Abstract
IMPORTANCE
Relapse is highly prevalent following substance abuse treatments, highlighting the need for improved aftercare interventions. Mindfulness-based relapse prevention (MBRP), a group-based psychosocial aftercare, integrates evidence-based practices from mindfulness-based interventions and cognitive-behavioral relapse prevention (RP) approaches.
OBJECTIVE
To evaluate the long-term efficacy of MBRP in reducing relapse compared with RP and treatment as usual (TAU [12-step programming and psychoeducation]) during a 12-month follow-up period.
DESIGN, SETTING, AND PARTICIPANTS
Between October 2009 and July 2012, a total of 286 eligible individuals who successfully completed initial treatment for substance use disorders at a private, nonprofit treatment facility were randomized to MBRP, RP, or TAU aftercare and monitored for 12 months. Participants medically cleared for continuing care were aged 18 to 70 years; 71.5% were male and 42.1% were of ethnic/racial minority.
INTERVENTIONS
Participants were randomly assigned to 8 weekly group sessions of MBRP, cognitive-behavioral RP, or TAU.
MAIN OUTCOMES AND MEASURES
Primary outcomes included relapse to drug use and heavy drinking as well as frequency of substance use in the past 90 days. Variables were assessed at baseline and at 3-, 6-, and 12-month follow-up points. Measures used included self-report of relapse and urinalysis drug and alcohol screenings.
RESULTS
Compared with TAU, participants assigned to MBRP and RP reported significantly lower risk of relapse to substance use and heavy drinking and, among those who used substances, significantly fewer days of substance use and heavy drinking at the 6-month follow-up. Cognitive-behavioral RP showed an advantage over MBRP in time to first drug use. At the 12-month follow-up, MBRP participants reported significantly fewer days of substance use and significantly decreased heavy drinking compared with RP and TAU.
CONCLUSIONS AND RELEVANCE
For individuals in aftercare following initial treatment for substance use disorders, RP and MBRP, compared with TAU, produced significantly reduced relapse risk to drug use and heavy drinking. Relapse prevention delayed time to first drug use at 6-month follow-up, with MBRP and RP participants who used alcohol also reporting significantly fewer heavy drinking days compared with TAU participants. At 12-month follow-up, MBRP offered added benefit over RP and TAU in reducing drug use and heavy drinking. Targeted mindfulness practices may support long-term outcomes by strengthening the ability to monitor and skillfully cope with discomfort associated with craving or negative affect, thus supporting long-term outcomes.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01159535
Despite decades of research, the high prevalence of and relapse to substance use disorders (SUDs) continues to challenge the field. Approximately 10.6% of US individuals with SUDs seek treatment,1 and 40% to 60% relapse within 1 year.2 This lack of treatment engagement and chronic relapsing nature3 highlight the need for further attention to and options for treatment of SUDs.
Relapse prevention (RP) therapy,4 a widely studied and implemented approach,5 posits that interactions between individual factors (eg, motivation and coping) and environmental factors (eg, social influences and access to substances) increase relapse risk.6 Relapse prevention offers a framework for identifying situations that precipitate relapse and teaches cognitive and behavioral skills to reduce risk. Reviews and meta-analyses7–12 show evidence for effectiveness relative to no-treatment control conditions.
Despite supporting evidence, potential shortcomings of RP have been identified, including focus on avoidance-based goals (ie, avoiding high-risk situations)13 vs approach-based goals14 and on controlling causes of negative affect or craving vs learning to tolerate these states.15 The latter point may be particularly disadvantageous because negative affect has been shown3,14 to be a primary predictor of relapse, and exposure to and acceptance of such states may be mechanisms of behavioral change.15–19 Finally, RP places relatively little emphasis on individual needs, values, and issues that may underlie problematic behavior.14,20 It has thus been proposed that to increase effectiveness, treatment must address avoidance of affective or cognitive discomfort,20 provide skills to tolerate these states, and identify underlying functions of substance use.21
Mindfulness-Based Treatment
Mindfulness involves attending to experiences on a moment-to-moment basis with intention to cultivate nonjudgmental, nonreactive states of awareness.22 Mindfulness-based treatments teach patients to remain in contact with and relate differently to challenging affective or physical states,23 use alternatives to avoidant-based coping, recognize underlying reasons for maladaptive behaviors, and identify and increase contact with natural contingencies.21
Mindfulness training has been associated with reductions in anxiety,24–26 disordered eating,27,28 and depressive relapse,29–31 and a growing body of literature23,32–36 supports its efficacy for SUD treatment. Complementing previous theory,20 integrating mindfulness practices into treatment may not only provide an alternative to standard RP, it may also enhance its efficacy. Mindfulness-based practices offer incremental training in awareness of environmental cues and internal phenomena, including cognitive and affective states that have previously triggered relapse,37–39 interrupting the habitual response of substance use.40 These practices may also function as exposure to internal experiences41 that often precipitate relapse, such as negative affect and craving. The resultant habituation may generalize to discomfort associated with a broader class of triggers. In contrast, RP practices often identify specific situations to avoid or present alternative coping strategies. Focus on internal experience vs external cues, however, may increase acceptance and tolerance of substance use cues and associated internal distress, decrease subjective urgency to alleviate discomfort via substance use,32,42,43 and decouple negative affect and substance use.23
Mindfulness-Based Relapse Prevention
Mindfulness-based relapse prevention (MBRP)44 integrates evidence-based practices to decrease the probability and severity of relapse for patients in SUD aftercare. The program draws on select components of RP, such as identifying individual risk factors and common antecedents of relapse.4,45 However, based on mindfulness-based stress reduction46 and mindfulness-based cognitive therapy for depression,30 at its foundation are formal practices, such as sitting meditation, which increase awareness of and exposure to emotional and cognitive experience, and briefer informal mindfulness practices to increase awareness and behavioral flexibility in daily life.
Previous research32 has shown that, compared with 12-step and psychoeducation-based treatment as usual (TAU) for SUDs, MBRP is associated with decreased craving and increased acceptance and awareness during a 4-month follow-up period and with decreased substance use during a 2-month follow-up period.
Aims and Hypotheses
Although supported by similar studies33,47 of mindfulness-based SUD treatment, the present study is, to our knowledge, the first randomized clinical trial to assess the relative efficacy of MBRP, RP, and TAU on 12-month SUD outcomes. Given prior research,32,35,48 we hypothesized that participants in MBRP and RP would show significant improvement on main outcomes compared with those in TAU, and MBRP participants would better maintain treatment gains over time compared with those in TAU or RP.
