Abstract
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease affecting spinal cord motoneurons (MN) with an associative connection to Frontotemporal Lobar Dementia (FTLD). The endoplasmic reticulum (ER) bound Sigma-1 Receptor (S1R) chaperone protein localizes to specialized ER cisternae within 10 nm of the plasma membrane in spinal cord ventral horn cholinergic post synaptic C-terminals. Removal of the S1R gene in the Superoxide Dismutase-1 (SOD-1) mouse model of ALS exacerbated the neurodegenerative condition and resulted in a significantly reduced longevity when compared to the SOD-1/S1R wild type (WT) mouse. The proposed amelioration of the ALS phenotype by the S1R is likely due to a “brake” on excitation of the MN as evidenced by a reduction in action potential generation in the MN of the WT when compared to the S1R KO mouse MN. Although the precise signal transduction pathway(s) regulated by the S1R in the MN has/have not been elucidated at present, it is likely that direct or indirect functional interactions occur between the S1R in the ER cisternae with voltage gated potassium channels and/or with muscarinic M2 receptor signaling in the post synaptic plasma membrane. Possible mechanisms for regulation of MN excitability by S1R are discussed.
Keywords: Sigma-1 receptor, ALS, C-terminals, Motoneurons
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease primarily targeting spinal cord motoneurons (MN) (1,2). Often it associates with other neurodegenerative diseases such as Frontotemporal Lobar Dementia (FTLD). ALS can occur due to genetic mutations or environmental factors. Familial cases represent only about 10 percent of ALS cases and these are linked to mutations in particular proteins that eventually result in dysfunction and death of MN (2). Mutations of more than 100 genes result in the establishment of ALS (3), including increasing expansion of GGGGCC hexanucleotide repeats and mutations in superoxide dismutase-1 protein (SOD1), the TAR-DNA binding protein,TDP43, and the RNA-binding protein, FUS. Usually ALS is diagnosed in humans after the age of 40, but in some cases it can occur in juveniles (4,5). There is no cure for ALS and the best known protector, riluzole, which, in part, reduces levels of the excitatory neurotransmitter, glutamate, from neuronal synapses (6,7) can only extend human life for a few months.
Synaptic innervation of MN is complex (8). Synapses involving all known major neurotransmitters innervate MN and have been classified as S, M, T, F, P and C-boutons/terminals (9) (boutons refer to presynaptic; terminals refer to postsynaptic). Presynaptic C-boutons originate from interneurons whose cell bodies are located close to the central canal of the spinal cord, between laminae VII and X (10). Diverse and even controversial changes of C-bouton morphology have been reported in animal models of ALS and spinal cord injury (11–14). C-terminals are cholinergic postsynaptic sites with a unique ultrastructure seen at the electron microscopy level. They are referred to as “C” because of the subsurface cisternae of smooth endoplasmic reticulum adjacent to the plasma membrane (PM) and are giant synapses (2–7 μm in diameter, but somewhat vary depend on the species) found only on soma and proximal dendrites of MN. The presence of such subsurface cisternae in synaptic terminals larger than 2 μm can serve as a marker for MNs (15).
2. The S1R and neurodegenerative diseases
The Sigma-1 Receptor (S1R) chaperone protein (16) has been shown to be a target for the treatment of a variety of chronic neurological diseases including amnesia (17), pain (18), depression (19), Alzheimers (20), Parkinsons (21), and Huntingtons (22) diseases, schizophrenia (23), stroke (24) and degeneration of retinal neurons (25,26). Recently, ALS was added to this list (4). It is remarkable that MN express S1R at the highest levels in the CNS (27, 28). Mutations of the S1R have been found to result in the establishment of ALS in humans (4). In contrast, genetic knockout of the S1R in mice did not produce an ALS phenotype (29). S1R knockout mice on an ALS background, however, showed a faster onset of disease and decreased longevity (30) while application of S1R ligands significantly extended the lifespan of ALS model mice (31–33).
3. Structural and pharmacological properties of S1R
Initially the S1R was identified by specific binding to SKF 10047 (34) and was classified as an opioid-like receptor, but later experiments showed that the S1R has pharmacological binding properties distinct from opioid receptors (35). Another sigma receptor, the Sigma-2 receptor, has been identified by binding to ditolylgunaidine (DTG) (36) although it has not yet been cloned. The cloned S1R is a protein of 223 amino acids containing two transmembrane sequences with both the N and C termini on the same side of the membrane (16,37). The S1R in its purified form, in vitro, and also the S1R in vivo, exists in monomeric, dimeric, tetrameric and higher oligomeric forms (38,39). It has been suggested that the functional activities of the S1R are linked to S1R agonist and antagonist alteration of the equilibria between these various forms (38). The C-terminus (approximately 110 amino acids) in either the dimeric and/or monomeric form(s) is likely to determine the manner by which the overall chaperone functions of the S1R are established with various “client” proteins (37,40–44).
