelinating diseases case ascertainment
IIDD diagnoses were identified using ICD-9 codes and the following search terms: demyelinating disease, multiple sclerosis (MS), radiologically isolated syndrome (RIS), clinically isolated syndrome (CIS), single attack multiple sclerosis (SAMS), relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS), single attack progressive multiple sclerosis (SAPMS), primary progressive multiple sclerosis (PPMS), acute disseminated encephalomyelitis (ADEM), transverse myelitis (TM), optic neuritis (ON), neuromyelitis optica (NMO), Balo’s concentric sclerosis, Marburg, fulminant multiple sclerosis, and tumefactive multiple sclerosis.
Patients diagnosed with IIDDs before and after the onset of IBD symptoms were included. Each case of IIDD was carefully reviewed and confirmed by a single neurologist with subspecialty training in MS. Based on clinical, MRI, CSF and other laboratory findings, patients were classified into the different IIDD categories (Figure 2). McDonald (30, 31) criteria were used to establish the diagnosis of IIDDs. MS cases were further classified as RIS, CIS, SAMS, RRMS, SPMS, SAPMS, and PPMS. Only cases confirmed by an expert neurologist (M.N.) were included in the study. Unconfirmed cases (those diagnosed elsewhere and not confirmed at the Mayo Clinic) were excluded (Figure 1). Each confirmed case of IIDD was reviewed by a gastroenterologist (K.D.) to confirm the diagnosis of UC or CD by endoscopic, histologic, and radiographic criteria.
Figure 2.
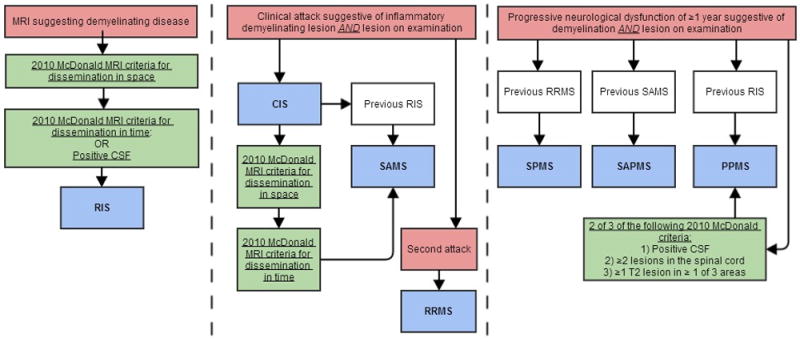
Diagnostic algorithm of IIDDs using 2010 McDonald criteria.
Central nervous system (CNS), Gadolinium (Gd), cerebrospinal fluid (CSF), radiologically isolated syndrome (RIS), clinically isolated syndrome (CIS), single attack multiple sclerosis (SAMS), relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS), single attack progressive multiple sclerosis (SAPMS), primary progressive multiple sclerosis (PPMS)
Demographic and clinical data collection
Clinical data was collected on all patients with confirmed diagnoses including age, sex, IBD subtype, disease location, disease behavior, disease activity at IBD diagnosis, development of first neurological symptoms and/or demyelination on MRI related to IIDD, other extraintestinal manifestations, and smoking status. Clinical IBD disease activity was grouped into mild, moderate, severe, and remission based on the American College of Gastroenterology guidelines.(32, 33) Detailed data was collected including the type of anti-TNFα agent received, treatment duration, concomitant therapy, discontinuation or continuation of anti-TNFα therapy with the development of neurological symptoms, and disease activity of both IBD and IIDD after discontinuation of anti-TNFα therapy. A personal history and family history of demyelinating diseases, IBD, and autoimmune diseases were recorded. Time to Expanded Disability Status Scale of 6 (EDSS6), (unilateral assistance required to walk 100 m) was recorded in all IIDD patients with a history of IBD, if this endpoint had been reached by the last follow-up. The EDSS scale ranges from 0-10, with 0 indicating a normal neurological exam and 10 indicating death due to MS.
