Abstract
Sirtuins (Sirts) regulate several cellular mechanisms through deacetylation of several transcription factors and enzymes. Recently, Sirt2 was shown to prevent the development of inflammatory processes and its expression favors acute Listeria monocytogenes infection. The impact of this molecule in the context of chronic infections remains unknown. We found that specific Sirt2 deletion in the myeloid lineage transiently increased Mycobacterium tuberculosis load in the lungs and liver of conditional mice. Sirt2 did not affect long-term infection since no significant differences were observed in the bacterial burden at days 60 and 120 post-infection. The initial increase in M. tuberculosis growth was not due to differences in inflammatory cell infiltrates in the lung, myeloid or CD4+ T cells. The transcription levels of IFN-γ, IL-17, TNF, IL-6 and NOS2 were also not affected in the lungs by Sirt2-myeloid specific deletion. Overall, our results demonstrate that Sirt2 expression has a transitory effect in M. tuberculosis infection. Thus, modulation of Sirt2 activity in vivo is not expected to affect chronic infection with M. tuberculosis.
Introduction
Sirtuins (Sirts) are a family of seven NAD+-dependent protein deacetylases in mammalians (Sirt1-Sirt7), distributed by different cellular compartments and enabling cells to deal with several stress conditions [1,2,3]. For example, Sirt1, 2 and 6 were shown to target several substrates involved in the cellular stress associated to inflammatory responses, such as the transcription factors NF-κB [4,5,6], AP-1 [7] and Foxp3 [8]. Specifically, Sirt2 shuttles between cytosol and nucleus effectively removing the acetyl group from lysine 310 of p65 subunit of NF-κB [5] and inhibiting the transcription of several inflammatory genes, including Il6, Il1β and Nos2, in activated macrophages [9,10,11,12]. Sirt2 also targets tubulin, inhibiting the microtubule-driven assembly of NLRP3 inflammasome, thus further supporting an anti-inflammatory role for this Sirt [13]. Indeed, Sirt2 suppresses inflammation in arthritis [14], colitis [12], in 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced ear edema [11] and in lipopolysaccharide (LPS)-induced brain inflammation [9]. Despite the evidence supporting a role of Sirt2 in regulating inflammation, the participation of this molecule in host-pathogen interactions only recently began to be unveiled. Infection with the intracellular pathogen Listeria monocytogenes induces translocation of Sirt2 from the cytosol to the nucleus where it deacetylates histone H3 on lysine 18, thus inducing subsequent gene repression [15]. This effect is mediated by the listerial virulence factor protein InlB, which is essential for in vivo infection by L. monocytogenes [15]. Although absence of Sirt2 clearly impaired L. monocytogenes infection [15], little is known on its impact in infections by other intracellular pathogens, such as Mycobacterium tuberculosis, the causative agent of tuberculosis.
A growing body of evidence highlights that the balance between the host immune response and M. tuberculosis factors is critical to the outcome of infection, with perturbations in the inflammatory profile potentially leading to a faster bacterial replication, accompanied by disease [16,17,18,19,20]. Because macrophages are within the first cells becoming into contact with M. tuberculosis during infection, the initial events dictating the innate immune response by myeloid cells not only impact the local and immediate inflammatory immune response, but also potentially shape the intensity and quality of the subsequent acquired immune response. Taking into consideration the role of Sirt2 in modulating the inflammatory response and the importance of this response in the context of M. tuberculosis infection, here we investigated whether the expression of Sirt2 in myeloid cells regulates the course and outcome of M. tuberculosis infection. Although Sirt2 initially impacted control of bacilli proliferation, this effect was attenuated at long-term. Overall, our results show that myeloid expression of Sirt2 is not critical in M. tuberculosis infection.
Materials and Methods
Ethics Statement
All animal experiments were performed in strict accordance with recommendations of the European Union Directive 2010/63/EU and previously approved by Portuguese National Authority for Animal Health–Direção Geral de Alimentação e Veterinária. Mice were euthanized by CO2 inhalation with efforts to minimize suffering.