Many synthetic compounds have been characterized as selective agonists or antagonists for the S1R, (16,45,46). Several endogenous compounds have been proposed to be S1R ligands, including the lipid, sphingosine and its derivatives (47), steroids such as DHEA, progesterone and pregnenolone sulfate (48), and the trace amine, N, N-dimethyl tryptamine (DMT) (49). It is very possible that various endogenous compounds regulate the functions of the S1R in different cells and tissues depend on their availability to specifically bind to the S1R (17).
4. Functions of S1R
The S1R has been shown to have a number of chaperone and client protein-related functions: activation or inhibition of plasma membrane excitability (50), modulation of G-protein coupled receptors (GPCRs) (51,52), and ER stress (53,54). Thus to date the literature shows that the S1R is a multi-tasking protein involved in a broad cell range of signaling pathways and functions (16,17). Langa et al (29) demonstrated that the Sigma-1 receptor knockout mice are fully fertile, viable, and showed no obvious behavioral phenotypes. This was an interesting result since the loss of the S1R was expected to cause loss of some crucial biological functions. The same group did subsequently show that S1R knockout mice had a diminished response to pain (55). In other studies two additional important functions have also been well documented; that is, modulation of ion channel activity (56,57) and ER stress (37). In ER stress, an unfolded protein response results from the accumulation of misfolded proteins in the endoplasmic reticulum lumen (58). The first response to ER stress is the activation of intracellular chaper-ones to refold the misfolded proteins. If the accumulation of unfolded proteins exceeds the ability of the chaperones to correct the condition, cellular apoptosis is triggered. Key proteins participating in the ER stress are BIP, IRE1, eIF1 and PERK (58). To date, the S1R has been shown to interact with BIP, PERK, and IRE1 and through these interactions has been proposed, in part, to alleviate ER stress (37,59,60). S1R interacts with the IP3 receptor 3 in mitochondrial-associated membranes (MAM) and modulates calcium flow from the ER into mitochondria, a process important for the regulation of bioenergetics (37). Through these mechanisms the S1R plays a secondary but critical role and acts as a chaperone/adaptor/modulator of the activities of interacting client proteins. To date the S1R itself has not been shown to have functional activities independent of other protein partners.
5. Specific connections of the S1R to ALS
The S1R is distributed in many mammalian tissues (61,62). In the central nervous system (CNS) the highest levels of Sigma-1 receptors are found in MN of the spinal cord and brainstem (27,28). The presence of the S1R in MN is particularly intriguing because these cells are highly vulnerable to destruction and no therapy is available to restore MN. Chemicals that induce toxicity and/or genetic mutations can result in the loss of MNs. It has been shown (although only in one family) that an E102Q autosomal-recessive mutation in the S1R results in juvenile ALS (4). Recent evidence has further linked overexpression of the E102Q S1R in Neuro2A cells to aggregation of the mutant S1R followed by reduction in mitochondrial ATP production and mislocalization of the TAR DNA binding protein, TDP43, from the nucleus to the cytoplasm. This effect of E102Q mutation could be rescued by addition of methyl pyruvate to maintain mitochondrial ATP production (63).
An autosomal recessive phenotype (required mutation on two alleles) implies that the lack of function of the S1R can establish ALS in humans. Also, mutation of the 3′ untranslated region of the Sigma-1 receptor gene in humans resulted in ALS and FTLD (64). In this case it was sufficient to have only one allele mutant for the appearance of disease. On the other hand S1R knockout mice (that might be predicted to show a “lack of function”) show no ALS development. The protective role of S1R in ALS, however, has been revealed in ALS model mice where the presence of a mutant superoxide dismutase 1 (SOD1 G*93A mouse model) and a lack of S1R (S1R KO), caused exacerbation of disease progression as revealed by behavioral and longevity experiments (Fig. 1). Moreover, to date, in two ALS animal mouse models, the specific S1R ligand, PRE084 and SA4503 extended lifespan and resulted in the maintenance of mouse motor skills and MN longevity. These data may demonstrate a difference between mutations of human and mouse S1Rs since when mice are stressed a S1R related phenotype is exhibited. The appearance of the S1R in the MN of ALS model mice has been somewhat controversial. Diseased MN lose their shape, decrease in size, and show decreases in synaptic coverage. Abundant S1R in the diseased MN that survive after fixation have been observed but the total number of Sigma-1 receptors in the spinal cord are reduced because of MN death. In contrast, using the same mouse model, Casas et al (14) found absence of Sigma-1 receptors in diseased lumbar MN although the use of specific markers to confirm MN identity were lacking.