Statistical Analysis
The baseline demographic and clinical characteristics of UC and CD patients with and without exposure to anti-TNFα agents who developed IIDDs were reported as percentages for categorical variables and medians with ranges for continuous variables. Risk of IIDDs in patients with and without anti-TNFα exposure were estimated for IBD, CD and UC groups separately, using Wilcoxon rank sum and Fisher exact tests as appropriate. The fraction of IIDDs in IBD patients with and without anti-TNFα therapy from 1996-2010 were expressed as percentages, with 95% confidence intervals (CI). The relative risk for development of IIDDs in IBD patients and number needed to harm were calculated. A p-value was set at < 0.05 for statistical significance.
Results
Case ascertainment
A total of 9095 IBD patients (4342 CD; 4753 UC) treated at Mayo Clinic in Rochester from the 5-state area between 01/01/1996 – 12/31/2010 were identified (Figure 1) (3425 patients exposed to anti-TNFα therapy and 5670 not exposed to anti-TNFα agents).
Ascertainment of confirmed cases of IBD and IIDD
Twelve IBD patients exposed to anti-TNFα therapy had suspected IIDD. Of these 12 IBD patients, six did not fulfill criteria for an IIDD diagnosis. The neurological diagnosis could not be confirmed in one patient diagnosed at an outside institution. The diagnosis of IIDD was confirmed at the Mayo Clinic in 5 patients (4 CD; 1 UC) (Figure 1). None of the patients exposed to anti-TNFα therapy had been diagnosed with IIDD prior to their IBD diagnosis.
Of the 61 IBD patients with no anti-TNFα exposure and a suspected IIDD diagnosis, an IBD diagnosis could not be confirmed in 5 patients, IIDD criteria were not met in 9 patients, 5 patients had neither IBD nor an IIDD, and 13 patients were diagnosed with IIDD elsewhere. After excluding these 32 patients, 19 patients (9 CD; 10 UC) developed an IIDD before the onset of IBD and 10 patients (5 CD; 5 UC) developed an IIDD after the onset of IBD (Figure 1).
Clinical Characteristics of IBD Patients with a confirmed diagnosis of IIDD, with and without anti-TNFα exposure
There were no statistically significant differences in the demographic or clinical characteristics among the CD and UC groups (Table 1). The median age at IBD diagnosis was 28.32 years (range, 11-48) for CD and 32.17 years (range, 13-49) for UC. The majority (80%) of patients were female. No patient had a prior personal history of demyelinating disease, although two patients had a first degree relative with a history of IIDD. The median follow up for IBD was 10.5 years (range, 5-39). At IBD diagnosis, the majority (67%) of UC patients had extensive colitis. The majority of CD patients had ileocolonic disease (56%) with inflammatory disease behavior (89%). Only one of six patients with UC who developed an IIDD received anti-TNFα therapy with concomitant immunomodulator therapy. Five of nine CD patients who developed an IIDD received anti-TNFα therapy monotherapy. One CD patient had exposure to both infliximab and adalimumab.
Table 1.
Clinical and demographic characteristics of IBD patients with a confirmed diagnosis of IIDD.
