Abstract
Hippocampal place fields show remapping between environments that contain sufficiently different contextual features, a phenomenon that may reflect a mechanism for episodic memory formation. Previous studies have shown that place fields remap to changes in the configuration of visual landmarks in an environment. Other experiments have demonstrated that remapping can occur with experience, even when the visual features of an environment remain stable. A special case of remapping may be trajectory coding, the tendency for hippocampal neurons to exhibit different firing rates depending upon recently visited or upcoming spatial locations. To further delineate the conditions under which different task features elicit remapping, we recorded from place cells in dorsal CA1 of hippocampus while rats switched between tasks that differed in memory demand and task structure; continuous spatial alternation (CA), delayed spatial alternation (DA), and tactile-visual conditional discrimination (CD). Individual hippocampal neurons and populations of simultaneously recorded neurons showed coherent remapping between CA and CD. However, task remapping was rarely seen between DA and CD. Analysis of individual units revealed that even though the population retained a coherent representation of task structure across the DA and CD tasks, the majority of individual neurons consistently remapped at some point during recording sessions. In contrast with previous studies, trajectory coding on the stem of the T-maze was virtually absent during all of the tasks, suggesting that experience with multiple tasks in the same environment reduces the likelihood that hippocampal neurons will represent distinct trajectories. Trajectory coding was, however, observed during the delay period of DA. Whether place fields change in response to task or trial type or remain stable within the same environment may depend on which aspects of the context are most salient or relevant to behavior.
Keywords: CA1, trajectory coding, remapping, conditional discrimination, spatial alternation
INTRODUCTION
The hippocampus plays a crucial role in both episodic memory (Squire, 1992; Eichenbaum et al., 1999; Fortin et al., 2002) and spatial cognition (Morris et al., 1982; Jarrard, 1993). One of the most striking and well-characterized behavioral correlates of hippocampal neurons is the coding of discrete spatial locations by place cells and the modulation of these spatial representations by contextual variables. Place cells, as first described by O’Keefe and Dostrovsky (1971), are pyramidal neurons in the hippocampus that fire maximally when an animal occupies a discrete location in space. the neuron’s place field. These neurons are known to exhibit radical changes in firing properties with sometimes subtle changes in the sensory features of an environment, as well as internal variables, such as previous experience and strategy switches, a property known as remapping (for review, see Colgin et al., 2008). Remapping may be a mechanism by which hippocampal neurons represent different contexts by encoding both the common features of the spatial layout of an environment and discrete behavioral events that occur within that environment, laying the framework for the formation of episodic memories (Leutgeb et al., 2005).
Remapping has been most commonly demonstrated in open-field environments, in which animals are not required to perform a specific task. In these experiments, hippocampal place fields normally remain stable between events in the same location if the visual layout of the environment is sufficiently similar, suggesting that hippocampal neurons can become bound to common visual features of a context (Muller and Kubie, 1987; Lever et al., 2002; Wills et al., 2005). Other studies have found that remapping can represent temporally distinct events that occur in the absence of any change in the visual configuration of the environment when some other aspect of experience is made salient; for example, place fields remap within an environment after rats have learned to associate a tone with a shock (Moita et al., 2004). Typically, populations of place cells remap coherently, however in some cases, manipulations of the recording environment have revealed subpopulations of place cells that are tied to one of multiple reference frames within an environment, a phenomenon that has been denoted “partial remapping” (Muller et al., 1991). The precise conditions that determine whether place fields remain stable or remap between temporally distinct events that occur in the same behavioral apparatus in the same location remain unknown.
A number of investigations have recorded from hippocampal neurons during memory-guided tasks in apparatuses that restrict the animal’s movements to a stereotyped motor pattern. Some, but not all, of these studies have found that neurons in hippocampus fire discriminatively to different trajectories, either selectively firing to an animal’s upcoming location (prospective coding) or past location (retrospective coding) in maze segments that are common between two or more possible journeys [(Frank et al., 2000; Wood et al., 2000; Ferbinteanu and Shapiro. 2003; Lee et al., 2006); but see (Lenck-Santini et al., 2001)]. When a delay interval is introduced between trials of a task that requires the animal to alternate continuously between goal arms of a T-maze to obtain reward, trajectory-specific coding shifts from the stem of the maze to the place in which the animal is confined during the delay period (Ainge et al., 2007; Pastalkova et al., 2008). Trajectory-specific firing rates in hippocampal neurons may represent a special form of remapping that occurs in response to task demand, representing each trajectory as a distinct event that occurs within the broader context of the recording session. This type of within-session context-specific hippocampal activity could influence mnemonic processes by distinguishing between behaviorally identical situations to guide appropriate future behavior (Smith and Mizumori, 2006).
Both trajectory coding and remapping have been theoretically linked to memory; however, both phenomena can be influenced by noncognitive experimental settings, such as structural manipulations of an environment (Bower et al., 2005). Furthermore, trajectory coding is present in some tasks that do not require the hippocampus (Ainge et al., 2007). Several recent studies have attempted to directly correlate trajectory coding with memory demand by comparing hippocampal cell firing characteristics across tasks that are behaviorally and structurally similar, bur mnemonically distinct (Ferbinteanu et al., 2011; Ainge et al., 2012; Griffin et al., 2012). Results from these experiments suggest that memory demand influences trajectory coding only under certain conditions (e.g., when tasks are run continuously vs. with a delay between trials).
To further delineate the possible links between remapping, trajectory coding, and memory, we recorded from CA1 of dorsal hippocampus while rats switched between three tasks in the same T-maze that differed in both structure (discrete-trials vs. continuous) and memory demand: (1) Continuous spatial alternation (CA), a nonhippocampal-dependent, continuously run task known to elicit trajectory coding in which the rat must alternate between left and right goal arms on successive trials with no delay period between trials (Wood et al., 2000; Lee et al., 2006); (2) delayed spatial alternation (DA), a hippocampal-dependent task that requires the rat to alternate between left and right goal arms on successive trials with a 20 s delay period between trials, during which neurons exhibit prospective trajectory coding (Ainge et al., 2007), and; (3) tactile-visual conditional discrimination (CD), a nonhippocampal-dependent task during which trajectory coding is rarely seen, in which the rat must choose a goal arm based on the appearance and texture of a floor insert cue that is placed on the floor of the T-maze pseudorandomly from trial to trial with a 20 s intertrial interval (ITI) between trials (Griffin et al., 2012).
MATERIALS AND METHODS
Subjects
Six, male, Long-Evans hooded rats (weighing 450–500 g) were individually housed and maintained on a 12 h light/dark cycle in a temperature and humidity-controlled colony room with ad libitum access to food and water. During the period of behavioral training and recording, rats were given four to five pellets of food per day after each testing session to maintain them at 90% of their free-feeding body weight.
Behavioral Training
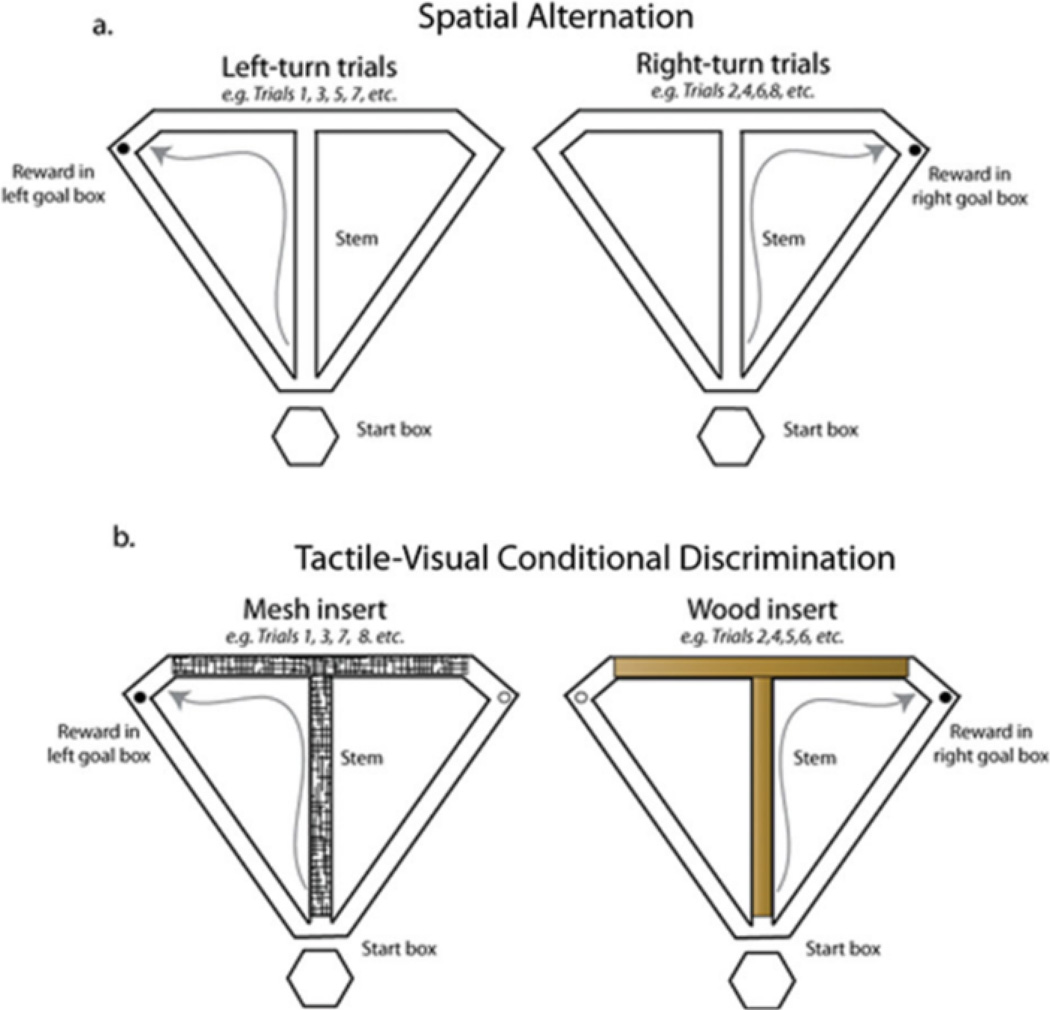
Rats were trained to perform two different tasks: the tactile-visual CD task and the CA task (see Fig. 1), Both tasks were conducted on a T-maze constructed of wood and painted black. The maze consisted of a central stem (116 × 10 cm), two goal arms (56.5 × 10 cm each), and two return arms (112 × 10 cm). A small plastic cup was located at the end of each goal arm for delivery of the chocolate sprinkle reward. The testing room was illuminated by a single compact florescent bulb and surrounded by black curtains with large visual cues attached. Before training on the tasks, each rat was handled by the experimenter and habituated to the testing room and maze for 1 week using the following procedure. For the first two days, the rats performed reward-zone training in which they were confined to the reward zone for 3 min, or until they ate the chocolate sprinkle reward, for six trials a day, with three trials performed in each reward zone. This procedure familiarized the rat with being placed on the maze and learning to eat out of the reward cups. For the next 3 days, each rat was placed at the base of the maze stem and encouraged to run up to the choice point, bur was blocked from entering either the right or the left goal arm. The rat then entered the unblocked goal arm to the reward zone to consume the food reward. Left and right trials were given in a pseudorandom sequence (Fellows, 1967). The rat was confined to a pedestal located at the base of the maze between trials, while the experimenter set up for the next trial. The following day, the rats were trained on both the CD and CA tasks, with CA training in the morning and CD training in the afternoon. The training sessions were at least 6 h apart and the rat was placed in its home cage between the two training sessions. For CD, wooden inserts covered on one side by black plastic mesh were placed on the floor of the maze stem and goal arms with either the bare wood or mesh side facing up. Rats were rewarded for making either a right or left turn, depending on the texture of the floor insert. Half of the rats were required to learn the “right on mesh, left on wood” rule and the remaining half learned the “left on mesh, right on wood” rule. The ITI was 20 s, during which the rat was confined to the pedestal using a large wooden blocker while the experimenter set up the next trial. To ensure that the rat did not anticipate the upcoming trial during the ITI by using auditory cues, the experimenter flipped-the maze inserts during each ITI, even between two consecutive identical trial types. Rats were given 24 trials per day (12 wood/12 mesh) in a pseudorandom sequence (Fellows, 1967). For the CA task, the pedestal was blocked and the rats were rewarded for traversing the maze in a “figure-8” pattern, traveling up the maze stem and choosing the right of left goal arm on alternate trials, and returning to the base of the stem via the return arm. Each rat performed 40–80 trials per day. Each training session was 25 min long, with more trials performed in the CA task due to the lack of a delay period between trials. Daily training sessions (5–7 days per week) commenced until the rat performed both tasks at 75% correct or above for two consecutive sessions.