Fig. 1.
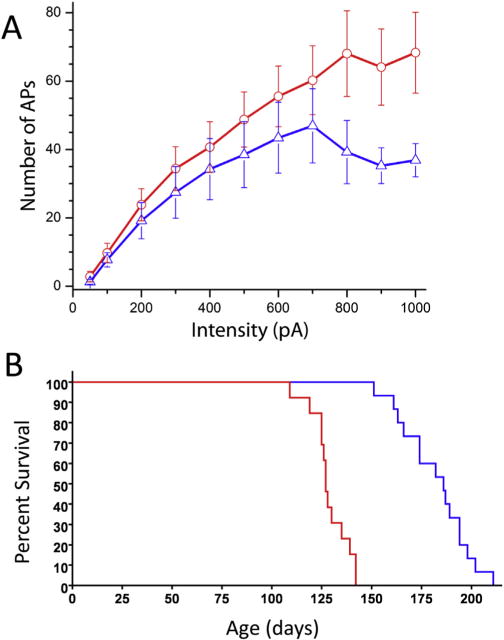
Sigma-1 receptor reduces motoneuron excitability and slows ALS progression. A. Frequency-current relationships in motoneurons of SR1 KO (red) and WT (blue) mice. A significant increase in the slope of FeI relationship was apparent in S1R KO mice at current intensities > 700 pA (p < 0.05). Bars are ± standard errors. Number of recordings per intensity is shown above/below standard error bars. B. Kaplane-Meyer end stage curve. Median survival of mice is 186.0 days for ALS S1R WT mice (blue), and 127.0 days for ALS S1R KO mice (red). p < 0.0001; χ2 = 32.29. Modified with copyright permission from: Mavlyutov et al., Neuroscience 2013; 240:129–34.
6. Subcellular localization and proposed function(s) of the S1R in motor neurons
The S1R can participate in many different intracellular pathways (16). To understand the mechanism(s) by which the S1R plays a neuroprotective role in MN (or generally in other cells), it is important to know where the S1R is localized in the cell. Using immunoelectron microscopy we previously showed that the S1R is localized in subsurface cisternae in C-terminals (Fig. 2). C-terminals contain Muscarinic type 2 acetylcholine receptors (M2AChR), voltage gated potassium channels (Kv2.1) and slow potassium (SK) channels located in the postsynaptic plasma membrane. The presence of subsurface cisternae in postsynaptic densities is believed to correlate with observed postsynaptic hyperpolarization (65–67). In this regard, Kv2.1 and SK channels are able to mediate after hyperpolarization of cells. Generally it has been proposed that differential expression of SK2.2 and SK2.3 channels in neurons distinguish fast or slow type MN (68). Miles et al (10). have shown in MN that activation of M2AChR results in inhibition of SK channels, a result that reduces after hyperpolarization and thus increases neuronal excitability. We have shown that the excitability of MN is higher in S1R knockout mice than in their wild type counterparts which is consistent with the idea that the absence of S1R prevents the activation of Kv2.1 and/or SK channels (30) (Fig. 2). Thus the S1R appears to act as a brake on the excitability of MN. Many potassium channels are modulated by S1R (44,69–71). To summarize: 1) in common to many mammalian cells, the S1R in MN is found in the ER; 2) the S1R is abundant in MN cholinergic postsynaptic sites; 3) the S1R is enriched in only C-terminals, when compared to neurons in general, including many of the neurons and interneurons of the spinal cord.
Fig. 2.