| CD N = 9 | UC N = 6 | P value | Total N = 15 | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Median age at IBD diagnosis, years (range) | 28.32 (11-48) | 32.17 (13-49) | 0.41 | 30.25 (11-49) |
| Female, n (%) | 8 (89%) | 4 (67%) | 0.53 | 12 (80%) |
| Previous personal history of autoimmune diseases, n (%) | 1 (11%) | 1 (17%) | 1.00 | 2 (13%) |
| Family history of IBD, n (%) | 3 (33%) | 0 | 0.23 | 3 (20%) |
| Family history of demyelinating diseases, n (%) | 1 (11%) | 1 (17%) | 1.00 | 2 (13%) |
| Smoking at diagnosis, n (%) | 3 (33%) | 0 | 0.23 | 3 (20%) |
| Ulcerative Colitis: Disease location, n (%) | ||||
| E1, proctitis | 1 (17%) | |||
| E2, left sided colitis | 1 (17%) | |||
| E3, extensive colitis | 4 (67%) | |||
| Crohn’s Disease: Disease location, n (%) | ||||
| L1, terminal ileal | 1 (11%) | |||
| L2, colon | 3 (33%) | |||
| L3, ileocolonic | 5 (56%) | |||
| Crohn’s Disease: Disease behavior, n (%) | ||||
| B1, inflammatory | 8 (89%) | |||
| B3p, perianal penetrating | 1 (11%) | |||
| Disease activity at diagnosis, n (%) | ||||
| Mild | 1 (11%) | 2 (33%) | 0.53 | 3 (20%) |
| Moderate | 6 (67%) | 4 (67%) | 1.00 | 10 (67%) |
| Severe | 2 (22%) | 0 | 0.49 | 2 (13%) |
| Extraintestinal manifestations, n (%) | ||||
| Skin | 2 (22%) | 0 | 0.49 | 2 (13%) |
| Oral | 2 (22%) | 0 | 0.49 | 2 (13%) |
| Anti-TNF alpha therapy, n (%) | ||||
| Infliximab | 3 (33%) | 1 (17%) | 0.60 | 4 (27%) |
| Adalimumab | 2 (22%) | 0 | 0.49 | 2 (13%) |
| Concomitant therapy, n (%) | ||||
| Azathioprine/6-mercaptopurine | 0 | 1(17%) | 0.40 | 1 (7%) |
| Corticosteroids | 1 (11%) | 0 | 1.00 | 1 (7%) |
| Antibiotics | 1 (11%) | 0 | 1.00 | 1 (7%) |
| Median followup in Gastroenterology, years (range) | 10.85 (5-32) | 7.35 (5-39) | 0.41 | 10.5 (5-39) |
Inflammatory bowel disease (IBD), Crohn’s disease (CD), Ulcerative colitis (UC)
Clinical Characteristics of IIDD
The clinical and neurological characteristics of the 15 IBD patients (9 CD; 6 UC) who developed an IIDD after their diagnosis of IBD are summarized according to their anti-TNFα exposure in Table 2. The median time from IBD diagnosis to onset of neurological symptoms and/or demyelination on MRI related to IIDD was 9.16 years (range, 0.07-24.89). IBD activity for both CD and UC at the time of neurological symptom onset and/or demyelination on MRI related to IIDD was mild (40%) and most patients were in remission (47%). The most common type of IIDD was PPMS (6/15), followed by RRMS (3/15), SAMS (2/15), SPMS (1/15), RIS (1/15), and undifferentiated IIDD (1/15).
Table 2.
Clinical characteristics of IBD patients with IIDD according to anti-TNFα exposure.
| (+)anti-TNFα N=5 | (-)anti-TNFα N=10 | P value | Total N=15 | |
|---|---|---|---|---|
| Inflammatory Bowel Disease, n (%) | ||||
| CD | 4 (80%) | 5 (50%) | 0.58 | 9 (60%) |
| UC | 1 (20%) | 5 (50%) | 0.58 | 6 (40%) |
| Median age at diagnosis of IBD, years (range) | 23.95 (14-30) | 32.17 (11-49) | 0.18 | 30.41 (11-49) |
| Idiopathic Inflammatory Demyelinating Disease, n (%) | ||||
| RIS | 1 (20%) | 0 | 0.33 | 1 (7%) |
| SAMS | 1 (20%) | 1 (10%) | 1.00 | 2 (13%) |
| RRMS | 1 (20%) | 3 (30%) | 1.00 | 4 (27%) |
| SPMS | 0 | 1 (10%) | 1.00 | 1 (7%) |
| PPMS | 1 (20%) | 5 (50%) | 0.58 | 6 (40%) |
| Undifferentiated IIDD | 1 (20%) | 0 | 0.10 | 1 (7%) |
| Median age at neurological symptom onset and/or demyelination on MRI, years (range) | 31.25 (19-48) | 38.90 (29-56) | 0.06 | 34.98 (19-56) |
| Median time from IBD diagnosis to neurological symptom onset and/or demyelination on MRI, years (range) | 9.16 (0.07-19.72) | 7.97 (3.34-24.89) | 0.62 | 9.16 (0.07-24.89) |