Animals
LysM-Cre+Sirt2fl/fl mice were obtained by crossing LysM-Cre mice (The Jackson Laboratory) with Sirt2-floxed mice used through an MTA with Johan Auwerx & Kristina Schoonjans Laboratory of Integrative and Systems Physiology, NCEM, Ecole Polytechnique de Lausanne (EPFL), Switzerland. Experimental mice were matched for sex and age and were infected at between 8 and 12 weeks of age.
Bacteria
M. tuberculosis H37Rv, originally from the Trudeau Institute Mycobacterial Collection and kindly provided by Dr. A. M. Cooper, was grown in Proskauer Beck medium containing 0.05% Tween 80 to mid-log phase and frozen in 1-mL aliquots at –80°C, as previously described [21].
Bone marrow derived macrophages
Bone marrow-derived macrophages (BMDM) were differentiated from bone marrow precursors cultured in complete DMEM (cDMEM, containing 10% FBS, 1% sodium pyruvate, 1% HEPES and 1% L-glutamine. all from GIBCO) supplemented with 20% of L929-cell conditioned media (LCCM), as previously described [22]. Briefly, total bone-marrow cells were cultured in microbiological Petri dishes (Sterilin) and kept at 37°C and 5% CO2. Cells were fed on day 4 with equal volume of cDMEM containing 20% LCCM. BMDM were recovered on day 7 of the culture, counted, seeded in 24 well-plates and used to infect with M. tuberculosis. IL-6 production in culture supernatants was measured by ELISA, according to the manufacture’s recommendations (eBiosciences).
Experimental infection and bacterial load determination
Mice were infected with M. tuberculosis H37Rv via the aerosol route using an inhalation exposure system (Glas-Col), as previously described [23]. The infection dose was confirmed by determining the number of viable bacteria in the lungs of 5 animals, 3 days after the aerosol infection. The initial infectious dose was Log101.942±0.106; Log102.00±0.030; and Log102.177±0.124, for three independent experiments performed. For bacterial load determination, mice were euthanized and the lungs were aseptically excised, individually homogenized, followed by plating serial dilution of the organ homogenate on nutrient 7H11 agar (BD Biosciences). Colony forming units were counted after 3 weeks of incubation at 37°C.
Flow Cytometry Analysis
For the analysis of surface markers, 1x106 lung cells were stained with antibodies anti-CD3-PerCPcy5.5 (clone 145-2C11),-CD4-APC-Cy7 (clone GK1.5) and-CD11b-PE (clone M1/70) (all from eBioscience);-CD11c-BV421 (clone N418) and-Ly6G-APC (clone 1A8) (Biolegend) and-Ly6C-PerCPCy5.5 (clone AL-21, Pharmingen). For intracellular staining, 2-3x106 lung cells were restimulated in vitro with a mixture of phorbol myristate acetate (PMA; 50ng/mL) and ionomycin calcium salt (500ng/mL), in the presence of brefeldin A (10μg/mL; all from Sigma-Aldrich) for 4 hours at 37°C. After restimulation, cells were fixed and stained for surface CD4 and intracellular IL-17, IFN-γ and TNF as described before [23]. Samples were acquired on a LSRII flow cytometry with Diva Software. All data were analyzed using FlowJo version 10 software. The total number of cells in each gate was calculated using the total number of cells determined by Countess Automated Cell Counter. The gating strategy followed is represented in S1 Fig.
Quantitative Real Time-PCR analysis
Total RNA from infected lungs was extracted with TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized and analyzed by real-time PCR as described previously [24]. Target gene mRNA expression was quantified using SYBR green (Fermentas) and specific oligonucleotides for each molecule and normalized to the ubiquitin mRNA levels.
Histological analysis
Anterior lobe of lungs were recovered from infected mice, fixed with 3.7% phosphate-buffered formalin, embedded in paraffin, sectioned in 3μm thickness sections and stained with hematoxylin and eosin (H&E). Immunofluorescence was performed on formalin-fixed tissue sections as previously described [24]. Briefly, antigens were unmasked and blocked with BSA and Fc-Block, and endogenous biotin was neutralized. Sections were probed with purified rabbit anti-NOS2 (M-19, Santa Cruz Biotechnology) followed by a secondary Alexa Fluor 568 goat anti-rabbit IgG (Invitrogen). Vectashield mounting medium with DAPI (Invitrogen) was used to detect nuclei. Images were obtained with an Olympus BX61 microscope and were recorded with a digital camera (DP70).