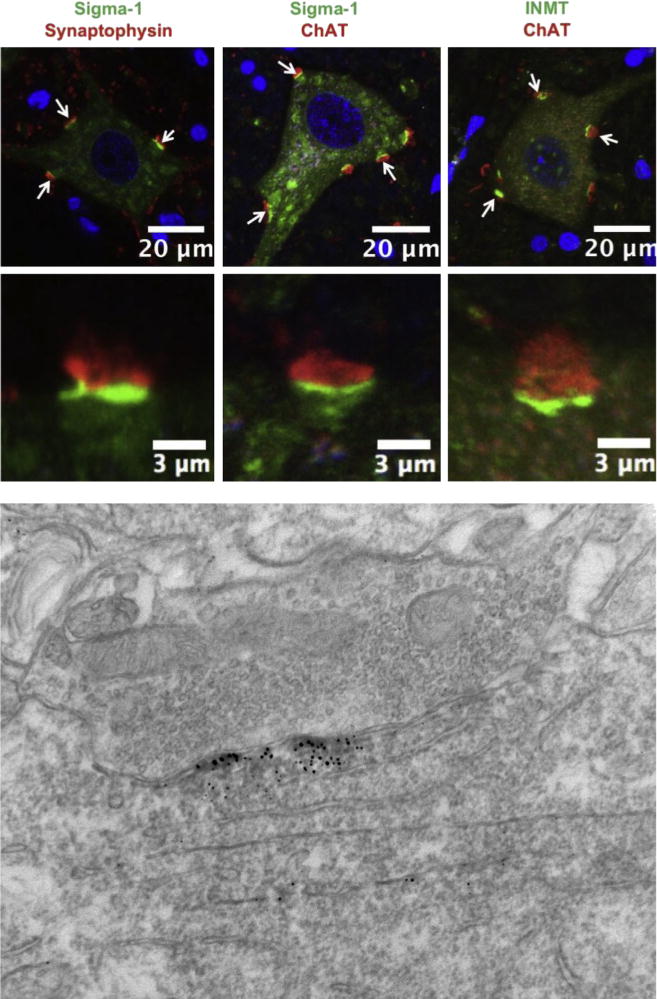
The sigma-1 receptor (green signal in the left and middle panels) and the DMT producing enzyme INMT (green in the right panel) are localized to postsynaptic sites of C-terminals and juxtaposed to presynaptic cholinergic (ChAT positive) boutons (red). To demonstrate that the sigma-1 receptor is juxtaposed only to cholinergic postsynaptic densities of MNs we performed double labeling with antibodies against synaptophysin (a universal marker for different types of chemical synapses). Notice that not all synaptophysin-positive synapses are juxtaposed to the sigma-1 receptor. Blue (DAPI stain) indicates cell nuclei. Lower panel shows localization of sigma-1 receptor in subsurface cistern of cholinergic postsynaptic site. Modified with copyright permission from: Mavlyutov et al., Neuroscience 2012; 206: 60–80.
7. Speculations regarding S1R mechanism(s) for protecting motor neurons (Fig. 3)
Fig. 3.
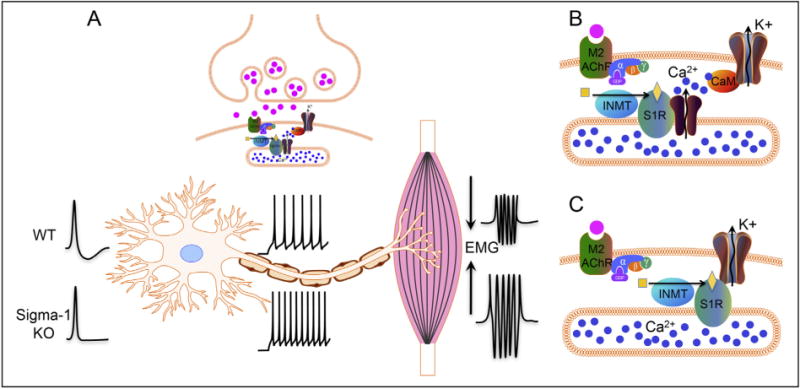
Diagram showing our hypothesis for the role of the S1R in C-terminals: A. The S1R regulates the activity of potassium channels located in postsynaptic sites of C-terminals. In WT animals the S1R will activate potassium channels resulting in a larger afterhyperpolarization that reduces the firing frequency in motoneurons. In sigma-1 KO animals, a reduced afterhyperpolarization results in a higher firing frequency and a stronger muscle contraction. B and C: Two possible mechanisms showing how S1R can regulate the activity of potassium channels in the postsynaptic plasma membrane. B. The enzyme INMT produces the ligand that activates the S1R localized in the subsurface cisternae. Active S1R results in a conductance increase in calcium channels, and the subsequent opening of potassium channels (SK2 and/or Kv2.1) through calmodulin (CaM), leading to an after-hyperpolarization. C. S1R can directly interact with and modulate potassium channels, resulting in an increased conductance of the SK2 and/or Kv2.1 channels in the plasma membrane of C-terminals.