| IBD Disease activity at neurological symptoms onset and/or demyelination on MRI, n (%) | ||||
| Mild | 2 (40%) | 4 (40%) | 1.00 | 6 (40%) |
| Moderate | 0 | 1 (10%) | 1.00 | 1 (7%) |
| Severe | 1 (20%) | 0 | 0.33 | 1 (7%) |
| Remission | 2 (40%) | 5 (50%) | 1.00 | 7 (47%) |
| Previous anti-TNF exposure, n (%) | 1 (20%) | |||
| Therapy at time of neurological symptom onset and/or demyelination on MRI, n (%) | ||||
| Infliximab | 2 (40%) | 0 | 0.10 | 2 (13%) |
| Adalimumab | 2 (40%) | 0 | 0.10 | 2 (13%) |
| Azathioprine/6-Mercaptopurine | 2 (40%) | 0 | 0.10 | 2 (13%) |
| 5-Aminosylicalates | 0 | 5 (50%) | 0.10 | 5 (33%) |
| No medications | 0 | 5 (50%) | 0.10 | 5 (33%) |
| Median time from first dose of anti-TNF to neurological symptom onset and/or demyelination on MRI, years (range) | 0.53 (0.01-11.37) | |||
| Median time from last dose of anti-TNF to neurological symptom onset and/or demyelination on MRI, years (range) | 0.05 (0.01-3.80) | |||
| Anti-TNF therapy discontinued due to IIDDS, (n %) | 3 (60%) | |||
| EDSS 6, (n%) | 1 (20%) | 4 (40%) | 0.60 | 5 (33%) |
| Median followup in Gastroenterology, years (range) | 10.36 (5-11) | 14.72 (5-39) | 0.22 | 10.5 (5-39) |
| Median followup in Neurology, years (range) | 0.556 (0.5-3) | 10.23 (1-23) | 0.027 | 3.37 (0.5-23) |
Inflammatory bowel disease (IBD), Crohn’s disease (CD), Ulcerative colitis (UC), anti-tumor necrosis factor alpha (anti-TNFα), idiopathic inflammatory-demyelinating disease (IIDD), single attack multiple sclerosis (SAMS), relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS), single attack progressive multiple sclerosis (SAPMS), primary progressive multiple sclerosis (PPMS), Expanded Disability Status Scale of 6 (EDSS 6)
Of the 5 patients who developed an IIDD and had exposure to an anti-TNFα agent (4 CD, 1 UC), three patients developed IIDD symptoms during anti-TNFα treatment, one patient developed demyelination on MRI related to IIDD without clinical symptoms during anti-TNFα treatment, and one patient had previous anti-TNFα exposure and developed progressive IIDD symptoms while on azathioprine monotherapy. Of those three patients on anti-TNFα treatment at symptom onset, two discontinued anti-TNFα therapy due to the development of IIDD: one patient had complete resolution of symptoms with corticosteroid treatment and the other patient had partial improvement of symptoms. A third patient discontinued anti-TNFα therapy due to worsening of underlying CD and subsequently underwent a total proctocolectomy with end ileostomy. This patient had persistent relapsing and remitting neurological disease despite discontinuation of anti-TNFα therapy. The fourth patient developed demyelinating lesions on MRI with no associated clinical symptoms. She remained asymptomatic and continued on anti-TNFα. A follow-up MRI showed additional new demyelinating lesions consistent with IIDD and she discontinued anti-TNFα therapy. Off anti-TNFα therapy, her MRI showed an overall minimally decrease in demyelinating lesions without new enhancing lesions. She continues to be asymptomatic.
One CD patient in the anti-TNFα exposed group and 4 UC patients in the anti-TNFα unexposed group reached an EDSS score of 6 within the last follow-up, (a score of 6 indicates a need for unilateral assistance required to walk 100 m). Ten of 19 patients (5 CD; 5 UC), who were diagnosed with IIDD prior to the onset of IBD, also reached an EDSS score of 6. The median duration of neurology follow-up for IIDD in patients with anti-TNFα exposure (0.556 years; range, 0.5-3) was shorter than in patients without anti-TNFα exposure (10.23 years; range, 1-23; p = 0.027).