Statistical Analysis
The results are given as means ± standard error of the mean (SEM) of at least five animals per experimental group, as indicated in the figure legends. Differences between groups were analyzed by unpaired Student’s t test using Graph Pad Prism 6 software, as indicated in the figure legends. Values were considered significant for p≤0.05.
Results
Ablation of myeloid Sirt2 transiently impacts the control of M. tuberculosis
Previous studies have demonstrated a role of Sirt2 both in inflammation [9,12,14] and in infection by the intracellular pathogen L. monocytogens [15]. Since the control of M. tuberculosis infection is dependent on the activation of macrophages, we questioned whether ablation of Sirt2 in the myeloid lineage would impact the course of M. tuberculosis infection. We infected control mice (Cre-Sirt2fl/fl) or myeloid-restricted Sirt2 deficient mice (Cre+Sirt2fl/fl) with this pathogen via the aerosol route and followed the bacterial burdens over time. At day 30 post-infection, we observed a significant increase of CFUs in the lungs of Cre+Sirt2fl/fl mice as compared to controls (difference of log0.305) (Fig 1A). However, by day 60 of infection and onwards bacterial burdens were similar in both groups (Fig 1A). A similar profile of bacterial burden was observed at day 30 post-infection in the liver (difference of log0.523), although the increased susceptibility of Cre+Sirt2 fl/fl mice was prolonged up to day 60 post-infection (difference of log0.305) (Fig 1B). Independently of the organ, the long-term control of M. tuberculosis was not compromised by the absence of myeloid Sirt2, as on day 120 post-infection bacterial burdens were similar in both groups of mice (Fig 1A and 1B). The difference observed in bacterial burdens on day 30 post-M. tuberculosis infection was not translated to a different histological pattern of the infected lungs (Fig 1C), nor to distinct NOS2 foci (Fig 1D). These data show that myeloid Sirt2 deficiency has a transient impact in bacterial burden without influencing the pathology caused at the site of infection.
Fig 1. Ablation of myeloid Sirt2 transiently impacts the control of M. tuberculosis.

(A) Lung and (B) liver M. tuberculosis burdens at days 30, 60 and 120 post-infection of Cre+Sirt2fl/fl mice (white circles) or Cre-Sirt2fl/fl (black circles). Represented are 3 independent experiments. The initial infectious dose was Log101.942±0.106; Log102.00±0.030; and Log102.177±0.124, for three independent experiments performed. *, p < 0.05; **, p < 0.01; determined by unpaired t-test; (C) Microscopic inflammatory lung lesions of M. tuberculosis-infected mice stained with hematoxylin-eosin. (D) NOS2 (red) and nuclei (blue) immunofluorescence staining, 30 days post-infection.
Absence of Sirt2 in the myeloid lineage does not impact the cellular responses to M. tuberculosis infection
Dynamics of cell infiltration to the lungs of infected animals is critical for the establishment of a protective immune response against M. tuberculosis [25]. Both myeloid cells, resident or recruited, and CD4+ T helper (Th) cells play a role in this process. To address whether the increased susceptibility of Cre+Sirt2fl/fl mice on day 30 post-infection was related to a different cellular response at the site of infection, we characterized the myeloid and T cell populations by flow cytometry (S1 Fig). We analyzed different myeloid populations with relevance during the course of infection, including alveolar macrophages (CD11b+CD11chi), neutrophils (CD11b+Ly6G+Ly6Cint) and inflammatory monocytes (CD11b+Ly6ChiLy6G-). As shown in Fig 2A, the number of cells in each myeloid population analyzed was similar independently of the expression of Sirt2 in myeloid cells. Similarly, the number of CD4+ T cells in the lung of infected animals was identical in Cre-Sirt2fl/fl and Cre+Sirt2fl/fl, as were the number of CD4+ T cells capable of producing IFN-γ, IL-17 or TNF, as measured by intracellular staining upon in vitro restimulation (Fig 2B). Of note, no cytokine-producing cells were detected in non-infected animals (data not shown). Thus, ablation of myeloid Sirt2 neither impacted the lung myeloid cellular populations observed at that time point, nor did it influence IFN-γ-, IL-17- and TNF-mediated protective T cell responses. These data suggest that the increased bacterial burden observed on day 30 post-infection in Cre+Sirt2fl/fl animals is not caused by alterations in these cellular mediators.