FIGURE 1.
Schematic of the tasks used in the current experiment A: Spatial alternation task. The rat must alternate visits to the left and right goal box to receive a chocolate sprinkle reward. For the CA version of the task, the rat runs continuously in a figure-8 pattern without visiting the start box. For the DA version of the task, the rat pauses in the start box for 20 s between trials. B: Tactile-visual CD task. On each trial, the maze floor is covered with a textured insert (smooth wood or plastic mesh) that signals which goal arm will contain food reward. Twenty-four trials are given in a pseudorandom sequence with an equal number of wood and mesh trials presented. The trials are separated by a 20 s ITI. [Color figure can be viewed in the online issue, which is available at wileyonlinclibrary.com.]
FIGURE 8.
Example neuron recorded from a CA-CD-CA0 session showing abrupt remapping of the place field on the left return arm. The trajectory of the rat on each left-turn trial is shown in gray with superimposed spike locations in black. This neuron has a place field on the left return arm in CA and CA0 but no field during CD. Interestingly, the neuron fires on the left return arm on the first trial of CD, is virtually silent during the remainder of the CD epoch, and fires robustly on the left return arm on the first trial and for the remainder of the CA0 epoch.
Surgery
Three of the six rats were implanted with recording micro-drives after reaching criterion on both the CA and CD tasks. Rats were given a preanesthetic dose of atropine (0.05 mg/kg) and anesthetized with isoflurane (1.5–3% in oxygen). The skull surface was exposed and cleaned. Six to seven small skull screws (4.0 mm long, shaft diameter 0.85 mm, and Fine Science Tools) were inserted near the skull ridge around the perimeter of the incision, and one ground screw was inserted posterior to the lambda skull suture. A hole was then drilled above dorsal hippocampus (4.0 posterior to bregma and 2.0 lateral to bregma) using a 2.7 mm diameter trephine (Fine Science Tools). The dura was removed and the brain surface was kept moist using gelfoam soaked in sterile saline. A microdrive containing 16 independently moveable tetrodes (composed of four 12.7-1 m-diameter nichrome wires. 150–300 kOhms at 1 kHz in gold solution) four reference electrodes and a ground wire were lowered onto the brain surface. After the microdrive was fixed to the skull with dental acrylic, each tetrode and reference wire was immediately advanced 1.13 mm into the brain. A subcutaneous injection of Banamine (2.5 mg/kg) was given 30 min prior to the end of surgery and Children’s Ibuprofen (30 mg/kg) was given in the drinking water for two days for pain relief. Rats were allowed to recover for a least one week before behavioral testing and recording began. All procedures were approved by the University of Delaware Institutional Animal Care and Use Committee.
Tetrode Adjustment and Recording
After the rats recovered from surgery, the reference electrodes were advanced until they were positioned just dorsal to the CA1 pyramidal cell layer of hippocampus, as evidenced by sharp waves but no ripple in the local field potential (Buzsaki, 1986; Chrobak and Buzsaki, 1996). The tetrodes were then advanced gradually over the course of 7–10 days until they reached stratum pyramidale, as evidenced by sharp waves, accompanied by large-amplitude ripples in the local field potential record and units with at least a 3:1 signal-noise ratio. Tetrode adjustments were made as the rats rested on a pedestal that could be rotated to untangle the tether if necessary. The rats were then trained on both the CA and CD tasks until they reached presurgical levels of performance. The interval between daily training sessions was gradually shorrened to 10–15 min to allow for recording across the two tasks. Recording sessions consisted of 40 trials of CA, followed by 24 trials of CD, followed by a second session of 40 trials of CA (CA0). Between sessions, the rat was confined to the ITI pedestal for 10–15 min. After several days of recording, the CA task was changed to a DA task, which imposed a delay between trials, during which the rat was confined to the pedestal. Similar to the CA-CD-CA0 design, these sessions consisted of a DA session (DA; 24 trials), followed by a CD session (24 trials), followed by a second DA session (DA0; 24 trials). The ITI was 20 s for both the DA and CD Tasks. The rats’ position data were recorded from a camera mounted above the maze that captured luminance (30 Hz) emitted from an array of light-emitting diodes. Neural signals were preamplified via unity-gain operational amplifiers located on the rat’s headstage. Signals were then differentially amplified against a reference wire, bandpass filtered (1 – 600 Hz for local field potentials and 0.6 – 6 kHz for units), and recorded using a 64-channel digital recording system (Digital Lynx, Neuralynx, Bozeman, MT).
Histology
At the conclusion of the experiment, rats were anesthetized with isoflurane and marking lesions were made by passing 10 1A of current through one wire of each tetrode and the reference electrodes. Rats were then returned to their home cages. After 24 h, rats were perfused transcardially with 0.9% saline, followed by 4% formalin, and the head was soaked in formalin for 2–3 days. After raising the tetrodes out of the brain, the brain was removed from the skull and placed in formalin and transferred to a 9% sucrose solution. After sinking, the brains were frozen and sectioned (40 1 m). The sections were mounted on slides and stained using cresyl violet (Paxinos and Watson, 2005). Tetrode tracks were reconstructed using the tetrode adjustment record and by visualizing the sections under a microscope.
Neural Data Analysis
Clusters were isolated offline using commercially available software (SpikeSort 3D, Neuralynx, Bozeman, MT). Pyramidal cells were distinguished from interneurons based on an average firing rate below 2 Hz and a spike duration greater than 0.3 ms (Ranck, 1973). A custom MATLAB script used the time of entry into the reward zones and the base of the stem to assign timestamps to the beginning and end of each trial. Correct trials were divided into right and left trial types. For both right and left trial types, the position data points were linearized into 55 consecutive 5-cm bins covering the stem, goal arm, and return arm (see Fig. 1) and total occupancy was calculated for each bin (Lee et al., 2006; Griffin et al., 2007, 2012). For each cluster, spikes were assigned to a position timestamp and x-y coordinates using a custom MATLAB script, Firing rate distributions were then calculated separately for right and left-turn trials by dividing the number of spikes emitted while the rat occupied each bin by the time spent in each bin. Firing rate distributions were smoothed using a Gaussian filter (full-width half-maximum = 4 bins). Place fields were defined as 4 or more adjacent bins with a firing rate of at least 10% of the peak bin firing rate. Thus, the field boundaries are defined as bins on either side of the field whose firing rate was < 10% of the peak bin-firing rate. In cases in which the field was located near the maze start or end, field boundaries were assigned as bins 1 and 55, respectively. For units with a place field on the maze, the center of mass (COM) was then calculated for each field and was used to assign the field to one of five maze zones: stem (bins 1–24), left or right goal arm (bins 25–36), and left or right return arm (bins 37–55)
To quantitatively define remapping at the single-unit level, left and right firing rate distributions were combined to make 86 spatial bins (bins 1–24: stem, bins 25–36: left goal arm, bins 37–55: left return arm, bins 56–67: right goal arm, and bins 68–86: right return arm). Firing rate distributions were calculated separately for each of the three epochs and compared across epochs using Pearson’s r. There were three correlations performed per neuron: For the CA-CD-CA0 sessions, correlation analyses were done between CA and CD, between CD and CA0, and between CA and CA0. Similarly, for DA-CD-DA0 sessions, correlation analyses were performed comparing the DA and CD epochs, the CD and DA0 epochs, and the DA and DA0 epochs. Units were assigned to the remapping category if the correlation analysis between any of the three epochs revealed that Pearson’s r was not statistically different from zero (Howell, 1997) and if there was a field present in one epoch that was not present on one or more of the other epochs. Remapping neurons were further categorized as “task” or “epoch” remapping. Neurons were considered to remap to task if there was a nonsignificant Pearson’s r value between the first epoch (CA or DA) and the second epoch (CD) and between the second epoch (CD) and the third epoch (CA0 or DA0), and a significant correlation between the first and third epochs. Neurons that showed other remapping patterns (i.e., a field that was present in CA and CD, but not CA0) were assigned to the “epoch remapping” category. Cumulative distribution functions of correlation values were then constructed for each epoch comparison for both CA-CD-CA0 and DA-CD-DA0 sessions. The distributions of spatial correlations were then compared using Kolmogorov–Smirnov tests.
The cluster waveform parameters, peak, energy, and valley, were compared before and after each recording session and units excluded if these parameters changed from the beginning to the end of the session. This procedure ensured that changes seen across epochs were not a result of recording instability (see Fig. 6c). Clusters were included in the analysis only if they exceeded an established isolation quality threshold (Schmitzer-Torbert et al., 2005). Cluster quality was calculated for each tetrode using SpikeSort 3D (Neuralynx, Version 2.5). Clusters with L-ratio values > 0.10, or an Isolation Distance < 15, were not included in the analyses. A separate analysis that included all clusters did not substantially change the results (data not shown).
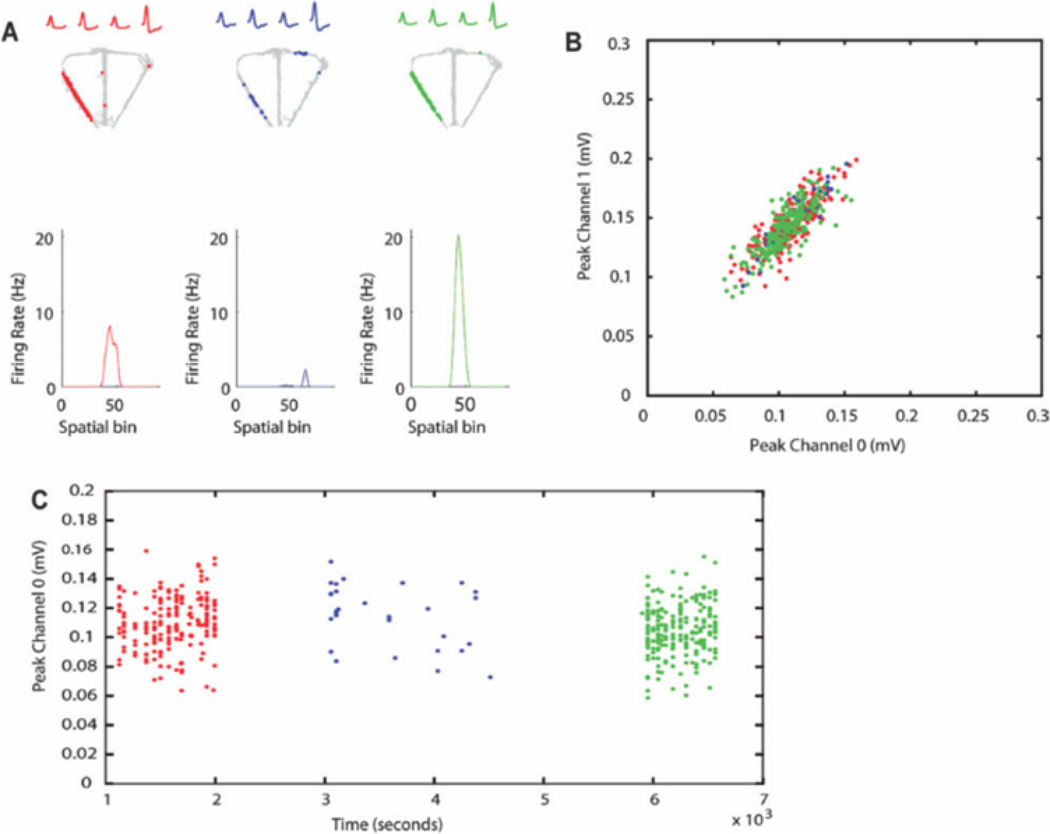
FIGURE 6.