Percent of IIDD
The observed percent of IIDDs in IBD patients with and without anti-TNFα therapy were; IBD: 0.146% and 0.176% (RR=0.828, 95%CI: 0.283-2.420; p=0.729); CD: 0.19% and 0.22% (RR=0.891, 95%CI: 0.240-3.314; p=0.863); UC: 0.073% and 0.148% (RR=0.493, 95%CI: 0.058-4.219; p=0.510). The number needed to harm one patient treated with anti-TNFα was 3333 (4202 for CD; 1335 for UC patients).
Discussion
In our study, anti-TNFα agents did not appear to impact the risk of IIDD in patients with UC or CD independently or as a combined comparator. Our study suggests that IBD patients treated with anti-TNFα agents are no more likely to develop IIDD than IBD patients not treated with anti-TNFα agents.
The prevalence of multiple sclerosis is increased in the IBD population compared to the general population.(12, 13) A recent population-based study in Manitoba (3879 UC patients and 4193 CD patients) reported a prevalence ratio of MS in IBD compared to the general population of 1.81 for UC (95% CI 1.35-2.42) and 1.69 for CD (95% CI 1.31–2.19).(12) A large cohort and cross-sectional study of 7988 CD and 12,185 UC patients showed a higher incidence and prevalence of optic neuritis, demyelination and/or MS in patients with IBD (UC: OR 1.75; 95% CI 1.28–2.39 and CD: OR 1.54; 95% CI 1.03–2.32) when compared to 80,666 healthy controls.(21) In a population based study from Denmark, MS occurs with increased frequency in IBD patients independent of treatment with anti-TNFα agents.(34)
In contrast to multiple sclerosis, there are no population based studies on the occurrence of IIDD in patients with IBD. There are only case reports and case series describing an association between anti-TNFα exposure and development of IIDDs in patients with IBD.(11, 23, 25, 27) However, there are no previously reported comparisons of the diagnosis of IIDD in patient with IBD with and without treatment with anti-TNFα agents.(7, 22, 24, 26, 28)
In the pre anti-TNFα era, population based studies showed a mild increase in the prevalence ratio of IIDDs in patients with UC compared to CD.(12, 13, 21) Interestingly, in the post anti-TNFα era there have been more cases of IIDDs reported in patients with CD.(22, 28) We also found a slightly higher, but not statistically significant difference in incidence of IIDDs in CD (0.21%) compared to UC (0.13%) patients. However, the number of IIDDs is too small to determine if this trend is due to greater use of anti-TNFα agents in patients with CD.
Our study demonstrates that the risk of IIDD in patients who do not have a history of demyelinating disease is small. Nevertheless, the short duration (median 0.53 years, range 0.01-11.37 years) of anti-TNFα use prior to neurological symptoms onset and/or demyelination on MRI related to IIDD suggests an association between anti-TNFα and IIDD. However, two of our 5 patients who developed IIDD after anti-TNFα treatment had persistent neurological symptoms despite stopping the anti-TNFα. In light of these findings, we recommend discontinuation of anti-TNFα agents with the development of neurological symptoms or with findings of demyelination on MRI. Our study did not include IBD patients with a personal history of IIDD who were treated with anti-TNFα agents. A double-blind, placebo-controlled phase II study showed that patients with RRMS treated with lenercept (anti-TNFα) had more exacerbations compared with patients receiving placebo (p = 0.007).(9) Therefore, we do not recommend anti-TNFα therapy in this population and anti-TNFα therapy should be used with caution in those patients with a first degree relative with IIDD. All patients on anti-TNFα therapy who develops neurological symptoms should be evaluated by an expert neurologist.
The strength of our study is the large number of IBD patients screened for IIDD in our 5-state regional area. This sampling allowed us to account for regional patient-referral patterns that could impact the study variables and capture a population with a high degree of ascertainment with regular follow-up. In addition, all cases of IIDD were confirmed by an expert neurologist and gastroenterologist. The greatest limitation of our study is the reliance on our administrative database to identify patients using ICD9 codes. Second, the power of this study is small due to the small number of IIDD cases. However, the sample size needed to detect a statistically significant difference in risk of developing IIDD in IBD patients with a power of 80% is 280250 IBD patients per anti-TNFα exposure group, which was not feasible in this single center study.