Fig 2. Absence of Sirt2 in myeloid cells does not impact lung cellular responses to M. tuberculosis.
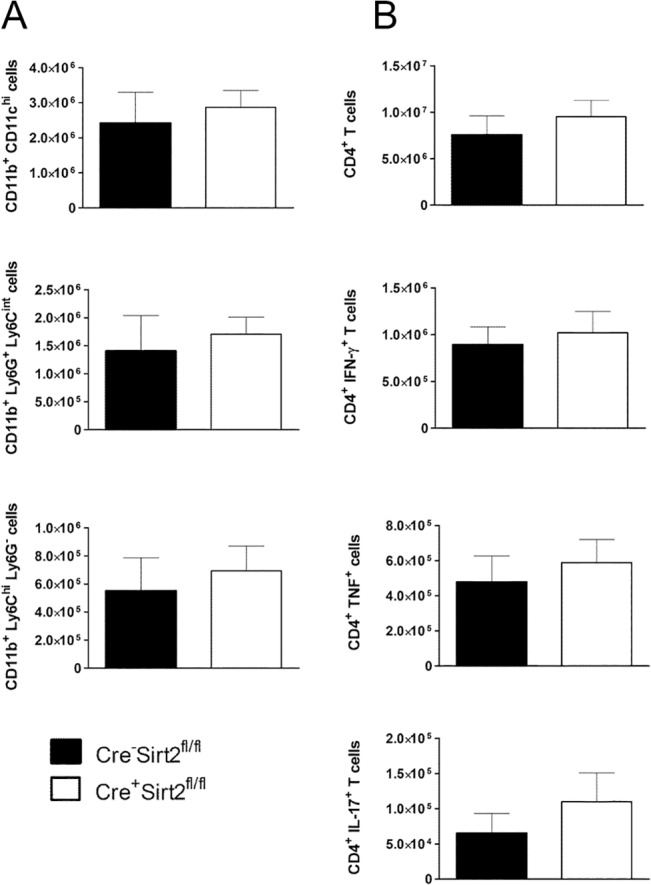
(A) Myeloid cell populations in lung 30 days post-infection were characterized by flow cytometry. (B) Flow cytometry analysis of total CD4+ T cells and IFN-γ, IL-17 and TNF production by CD4+ T cells restimulated with PMA and ionomycin in the presence of brefeldin A. Graphs show the mean ± SEM value of one representative experiment of at least two independent ones (n = 5). The gating strategies and representative plots are in S1 Fig. Significance was determined by the Student’s t-test.
Absence of myeloid Sirt2 does not impact the expression of protective molecules during M. tuberculosis infection
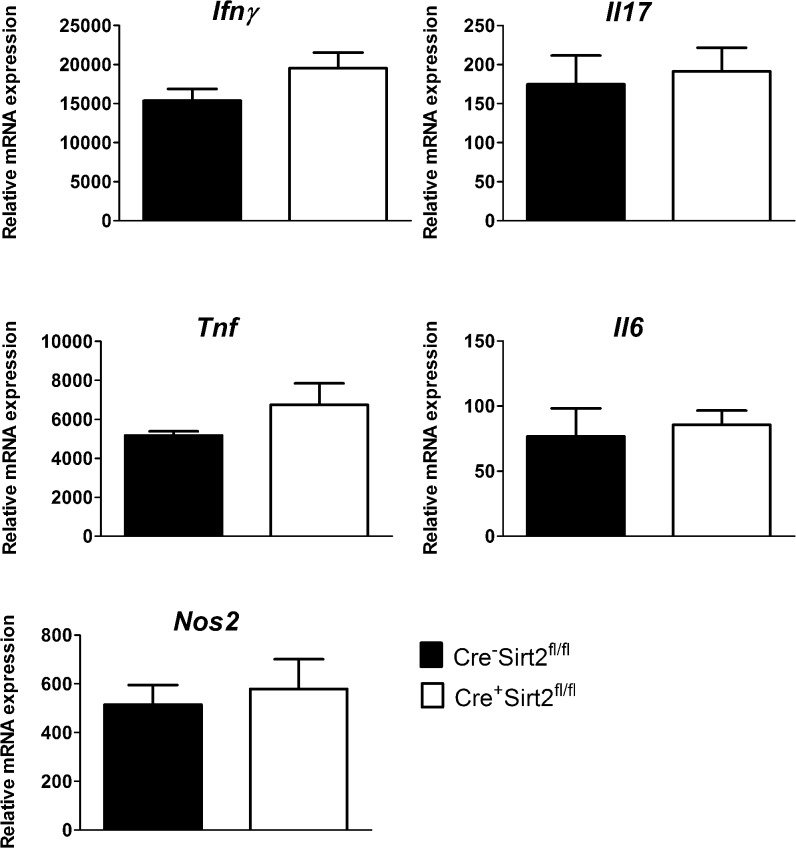
Considering the role of Sirt2 in the transcriptional control of macrophages responses [9,10], we next investigated if the difference observed in bacterial burden was due to distinct expression of protective molecules in the lungs of Cre-Sirt2fl/fl and Cre+Sirt2fl/fl. For that, total RNA of lung extracts was isolated, converted to cDNA and the transcription of Ifnγ, Il17, Tnf, Il6 and Nos2 measured by real-time PCR. These molecules have been shown to play important protective roles in the context of M. tuberculosis infection [25]. Transcription of some these genes has been previously described to be affected by Sirt2 [5,9,11]. In fact, we confirmed that ablation of Sirt2 in BMDM led to increased levels of IL-6 upon infection with M. tuberculosis (S2 Fig). Consistently with the cytometry (Fig 2B) and the immunofluorescence (Fig 1C) data, the transcription of Ifnγ, Il17, Tnf and Nos2 was similar in both groups at day 30 post-infection (Fig 3). Additionally, no difference was observed in the transcription of Il6 (Fig 3). Thus, the overall inflammatory response in the lung of myeloid-restricted Sirt2 deficient mice was similar to that of Sirt2-competent animals, despite a transient increase in the lung bacterial load.
Fig 3. The expression of inflammatory mediators in infected lungs is not altered in the absence of myeloid Sirt2.