Methods
Participants
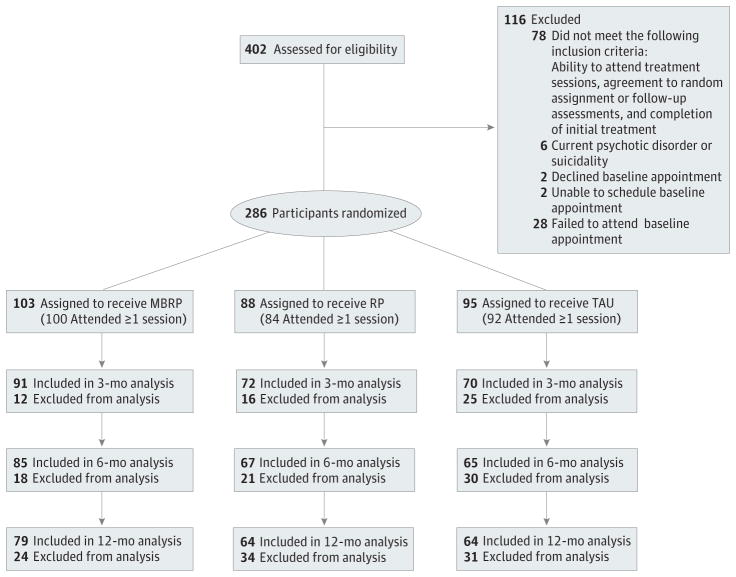
The study was conducted between October 2009 and July 2012. Participants (N = 286) were recruited from a 2-site private, nonprofit chemical dependency treatment agency offering medically supervised detoxification, inpatient treatment, intensive outpatient treatment, and standard aftercare (Figure). Patients at the agency typically attend approximately 1 year of aftercare after completing 28-day inpatient (60.3%) or 90-day intensive outpatient (39.7%) treatment. Study inclusion criteria were age 18 years or older, English fluency, medical clearance, ability to attend treatment sessions, agreement to random assignment and follow-up assessments, and completion of initial intensive outpatient or inpatient care. Exclusion criteria were current psychotic disorder, dementia, suicidality, imminent danger to others, or participation in previous MBRP trials. Individuals ineligible for (n = 84) or declining (n = 32) participation were excluded from study procedures and analyses (Figure).
Figure.
Study Flow From Screening to Analysis
Reasons for exclusion from analysis across all follow-up assessments for (1) mindfulness-based relapse prevention (MBRP): withdrew from the study, enrolled as inpatient, incarcerated, refused, and unable to contact; (2) standard relapse prevention (RP): withdrew from the study, enrolled as inpatient, incarcerated, refused, unable to contact, and died; and (3) treatment as usual (TAU): withdrew from the study, incarcerated, and unable to contact.
Design and Procedures
Recruitment and Screening
Participants were recruited through posted advertisements at the treatment facilities and information/recruitment sessions conducted by research staff. Interested individuals were screened via telephone or in person.
Measures and Assessment Procedures
Eligible individuals provided written informed consent, completed baseline assessment, and were randomly assigned to the treatment condition. When the requisite number was enrolled, a new cohort began. Treatment groups typically began within 2 weeks of baseline assessment. The MBRP and RP participants were removed from primary aftercare groups during the study intervention and returned following completion of the 8-week program. The TAU participants remained in standard aftercare alongside individuals not enrolled in the study.
All assessments were conducted in private sessions with research staff. Participants unable to attend onsite posttreatment assessments could complete all assessments online, with the exception of the Timeline Follow-back,49 which was administered via telephone with study staff. At baseline, participants reported for the 30 days before initial treatment admission and, for follow-up assessments, the period between the current and most recent assessment. Participants completed remaining web-based measures with research staff assistance available. All participants, including those in the TAU group, received $40 gift cards for each completed assessment, an additional $40 bonus for completion of all assessments, and $20 to $30 attendance bonuses. The TAU assessment windows were yoked to active treatment assessments. No adverse events were reported during the study. All study procedures were approved by the University of Washington Institutional Review Board.
Measures
Screening
Select sections of the Structured Clinical Interview for DSM-IV,50 a widely used diagnostic measure with demonstrated reliability and validity, were used to assess the presence of a psychotic disorder. The suicide assessment item from the Hamilton Depression Inventory,51 shown to be reliable and valid,52 was used to assess suicidality.
Individual and Treatment Characteristics
Age, sex, educational level, race, ethnicity, socioeconomic status, primary substance of abuse, prior treatment episodes,53 and total treatment hours attended during the study were assessed at baseline (Table 1). A total of 71.5% of the participants were male, 42.1% were of ethnic/racial minority, and the age ranged from 18 to 70 years. Abstinence (defined as no drug use and no drinking) at baseline and the end of treatment was assessed using the Addiction Severity Index.54 Substance dependence was measured using the Severity of Dependence Scale55 (SDS), validated across a wide range of substances (present study, α = .85–.91).
Table 1.