In conclusion, our findings suggest the number needed to harm (development of IIDD) one patient with exposure to anti-TNFα therapy is 3333 (4202 for CD; 1335 for UC patients). Based on these findings, anti-TNFα agents do not appear to substantially increase the risk of IIDD.
Acknowledgments
Grant Support:
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Dr. Novotna receives support from the European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123), the European Social Fund and the State Budget of the Czech Republic.
Abbreviations
- ADEM
Acute disseminated encephalomyelitis
- anti-TNFα
Anti-tumor necrosis factor-alpha
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CIS
Clinically isolated syndrome
- CD
Crohn’s disease
- EDSS 6
Expanded Disability Status Scale of 6
- FAERS
Food and Drug Administration Adverse Event Reporting System
- IIDD
Idiopathic inflammatory-demyelinating disease
- IBD
Inflammatory bowel disease
- IA
Iowa
- MN
Minnesota
- MS
Multiple sclerosis
- NMO
Neuromyelitis optica
- ND
North Dakota
- ON
Optic neuritis
- PPMS
Primary progressive multiple sclerosis
- RIS
Radiologically isolated syndrome
- RRMS
Relapsing-remitting multiple sclerosis
- SPMS
Secondary progressive multiple sclerosis
- SAMS
Single attack multiple sclerosis
- SAPMS
Single attack progressive multiple sclerosis
- SD
South Dakota
- SMR
Standardized morbidity ratio
- TM
Transverse myelitis
Footnotes
Guarantor of article: Laura Raffals, MD
Specific author contributions:
Kara M. De Felice – planning of study, data collection, data analysis, interpreting data, and drafting of manuscript. Approved final draft.
Martina Novotna – data collection, data analysis, interpreting data, and drafting of manuscript. Approved final draft.
Felicity T. Enders – statistical analysis. Approved final draft.
William A. Faubion – planning of study. Approved final draft.
William J. Tremaine – planning of study. Approved final draft.
Orhun H. Kantarci - planning of study, interpreting data, and drafting of manuscript. Approved final draft.
Laura E. Raffals – planning of study, interpreting data, and drafting of manuscript. Approved final draft.
Statement of Interests
Disclosures:
All authors have no conflicts of interest to disclose relevant to this research.
References
- 1.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007 Jan;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer S, Feagan B, Lichtenstein G, Mayer L, Schreiber S, Colombel J, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005 Dec 8;353(23):2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn W, Feagan B, Stoinov S, Honiball P, Rutgeerts P, Mason D, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–38. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, van Assche G, Reinisch W, Colombel J-FF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2013;146(1):85. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Lees CW, Ali AI, Thompson AI, Ho GT, Forsythe RO, Marquez L, et al. The safety profile of anti-tumour necrosis factor therapy in inflammatory bowel disease in clinical practice: analysis of 620 patient-years follow-up. Aliment Pharmacol Ther. 2009 Feb 1;29(3):286–97. doi: 10.1111/j.1365-2036.2008.03882.x. [DOI] [PubMed] [Google Scholar]
- 8.van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996 Dec;47(6):1531–4. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 9.TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1999 Aug 11;53(3):457–65. [PubMed] [Google Scholar]
- 10.Mir Subias A, Garcia-Lopez S, Sebastian Torres B, Ollero Domenche L, Garcia Gamez A, Gomollon F. Multiple sclerosis as an adverse effect of anti-tumor necrosis factor agents: an infrequent but important complication of infliximab in Crohn’s disease. Gastroenterol Hepatol. 2013 Feb;36(2):81–5. doi: 10.1016/j.gastrohep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CW, Jr, Weinshenker BG, Sandborn WJ. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis. 2004 Jan;10(1):28–31. doi: 10.1097/00054725-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005 Sep;129(3):827–36. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Hunter SF, Thollander MS, Loftus EV, Jr, Melton LJ, 3rd, O’Brien PC, et al. Concurrence of inflammatory bowel disease and multiple sclerosis. Mayo Clin Proc. 2000 Aug;75(8):802–6. doi: 10.4065/75.8.802. [DOI] [PubMed] [Google Scholar]