The S1R in C-terminals is in close physical proximity to Kv2.1 and SK channels making these two channels suitable candidates for interaction with S1R in postsynaptic membrane of MN. At the moment, it is unclear how the S1R might activate Kv2.1 and/or SK channels and thus decrease excitability. It has been shown in other neuronal cells that S1R can modulate activities of SK channels and a variety of Kv type channels (44,71–73). Moreover, it has been shown that S1R can also form a complex with a variety of G-protein coupled receptors (GPCRs) and can modulate their activities (51,52). An accumulation of literature indicates that inhibition of M2AChR and/or activation of Kv2.1 and/or SK channels in C-terminals should contribute toward reduction of MN excitability (10,74,75). Thus it remains to be elucidated whether, in MN and possibly other neurons, the S1R can inhibit the M2AChR directly or indirectly interfere with Gi signaling with a resultant decrease in neuronal excitability. As previously stated, M2AChR, Kv2.1, and SK channels are all known to be located in the plasma membrane (PM) (75), while the S1R is located in the subsurface cisternae of C-terminals (28). Physical proximity of the PM and the cisternae in C-terminals (distance of less than 10 nM) make direct molecular interaction possible for proteins located in adjacent membranes. Examples of such interactions between proteins located in the PM and subsurface cisternae can be found in skeletal muscle, where the dihydropyridine receptor in the PM directly interacts with the ryanodine receptor in cisternae, or in cardiomyocytes where the calcium channel, Orai, located in the PM and STIM-1 located in cisternae, close to the PM, interact in a functionally relevant manner (76). In both examples these interactions are important for enhanced calcium flow. Subsurface cisternae have been considered as powerful calcium capacitors (77). Perhaps relevant to this fact is the interaction of S1R with the inositol triphosphate type 3 receptor (IP3R3) to enhance calcium flow to mitochondria (37). Activities of both Kv2.1 and SK channels are modulated by calcium, ether directly or indirectly through Ca/calmodulin/calcineurin mechanisms (78,79) (Fig. 3). It is possible that the S1R by chaperoning IP3Rs can also indirectly influence these potassium channels via regulation of calcium release from the cisternae. Thus it is reasonable to consider that modulation of potassium channels in C-terminals may partly underlie the mechanism(s) by which the S1R can reduce excitability of MN. Other possible mechanisms for S1R protection of MN certainly exist; for example, recently Esquerda and colleagues have shown that the growth factor Neuregulin-1 that protects MN loss in ALS co-localizes with the S1R in C-terminal cisternae (80) further expanding a possible role for S1R in MN as a Neuregulin-1 interacting protein.
8. Other possible S1R interactions in C-terminals
1) The S1R, Indole(ethyl)amine N-MethylTransferase (INMT) and N,N-Dimethyltryptamine (DMT)
As indicated previously, several endogenous compounds have been shown to bind to the S1R and thus may provide regulation of S1R activities (16). Although the affinity of these compounds for the S1R varies considerably, activation of the S1R may depend on the cellular environments and the local concentrations in various mammalian tissues. We have shown that N,N-dimethylytrptamine (DMT) is an agonist for the S1R (49). The relationship between DMT and the S1R was further reinforced by the demonstration that the enzyme, Indole(ethyl)amine N-methyltransferase (INMT) that converts the amino acid tryptophan decarboxylation product, tryptamine, into DMT, co-localized with the S1R in C-terminals of the primate MN (81) (Fig. 2). This paradigm shifting observation of co-localization of S1R and INMT to produce DMT, may be functionally very important to provide high local concentrations of the agonist due to the close juxtaposition with the S1R. This co-localization may further be relevant because the affinity of DMT towards S1R as determined by in vitro experiments is relatively low, in the micromolar range (49).
2) S1R, Indole(ethyl)amine N-MethylTransferase INMT) and Thio-EtherMethylTransferase (TEMT)
Recent data indicate (82) that, in contrast to humans, in mice the primary function of INMT (also known as mouse TEMT) is NOT methylation of tryptamine, but rather methylation of sulfur, selenium and tellurium containing compounds, presumably for detoxification purposes (83) and for reduction of oxidative stress (84,85). In addition to its ability to methylate tryptamine, recombinant; human INMT (hINMT) can also methylate thiol containing compounds (such as the endogenous compound, cysteamine and the synthetic substrate, 2-(methylthio) ethylamine) due to an alternate TEMT enzyme activity (82). This observation reinforces the report (63) that the S1R mutation E102Q that underlies juvenile ALS (4) causes cytoplasmic aggregation of S1R and oxidative stress in Neuro2A cells via mitochondrial disruption of ATP synthesis. The potential oxidative stress reducing properties of the TEMT activity of INMT is also in complete accord with previous demonstrations that the S1R reduces oxidative stress in cells (25,54,86). It is therefore reasonable to conclude that the co-localization of S1R with INMT/TEMT (81) in primate and human MN may provide protection by the S1R through both enzyme activities (i.e. formation of DMT and reduction in oxidative stress via methylation of thiols and trace metals).