Verification of recording stability across the three epochs. AI Trajectory of the rat (gray) with superimposed spike locations during CA (red), CD (blue), and CA0 (green). The mean waveforms recorded on the four channels of the tetrode are shown at the top of each plot and the linearized firing rate distribution is shown below. B: Plot of the peak of each waveform on channel 0 against the peak of each waveform on channel 1 of the tetrode. Colors are as indicated in A. Notice that the clusters recorded in each epoch show a high degree of overlap. C: Plot of the peak waveform on channel 0 over the entire recording session. Colors are as indicated in A. Notice that the waveform amplitude does not deviate substantially across the session. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To calculate remapping in populations of simultaneously recorded neurons, population firing rate vectors were constructed for each of the 86 (left and right trials combined) spatial bins using all recorded neurons from each session type. Three separate correlation matrices were constructed by computing the Pearson’s r between the population vectors for Epochs 1 and 2, Epochs 2 and 3, and Epochs 1 and 3. A hand of high-correlation along the diagonal of a correlation matrix indicates that there is little to no remapping across epochs. Therefore, an absence of a correlation hand along the diagonal of the matrix would be evidence of population remapping across epochs. An advantage of this analysis over single-neuron analysis is that the population coherence pattern can reveal regions of the maze where the population showed the most remapping.
The methods described so far identify neurons that show global remapping across tasks, defined as a place field that appears or disappears or moves to a different location across epochs. To identify neurons that show rate remapping, a rate change score was calculated by dividing the absolute value of the differences in the mean firing rates between two epochs by the sum of the mean firing rates for the two epochs (See Leutgeb et al., 2005). To exclude low-firing rate neurons, rate change scores were only calculated for neurons that exhibited mean firing rates above 0.25 Hz in one of the epochs. The rate change scores were calculated for epoch 1 vs. epoch 2, epoch 2 vs. epoch 3, and epoch 1 vs. epoch 3 for both session types. Scores of 0.5 and above (a threefold difference in firing rate) were taken as an indication of rate remapping.
Units with a place field on the maze stem were analyzed further to determine the proportion of stem-firing units that exhibited trajectory coding. The mean stem firing rate was compared between left and right correct trials by calculating a discrimination index: Dl = abs (FRL, – FRR)/(FRL + FRR), where FRL is the mean firing rate on left correct trials and FRR is the mean firing rate on right correct trials. To determine if units exhibited significant trajectory coding, the trial-by-trial field firing rate was compared between left and right correct trials using a Kolmogorov-Smirnov test. The procedure for constructing the firing rate histograms, including binning the maze and comparing firing rates on left and right trials, was identical between the CD, CA, and DA tasks.
To analyze the activity of cells during the ITI, perievent time histograms (PETHs) were constructed using a custom MAT-LAB program for DA-CD-DA0 sessions only. The PETHs were triggered by the rat entering and leaving the ITI pedestal. For each neuron that emitted >20 spikes during at least one of the three epochs, the delay period firing was sorted based on whether the upcoming trial was a right or left trial, which would indicate prospective firing. The firing rates were then compared using a Kolmogorov-Smirnov test. Due to inadequate numbers of error trials, only correct trials were analyzed.
RESULTS
Behavioral Results
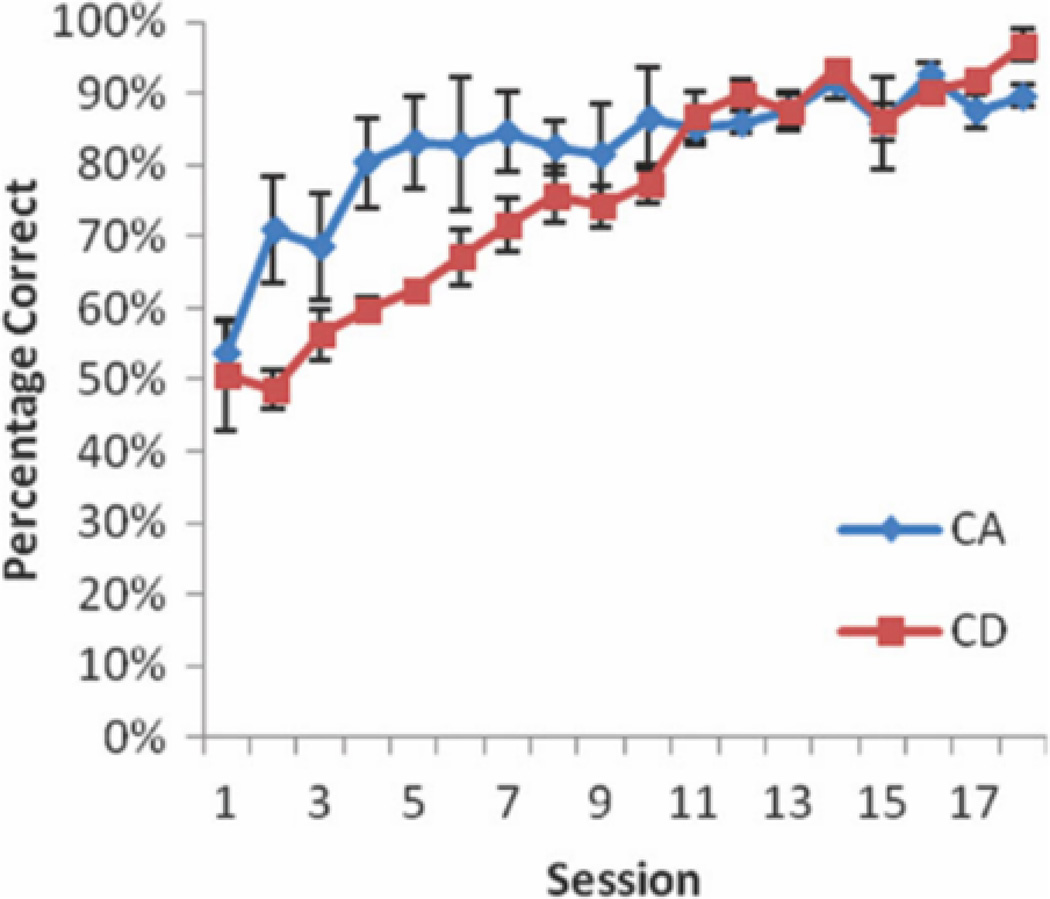
Six rats were trained to perform the CA task in the morning and the CD task in the afternoon, with sessions separated by at least 6 h. The CA task was learned marginally faster (M = 5.33, SD = 1.37) than the DA task (M = 9.33, SD = 3.14), as measured by number of days to reach 75% correct for two consecutive days, t(5) = 2.24, P = 0.076. Importantly, however, after 10 training sessions, performance for all rats was above 80% correct for both tasks. Three of the six rats that underwent the dual-task training procedure were then implanted with recording microdrives. After recovery from surgery, the rats were retrained on both tasks. The time between tasks was gradually shortened to 15 min and a second epoch of CA (CA0) was added. Recording sessions consisted of 18–24 CA trials, followed by 18–24 CD trials, followed by a second set of 18–24 CA trials (CA-CD-CA0; Fig. 2). All three rats showed high levels of accuracy on the CA and CD epochs on all CA-CD-CA0 sessions (CA: M = 91%, SD = 0.06, CD: M = 93%, SD = 0.073, CA0 M = 94%, and SD = 0.06). After several recording sessions, the alternation task was changed to a discrete-trials task by including a 20-s delay (DA) between trials. This change made the CD and DA tasks structurally similar, with the only difference being the task strategy/memory demand. Sessions were run in a similar pattern as CA-CD-CA0 sessions, with an epoch of DA trials, followed by an epoch of CD trials, followed by a second epoch of DA trials (DA-CD-DA0). Performance accuracy dropped for the DA epochs, but not the CD epochs for all three rats from the last CA-CD-CA0 session to the first DA-CD-DA0 session (CA-DA: M = −18%, SD = 0.03, CD-CD: M = 7%, SD = 0.06; CA0-DA0: M = −36%, and SD = 0.11). DA-CD-DA0 sessions in which performance accuracy was below 70% for any of the three epochs were excluded from further analysis.
FIGURE 2.