- 14.Green C, Elliott L, Beaudoin C, Bernstein CN. A population-based ecologic study of inflammatory bowel disease: searching for etiologic clues. Am J Epidemiol. 2006 Oct 1;164(7):615–23. doi: 10.1093/aje/kwj260. discussion 24-8. [DOI] [PubMed] [Google Scholar]
- 15.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–67. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006 Dec 20;296(23):2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 17.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006 Nov;118(5):1950–61. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westall FC. Molecular mimicry revisited: gut bacteria and multiple sclerosis. J Clin Microbiol. 2006 Jun;44(6):2099–104. doi: 10.1128/JCM.02532-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcellos LF, Kamdar BB, Ramsay PP, DeLoa C, Lincoln RR, Caillier S, et al. Clustering of autoimmune diseases in families with a high-risk for multiple sclerosis: a descriptive study. Lancet Neurol. 2006 Nov;5(11):924–31. doi: 10.1016/S1474-4422(06)70552-X. [DOI] [PubMed] [Google Scholar]
- 20.Geissler A, Andus T, Roth M, Kullmann F, Caesar I, Held P, et al. Focal white-matter lesions in brain of patients with inflammatory bowel disease. Lancet. 1995 Apr 8;345(8954):897–8. doi: 10.1016/s0140-6736(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 21.Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005 Sep;129(3):819–26. doi: 10.1053/j.gastro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Deepak P, Stobaugh D, Sherid M, Sifuentes H, Ehrenpreis E. Neurological events with tumour necrosis factor alpha inhibitors reported to the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther. 2013;38(4):388–96. doi: 10.1111/apt.12385. [DOI] [PubMed] [Google Scholar]
- 23.Dubcenco E, Ottaway CA, Chen DL, Baker JP. Neurological symptoms suggestive of demyelination in Crohn’s disease after infliximab therapy. Eur J Gastroenterol Hepatol. 2006 May;18(5):565–6. doi: 10.1097/00042737-200605000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009 Apr;58(4):501–8. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 25.Freeman HJ, Flak B. Demyelination-like syndrome in Crohn’s disease after infliximab therapy. Can J Gastroenterol. 2005 May;19(5):313–6. doi: 10.1155/2005/358658. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell S, Murphy S, Anwar MM, O’Sullivan M, Breslin N, O’Connor HJ, et al. Safety of infliximab in 10 years of clinical practice. Eur J Gastroenterol Hepatol. 2011 Jul;23(7):603–6. doi: 10.1097/MEG.0b013e3283479125. [DOI] [PubMed] [Google Scholar]
- 27.Ouakaa-Kchaou A, Gargouri D, Trojet S, Hefaiedh R, Elloumi H, Kochlef A, et al. Retrobulbar optic neuritis associated with infliximab in a patient with Crohn’s disease. J Crohns Colitis. 2009 Jun;3(2):131–3. doi: 10.1016/j.crohns.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Casals M, Roberto Perez A, Diaz-Lagares C, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010 Jan;9(3):188–93. doi: 10.1016/j.autrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Van Assche G, Lewis J, Lichtenstein G, Loftus E, Ouyang Q, Panes J, et al. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: safety. Am J Gastroenterol. 2011;106(9):1594. doi: 10.1038/ajg.2011.211. [DOI] [PubMed] [Google Scholar]
- 30.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. ss. Ann Neurol. 2001 Jul;50(1):121–7. doi: 10.1002/ana.1032. Guideline Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 31.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005 Dec;58(6):840–6. doi: 10.1002/ana.20703. Review. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein G, Hanauer S, Sandborn W Practice Parameters Committee of American College of G. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104(2):465. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 33.Kornbluth A, Sachar D Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 34.Andersen NN, Caspersen S, Jess T, Munkholm P. Occurrence of demyelinating diseases after anti-TNFalpha treatment of inflammatory bowel disease: A Danish Crohn Colitis Database study. J Crohns Colitis. 2008 Dec;2(4):304–9. doi: 10.1016/j.crohns.2008.04.001. [DOI] [PubMed] [Google Scholar]