Descriptive Statistics at Baseline and During Treatment
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| TAU (n = 95) | RP (n = 88) | MBRP (n = 103) | ||
| Male sex | 69 (72.6) | 56 (63.6) | 76 (73.8) | .16 |
| Race/ethnicity | ||||
| Non-Hispanic white | 46 (48.4) | 43 (48.9) | 57 (55.3) | .38 |
| Black or African American | 22 (23.2) | 13 (14.8) | 26 (25.2) | |
| Native American | 4 (4.2) | 8 (9.1) | 6 (5.8) | |
| Asian | 1 (1.1) | 0 | 1 (1.0) | |
| Native Hawaiian/Pacific Islander | 1 (1.1) | 1 (1.1) | 0 | |
| Mixed | 9 (9.5) | 12 (13.6) | 6 (5.8) | |
| Other, not specified | 2 (2.1) | 3 (3.4) | 0 | |
| Hispanic or Latino/Latina | 13 (13.7) | 10 (11.4) | 9 (8.7) | |
| Unemployed | 62 (65.3) | 53 (60.2) | 67 (65.0) | .69 |
| Educational level | ||||
| Did not complete high school | 12 (12.6) | 7 (8.0) | 7 (6.8) | .27 |
| High school graduate/GED | 45 (47.4) | 33 (37.5) | 52 (50.5) | |
| Some college | 22 (23.2) | 24 (27.3) | 23 (22.3) | |
| College degree | 16 (16.8) | 24 (27.3) | 21 (20.4) | |
| Age, mean (SD), y | 37.2 (10.8) | 38.9 (10.9) | 39.1 (10.9) | .42 |
| Alcohol use only | 14 (14.7) | 9 (10.2) | 16 (15.5) | .08 |
| Polysubstance use | 79 (83.2) | 75 (85.2) | 81 (78.6) | .48 |
| Treatment hours completed, mean (SD) | 11.0 (7.5) | 12.4 (7.0) | 12.0 (6.6) | .40 |
| Treatment sessions attended, % | ||||
| ≥75 | 44 (46.3) | 43 (48.9) | 48 (46.6) | .35 |
| ≥50 | 17 (17.9) | 18 (20.5) | 16 (15.5) | .29 |
| ≥25 | 14 (14.7) | 13 (14.8) | 17 (16.5) | .76 |
| No. of prior treatment episodes, mean (SD) | 1.28 (1.18) | 1.72 (1.69) | 1.58 (1.52) | .14 |
| SDS baseline, mean (SD) | 8.52 (4.43) | 10.27 (3.67) | 9.52 (4.23) | .20 |
| Abstinence at baseline, ASI | 29 (30.5) | 32 (37.2) | 41 (41.6) | .35 |
| Abstinence during treatment, ASI | 44 (46.3) | 52 (59.1) | 65 (63.1) | .24 |
Abbreviations: ASI, Addiction Severity Index; GED, General Educational Development; MBRP, mindfulness-based relapse prevention; RP, relapse prevention; SDS, Severity of Dependence Scale; TAU, treatment as usual.
Outcome Measures
Substance use was assessed with the calendar-formatted Time-line Follow-back,49 which has demonstrated good reliability and validity56 (present study, α = .99). Urinalysis drug and alcohol screenings were obtained by the treatment agency from a subset of participants (n = 199) court mandated or otherwise indicated for testing (per agency policy) and showed 74.2% overall agreement with self-report. (The results showed that 20.8% were false-negatives [substance use endorsed and negative urinalysis], and 5.0% were false-positives [substance use denied and positive urinalysis test]. False-negatives may be explained by assessment timing; urinalyses did not necessarily immediately follow substance use occasions and thus may not have detected substances, although use was still reported by participants.)
Interventions
Treatment Conditions
All conditions were group format and held at the agency sites. Participants who lapsed during the intervention could remain in their groups and received additional support as needed.
The TAU program was abstinence based, primarily process oriented, and based on the Alcoholics/Narcotics Anonymous 12-step program.57,58 Weekly groups (n = 95) included facilitated recovery-oriented discussions in an open-group format (eg, communication and stress management). The TAU groups met 1 to 2 times weekly for 1½ hours.
The MBRP intervention was composed of 8 weekly, 2-hour sessions with 6 to 10 participants (n = 103) and 2 therapists. Each session had a central theme, such as the role of “automatic pilot” in addiction, mindfulness in high-risk situations, and balancing acceptance and action. Specifically, the first 3 weeks established a foundational awareness of physical, emotional, and cognitive phenomena. Additional sessions focused on mindfulness practices in the presence of relapse triggers and recognizing the role of thoughts in the relapse process. Final sessions emphasized balanced lifestyle, self-care and compassion, and social support. All sessions included 20- to 30-minute guided meditations, experiential skills-based practices, and discussion of practical applications.44 Participants received handouts and audio-recorded mindfulness exercises for assigned homework and tracking sheets to monitor daily craving and mood.
The RP intervention45,59 (n = 88) matched MBRP in time, format, size, location, and scope of assigned homework. Primary objectives included assessment of high-risk situations, cognitive and behavioral coping skills, problem solving, goal setting, self-efficacy, and social support. Participants monitored daily craving and mood.
Treatment Adherence
Treatment adherence to RP and MBRP was established via weekly supervision and review of audio-recorded sessions. Competence for MBRP delivery was established by ratings of a randomly selected 50% of sessions from 8 MBRP cohorts, each rated by 2 of 3 independent raters (S.B., J.G., and N.C.). A13-item competence scale, modeled on the Yale Adherence and Competence Scale,60 Mindfulness-Based Cognitive Therapy Adherence Scale,61 and a previously developed MBRP adherence and competence scale,62 contained Likert-scale items (1, very poor, to 7, excellent) reflecting core processes described in the treatment manual.
Raters attended practice and review meetings until acceptable reliability was achieved, with regular recalibration sessions to prevent drift. Using 1-way random-effects models, interrater consistency was adequate for mean ratings of competence (intraclass correlation coefficient, 0.77), with mean (SD) competence rated between adequate and good (4.63 [0.42]).
Therapists
The TAU therapists were licensed chemical dependency counselors with varying professional degrees and out-patient aftercare experience. The MBRP therapists had doctoral degrees in clinical psychology (n = 4; including S.B., J.G., and N.C.), were in a doctoral training program (n = 1), or had master’s degrees (n = 5). All had an established personal mindfulness practice, including attendance at intensive mindfulness meditation retreats. The RP therapists had doctoral degrees in clinical psychology (n = 6; including K.W. and S.E.C.), were in a doctoral training program (n = 1; including S.H.H.), or had master’s degrees (n = 2). All study therapists had experience in cognitive-behavioral and group-based interventions and participated in a 2-day intensive training followed by ongoing training and weekly supervision with RP or MBRP experts.
Statistical Analysis
Intent-to-treat analyses were conducted using sample size–weighted orthogonal contrasts2 between RP and MBRP vs TAU (contrast 1) and MBRP vs RP (contrast 2). Primary outcomes were time to first lapse to drug use or heavy drinking across the 12-month period following the end of the intervention and days of drug use and heavy drinking in the 90 days before follow-ups occurring 3, 6, and 12 months after the end of the intervention. Heavy drinking was defined as 4 or more drinks per occasion for women and 5 or more for men.63 Covariates for all models included age, baseline severity of dependence, treatment hours, treatment history, and treatment site.