Behavioral results. Rats (N 56) were trained on CA in the morning and CD in the afternoon (6 h later). The plot shows the percentage of correct trials for the CA and CD tasks over 18 training sessions. Rats learned CA marginally faster than the CD task However, by the 10th training session, all rats showed high levels of accuracy on both tasks. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Within-Session Remapping Across the Alternation and CD Tasks
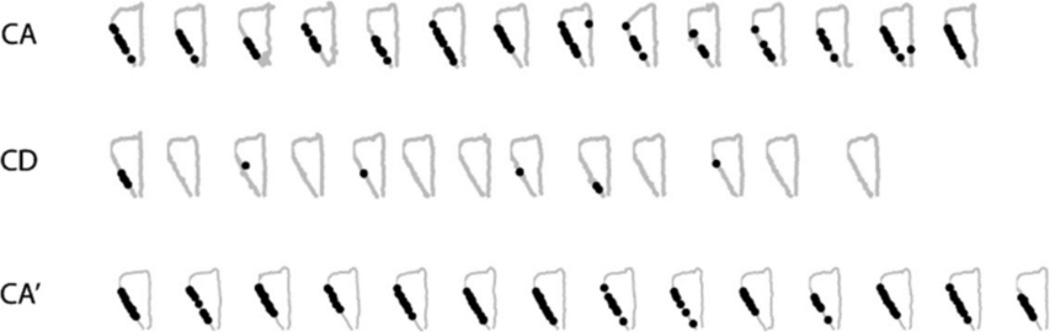
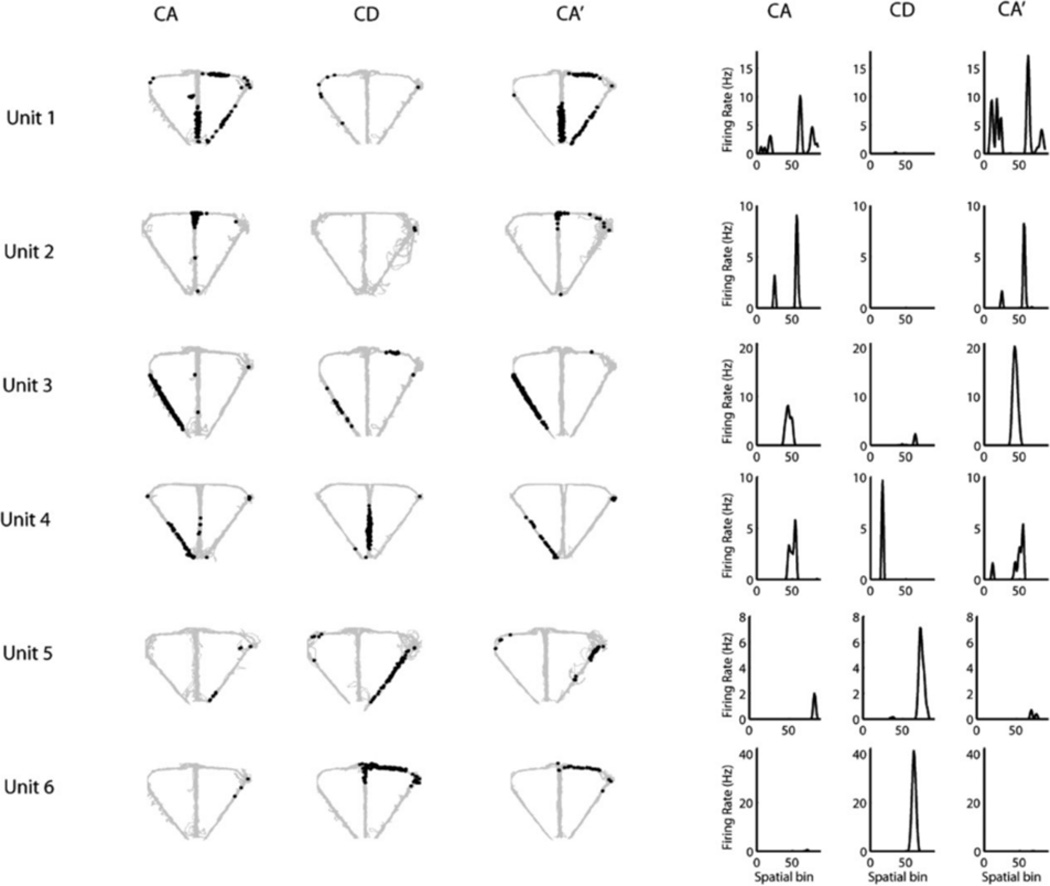
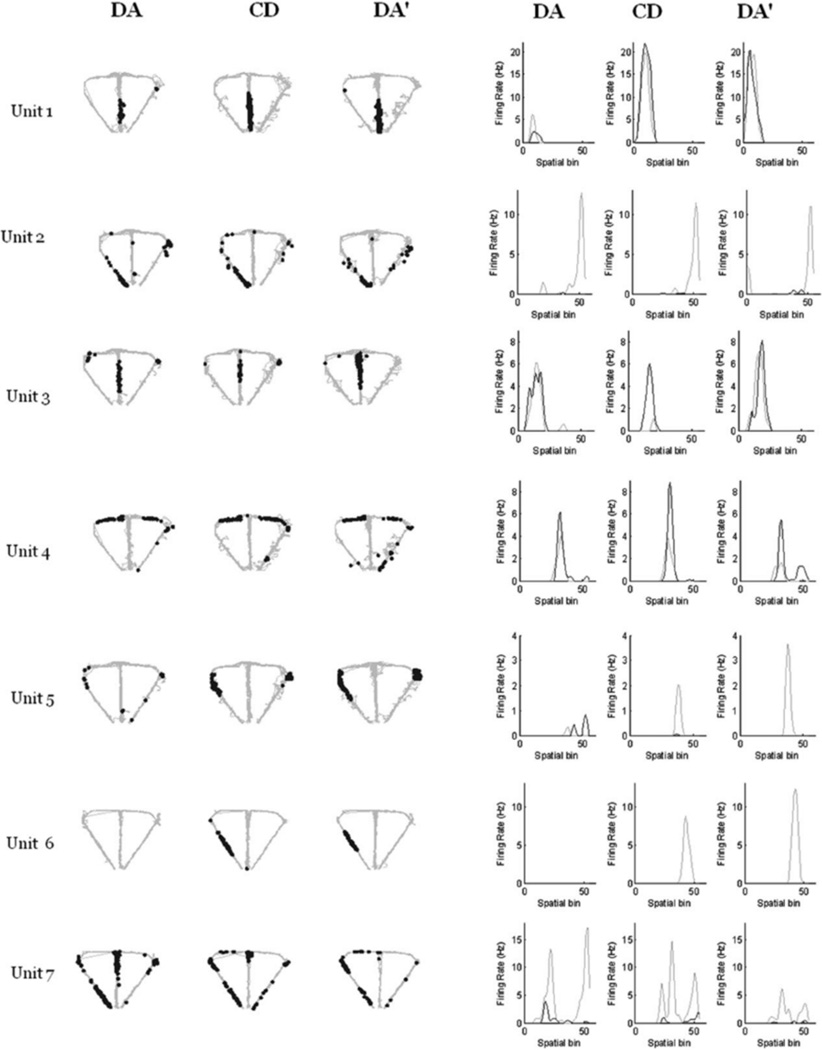
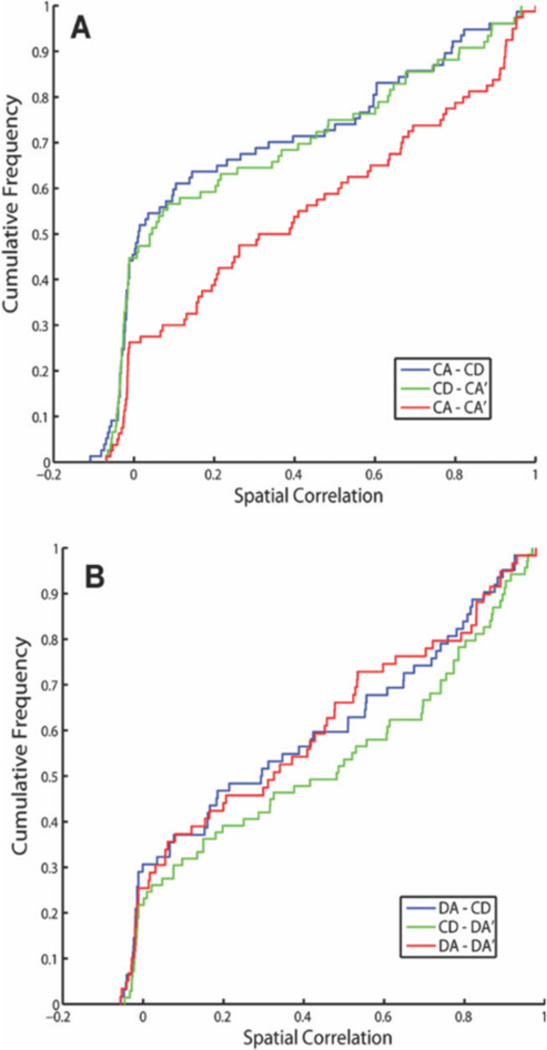
We recorded a total of 349 pyramidal neurons from the CA1 pyramidal cell layer of dorsal hippocampus of 3 rats, 195 (rat 1: 76, rat 2: 38, and rat 3: 81) in CA-CD-CA0 sessions and 154 (rat 1: 127, rat 2: 21, and rat 3: 6) in the DA-CD-DA0 sessions. After excluding clusters that did not meet the cluster isolation quality threshold, there were 201 pyramidal neurons included in the analysis, 105 (rat 1: 60, rat 2: 29, and rat 3: 16) in CA-CD-CA0 sessions and 96 (rat 1: 86, rat 2: 10, and rat 3: 0) in DA-CD-DA0 sessions. As shown in Figure 3, many of the hippocampal neurons with place fields on the maze showed remapping across epochs, with remapping defined as a shift in the location of the firing field across the three epochs, the appearance/disappearance of a field from epoch to epoch (global remapping) or a significant difference in mean firing rate across the three epochs (rare remapping, See Leutgeb et al., 2005). Global remapping patterns were divided into two categories: task remapping and epoch remapping. Units were categorized as task-remapping if they remapped between the CA and CD epochs, but showed no remapping between the CA and CA0 epoch. A common pattern of task remapping, shown in Figure 3, was that units had a firing fields in CA and CA0 and not CD (Neurons 1, 2, and 3) or showed the opposite pattern of having a firing field only in CD (Neurons 4, 5, and 6), Task remapping indicates that the neuron is sensitive to the task being performed because its spatial firing field is similar in CA and CA0 and different for CD. Units showing other remapping patterns across epochs (having a firing field in either the CA or CA0 epoch only, in the CA and CD epochs only, in the CD and CA0 epochs only) were grouped together and categorized as epoch-remapping units. Epoch remapping indicates that neurons were sensitive to a variable other than task (i.e. passage of time; previous experience on the maze within and between sessions, etc.). For the CA-CD-CA0 sessions, of the 59 neurons that had firing fields on the maze, 47 (80%) showed remapping with 23 (49%) of remapping neurons showing epoch remapping and 24 (51%) showing task remapping. For the DA-CD-DA0 sessions, of the 54 neurons that had firing fields on the maze, 44 (81%) showed remapping with 32 (73%) of remapping neurons showing epoch remapping and 12 (27%) showing task remapping. These data show that approximately equal proportions of remapping neurons recorded in CA-CD-CA0 sessions exhibited task remapping and epoch remapping, whereas more remapping neurons recorded in DA-CD-DA0 sessions showed epoch remapping than task remapping (X2 (1) =5.37. P = 0.02). Even though there were more total neurons recorded from rat 1 than rats 2 and 3, remapping patterns did not differ across the 3 rats for either session type (CA-CD-CA0 sessions: X2 (4) =2.9, P = 0.575; DA-CD-DA0 sessions: X2 (2) =1.8, P = 0.406). The most common pattern of epoch remapping in both session types was a field that was present in only epoch 1 or only epoch 3. This pattern was seen is 26% of epoch remapping neurons in CA-CD-CA0 sessions and 34% of epoch-remapping neurons in DA-CD-DA0 sessions. The next common pattern of epoch remapping (as seen in Fig. 4, units 5, 6, and 7) was the maintenance of a place field across epochs 1 and 2 or across epochs 2 and 3 (17% and 13% of epoch-remapping neurons, respectively for CA-CD-CA0 sessions and 9 and 31% of epoch-remapping neurons. respectively for DA-CD-DA0 sessions). Figure 5 shows the distributions of spatial correlation values between epochs for all of the neurons included in the analysis. For neurons recorded during CA-CD-CA0 sessions, the distribution of correlation values between CA and CD and CD and CA0epochs (blue and green curves in Fig. 5a) were significantly different than the distribution of correlation values between CA and CA0 (red curve in Fig. 5a; KS rest, P = 0.0007 and 0.0035, respectively). The distributions of spatial correlation values for neurons recorded during the DA-CD-DA0 sessions did not differ significantly. By examining the curves in Figure 5a, it is clear that a large proportion of neurons showed low-correlation values between the CA and CD epochs and between the CD and CA0 epochs, indicating that there were a large number of task remapping neurons in the CA-CD-CA0 sessions, The overlap of all three of the spatial correlation curves in Figure 5b indicates that there was an absence of task remapping in the DA-CD-DA0 sessions.
FIGURE 3.