3) S1R, Neuregulin-1 and erbB receptors
The S1R co-localizes in mouse MN C-terminal cisternae with Neuregulin-1 (80). Neuregulin 1 is a growth factor acting on erbB receptors which are found on C-boutons. To reach the presynaptic membrane from the subsurface cisternae, neuregulin 1 is sorted in an exosomal pathway (80). Levels of Neuregulin-1 are known to be reduced in MN of human ALS patients and also in SOD1G93A mice (87). Although a molecular interaction between S1R and Neuregulin-1 has not been established, it is tempting to speculate that the S1R serves to chaperone Neuregulin-1 to the subsurface cisternae of MN. Indeed, the S1R has been also shown to regulate post-translational processing of growth factors (88).
9. Conclusions
The protective roles of S1R in MN of mouse models of ALS have been confirmed. Although the precise molecular mechanisms underlying these protective roles have not been clearly elucidated at present, the multi functional nature of the S1R provides an attractive target for treating ALS. Future human trials will be needed to show if targeting S1R with selective S1R agonists, perhaps in combination with appropriate drugs that regulate other ALS ameliorating signaling pathways, can synergistically protect MN in Amyotrophic Lateral Sclerosis.
Acknowledgments
This work was supported by National Institutes of Health Grant Number NIH NS075820 (AER), the UW McPherson Eye Research Institute Retina Research Foundation Edwin and Dorothy Gamewell Professorship (AER), NIH DK081634 (MLE), and NIH RO1EY022678 (L-WG).
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Peer review under responsibility of Japanese Pharmacological Society.
References
- 1.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel O, Powell JF, Andersen PM, Al-Chalabi A. ALSoD: a user-friendly online bioinformatics tool for amyotrophic lateral sclerosis genetics. Hum Mutat. 2012;33:1345–1351. doi: 10.1002/humu.22157. [DOI] [PubMed] [Google Scholar]
- 4.Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70:913–919. doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- 5.Marin B, Kacem I, Diagana M, Boulesteix M, Gouider R, Preux PM, et al. Juvenile and adult-onset ALS/MND among Africans: incidence, phenotype, survival: a review. Amyotroph Lateral Scler. 2012;13:276–283. doi: 10.3109/17482968.2011.648644. [DOI] [PubMed] [Google Scholar]
- 6.Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17:1942–1959. doi: 10.2174/092986710791163939. [DOI] [PubMed] [Google Scholar]
- 7.Vucic S, Lin CS, Cheah BC, Murray J, Menon P, Krishnan AV, et al. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361–1370. doi: 10.1093/brain/awt085. [DOI] [PubMed] [Google Scholar]
- 8.Wyckoff RW, Young JZ. The motorneuron surface. Proc R Soc Lond, B, Biol Sci. 1956;144:440–450. doi: 10.1098/rspb.1956.0002. [DOI] [PubMed] [Google Scholar]
- 9.Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- 10.Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pullen AH, Athanasiou D. Increase in presynaptic territory of C-terminals on lumbar motoneurons of G93A SOD1 mice during disease progression. Eur J Neurosci. 2009;29:551–561. doi: 10.1111/j.1460-9568.2008.06602.x. [DOI] [PubMed] [Google Scholar]
- 12.Herron LR, Miles GB. Gender-specific perturbations in modulatory inputs to motoneurons in a mouse model of amyotrophic lateral sclerosis. Neuroscience. 2012;226:313–323. doi: 10.1016/j.neuroscience.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Nagao M, Misawa H, Kato S, Hirai S. Loss of cholinergic synapses on the spinal motor neurons of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1998;57:329–333. doi: 10.1097/00005072-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Casas C, Herrando-Grabulosa M, Manzano R, Mancuso R, Osta R, Navarro X. Early presymptomatic cholinergic dysfunction in a murine model of amyotrophic lateral sclerosis. Brain Behav. 2013;3:145–158. doi: 10.1002/brb3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deardorff AS, Romer SH, Sonner PM, Fyffe RE. Swimming against the tide: investigations of the C-bouton synapse. Front Neural Circuits. 2014;8:106. doi: 10.3389/fncir.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem Pharmacol. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 19.Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villard V, Espallergues J, Keller E, Vamvakides A, Maurice T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (sigma1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J Psychopharmacol. 2011;25:1101–1117. doi: 10.1177/0269881110379286. [DOI] [PubMed] [Google Scholar]
- 21.Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137:1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- 22.Hyrskyluoto A, Pulli I, Tornqvist K, Ho TH, Korhonen L, Lindholm D. Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-kappaB pathway. Cell Death Dis. 2013;4:e646. doi: 10.1038/cddis.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishiguro H, Ohtsuki T, Toru M, Itokawa M, Aoki J, Shibuya H, et al. Association between polymorphisms in the type 1 sigma receptor gene and schizophrenia. Neurosci Lett. 1998;257:45–48. doi: 10.1016/s0304-3940(98)00797-6. [DOI] [PubMed] [Google Scholar]