RNA was extracted from the lung tissue after 30 days of infection and the expression of Ifnγ, Il17, Tnf, Il6 and Nos2 was analyzed by real-time PCR and normalized to the expression of ubiquitin. Data shows the mean ± SEM value (n = 5–6) and the significance was determined by the Student’s t-test. The data are representative of two independent experiments.
Discussion
The Sirt family is composed by several evolutionarily conserved protein deacetylases that regulate many cellular processes including metabolism, cell cycle, and longevity [26]. Additionally, a role for Sirt in infection is emerging. Indeed, a function for specific Sirts in infection with Herpesvirus, Hepatitis virus and HIV has been described [27,28,29,30,31] and broad-range antiviral properties have been recently reported to all seven Sirt [32]. As for bacterial infection, myeloid Sirt1 expression was shown to have little influence in Gram-negative toxin-induced shock or Gram-positive bacteremia [33], whereas ablation of Sirt2 profoundly changed the outcome of infection with L. monocytogenes [15]. In this study, we expanded the research on the role of Sirt2 in infection, by investigating the impact of myeloid Sirt2 expression in M. tuberculosis infection. Since M. tuberculosis is an intracellular pathogen whose control is dependent on the activation of macrophages [25], we addressed this issue using myeloid-restricted Sirt2 deficient mice. We found that ablation of Sirt2 in the myeloid lineage had a transient effect in the outcome of infection, with higher bacterial burdens detected on day 30 post infection in the absence of Sirt2. However, this effect was not sustained over time, pointing to a minor role of myeloid Sirt2 in tuberculosis. Our data also show that the increased susceptibility observed was not related to differential myeloid and T cell responses at this time-point. It is possible that either a more subtle immune alteration on the course of infection or a non-immune mechanism, such as metabolic variations commanded by the absence or Sirt2, underlie our observations. In fact, recent studies show that Sirt2 affects the activity of phosphoglycerate mutase (PGAM), a glycolytic enzyme, preventing the Warburg effect in cancer cells [34]. IFNγ-activated macrophages in mycobacterial granulomas undergo same metabolic changes with increased glucose uptake [35], which can be affected in Kuppfer cells by Sirt2 deletion.