Six example units showing task remapping during CA-CD-CA0 sessions. The left panel shows the trajectory of the rat (gray) with superimposed spike locations (black) during the first CA epoch (left), CD epoch (middle) and second CA epoch (CA0, right). The right panel shows the corresponding firing rate distributions, with left and right trials combined to make 86 spatial bins (bins 1–24: stem, bins 25–36: left goal arm, bins 37–55: left return arm, bins 56–67: right goal arm, and bins 68–86: right return arm). Units 1, 2, and 3 had fields that were present in CA and CA0 and absent in CD and units 4, 5, and 6 showed the opposite pattern. Note that some firing fields shifted to a new location between CA and CD and back to the original location for CA0 (Unit 4).
FIGURE 4.
Seven example neurons recorded during DA-CD-DA0 sessions. The left panel shows the trajectory of the rat (gray) with superimposed spike locations (black) during the first DA epoch (DA, left), CD epoch (middle) and second DA epoch (DA0, right). The right panel shows the corresponding firing rate distributions across 55 spatial bins for right (black line) and left (gray line) trials (bins 1–24: stem, bins 25–36: left or right goal arm, and bins 37–55: left or right return arm). Units 1–4 showed no remapping across the session and units 5–7 showed epoch remapping. Units 5 and 6 showed little or no firing in the first DA epoch, but a firing field appeared during CD and remained during DA0. Unit 7 showed the opposite pattern: there was a firing field present on the maze stem during DA that was compressed in size during CD and disappeared during DA0. Note that Unit 7 had a subfield on the left return arm that remained stable across all three epochs, while the stem field remapped.
FIGURE 5.
Distributions of correlation values are shown using a cumulative density function for each across-epoch comparison for CA-CD-CA0 sessions (A) and DA-CD-DA0 sessions (B). Note that the CA-CD and CD-CA0 (blue and green) curves overlap and are significantly different than the CA-CA0 curve indicating that a large proportion of neurons in the CA-CD-CA0 sessions exhibited task remapping. In contrast, all three curves overlap in B, suggesting a low incidence of task remapping in the DA-CD-DA0 sessions. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 6a shows the raw spiking plots and linearized firing rate distributions across epochs from a representative task-remapping neuron recorded during a CA-CD-CA0 session (Unit 3 from Fig. 3). The insets above each plot show the average spike waveforms recorded during each epoch. The clusters from each epoch showed a high degree of overlap as shown in Figure 6b and the recording was stable as shown by the consistency of the peak spike waveform across time shown in Figure 6c. Moreover, Table 1 shows that there was no relationship between the degree of remapping and cluster isolation quality for either session type. Together, these findings show that the high proportion of remapping neurons observed cannot be attributed to poor cluster isolation or lack of recording stability.
TABLE 1.
Relationship Between Cluster Isolation Quality and the Degree of Remapping
| Epoch 1 vs. 2 | Epoch 2 vs. 3 | Epoch 1 vs. 3 | |
|---|---|---|---|
| CA CD CA0 sessions | |||
| L-ratio | 0.025 (0.800) | 0.048 (0.620) | −0.082 (0.410) |
| Isolation distance | 0.050 (0.610) | −0.046 (0.640) | 0.073 (0.460) |
| DA CD DA0 sessions | |||
| L-ratio | −0.136 (0.188) | −0.119 (0.250) | −0.106 (0.306) |
| Isolation distance | −0.010 (0.920) | 0.070 (0.499) | −0.019 (0.856) |
For CA-CD-CA0 sessions, there was no relationship between remapping and field location. However for DA-CD-DA0 sessions, there was a significant relationship between remapping and field location (v2(4) = 11.61, P = 0.02). The significant result was largely driven by the fact that all of the fields that were located on the stem remapped, with 12/14 (86%) showing epoch remapping, and 2/14 (14%) showing task remapping.
To determine whether the tendency for fields to remap varied by field location, the proportion of neurons that exhibited remapping was compared between neurons whose main firing field was on the maze stem, the return arms, and goal arms (see Table 2). For CA-CD-CA0 sessions, there was no relationship between the number of place fields showing remapping and the field location (X2 (4) = 5.12, P = 0.276). For DA-CD-DA sessions, there was a significant relationship between type of remapping (task, epoch, or no remapping) and place field location (stem, return arm, or goal arm; X2 (4) = 11.61, P = 0.02). The significant result was largely driven by the fact that all of the fields that were located on the stem remapped, with 12/14 (86%) showing epoch remapping, and 2/14 (14%) showing task remapping.
TABLE 2.
Number of Neurons that Exhibited Remapping Separated by Field Location and Session Type
| Task remapping |
Epoch remapping |
No remapping | |
|---|---|---|---|
| CA CD CA0 sessions | |||
| Stem | 9 | 7 | 2 |
| Goal arm | 4 | 1 | 2 |
| Return arm | 8 | 9 | 8 |
| DA CD DA0 sessions | |||
| Stem | 2 | 12 | 0 |
| Goal arm | 6 | 6 | 2 |
| Return arm | 4 | 11 | 8 |
For CA-CD-CA0 sessions, there was no relationship between remapping and field location. However for DA-CD-DA0 sessions, there was a significant relationship between remapping and field location (X2(4) = 11.61, P = 0.02). The significant result was largely driven by the fact that all of the fields that were located on the stem remapped, with 12/14 (86%) showing epoch remapping, and 2/14 (14%) showing task remapping.
Because DA-CD-DA0 sessions were run after extensive training on CA-CD-CA0 version of the task, it was necessary to determine whether the degree of experience on the maze might have accounted for the differences between the CA-CD-CA0 sessions and DA-CD-DA0 sessions in epoch and task remapping. Therefore, the number of task remapping and epoch remapping clusters and clusters that showed no remapping was compared between the first and last sessions for each rat. Only two rats were used in this analysis due to the low (< 4) clusters per session that had firing fields on the maze in the third rat. Early and late CA-CD-CA0 and DA-CD-DA0 sessions were compared for Rat 1 (CA-CD-CA0 sessions: X2 (1) = 3.6, P = 0.058, DA-CD-DA0 sessions: X2 (1) = 0.154, P = 0.694); and early and late CA-CD-CA0 sessions were compared for Rat 2 (X2 (1) = 0.51, P = 0.48). There was no relationship between degree of experience on the maze and the proportion of neurons showing remapping, suggesting that degree of experience with the tasks did not influence the difference observed in the remapping pattern between session types.
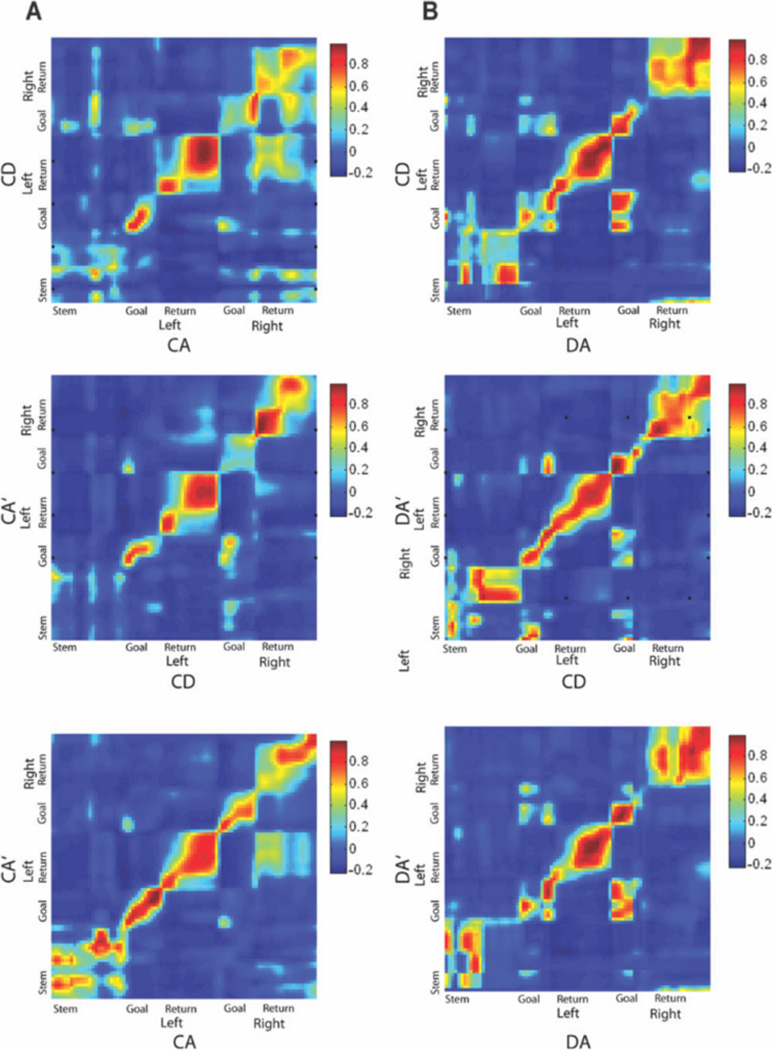
Previous studies have shown that external changes in an environment induce an abrupt change in spatial representations that are coherent across the population (Wills et al., 2005). To examine whether task remapping follows this same pattern, we first examined the population coherence across epochs for CA-CD-CA0 and DA-CD-DA0 sessions. We then examined whether remapping occurred abruptly or if spatial representations changed slowly and systemically across trials after a task change. To investigate whether populations of simultaneously recorded neurons remapped coherently across epochs, we constructed correlation matrices of population firing rate vectors for each session and compared them across epochs by constructing correlation matrices. If the population of recorded neurons represents two epochs similarly, there will be a high-correlation band along the diagonal of the correlation matrix plot. A correlation matrix for all neurons recorded during the CA-CD-CA0 sessions is shown in Figure 7a. Population coherence was high between the CA and CA0 epochs in all parts of the maze. However, a comparison between CA and CD epochs and between CD and CA0 epochs showed high coherence only in the goal arms and return arms, but lower coherence in the stem area of the maze as indicated by the lack of the red band for the stem region of the maze. Figure 7b shows a correlation matrix for all neurons recorded in the DA-CD-DA0 sessions. Population coherence was high across all three epochs and in all regions of the maze, including the maze stem, indicating an absence of task remapping at the population level. Interestingly, the population coherence plots also show a moderate level of coherence between corresponding regions of left and right turn trials, most notably between the left and right goal zones on the DA-CD-DA0 sessions and between the left and right return arms during the CA-CD-CA0 sessions.
FIGURE 7.
Correlation matrices between population firing rate vectors for all units recorded during CA-CD-CA0 sessions (A) and all units recorded during DA-CD-DA0 sessions (B). The abissa and ordinate represent spatial bins on left and right trials, with the maze zones indicated next to each axis. For the CA-CD-CA0 session, the population of recorded neurons was coherent between CA and CA0 epochs, but lost coherence between CA and CD and between CD and CA0 on the maze stem. For the DA-CD-DA0 session, the population of recorded neurons was coherent across all three epochs in all regions of the maze. Note that regions of high correlation also appeared between the right and left return arms in A and the right and left goal zones in both A and B. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To investigate whether task remapping occurs abruptly with a task change, we examined the firing characteristics of remapping neurons on individual trials. A representative neuron is shown in Figure 8. When the task was changed from CA to CD, there was an abrupt change in the firing field. The neuron had a robust firing field in CA, fired on the first trial of CD, but was silent during the remainder of the CD epoch. When the task was switched back to CA (CA0), the firing field reappeared on the first trial and fired robustly throughout the remainder of the epoch.