- 24.Ajmo CT, Jr, Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- 25.Mavlyutov TA, Nickells RW, Guo LW. Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis. 2011;17:1034–1043. [PMC free article] [PubMed] [Google Scholar]
- 26.Aamodt K, Abrahantes Quintana A, Adamova D, Adare AM, Aggarwal MM, Aglieri Rinella G, et al. Centrality dependence of the charged-particle multiplicity density at midrapidity in Pb-Pb collisions at sqrt[s(NN)] = 2.76 TeV. Phys Rev Lett. 2011;106:032301. doi: 10.1103/PhysRevLett.106.032301. [DOI] [PubMed] [Google Scholar]
- 27.Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+) 3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 30.Mavlyutov TA, Epstein ML, Verbny YI, Huerta MS, Zaitoun I, Ziskind-Conhaim L, et al. Lack of Sigma-1 receptor exacerbates ALS progression in mice. Neuroscience. 2013;240:129–134. doi: 10.1016/j.neuroscience.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peviani M, Salvaneschi E, Bontempi L, Petese A, Manzo A, Rossi D, et al. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol Dis. 2014;62:218–232. doi: 10.1016/j.nbd.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Mancuso R, Olivan S, Rando A, Casas C, Osta R, Navarro X. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurother: J Am Soc Exp Neurother. 2012;9:814–826. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono Y, Tanaka H, Takata M, Nagahara Y, Noda Y, Tsuruma K, et al. SA4503, a sigma-1 receptor agonist, suppresses motor neuron damage in in vitro and in vivo amyotrophic lateral sclerosis models. Neurosci Lett. 2014;559:174–178. doi: 10.1016/j.neulet.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- 35.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 36.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 38.Gromek KA, Suchy FP, Meddaugh HR, Wrobel RL, LaPointe LM, Chu UB, et al. The oligomeric states of the purified Sigma-1 receptor are stabilized by ligands. J Biol Chem. 2014;289:20333–20344. doi: 10.1074/jbc.M113.537993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavlyutov T, Chu U, Yag J, Fox BG, Gromek K, Primm J, et al. Oligomerization of the sigma-1 receptor. Neurosci Soc Abstr. 2013 [Google Scholar]
- 40.Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J Biol Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balasuriya D, Stewart AP, Crottes D, Borgese F, Soriani O, Edwardson JM. The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry. J Biol Chem. 2012;287:37021–37029. doi: 10.1074/jbc.M112.382077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balasuriya D, Stewart AP, Edwardson JM. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J Neurosci. 2013;33:18219–18224. doi: 10.1523/JNEUROSCI.3360-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carnally SM, Johannessen M, Henderson RM, Jackson MB, Edwardson JM. Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels. Biophys J. 2010;98:1182–1191. doi: 10.1016/j.bpj.2009.12.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balasuriya D, D’sa L, Talker R, Dupuis E, Maurin F, Martin P, et al. A direct interaction between the sigma-1 receptor and the hERG voltage-gated K+ channel revealed by atomic force microscopy and homogeneous time-resolved fluorescence (HTRF(R)) J Biological Chem. 2014;289:32353–32363. doi: 10.1074/jbc.M114.603506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robson MJ, Noorbakhsh B, Seminerio MJ, Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Curr Pharm Des. 2012;18:902–919. doi: 10.2174/138161212799436601. [DOI] [PubMed] [Google Scholar]
- 46.Berardi F, Abate C, Ferorelli S, Colabufo NA, Perrone R. 1-Cyclohexylpiperazine and 3,3-dimethylpiperidine derivatives as sigma-1 (sigma1) and sigma-2 (sigma2) receptor ligands: a review. Cent Nerv Syst Agents Med Chem. 2009;9:205–219. doi: 10.2174/1871524910909030205. [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol. 2009;609:19–26. doi: 10.1016/j.ejphar.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 49.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim FJ, Kovalyshyn I, Burgman M, Neilan C, Chien CC, Pasternak GW. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, et al. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, et al. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012;682:12–20. doi: 10.1016/j.ejphar.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 56.Crottes D, Guizouarn H, Martin P, Borgese F, Soriani O. The sigma-1 receptor: a regulator of cancer cell electrical plasticity? Front Physiol. 2013;4:175. doi: 10.3389/fphys.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, et al. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000;526:527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omi T, Tanimukai H, Kanayama D, Sakagami Y, Tagami S, Okochi M, et al. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014;5:e1332. doi: 10.1038/cddis.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori T, Hayashi T, Hayashi E, Su TP. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8:e76941. doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferris CD, Hirsch DJ, Brooks BP, Snyder SH. Sigma receptors: from molecule to man. J Neurochem. 1991;57:729–737. doi: 10.1111/j.1471-4159.1991.tb08213.x. [DOI] [PubMed] [Google Scholar]
