In follow-up experiments in our laboratory, we have found that errors in analyzing the unpurified 1H NMR spectra led us to incorrectly report the crude diastereomer ratios for the lactone products. The correct ratios are listed below. To address this issue, we have developed an alternative experimental procedure that generally results in improved stereoselectivity. The reduction is conducted at room temperature, then the reaction mixture is warmed to 70 °C to achieve lactonization. For example, in an aggregate of two trials lactone 2a was obtained in 84% yield, >30:1 dr and 95:5 er when the reduction was conducted at ambient temperature for 24 h followed by heating for 16 h at 70 °C. Lactone 2d was similarly prepared in 98% yield (dr >30:1 dr, er 95:5, two runs). Using α-keto ester 1h as a substrate, the major diastereomer of lactone 2h was isolated in 78% yield (crude dr = 7:1, er 95:5, two runs) using this updated procedure. We regret the error and suggest using this revised protocol for optimal results. The authors thank Blane Zavesky and Guy Goodman of these laboratories for their work on the revised protocol.
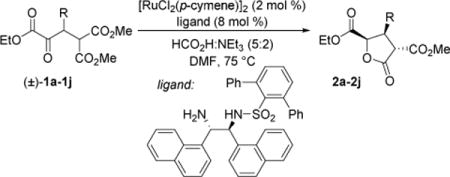
| |
|
| |
| R | dr |
|
| |
| C6H5 (2a) | 8–14:1 |
| 4-ClC6H4 (2b) | 14:1 |
| 4-MeC6H4 (2c) | 11:1 |
| 4-MeOC6H4 (2d) | 10:1 |
| 4-NCC6H4 (2e) | 17:1 |
| 2-MeC6H4 (2f) | 6:1 |
| benzo[d][1,3]dioxol-5-yl (2g) | 20:1 |
| fur-2-yl (2h) | 6:1 |
| N-Ts-indol-3-yl (2i) | 25:1 |
| N-Boc-indol-3-yl (2j) | 20:1 |
|
| |
Contributor Information
Kimberly M. Steward, University of North Carolina at Chapel Hill, Chemistry
Emily C. Gentry, University of North Carolina at Chapel Hill, Chemistry
Jeffrey S. Johnson, University of North Carolina, Department of Chemistry