An analysis of rate remapping was also conducted by calculating the percentage of neurons with rate difference scores above 0.5 (a threefold difference in mean firing rate) across each of the three epochs for both CA-CD-CA0 sessions and DA-CD-DA0 sessions. For CA-CD-CA0 sessions, 21.4% of neurons showed rate remapping between the CA and CD epochs, 20.8% of neurons showed rate remapping between CD and CA0 epochs and 8.1% showed rate remapping between CA and CA0 epochs. For the DA-CD-DA0 sessions, 12% showed rate remapping between DA and CD, 7.1% showed rate remapping between CD and DA0 and 4% showed rate remapping between CD and DA0 epochs. Thus, rate remapping was more common when the tasks differed across epochs than when the tasks were the same across epochs.
Trajectory Coding Was Extremely Rare in Both CA-CD-CA0 and DA-CD-DA0 Sessions
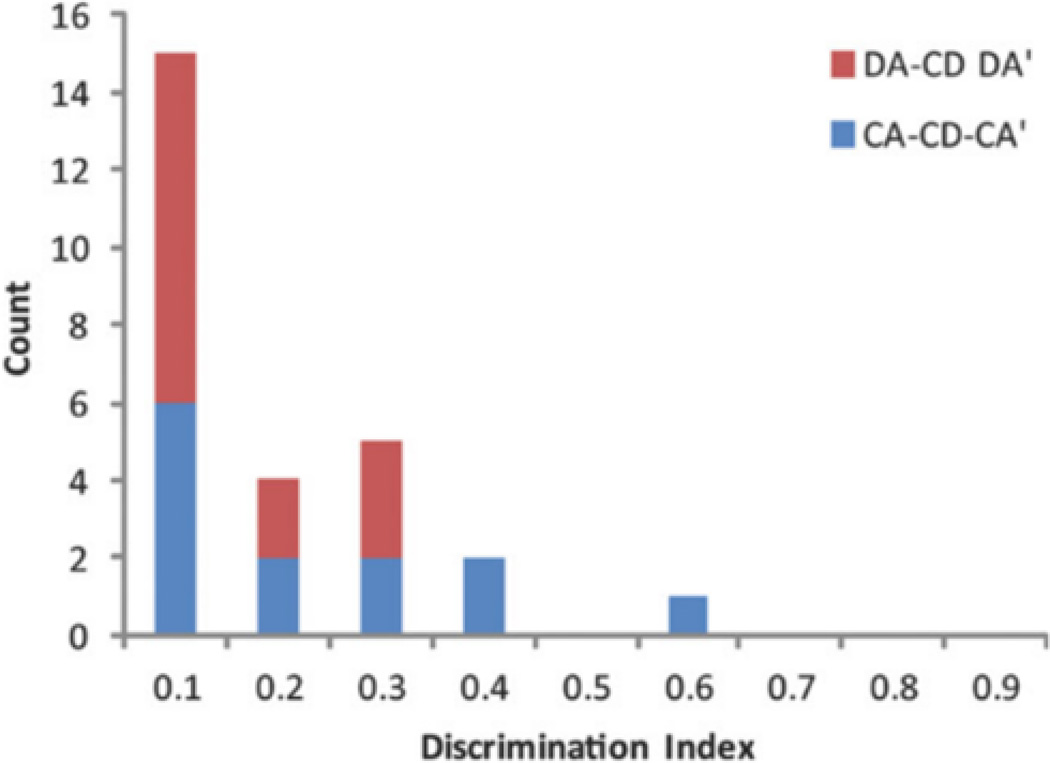
For neurons with a firing field on the stem of the T-maze, the mean field-firing rate per trial was compared between left and right trials using a Kolmogorov-Smirnov test. There were 27 stem-firing neurons, 13 in the CA-CD-CA0 sessions, and 14 in the DA-CD-DA0 sessions. One rat was excluded from the trajectory-coding analysis because there was not sufficient overlap between left and right stem trajectories. Trajectory coding was extremely rare in both CA-CD-CA0 and DA-CA-DA0 sessions (Fig. 9a). In fact, there was only 1 neuron from each session type that met the criteria for the trajectory-coding category. Interestingly, both of these trajectory-coding neurons had a firing field centered on the choice point of the T-maze.
FIGURE 9.
Trajectory coding was rare in CA-CD-CA0 and DA-CD-DA0 sessions. Distribution of discrimination indices (DI) for all neurons with firing fields located of the maze stem for CA-CD-CA0 sessions and for DA-CD-DA0 sessions. DI values range from 0, meaning no trial-specific firing to 1, meaning perfect discrimination between left and right trials. Note that the distribution is skewed heavily to the left, indicating that trajectory coding was extremely rare in the population of recorded neurons. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Delay-Period Firing Predicts the Upcoming Goal-Arm Choice on DA
We then investigated whether the firing rate in the delay period predicts the upcoming trial choice selectively for DA by comparing firing rates based on whether the upcoming goal arm choice was right or left. Of the 74 neurons recorded during the DA-CD-DA0 sessions that emitted at least 20 spikes in the session, 16 neurons (22%) showed a differential firing rates on the DA epochs depending upon the goal arm selection on the upcoming trial (see Fig. 10). The demonstration that prospective activity is seen during the delay period of the working-memory dependent DA task strongly suggests that the firing rate during the delay period can be used as a prospective memory signal.
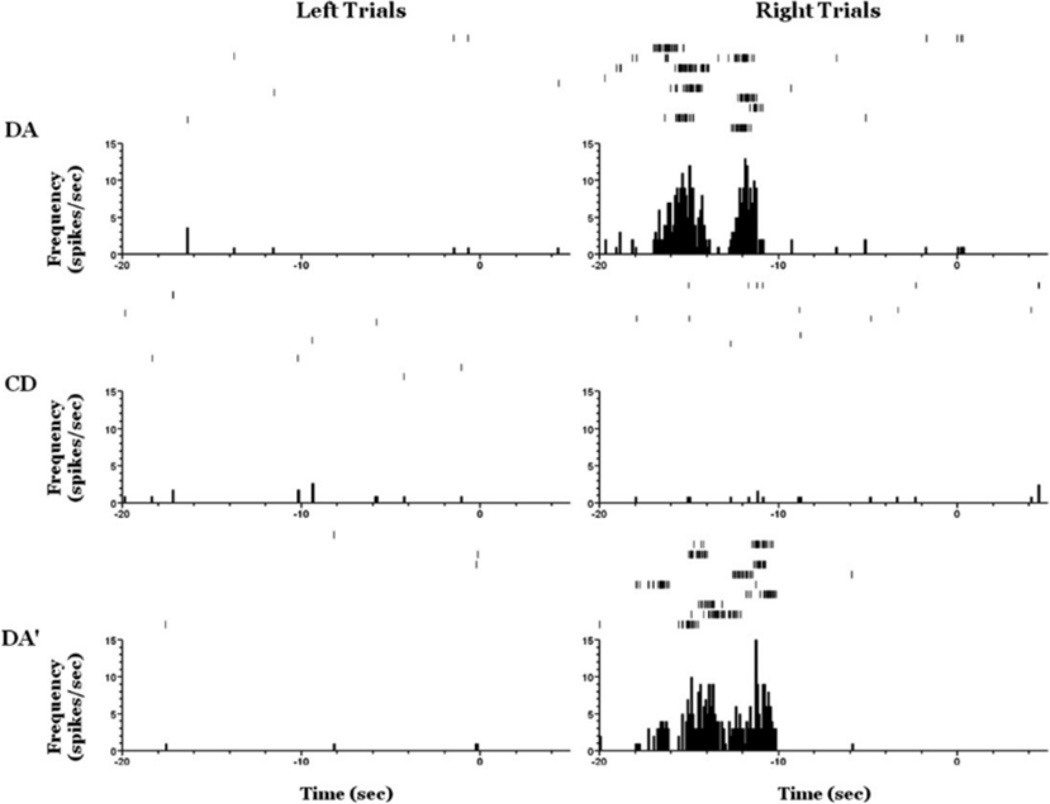
FIGURE 10.
An example neuron showing prospective coding during the delay period on both DA epochs but not the CD epoch of a DA-CD-DA0 session. Raster plots are shown on the top of each panel and perievent time histograms on the bottom, t(0) 5 trial start. Note that there is a significantly greater firing rate in the delay period preceding right trials than that preceding left trial for both the DA and the DA0 epochs and not the CD epoch.
DISCUSSION
Consistent with previous studies (Markus et al., 1995; Ferbinteanu et al., 2011), our results demonstrate that hippocampal place fields can remap to flexibly represent changes in task (task remapping), as a subpopulation of hippocampal neurons with place fields on the T-maze showed high spatial correlation between alternation epochs (same task), and low spatial correlation between either alternation epoch and the CD epoch (different tasks). This result indicates that place cells are sensitive to changes in behavioral strategy even in the absence of changes to the recording environment and behavioral apparatus. A separate subpopulation of place fields showed no remapping across epochs, suggesting that these fields remained tied to the physical layout of the recording environment, even when the task was altered. A third distinct subpopulation of neurons showed remapping that was not associated with a specific task, displaying low spatial correlation between any of the three epochs (epoch remapping). For example, a neuron with a place field on the left return arm of the maze during the first alternation epoch may have shifted its place field to the stem of the maze during the CD epoch, but failed to shift back to the left return arm during the second alternation epoch, as would be expected if task strategy alone was driving remapping. This type of remapping indicates that subpopulations of hippocampal neurons are sensitive to changes in experience, distinctly representing different epochs even when the rat is performing the same task in the same recording environment on the same behavioral apparatus. Epoch remapping may represent a mechanism by which the hippocampus is able to distinguish distinct events that occur in the same location, a critical component of episodic memory (Tulving, 1972). Observing three separate remapping patterns (no remapping, epoch remapping and task remapping) within the same population of neurons is an example of partial remapping, in which manipulations of the recording environment reveal subpopulations of place cells that are tied to separate reference frames (Gothard et al., 1996; Shapiro et al., 1997; Tanila et al., 1997; Skaggs and McNaugton, 1998; Zinyuk et al., 2000; Knierim, 2002; Anderson and Jeffery, 2003; Paz-Villagran et al., 2004). The partial remapping observed between the three behavioral epochs indicates that the epoch-unique place field representations occur in conjunction with a stable spatial code (place fields that did not remap between epochs), and task code (task remapping place fields). Even though all three subpopulations of place fields were observed in both CA-CD-CA0 and DA-CD-DA0 session types, the relative proportion of neurons that displayed activity that was modulated by space, task, or epoch was different between CA-CD-CA0 and DA-CD-DA0 sessions.