- 62.Su TP. Sigma receptors. Putative links between nervous, endocrine and immune systems. Eur J Biochem. 1991;200:633–642. doi: 10.1111/j.1432-1033.1991.tb16226.x. [DOI] [PubMed] [Google Scholar]
- 63.Tagashira H, Shinoda Y, Shioda N, Fukunaga K. Methyl pyruvate rescues mitochondrial damage caused by SIGMAR1 mutation related to amyotrophic lateral sclerosis. Biochim Biophys Acta. 2014;1840:3320–3334. doi: 10.1016/j.bbagen.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Luty AA, Kwok JB, Dobson-Stone C, Loy CT, Coupland KG, Karlstrom H, et al. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol. 2010;68:639–649. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- 65.Henkart M, Landis DM, Reese TS. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol. 1976;70:338–347. doi: 10.1083/jcb.70.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henkart M. Identification and function of intracellular calcium stores in axons and cell bodies of neurons. Fed Proc. 1980;39:2783–2789. [PubMed] [Google Scholar]
- 67.Fujimoto S, Yamamoto K, Kuba K, Morita K, Kato E. Calcium localization in the sympathetic ganglion of the bullfrog and effects of caffeine. Brain Res. 1980;202:21–32. [PubMed] [Google Scholar]
- 68.Deardorff AS, Romer SH, Deng Z, Bullinger KL, Nardelli P, Cope TC, et al. Expression of postsynaptic Ca2þ-activated Kþ (SK) channels at C-bouton synapses in mammalian lumbar -motoneurons. J Physiol. 2013;591:875–897. doi: 10.1113/jphysiol.2012.240879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 71.Kinoshita M, Matsuoka Y, Suzuki T, Mirrielees J, Yang J. Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res. 2012;1452:1–9. doi: 10.1016/j.brainres.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aydar E, Onganer P, Perrett R, Djamgoz MB, Palmer CP. The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 2006;242:245–257. doi: 10.1016/j.canlet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 73.Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deardorff AS, Romer SH, Deng Z, Bullinger KL, Nardelli P, Cope TC, et al. Expression of postsynaptic Ca2þ-activated Kþ (SK) channels at C-bouton synapses in mammalian lumbar alpha-motoneurons. J Physiol. 2012;591:875–897. doi: 10.1113/jphysiol.2012.240879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clapham DE. A STIMulus package puts orai calcium channels to work. Cell. 2009;136:814–816. doi: 10.1016/j.cell.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 77.Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 80.Gallart-Palau X, Tarabal O, Casanovas A, Sabado J, Correa FJ, Hereu M, et al. Neuregulin-1 is concentrated in the postsynaptic subsurface cistern of C-bouton inputs to alpha-motoneurons and altered during motoneuron diseases. Faseb J. 2014;28:3618–3632. doi: 10.1096/fj.13-248583. [DOI] [PubMed] [Google Scholar]
- 81.Mavlyutov TA, Epstein ML, Liu P, Verbny YI, Ziskind-Conhaim L, Ruoho AE. Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N,N′-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience. 2012;206:60–68. doi: 10.1016/j.neuroscience.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mavlyutov TA, Chu UB, Schulman A, Baker E, Raj R, Epstein ML, et al. Methylation of a thiols and thioethers by human indolethylamine-n-methyl transferase. Soc Neurosci Abstr. 2014 [Google Scholar]
- 83.Mozier NM, McConnell KP, Hoffman JL. S-adenosyl-L-methionine:thioether S-methyltransferase, a new enzyme in sulfur and selenium metabolism. J Biol Chem. 1988;263:4527–4531. [PubMed] [Google Scholar]
- 84.Steinbrenner H, Sies H. Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys. 2013;536:152–157. doi: 10.1016/j.abb.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 85.Dharmasena A. Selenium supplementation in thyroid associated ophthalmopathy: an update. Int J Ophthalmol. 2014;7:365–375. doi: 10.3980/j.issn.2222-3959.2014.02.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai SY, Rothman RK, Su TP. Insights into the Sigma-1 receptor chaperone’s cellular functions: a microarray report. Synapse. 2012;66:42–51. doi: 10.1002/syn.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song F, Chiang P, Wang J, Ravits J, Loeb JA. Aberrant neuregulin 1 signaling in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2012;71:104–115. doi: 10.1097/NEN.0b013e3182423c43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66:630–639. doi: 10.1002/syn.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]