Cox proportional hazards regression modeling was used to assess treatment group differences in lapse to drug use and heavy drinking after controlling for covariates. Generalized linear models, specifically, negative binomial hurdle regression models, were used to examine associations between treatment group and drug use and heavy drinking days in the 90-day period before the 3-, 6-, and 12-month follow-up dates. The negative binomial hurdle model is useful when the outcome is a count (ie, days of use) and there is an inflation of zero values,64 and simultaneously estimates the count outcome as a logistic (eg, drug use vs no drug use, with the logistic portion predicting no drug use) and as a truncated count regression (eg, days of use). Parameters were estimated using maximum likelihood estimation, which provides the estimated variance-covariance matrix for the available data. Maximum likelihood is a preferred method for estimation when some data are missing, assuming that data are missing at random.65
Results
Descriptive Analyses
Participant characteristics are presented in Table 1. No significant treatment group differences were found on baseline demographic measures or abstinence status (defined as any drug or alcohol use) at baseline. There were, however, significant baseline differences on the SDS, with TAU participants reporting lower severity than RP or MBRP participants. We thus included baseline SDS scores as a covariate in all models. Regarding skills use, the majority of MBRP participants (88.3%) and all RP participants (100%) reported using skills taught in sessions at least once a week after completion of the course, and 67.6% of MBRP and 100% of RP participants gave similar reports at the 12-month follow-up.
Follow-up completion rates were not significantly different across treatment groups. Attrition analyses revealed that participants with missing data at the 12-month follow-up were significantly older (P < .02). Missing data at 3, 6, or 12 months were not significantly associated with other demographic measures or studied variables (eg, substance use) (all P > .06). Given these findings, we included age as a covariate predictor in all analyses. A previous study23 found that treatment hours were significantly related to outcomes following MBRP. We thus included treatment hours and treatment history as covariates. Follow-up completion rates and drug use and heavy drinking at the 3-, 6-, and 12-month follow-ups are provided in Table 2.
Table 2.
Outcome Variable Findings at Follow-up
| Characteristic | TAU (n = 95) | RP (n = 88) | MBRP (n = 103) |
|---|---|---|---|
| Sample size % completed, No. (%) | |||
| 3 mo | 82 (86.3) | 80 (90.9) | 95 (92.2) |
| 6 mo | 77 (81.1) | 75 (85.2) | 89 (86.4) |
| 12 mo | 76 (80.0) | 72 (81.8) | 83 (80.6) |
| Drug use days, TLFB, mean (SD) | |||
| 3 mo | 5.23 (15.43) | 2.09 (10.65) | 3.92 (16.24) |
| 6 mo | 5.81 (19.11) | 1.71 (10.77) | 2.73 (12.00) |
| 12 mo | 4.63 (16.03) | 6.09 (19.05) | 3.06 (15.08) |
| Any drug use, TLFB, No. (%) | |||
| 3 mo | 20 (21.0) | 11 (12.5) | 14 (13.6) |
| 6 mo | 20 (21.0) | 7 (8.0) | 10 (9.7) |
| 12 mo | 13 (13.7) | 15 (17.0) | 9 (8.7) |
| Heavy drinking days, TLFB, mean (SD) | |||
| 3 mo | 2.64 (10.64) | 2.13 (7.75) | 1.99 (8.06) |
| 6 mo | 2.61 (9.93) | 1.13 (5.96) | 1.63 (8.53) |
| 12 mo | 4.65 (14.93) | 3.89 (12.17) | 1.44 (7.66) |
| Any heavy drinking, TLFB, No. (%) | |||
| 3 mo | 19 (20.0) | 18 (20.5) | 12 (11.7) |
| 6 mo | 15 (15.8) | 8 (9.1) | 8 (7.8) |
| 12 mo | 19 (20.0) | 17 (19.3) | 8 (7.8) |
Abbreviations: MBRP, mindfulness-based relapse prevention; RP, relapse prevention; TAU, treatment as usual; TLFB, Timeline Follow-back.
Survival Analyses
Cox proportional hazards regression models were used to estimate hazard ratios for relapse to substance use and heavy drinking during the 12-month follow-up, with treatment group, age, treatment site, treatment history, treatment hours, and baseline SDS as covariates.
As evidenced by hazard ratios3 (ie, risk of lapse given the treatment condition and other covariates) (Table 3), nearly all covariates were significant predictors of each of the outcomes. Compared with TAU, the MBRP and RP groups showed a 54% decreased risk of relapse to drug use and a 59% decreased risk of relapse to heavy drinking. Compared with RP, the MBRP group showed a 21% increase in relapse risk to first drug use; the RP and MBRP groups did not differ significantly on time to the first heavy drinking day.
Table 3.
Results From Cox Proportional Hazards Regression Models for Time to First Lapse
| Covariatea | B (SE) | HR (95% CI for Hazard Odds) |
|---|---|---|
| Time to first drug use day | ||
| Contrast 1: TAU (−) vs RP/MBRP (+) | −0.77 (0.05)b | 0.46 (0.42–0.51) |
| Contrast 2: RP (−) vs MBRP (+) | 0.19 (0.05)b | 1.21 (1.10–1.33) |
| Age | −0.05 (0.002)b | 0.95 (0.95–0.96) |
| Treatment history | −0.04 (0.01)b | 0.96 (0.94–0.98) |
| SDS baseline | 0.17 (0.005)b | 1.18 (1.17–1.19) |
| Treatment hours | −0.05 (0.003)b | 0.95 (0.95–0.96) |
| Treatment site, coded 0, 1 | 0.48 (0.04)b | 1.61 (1.50–1.73) |
| Time to first heavy drinking day | ||
| Contrast 1: TAU (−) vs RP/MBRP (+) | −0.89 (0.05)b | 0.41 (0.37–0.46) |
| Contrast 2: RP (−) vs MBRP (+) | 0.02 (0.06) | 0.72 (0.91–1.15) |
| Age | 0.01 (0.002)b | 1.01 (1.01–1.02) |
| Treatment history | 0.07 (0.01)b | 1.08 (1.05–1.10) |
| SDS baseline | 0.08 (0.006)b | 1.08 (1.07–1.09) |
| Treatment hours | 0.001 (0.003) | 1.001 (0.99–1.01) |
| Treatment site, coded 0, 1 | −0.71 (0.05)b | 0.49 (0.45–0.54) |
Abbreviations: B, unstandardized regression coefficient; HR, hazard ratio; MBRP, mindfulness-based relapse prevention; RP, relapse prevention; SDS, Severity of Dependence Scale; TAU, treatment as usual.