It is notable the fact that ablation of Sirt2 deeply affected the outcome of L. monocytogenes infection [15], whereas in the case of M. tuberculosis such was not the case. This could be due to the fact that L. monocytogenes exploits the activity of the host Sirt2 through the expression of the bacterial virulence factor InlB, for which no homologue is found in M. tuberculosis.
One third of the world’s population is estimated to be infected with M. tuberculosis and reactivation of tuberculosis is known to occur when the delicate balance established between the host and the pathogen is broken. A great number of clinical trials are reported with either Sirt inhibitors or activators, for example in the context of metabolic or neurodegenerative diseases [36]. Therefore, the clarification of the role of Sirt2 and other Sirts in infection is relevant for future clinical applications.
Supporting Information
(TIF)
BMDM were generated from Cre+Sirt2fl/fl mice or Cre-Sirt2fl/fl and left uninfected (NI) or infected with M. tuberculosis at a multiplicity of infection of 2 bacteria:1 cell for 24 hours. Supernatants were recovered and the amount of IL-6 quantified by immunoassay. The significance was determined by the Student’s t-test. ***p<0.001.
(TIF)
Acknowledgments
The authors are grateful to Dr Rui Appelberg for helpful discussions. The authors thank the personnel at the ICVS animal house facility for excellent animal husbandry.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by Fundação para a Ciência e Tecnologia, Portugal and cofunded by Programa Operacional Regional do Norte (ON.2–O Novo Norte), Quadro de Referência Estratégico Nacional (QREN), through the Fundo Europeu de Desenvolvimento Regional (FEDER). Project grants: PTDC/SAU-MII/101977/2008 (to AGC) and PTDC/BIA-BCM/102776/2008 (to MS). LMT was supported by FCT Grant SFRH/BPD/77399/201. MS is an Associate-FCT Investigator fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, et al. (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140: 280–293. 10.1016/j.cell.2009.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scher MB, Vaquero A, Reinberg D (2007) SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21: 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, et al. (2009) Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324: 1289–1293. 10.1126/science.1169956 [DOI] [PubMed] [Google Scholar]
- 4. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, et al. (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123: 4251–4258. 10.1242/jcs.073783 [DOI] [PubMed] [Google Scholar]
- 6. Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, et al. (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136: 62–74. 10.1016/j.cell.2008.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, et al. (2010) SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem 285: 7097–7110. 10.1074/jbc.M109.038604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, et al. (2010) Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115: 965–974. 10.1182/blood-2009-02-207118 [DOI] [PubMed] [Google Scholar]
- 9. Pais TF, Szego EM, Marques O, Miller-Fleming L, Antas P, et al. (2013) The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J 32: 2603–2616. 10.1038/emboj.2013.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee AS, Jung YJ, Kim D, Nguyen-Thanh T, Kang KP, et al. (2014) SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages. Biochem Biophys Res Commun 450: 1363–1369. 10.1016/j.bbrc.2014.06.135 [DOI] [PubMed] [Google Scholar]
- 11. Kim MJ, Kim DW, Park JH, Kim SJ, Lee CH, et al. (2013) PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radic Biol Med 63: 432–445. 10.1016/j.freeradbiomed.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 12. Lo Sasso G, Menzies KJ, Mottis A, Piersigilli A, Perino A, et al. (2014) SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One 9: e103573 10.1371/journal.pone.0103573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Misawa T, Takahama M, Kozaki T, Lee H, Zou J, et al. (2013) Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 14: 454–460. 10.1038/ni.2550 [DOI] [PubMed] [Google Scholar]
- 14. Lin J, Sun B, Jiang C, Hong H, Zheng Y (2013) Sirt2 suppresses inflammatory responses in collagen-induced arthritis. Biochem Biophys Res Commun 441: 897–903. 10.1016/j.bbrc.2013.10.153 [DOI] [PubMed] [Google Scholar]
- 15. Eskandarian HA, Impens F, Nahori MA, Soubigou G, Coppee JY, et al. (2013) A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 341: 1238858 10.1126/science.1238858 [DOI] [PubMed] [Google Scholar]