Remapping at the Population and Single-Neuron Level
During CA-CD-CA0 sessions, both structure and strategy used by the rat differ between the two tasks. During CA epochs, the ITI pedestal is physically occluded from the T-maze and is never visited by the rat. Thus, the rat is required to go up the maze stem, choose a goal arm, go down a return arm, and immediately go back up the stem for the start of the next trial. During CD epochs, the rat is placed on the ITI pedestal, and the wooden blocker separating the pedestal from the maze is lifted at the start of the trial. The rat must then move up the stem, choose a goal arm, and go down a return arm. Instead of immediately going back up the stem as in CA, the rat must then go back to the ITI pedestal where it is confined until the start of the next trial. This change in task structure not only allows the rat to occupy the ITI pedestal, which is inaccessible during CA, but also alters the motor pattern used by the rat to complete the task, as the insertion of an ITI interrupts time spent on the maze, presumably making each trial more discrete. In addition to the change in structure between the two tasks, rats must use a working memory-dependent strategy (remembering which goal arm was visited on the previous trial) during CA, and a nonworking memory-dependent strategy during CD (the rat cannot make a decision about which goal arm to visit until the start of each trial when the floor insert cue is pseudorandomly presented). During DA-CD-DA0 sessions, the structure of the tasks is similar. During both tasks, the rat must wait on the ITI pedestal until the trial starts, and then move up the maze stem, choose a goal arm, and go down a return arm to the ITI pedestal where it waits until the next trial. However, in a similar manner to CA-CD-CA0 sessions, the strategy used by the rat differs between the two tasks. DA is high in working memory demand because the rat must use information gained on a previous trial to choose the correct goal arm on the upcoming trial. In contrast, CD is low in working memory demand because the floor insert cues are presented pseudorandomly, thus ensuring that the goal arm chosen on the previous trial cannot predict the correct goal arm on the upcoming trial. During CA-CD-CA0 sessions, population coherence was high between the two alternation epochs, but low between the CD epoch and either alternation epoch, especially in the maze stem where the rat must use the floor inserts (CD) or memory of the previous goal arm choice (CA) to guide its next goal arm choice. In contrast, coherence was high between all three epochs during DA-CD-DA0 sessions. This indicates that, as a population, hippocampal neurons represented the structure of the task similarly. Interestingly, however, analysis of individual units revealed that the majority of place fields observed during both CA-CD-CA0 and DA-CD-DA0 sessions remapped between epochs, representing the two alternation epochs differently even though the task, location, and performance of the rat remained consistent. This result suggests that populations of hippocampal neurons in CA1 reflect the physical similarities between environments, but that individual neurons within that population can remap between similar environments in a manner that is not consistent across the population. For example, one hippocampal place cell may display a firing field that is similar between the DA and CD epochs, but remap during the DA0 epoch. Another place field may display similarity between the CD and DA0 sessions, but remap during the DA epoch. Yet another place field may be similar between the DA and DA0 epochs, but remap during the CD epoch. Even though each individual place field is remapping during one epoch, the representation of the population across the three epochs would be highly coherent because two out of the three place fields remain coherent between any given pair of epochs. This finding is in contrast to previous studies that have shown that individual place field remapping is most likely to occur when either salient features of the environment or the location of the recording apparatus changes (Muller and Kubie, 1987; Leutgeb et al., 2005; Wills et al., 2005). During CA-CD-CA0 sessions, roughly half of the place fields that remapped showed task remapping, and half displayed epoch remapping. This finding is consistent with the activity of the total population of hippocampal neurons recorded during CA-CD-CA0 sessions, which showed high coherence between the alternation epochs, but low coherence between the CD and either one of the alternation epochs. During DA-CD-DA0 sessions, however, a smaller proportion (27%) of place fields displayed task remapping, with 73% of remapping place fields showing variability between alternation epochs.
The majority of remapping observed in both CA-CI-CA0 and DA-CD-DA0 sessions involved a substantial shift in place field location. This type of remapping manifested itself in several ways; a hippocampal neuron that fired on one part of the maze (e.g., the stem) began firing while the rat traversed a different location on the maze (e.g., the left return arm) between epochs; a hippocampal neuron that did not fire during one epoch fired robustly during another epoch; and a hippocampal neuron that fired during one epoch ceased to fire during another epoch. Rate remapping, which is operationally defined as a significant change in mean firing rate without a shift in place field location across epochs, was rate in this recorded population of neurons. Previous studies have shown that rate remapping is more likely to occur when changes in the recording environment are relatively subtle (Leutgeb et al., 2005; Fyhn et al., 2007); in light of this, rate remapping has been proposed to represent changes in experience that occur on top of a stable spatial code. Another set of evidence has demonstrated that global remapping can be induced as a function of experience, even in the absence of changes to the environment (Bostock et al., 1991; Moira et al., 2004). Even though minor sensory changes (insertion of floor insert cue between alternation and CD epochs), or no changes at all (between alternation epochs) were introduced in this study across behavioral sessions, the majority of remapping place fields showed a change in firing location on the maze. Furthermore, even though a low overall proportion of place fields displayed rate remapping between epochs, this proportion was higher between CD and alternation epochs (differences in task strategy and sensory input) and lower between the two alternation epochs (no differences in task strategy or sensory input). These data provide further evidence that place field shifting can reflect changes in experience that occur in the same location, even in the absence of any variation in behavior or external environment, especially when those changes in experience are particularly salient or have been exaggerated during training.
Place field remapping has most often been demonstrated in open-field environments, in which hippocampal neurons are recorded while rats forage for food placed randomly around the recording environment. In these experiments, hippocampal neurons most often become bound to salient visual aspects of the recording arena; for example, the color and position of a cue card on the wall or the physical shape of the behavioral apparatus (Muller and Kuhie. 1987; Wills et al., 2005). When the arrangement of visual cues in the environment is sufficiently similar between different sessions in the recording arena, place fields show a high degree of coherence between those sessions. Recognizing salient visual cues and generalizing them across contexts may be particularly important when rats are foraging in an open-field, as nonstereotyped movement throughout the arena creates multiple spatial reference frames (See Gothard et al., 1996), and thus a presumably greater need to rely on stable landmark cues for navigation. Conversely, the T-maze recording sessions in the current study required the animals to move through the T-maze in a goal-directed, stereo-typed manner on every trial, effectively lowering the number of possible reference frames during task performance and hence decreasing the need to rely on distal visual landmarks for successful navigation. Instead of remaining bound to visual cues in the recording room, hippocampal neurons may be engaged in other aspects of the event, such as retaining pertinent task-related information that may be necessary for successful alternation performance. In this instance, successive visits to the T-maze recording room would not necessarily result in high place field coherence because hippocampal neurons would have never become tied to the visual layout of the environment.
In contrast to the place field stability normally seen between foraging sessions in the same open-field environment, the lack of place field coherence between alternation epochs in the. T-maze suggests that the hippocampus may be resolving interference that occurs between episodes that rate place in the same location. The resolution of contextual interference is necessary for the formation of episodic memories, which can contain overlapping features of an environment, but must be separated into distinct events that occur at specific points in rime. Our results suggest that, via remapping, a large proportion hippocampal neurons can differentiate between two events that occur in the absence of any physical change in the recording environment or shift in task performance on the part of the animal. Whether hippocampal place fields show high or low consistency between visits to the same location may depend on the relevant aspects of an animal’s experience. When the arrangement of visual cues is especially salient or is necessary to inform behavior, hippocampal neurons may be more likely to form stable place fields that are linked to the physical layout of the environment; in contrast, when visual cues are not particularly salient or necessary for purposeful behavior, hippocampal neurons may remain engaged in aspects that are unique to an event, and thereby represent temporally distinct epochs differently even when the majority of features between those epochs remain the same.
Although the majority of hippocampal neurons during both CA-CD-CA0 and DA-CD-DA0 sessions differentiated between the two alternation epochs, a proportion of place fields during CA-CD-CA0 sessions did remain tied to task, remapping only between the alternation and CD epochs. This proportion was much larger during CA-CD-CA0 sessions compared with DA-CD-DA0 sessions (51 and 27%, respectively), One factor that determines whether task remapping will occur may be the memory system that is employed across tasks. Asymptotic performance of the continuous alternation (CA) and CD tasks does not depend on the dorsal hippocampus (Ainge et al. 2007; Hallock and Griffin, unpublished observations). In contrast, both excitotoxic lesions and pharmacological inactivation of the dorsal hippocampus produce deficits in delayed alternation (DA) performance (Ainge et al., 2007; Czerniawski et al., 2009). Even though rats use different strategies to solve the CA and CD tasks, hippocampal-dependent memory demand is consistently low during CA-CD-CA0 sessions, presumably allowing a greater number of neurons to respond to physical features of the environment, Conversely, hippocampal-dependent memory demand varies during DA-CD-DA0 sessions, selectively increasing during DA and DA0 epochs, and decreasing during the CD epoch. The higher proportion of epoch remapping seen during DA-CD-DA0 sessions as compared with CA-CD-CA0 sessions may therefore reflect an allocation of cognitive resources in the hippocampus; when the hippocampus is necessary for the successful completion of a task, hippocampal neurons may be more likely to encode behaviorally relevant information (such as prospective and retrospective journey coding), and less likely to become sensitive to the spatial arrangement of the recording environment, thus remapping even when the recording environment does not change.
Alternatively, task remapping may reflect the insertion of a floor insert cue during CD epochs. Previous results have demonstrated that place fields are sensitive to local cues in the recording environment (Shapiro et al., 1997; Lee et al., 2004). Therefore, it is reasonable to interpret the finding that subpopulations of place fields in the current study remapped to task as a sensitivity to the addition of the wood and mesh floor inserts rather than a change in memory demand per se. Indeed, an analysis of the portions of the maze in which place field remapping occurred demonstrated that hippocampal neurons with place fields in the stem of the T-maze were more likely to remap than hippocampal neurons with place fields on other parts of the maze. However, two lines of evidence suggest that the maze floor inserts alone did not drive the remapping seen in this study. The first line of evidence is that a relatively small proportion of the total number of place fields observed displayed remapping that was consistent with the presence of the floor inserts during DA-CD-DA0 sessions. If the remapping between epochs during DA-CD-DA0 sessions was driven primarily by the presentation of the cue, the expectation would be that place fields would remap only when the floor inserts were presented. Instead, only 27% of place fields that remapped showed variability that could have been tied directly to cue presentation. Instead, a much greater proportion (73%) of remapping place fields showed variability that was not consistent with any one task. These place fields often remapped between the DA and DA0 epochs, during which the rat performed the same task in the same environment (in the absence of floor inserts). The second line of evidence is the proportion of task remapping place fields was different between CA-CD-CA0 and DA-CD-DA0 sessions, In both session types, one task (CD) contains floor insert cues, and the other task (CA or DA) does not. If the floor inserts were the most salient aspect of the recording environment, it would be expected that equal proportions of neurons between the two session types would have shown task remapping. Instead, a higher proportion of task remapping neurons was seen during CA-CD-CA0 sessions, suggesting that other differences between the two tasks, such as changes in motor pattern or differences in task strategy, contributed to the task sensitivity of this subpopulation of hippocampal neurons. Although it is likely that the wood and mesh floor inserts influenced place field remapping to a degree, the presence of the inserts cannot account for the large proportion of epoch remapping and the differences in type of remapping between the two behavioral session types.
Our data suggest that task-based remapping is more likely to occur when the physical structure of the tasks is different (discrete vs. continuous) or when the hippocampus is not necessary for successful task performance, thereby allowing a greater number of hippocampal neurons to respond to the physical features of the environment. This finding suggests that different populations of hippocampal neurons can flexibly create similar representations of distinct events based on common visual features of an environment when those features are made relevant, or create discrete representations of different events that occur in similar locations when the need to reduce interference is favorable for the successful performance of a task. The data collected in this study therefore support the view that the hippo-campus processes both the spatial and temporal components of episodes in parallel (Eichenbaum et al., 1999). Recent studies have lent further evidence to this claim by showing that the activity of hippocampal neurons can be modulated by the passage of time in the same location if temporal processing is relevant to task performance (Manns et al. 2007; MacDonald et al. 2011).