Contrast 1 was coded with TAU as negative and RP/MBRP as positive; contrast 2 was coded with RP as negative and MBRP as positive.
P < .05.
Generalized Linear Models
Results from the generalized linear models are provided in Table 4, and the main outcomes by treatment group are summarized here. There were no significant treatment group differences on drug use days, any drug use, heavy drinking days, or any heavy drinking at the 3-month follow-up.
Table 4.
Results From Negative Binomial Hurdle Models
| Covariatea | Days of Use Among Those Who Used
|
Logistic Portion Predicting Nonuse and Nonheavy Drinking
|
||
|---|---|---|---|---|
| B (SE) | IRRb | B (SE) | ORb | |
| 3-mo Drug use days | ||||
|
| ||||
| Contrast 1: TAU (−) vs RP/MBRP (+) | −0.06 (0.18) | 0.94 | 0.11 (0.12) | 1.12 |
|
| ||||
| Contrast 2: RP (−) vs MBRP (+) | 0.48 (0.25) | 1.61 | −0.02 (0.12) | 0.98 |
|
| ||||
| Age | −0.07 (0.04) | 0.94 | 0.01 (0.02) | 1.01 |
|
| ||||
| Treatment history | 0.06 (0.36) | 1.06 | −0.10 (0.09) | 0.90 |
|
| ||||
| SDS baseline | −0.03 (0.18) | 0.97 | −0.06 (0.04) | 0.94 |
|
| ||||
| Treatment hours | −0.002 (0.05) | 0.99 | 0.07 (0.02)c | 1.08c |
|
| ||||
| Treatment site, coded 0, 1 | −1.47 (1.15) | 0.23 | −0.58 (0.32) | 0.56 |
|
| ||||
| 6-mo Drug use days | ||||
|
| ||||
| Contrast 1: TAU (−) vs RP/MBRP (+) | −0.44 (0.44) | 0.65 | 0.24 (0.12)c | 1.28c |
|
| ||||
| Contrast 2: RP (−) vs MBRP (+) | 0.52 (0.69) | 1.68 | −0.05 (0.18) | 0.95 |
|
| ||||
| Age | −0.02 (0.15) | 0.98 | 0.000 (0.02) | 1.00 |
|
| ||||
| Treatment history | −0.25 (0.88) | 0.78 | −0.08 (0.09) | 0.92 |
|
| ||||
| SDS baseline | 0.21 (0.13) | 1.23 | −0.07 (0.04) | 0.93 |
|
| ||||
| Treatment hours | −0.01 (0.06) | 0.99 | 0.07 (0.03)c | 1.08c |
|
| ||||
| Treatment, coded 0, 1 | −0.79 (0.80) | 0.44 | 0.47 (0.35) | 1.59 |
|
| ||||
| 12-mo Drug use days | ||||
|
| ||||
| Contrast 1 TAU (−) vs RP/MBRP (+) | −0.24 (0.15) | 0.79 | 0.09 (0.16) | 1.09 |
|
| ||||
| Contrast 2: RP (−) vs MBRP (+) | −0.37 (0.16)c | 0.69c | 0.21 (0.18) | 1.24 |
|
| ||||
| Age | −0.09 (0.04)c | 0.91c | 0.02 (0.02) | 1.02 |
|
| ||||
| Treatment history | −0.29 (0.28) | 0.75 | 0.19 (0.17) | 1.22 |
|
| ||||
| SDS baseline | 0.11 (0.06) | 1.12 | −0.15 (0.09) | 0.86 |
|
| ||||
| Treatment hours | −0.02 (0.02) | 0.98 | 0.01 (0.02) | 1.01 |
|
| ||||
| Treatment site, coded 0, 1 | −0.25 (0.42) | 0.78 | 0.30 (0.49) | 1.35 |
|
| ||||
| 3-mo Heavy drinking days | ||||
|
| ||||
| Contrast 1: TAU (−) vs RP/MBRP (+) | 0.33 (0.47) | 1.40 | 0.12 (0.11) | 1.12 |
|
| ||||
| Contrast 2: RP (−) vs MBRP (+) | −0.02 (0.30) | 0.98 | 0.25 (0.15) | 1.28 |
|
| ||||
| Age | −0.05 (0.02)c | 0.95c | −0.03 (0.02) | 0.97 |
|
| ||||
| Treatment history | 0.49 (0.78) | 1.64 | −0.13 (0.12) | 0.88 |
|
| ||||
| SDS baseline | −0.16 (0.31) | 0.85 | −0.04 (0.05) | 0.96 |
|
| ||||
| Treatment hours | −0.20 (0.11) | 0.82c | 0.09 (0.02)c | 1.09c |
|
| ||||
| Treatment site, coded 0, 1 | −1.81 (1.03) | 0.16c | −1.03 (0.36)c | 0.36c |
|
| ||||
| 6-mo Heavy drinking days | ||||
|
| ||||
| Contrast 1: TAU (−) vs RP/MBRP (+) | −0.37 (0.09)c | 0.69 | 0.23 (0.09)c | 1.26c |
|
| ||||
| Contrast 2: RP (−) vs MBRP (+) | 0.57 (0.32) | 1.78 | −0.06 (0.19) | 0.94 |
|
| ||||
| Age | −0.06 (0.04) | 0.95 | −0.01 (0.02) | 0.99 |
|
| ||||
| Treatment history | −0.04 (0.23) | 0.96 | −0.04 (0.14) | 0.97 |
|
| ||||
| SDS baseline | 0.13 (0.12) | 1.14 | −0.03 (0.04) | 0.97 |
|
| ||||
| Treatment hours | −0.04 (0.02) | 0.96 | 0.02 (0.04) | 1.02 |
|
| ||||
| Treatment site, coded 0, 1 | 0.21 (0.41) | 1.23 | −0.89 (0.61) | 0.41 |
|
| ||||
| 12-mo Heavy drinking days | ||||
|
| ||||
| Contrast 1: TAU (−) vs RP/MBRP (+) | −0.04 (0.16) | 0.97 | 0.17 (0.12) | 1.19 |
|
| ||||
| Contrast 2: RP (−) vs MBRP (+) | 0.37 (0.39) | 1.45 | 0.43 (0.11)c | 1.51c |
|
| ||||
| Age | 0.11 (0.04)c | 1.11c | −0.01 (0.02) | 0.99 |
|
| ||||
| Treatment history | −0.37 (0.20) | 0.69 | 0.05 (0.15) | 1.05 |
|
| ||||
| SDS baseline | 0.31 (0.08)c | 1.36c | 0.03 (0.04) | 1.03 |
|
| ||||
| Treatment hours | 0.02 (0.02) | 1.02 | −0.004 (0.04) | 0.99 |
|
| ||||
| Treatment site, coded 0, 1 | 1.13 (0.40)c | 3.09c | −0.45 (0.38) | 0.64 |
Abbreviations: B, unstandardized regression coefficient; IRR, incidence rate ratio; MBRP, mindfulness-based relapse prevention; OR, odds ratio; RP, relapse prevention; SDS, Severity of Dependence Scale; TAU, treatment as usual.