Epoch remapping in CA1 may reflect a mechanism by which the hippocampus disambiguates events that rate place in the same location, representing the accumulation of experience over a behavioral session, such that each epoch is a combination of both present and past events on the maze, which may occur as a result of either low sensory input from the recording environment or an increase in hippocampal-dependent memory demand (Leutgeb et al., 2004). Alternatively, epoch remapping may result from a lack of attention to the physical features of a context. During CA-CD-CA0 sessions, hippocampal-dependent memory demand is low during all behavioral epochs. Thus, epoch remapping could simply reflect a lack of attention produced by the reflexive nature of the rats’ behavior. The majority of place fields during CA-CD-CA0 sessions showed either no remapping, or remapping to task, indicating that these hippocampal neurons were sensitive to some physical aspect of the recording environment or behavioral apparatus, Conversely, when rats switched between hippocampal-dependent and non hippocampal-dependent strategies during DA-CD-DA0 sessions, the majority of place fields on the maze showed epoch remapping. if epoch remapping reflected the automatization of behavior, and thus lack of attention to context, the expectation would be that a large proportion of neurons would show epoch remapping during CA-CD-CA0 sessions, and a smaller proportion would show epoch remapping during DA-CD-DA0 sessions, Contrary to this expectation, the opposite pattern was observed, suggesting that epoch remapping is not solely a product of a lack of attention to context on the part of the animal. During CA-CD-CA0 sessions, the lack of necessity for task performance on the part of the hippocampus could drive attentional processing on the part of the animal toward the physical configuration of the environment. Conversely, the requirement for hippocampal engagement during DA-CD-DA0 sessions could shift attentional focus on the part of the animal away from the configuration of the environment.
Trajectory Coding on the Maze Stem and During the Delay Period
A number of experiments have shown that when rats alternate continuously on a modified T-maze, a significant proportion of place cells with place fields on the stem of the maze exhibit trial-unique activity, displaying a higher peak firing rate during left turn trials than right turn trials or vice versa (Wood et al., 2000; Lee et al., 2006; Griffin et al., 2012). One goal of the current study was to examine what would happen to these “trajectory coding” neurons as rats used a CA strategy, switched to a CD strategy (a task in which trial-specific activity on the maze stem is not observed), and then switched back to a CA strategy within a single recording session on the same T-maze. Our hypothesis was that place cells would exhibit trajectory coding selectively during the first and second CA epochs, but cease to demonstrate trajectory-specific variations in firing rate during the CD epoch. Contrary to our predictions, trajectory coding was virtually absent during both CA-CD-CA0 and DA-CD-DA0 sessions, with only one neuron from each session type displaying differences in firing rate to right or left turn trials.
Place cell “directionality” is most often seen during tasks that restrict an animal’s movements to a linear trajectory. For example, on specific arms of a radial arm maze, place cells demonstrate differential firing rates when a rat passes through the place field on an outward journey (toward the end of the arm) compared with passes through the field on an inward journey (toward the center of the maze), and vice versa (McNaughton et al., 1983). Task strategy can also influence the directionality of place cells within an environment, with a larger proportion of hippocampal neurons showing trajectory-dependent firing rates when rats visit locations sequentially along the periphery of an open cylinder, as opposed to when rats are allowed to forage randomly in the cylinder (Markus et al., 1995). In a plus maze, place cells code for both the previous and future location of the rat, displaying variations in firing rate when the animal has come from a particular start arm, or is going to a particular goal arm (Ferbinteanu and Shapiro, 2003). In light of these findings, trajectory coding has been proposed to represent a mechanism wherein the retrieval of stored information in the hippocampus is used to guide appropriate future behavior, a component of episodic memories (Hasselmo and Eichenbaum, 2005).
In addition to the studies that have attempted to link trajectory coding with memory, other lines of research have shown that trajectory coding can be influenced by the physical layout of the environment and the previous experience of the animal. Trajectory-dependent place cell firing is seen less when rats run on a circular track in a cue-rich environment, as opposed to a cue-impoverished environment (Battaglia et al., 2004). Bower et al., (2005) showed that trajectory coding was not necessary for the performance of a presumably hippocampal-dependent task, and that journey-dependent place cell firing only appeared when harriers were presented in the recording arena during training, suggesting that place cell directionality can be influenced by an animal’s prior experience. Recent studies have supported these findings by demonstrating that trajectory coding on the stem of a T-maze that signals the journey of the rat during an alternation task does not respond to the introduction of a visual or tactile cue on the maze stem that signals reward location, indicating that memory retrieval and trajectory coding can occur independently of one another (Ainge et al., 2012; Griffin et al., 2012). Furthermore, when rats switch between a hippocampal-dependent spatial navigation task and a non hippocampal-dependent cue-approach task in a plus maze, both prospective and retrospective trajectory coding is seen in equal proportion during both tasks, arguing against the interpretation that trajectory coding is a reflection of hippocampal-dependent episodic memories (Ferbinteanu et al., 2011).
The results of the current study are in agreement with the findings that trajectory coding in the hippocampus is not necessary the successful task performance, as tars performed CA at a high level (>80% correct choices) during CA-CD-CA0 sessions, even though the proportion of neurons that showed trajectory coding in the maze stem was extremely low. This finding supports the conclusion that trajectory coding can be heavily influenced by the experiences of an animal in a particular environment, decreasing, as the environment is associated with more than one task. As an animal is required to learn more than one task on the T-maze, place cells that might have previously signaled the animal’s trajectory may be required to represent other aspects of the environment in the face of the increasing need to disambiguate events that occur in the same location.
A subset of neurons recorded during DA-CD-DA0 sessions exhibited trial-specific activity during the ITI of the DA epochs, displaying differences in firing rate that were dependent on the rats’ trajectory on the upcoming trial. In contrast to CA and CD, DA performance is high in working memory demand and is hippocampal-dependent (Ainge et al., 2007). Our results are consistent with other investigations showing that hippocampal neurons show prospective coding during the delay period of spatial alternation tasks (Ainge et al., 2007; Pastalkova et al., 2008; Gill et al., 2011). Even though hippocampal neurons represented alternation epochs as distinct events during DA-CD-DA0 sessions, a proportion of neurons still represented trial-unique information during both alternation epochs, indicating that hippocampal neurons are capable of signaling the prospective location of the rat when working-memory demand is increased, even when the rat associates the maze with two tasks. The prospective coding seen during the DA-CD-DA0 sessions may thus represent a true hippocampal-dependent memory signal that is maintained throughout an animal’s experience on the maze.
CONCLUSIONS
The majority of hippocampal place fields observed when rats switched between two tasks in a T-maze represented epochs differently, even when the task was the same (in the case of alternation epochs). This finding indicates that hippocampal neurons can flexibly differentiate between distinct events that occur in the same location, possibly when a reduction in interference that occurs from shared features between events aids in the performance of a specific task. When both the task and structure of the task (i.e., continuously run vs. delay period between trials) changed between epochs, the population of hippocampal neurons exhibited task remapping, representing the alternation epochs similarly, but remapping during the CD epoch. When physical differences in the environment are especially salient or when hippocampal-dependent memory demand is low, hippocampal neurons may remain tied to the physical layout of the environment. Together, the data suggest that subpopulations of hippocampal neurons can process both the common and discrete elements of events that take place in the same location in parallel.
A very small proportion of hippocampal neurons with place fields on the stem of the maze fired differentially to the trajectory of the rat. Despite the rarity of trajectory coding, rats performed both tasks at levels significantly above chance. Hippocampal neurons that may have discriminated between left and right turn trials on the maze stem during CA epochs may become tied to other aspects of an event when the maze is associated with multiple contexts. A larger proportion (22%) of hippocampal neurons with place fields on the delay pedestal fired differentially to the prospective trajectory of the rat during DA epochs within DA-CD-DA0 sessions. When hippocampal-dependent memory demand is increased, trial-unique firing of hippocampal neurons may be necessary for successful task performance, even when the maze becomes affiliated with different tasks.
Acknowledgments
The authors would like to thank Kathryn Cline for technical assistance and Eric Zilli for assistance with data analysis.
REFERENCES
- Ainge JA, van der Meer MAA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in alternation behavior. Hippocampus. 2007;17:988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Tamosiunaite M, Worgotter F, Dudchenko PA. Hippocampal place cells encode intended destination, and not a discriminative stimulus, in a conditional T-maze task. Hippocampus. 2012;22:534–543. doi: 10.1002/hipo.20919. [DOI] [PubMed] [Google Scholar]
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Local sensory cues and place cell directionality: Additional evidence of prospective coding in the hippocampus. J Neurosci. 2004;24:4541–4550. doi: 10.1523/JNEUROSCI.4896-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- Bower MR, Euston DR, McNaughton BL. Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements. J Neurosci. 2005;25:1313–1323. doi: 10.1523/JNEUROSCI.2901-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: Their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–447. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood ER, Shapiro M, Tanila H. The hippocampus, place cells, and memory: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Fellows BJ. Chance stimulus sequences for discrimination tasks. Psychol Bull. 1967;67:87–92. doi: 10.1037/h0024098. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shirvalkar P, Shapiro ML. Memory modulates journey-dependent coding in the rat hippocampus. J Neurosci. 2011;31:9135–9146. doi: 10.1523/JNEUROSCI.1241-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KA, Eichenbaum H. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser M, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Gill PR, Mizumori SJ, Smith DM. Hippocampal episode fields develop with learning. Hippocampus. 2011;21:1240–1249. doi: 10.1002/hipo.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Dynamics of mismatch correction in the hippocampal ensemble code for space: Interaction between path integration and environmental cues. J Neurosci. 1996;16:8027–8040. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci. 2007;27:2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, Owens CB, Peters GJ, Adelman PC, Cline KM. Spatial representations in dorsal hippocampal neurons during a tactile-visual conditional discrimination task. Hippocampus. 2012;22:299–308. doi: 10.1002/hipo.20898. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. Belmont, CA: Wadsworth Publishing Company; 1997. [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci. 2002;22:6254–6264. doi: 10.1523/JNEUROSCI.22-14-06254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Lee I, Griffin AL, Zilli EA, Eichenbaum H, Hasselmo ME. Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron. 2006;51:639–650. doi: 10.1016/j.neuron.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Save E, Poucet B. Place-cell firing does not depend on the direction of turn in a Y-maze alternation task. Eur J Neurosci. 2001;13:1055–1058. doi: 10.1046/j.0953-816x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser M, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O’Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- Moita MAP, Rosis S, Zhou Y, LeDoux JE, Blair HT. Putting fear in its place: Remapping of hippocampal place cells during fear conditioning. J Neurosci. 2004;24:7015–7023. doi: 10.1523/JNEUROSCI.5492-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Bostock EM, Taube JS, Quirk GJ. Spatial firing correlates of neurons in the hippocampal formation of freely moving rats. In: Paillard J, editor. Brain and Space. New York, NY: Oxford University Press; 1991. pp. 296–333. [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos J, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. New York: Elsevier; 2005. [Google Scholar]
- Paz-Villagran V, Save E, Poucet B. Independent coding of connected environments by place cells. Eur J Neurosci. 2004;20:1379–1390. doi: 10.1111/j.1460-9568.2004.03570.x. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience. 2005;131:1–11. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: Dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci. 1998;18:8455–8466. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: The hippocampal role in context processing. J Neurosci. 2006;26:3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tanila H, Shapiro ML, Eichenbaum H. Discordance of spatial representations in ensembles of hippocampal place cells. Hippocampus. 1997;7:613–623. doi: 10.1002/(SICI)1098-1063(1997)7:6<613::AID-HIPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic; 1972. pp. 382–403. [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O’Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Zinyuk L, Kubik S, Kaminsky Y, Fenton AA, Bures J. Understanding hippocampal activity by using purposeful behavior: Place navigation induces place cell discharge in both task-relevant and task-irrelevant spatial reference frames. Proc Natl Acad Sci USA. 2000;97:3771–3776. doi: 10.1073/pnas.050576397. [DOI] [PMC free article] [PubMed] [Google Scholar]