Contrast 1 was coded with TAU as negative and RP/MBRP as positive; contrast 2 was coded with RP as negative and MBRP as positive.
The IRR can be interpreted as percentage increase (above 1.0) or decrease (below 1.0) in heavy drinking or drug use days for a 1-unit increase in the predictor (with other predictors in the model held constant). The OR can be interpreted as the increase (above 1.0) or decrease (below 1.0) in the odds of not using or not engaging in heavy drinking (with other predictors in the model held constant).
P < .05.
6-Month Follow-up
For the censored count regression portion of the negative binomial hurdle model, there was a significant main effect of treatment contrast 1 on number of heavy drinking days. Among participants who drank heavily, RP and MBRP participants reported 31% fewer days of heavy drinking compared with those assigned to TAU. For the logistic portion of the model, RP and MBRP participants, as compared with TAU participants, had a significantly higher probability of abstinence from drug use and significantly higher probability of not engaging in heavy drinking. There were no significant differences between RP and MBRP at the 6-month follow-up.
12-Month Follow-up
At the 12-month follow-up, there was a significant main effect of treatment contrast 2 on number of drug use days (among those who used drugs) and probability of any heavy drinking. Among participants who reported substance use, the MBRP participants, compared with the RP participants, reported 31% fewer drug use days and a significantly higher probability of not engaging in any heavy drinking.
Discussion
To our knowledge, the present study was the first to assess the relative efficacy of MBRP, RP, and 12-step–oriented TAU programs on participants in an SUD aftercare program during a 1-year follow-up period. Across all 3 groups, the rates of substance use and heavy drinking were much lower compared with those of other SUD treatment studies.66–68 This finding is consistent with previous research32 conducted in the same treatment agency, which found substance use rates below 30% at the 4-month follow-up. This may be the result of both continued participation in ongoing aftercare and urinalysis testing.
Between-group differences were not found at the 3-month follow-up. At the 6-month follow-up, however, both RP and MBRP participants had a significantly reduced risk of relapse to drug use and heavy drinking compared with TAU participants, with RP showing an advantage over MBRP in time to first drug use. Among participants reporting alcohol use, MBRP and RP participants reported significantly fewer days of heavy drinking compared with TAU participants. At the 12-month follow-up, MBRP participants reported significantly fewer drug use days and higher probability of not engaging in heavy drinking compared with RP participants. These findings suggest that the treatments may be equally effective at 3 months’ follow-up; both MBRP and RP, compared with TAU, blunt the probability and severity of relapses at the 6-month follow-up, with RP delaying time to first drug use; and MBRP may have a more enduring effect thereafter.
Such longer-term MBRP effects may be explained by the participants’ improved ability to recognize and tolerate discomfort associated with craving or negative affect.23,69–73 Although focus on modifying responses to distressing symptoms through acceptance-based practices has been studied in other populations,74,75 it has been understudied in SUDs. The MBRP intervention integrates empirically tested cognitive behavioral and mindfulness-based approaches to increase awareness of individual internal and environmental events that precipitate relapse and alter responses to craving and negative affect via exposure-based processes facilitated through mindfulness practice. Continued practice over time can strengthen the ability to monitor and address factors contributing to an individual’s well-being, thus supporting long-term outcomes. Previous studies have assessed mindfulness-based SUD treatment; however, only one smoking cessation trial48 has compared mindfulness training with an empirically supported control condition. To our knowledge, the present study is the first to assess 12-month longitudinal outcomes of mindfulness meditation–based treatment vs an active evidence-based treatment for SUDs.
The following limitations are noteworthy. There were several differences between TAU and the active treatment groups, including therapist training and assignment of homework. However, RP and MBRP interventions were matched on time, structure, and therapist training, differing only in the intervention delivered, thus offering a rigorous test of MBRP. Another limitation is the self-report measures of main treatment outcomes and the limited urinalysis data, although research has shown76,77 that self-reported substance use and urinalysis documentation are often not significantly different.
Conclusions
The present randomized trial offers evidence that RP and MBRP are beneficial aftercare interventions compared with typical 12-step aftercare treatment. In addition, MBRP resulted in significantly less drug use and a lower probability of any heavy drinking than RP at a 12-month follow-up. These findings suggest that MBRP may support longer term sustainability of treatment gains for individuals with substance-use disorders.
Acknowledgments
Funding/Support: The National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) sponsored this research, which provided participant incentives, partial support for researchers (Drs Bowen, Witkiewitz, Clifasefi, Grow, Chawla, Collins, and Larimer), and all of the costs associated with conducting the study. National Institutes of Health supplemental grants (UL1RR025014 and 1K18DA031464-01) provided partial support for Dr Lustyk. Ms Carroll was partially supported by NIH supplemental grant UL1RR025014 awarded to Dr Lustyk. Ms Hsu was supported by NIH/National Institute of Alcohol Abuse and Alcoholism F31 fellowship AA 019608-03. Some salary support was provided through NIDA grant 5R01DA025764-02 for Ms Harrop through a consortium agreement to Recovery Centers of King County (RCKC). Recovery Centers of King County has also sponsored project research efforts among their administrators and staff in line with the consortium agreement. Additionally, RCKC provided space for participant groups and baseline and follow-up interviews.
Footnotes
Conflict of Interest Disclosures: Drs Bowen, Grow, and Chawla conduct MBRP trainings for which they receive monetary incentives, although the findings presented in this article have not yet been presented as part of these trainings. No other disclosures were reported.
Role of the Sponsor: The NIH/NIDA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We dedicate this article to G. Alan Marlatt, PhD, whose innovative approaches and dedication to the field of addictive behaviors transformed many aspects of the study of addictive behaviors. Dr Marlatt died March 14, 2011. The following individuals located and interviewed study participants, entered data, and assisted with other necessary office tasks: Michelle Chan, BS, Matt Enkema, BA, and Sara Hoang, BS (University of Washington); Beth Dana, MS (Antioch University); David Dunkley, BFA (Cornish College of the Arts); Sarah Sanderson, BA (University of Washington–Tacoma); and Elizabeth Shilling, BA (Seattle Pacific University). These individuals received no additional financial compensation for their services. We acknowledge the RCKC for their collaboration. We also acknowledge all of our study therapists.
Author Contributions: Drs Bowen and Witkiewitz had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bowen, Witkiewitz, Clifasefi, Grow, Chawla, Collins, Larimer.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bowen, Witkiewitz, Clifasefi, Grow, Chawla, Hsu, Carroll, Harrop, Collins, Lustyk.
Critical revision of the manuscript for important intellectual content: Bowen, Witkiewitz, Clifasefi, Grow, Hsu, Carroll, Collins, Lustyk, Larimer.
Statistical analysis: Bowen, Witkiewitz, Collins.
Obtained funding: Bowen, Witkiewitz, Clifasefi, Grow, Collins, Lustyk, Larimer.
Administrative, technical, or material support: Witkiewitz, Clifasefi, Grow, Carroll, Harrop, Lustyk.
Study supervision: Bowen, Clifasefi, Chawla, Collins, Larimer.
Contributor Information
Sarah Bowen, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Katie Witkiewitz, Psychology Department, University of New Mexico, Albuquerque.
Seema L. Clifasefi, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Joel Grow, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Neharika Chawla, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Sharon H. Hsu, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Haley A. Carroll, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Erin Harrop, Psychology Department, Addictive Behaviors Research Center, Seattle, Washington.
Susan E. Collins, Department of Psychiatry and Behavioral Sciences, University of Washington–Harborview Medical Center.
M. Kathleen Lustyk, Psychology Department, Seattle Pacific University, Seattle, Washington.
Mary E. Larimer, Center for the Study of Health and Risk Behaviors, Psychiatry and Behavioral Sciences, University of Washington, Seattle.
References
- 1.Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS): 2005: Discharges From Substance Abuse Treatment Services: DASIS Series: S-41. Rockville, MD: Dept of Health & Human Services; 2008. Dept of Health & Human Services publication No. (SMA) 08-4314. [Google Scholar]
- 2.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 3.Connors GJ, Maisto SA, Donovan DM. Conceptualizations of relapse: a summary of psychological and psychobiological models. Addiction. 1996;91(12 suppl):S5–S13. [PubMed] [Google Scholar]
- 4.Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 1985. [Google Scholar]
- 5.Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- 6.Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- 7.Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol. 1996;4:46–54. [Google Scholar]
- 8.Irvin JE, Bowers CA, Dunn ME, Wang MC. Efficacy of relapse prevention: a meta-analytic review. J Consult Clin Psychol. 1999;67(4):563–570. doi: 10.1037//0022-006x.67.4.563. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med. 2006;166(8):828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- 10.Baker A, Boggs TG, Lewin TJ. Randomized controlled trial of brief cognitive-behavioural interventions among regular users of amphetamine. Addiction. 2001;96(9):1279–1287. doi: 10.1046/j.1360-0443.2001.96912797.x. [DOI] [PubMed] [Google Scholar]
- 11.Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 13.Larimer ME, Palmer RS, Marlatt GA. Relapse prevention: an overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health. 1999;23(2):151–160. [PMC free article] [PubMed] [Google Scholar]
- 14.Thakker J, Ward T. Relapse prevention: a critique and proposed reconceptualisation. Behav Change. 2010;27(3):154–175. [Google Scholar]
- 15.Foa EP, Kozak MJ. Obsessive-compulsive disorder. In: Lindemann CG, editor. Handbook of the Treatment of the Anxiety Disorders. 2. Lanham, MD: Jason Aronson; 1996. pp. 139–171. [Google Scholar]
- 16.Greenberg LS. Integrating an emotion-focused approach to treatment into psychotherapy integration. J Psychother Integration. 2002;12(2):154–189. [Google Scholar]
- 17.Adele M, Feldman G. Clarifying the construct of mindfulness in the context of emotion regulation and the process of change in therapy. Clin Psychol Sci Pract. 2004;11(3):255–262. [Google Scholar]
- 18.Samoilov A, Goldfried MR. Role of emotion in cognitive-behavioral therapy. Clin Psychol Sci Pract. 2000;7:373–385. [Google Scholar]
- 19.Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behav Res Ther. 1999;37(suppl 1):S53–S77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 20.Ward T. Relapse prevention: critique and reformulation. J Sex Aggress. 2000;5(2):118–133. [Google Scholar]
- 21.Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, editor. Mindfulness-Based Treatment Approaches: A Clinician’s Guide. 2. San Diego, CA: Elsevier Academic Press; In press. [Google Scholar]
- 22.Marlatt GA, Kristeller JL. Mindfulness and meditation. In: Miller WR, editor. Integrating Spirituality Into Treatment. Washington, DC: American Psychological Association; 1999. pp. 67–84. [Google Scholar]
- 23.Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. J Consult Clin Psychol. 2010;78(3):362–374. doi: 10.1037/a0019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabat-Zinn J, Massion AO, Kristeller JL, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 25.Orsillo SM, Roemer L, Barlow DH. Integrating acceptance and mindfulness into existing cognitive-behavioral treatment for GAD: a case study. Cognit Behav Pract. 2003;10(3):222–230. [Google Scholar]
- 26.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristeller JL, Hallett CB. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- 28.Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord. 2011;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- 29.Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- 30.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, NY: Guilford Press; 2002. [Google Scholar]
- 31.Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowen S, Chawla N, Collins SE, et al. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brewer JA, Sinha R, Chen JA, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30(4):306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zgierska A, Rabago D, Chawla N, Kushner K, Koehler R, Marlatt A. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30(4):266–294. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: results of a randomized controlled pilot trial. J Psychoactive Drugs. 2010;42(2):177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieten C, Astin JA, Buscemi R, Galloway GP. Development of an acceptance-based coping intervention for alcohol dependence relapse prevention. Subst Abus. 2010;31(2):108–116. doi: 10.1080/08897071003641594. [DOI] [PubMed] [Google Scholar]
- 37.Marlatt GA. Buddhist philosophy and the treatment of addictive behavior. Cognit Behav Pract. 2002;9(1):44–49. [Google Scholar]
- 38.Teasdale JD. The relationship between cognition and emotion: the mind-in-place in mood disorders. In: Clark DM, Fairbum CG, editors. Science and Practice of Cognitive Behaviour Therapy. Oxford, England: Oxford University Press; 1997. pp. 67–93. [Google Scholar]
- 39.Teasdale JD, Segal ZV, Williams JMG. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav Res Ther. 1995;33(1):25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- 40.Breslin CF, Zack M, McMain S. An information-processing analysis of mindfulness: implications for relapse prevention in the treatment of substance abuse. Clin Psychol Sci Pract. 2002;9:275–299. [Google Scholar]
- 41.Otto MW, Powers MB, Fischmann D. Emotional exposure in the treatment of substance use disorders: conceptual model, evidence, and future directions. Clin Psychol Rev. 2005;25(6):824–839. doi: 10.1016/j.cpr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Bowen S, Kurz AS. Between-session practice and therapeutic alliance as predictors of mindfulness after mindfulness-based relapse prevention. J Clin Psychol. 2012;68(3):236–245. doi: 10.1002/jclp.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu SH, Collins SE, Marlatt GA. Examining psychometric properties of distress tolerance and its moderation of mindfulness-based relapse prevention effects on alcohol and other drug use outcomes. Addict Behav. 2013;38(3):1852–1858. doi: 10.1016/j.addbeh.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Bowen S, Chawla N, Marlatt GA. Mindfulness-Based Relapse Prevention for Addictive Behaviors: A Clinician’s Guide. New York, NY: Guilford Press; 2010. [Google Scholar]
- 45.Daley DC, Marlatt GA. Overcoming Your Alcohol or Drug Problem: Effective Recovery Strategies: Therapist Guide. 2. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 46.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Delacorte Press; 1990. [Google Scholar]
- 47.Zgierska A, Rabago D, Zuelsdorff M, Coe C, Miller M, Fleming M. Mindfulness meditation for alcohol relapse prevention: a feasibility pilot study. J Addict Med. 2008;2(3):165–173. doi: 10.1097/ADM.0b013e31816f8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1–2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobell LC, Sobell MB. Timeline Follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZA, John P, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Vol. 12. Totowa, NJ: Humana Press; 1992. p. 228. [Google Scholar]
- 50.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press Inc; 1997. [Google Scholar]
- 51.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dozois DJA. The psychometric characteristics of the Hamilton Depression Inventory. J Pers Assess. 2003;80(1):31–40. doi: 10.1207/S15327752JPA8001_11. [DOI] [PubMed] [Google Scholar]
- 53.Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 54.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 55.Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction. 1995;90(5):607–614. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- 56.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 57.Alcoholics Anonymous. Twelve Steps and Twelve Traditions. New York, NY: Alcoholics Anonymous World Services; 1952. [Google Scholar]
- 58.Alcoholics Anonymous. Living Sober. New York, NY: Alcoholics Anonymous World Services; 1975. [Google Scholar]
- 59.Monti PM, Kadden RM, Rohsenow DJ, Cooney NL, Abrams DB. Treating Alcohol Dependence: A Coping Skills Training Guide. 2. New York, NY: Guilford Press; 2002. [Google Scholar]
- 60.Carroll K, Nich C, Rounsaville B, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. 2000;57(3):225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 61.Segal Z, Teasdale J, Williams J, Gemar M. The Mindfulness-Based Cognitive Therapy Adherence Scale: inter-rater reliability, adherence to protocol and treatment distinctiveness. Clin Psychol Psychother. 2002;9(2):131–138. [Google Scholar]
- 62.Chawla N, Collins S, Marlatt G, et al. The Mindfulness-Based Relapse Prevention Adherence and Competence Scale: development, interrater reliability, and validity. Psychother Res. 2010;20(4):388–397. doi: 10.1080/10503300903544257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.NIIDA NIoAAaA. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- 64.Atkins DC, Baldwin SA, Zheng C, Gallop RJ, Neighbors C. A tutorial on count regression and zero-altered count models for longitudinal substance use data. Psychol Addict Behav. 2013;27(1):166–177. doi: 10.1037/a0029508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 66.Byrne SA, Petry N. Concurrent alcohol dependence among methadone-maintained cocaine abusers is associated with greater abstinence. Exp Clin Psychopharmacol. 2011;19(2):116–122. doi: 10.1037/a0022795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laudet A, Stanick V, Sands B. An exploration of the effect of on-site 12-step meetings on post-treatment outcomes among polysubstance-dependent outpatient clients. Eval Rev. 2007;31(6):613–646. doi: 10.1177/0193841X07306745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reif S, George P, Braude L, et al. Residential treatment for individuals with substance use disorders: assessing the evidence [published online January 21, 2014] Psychiatr Serv. doi: 10.1176/appi.ps.201300242. [DOI] [PubMed] [Google Scholar]
- 69.Bowen S, Marlatt A. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychol Addict Behav. 2009;23(4):666–671. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- 70.Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211–237. [Google Scholar]
- 71.Gifford EV, Kohlenberg BS, Hayes SC, et al. Acceptance-based treatment for smoking cessation. Behav Ther. 2004;35(4):689–705. [Google Scholar]
- 72.Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111(1):180–185. [PubMed] [Google Scholar]
- 73.Hernández-López M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychol Addict Behav. 2009;23(4):723–730. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- 74.Dahl J, Wilson KG, Nilsson A. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: a preliminary randomized trial. Behav Ther. 2004;35(4):785–801. [Google Scholar]
- 75.Levitt JT, Brown TA, Orsillo SM, Barlow DH. The effects of acceptance versus suppression of emotion on subjective and psychophysiological response to carbon dioxide challenge in patients with panic disorder. Behav Ther. 2004;35(4):747–766. [Google Scholar]
- 76.Jain RAKA. Self-reported drug use and urinalysis results. Indian J Physiol Pharmacol. 2004;48(1):101–105. [PubMed] [Google Scholar]
- 77.Digiusto E, Seres V, Bibby A, Batey R. Concordance between urinalysis results and self-reported drug use by applicants for methadone maintenance in Australia. Addict Behav. 1996;21(3):319–329. doi: 10.1016/0306-4603(95)00064-x. [DOI] [PubMed] [Google Scholar]