- 16. Dorhoi A, Reece ST, Kaufmann SH (2015) For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev 240: 235–251. [DOI] [PubMed] [Google Scholar]
- 17. Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, et al. (2014) Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511: 99–103. 10.1038/nature13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobin DM, Ramakrishnan L (2013) TB: the Yin and Yang of lipid mediators. Curr Opin Pharmacol 13: 641–645. 10.1016/j.coph.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torrado E, Cooper AM (2013) Cytokines in the balance of protection and pathology during mycobacterial infections. Adv Exp Med Biol 783: 121–140. 10.1007/978-1-4614-6111-1_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orme IM, Robinson RT, Cooper AM (2015) The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 16: 57–63. 10.1038/ni.3048 [DOI] [PubMed] [Google Scholar]
- 21. Carmona J, Cruz A, Moreira-Teixeira L, Sousa C, Sousa J, et al. (2013) Strains Are Differentially Recognized by TLRs with an Impact on the Immune Response. PLoS One 8: e67277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teixeira-Coelho M, Guedes J, Ferreirinha P, Howes A, Pedrosa J, et al. (2014) Differential post-transcriptional regulation of IL-10 by TLR2 and TLR4-activated macrophages. Eur J Immunol 44: 856–866. 10.1002/eji.201343734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cruz A, Torrado E, Carmona J, Fraga AG, Costa P, et al. (2015) BCG vaccination-induced long-lasting control of Mycobacterium tuberculosis correlates with the accumulation of a novel population of CD4(+)IL-17(+)TNF(+)IL-2(+) T cells. Vaccine 33: 85–91. 10.1016/j.vaccine.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 24. Teixeira-Coelho M, Cruz A, Carmona J, Sousa C, Ramos-Pereira D, et al. (2010) TLR2 deficiency by compromising p19 (IL-23) expression limits Th 17 cell responses to Mycobacterium tuberculosis. Int Immunol 23: 89–96. 10.1093/intimm/dxq459 [DOI] [PubMed] [Google Scholar]
- 25. Cooper AM (2009) Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27: 393–422. 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R (2012) Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 11: 443–461. 10.1038/nrd3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He M, Gao SJ (2014) A novel role of SIRT1 in gammaherpesvirus latency and replication. Cell Cycle 13: 3328–3330. 10.4161/15384101.2014.968431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon HS, Brent MM, Getachew R, Jayakumar P, Chen LF, et al. (2008) Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe 3: 158–167. 10.1016/j.chom.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q, He M, Zhou F, Ye F, Gao SJ (2014) Activation of Kaposi's sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle. J Virol 88: 6355–6367. 10.1128/JVI.00219-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu JW, Sun LJ, Liu W, Zhao YH, Kang P, et al. (2013) Hepatitis C virus core protein induces hepatic metabolism disorders through down-regulation of the SIRT1-AMPK signaling pathway. Int J Infect Dis 17: e539–545. 10.1016/j.ijid.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 31. Ren JH, Tao Y, Zhang ZZ, Chen WX, Cai XF, et al. (2014) Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J Virol 88: 2442–2451. 10.1128/JVI.02861-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koyuncu E, Budayeva HG, Miteva YV, Ricci DP, Silhavy TJ, et al. (2014) Sirtuins are evolutionarily conserved viral restriction factors. MBio 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crotty Alexander LE, Marsh BJ, Timmer AM, Lin AE, Zainabadi K, et al. (2013) Myeloid cell sirtuin-1 expression does not alter host immune responses to Gram-negative endotoxemia or Gram-positive bacterial infection. PLoS One 8: e84481 10.1371/journal.pone.0084481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsusaka T, Guo T, Yagura T, Inoue T, Yokode M, et al. (2014) Deacetylation of phosphoglycerate mutase in its distinct central region by SIRT2 down-regulates its enzymatic activity. Genes Cells 19: 766–777. 10.1111/gtc.12176 [DOI] [PubMed] [Google Scholar]
- 35.Appelberg R, Moreira D, Barreira-Silva P, Borges M, Silva L, et al. (2015) The Warburg effect in mycobacterial granulomas is dependent on the recruitment and activation of macrophages by interferon-gamma. Immunology. [DOI] [PMC free article] [PubMed]
- 36. Mellini P, Valente S, Mai A (2015) Sirtuin modulators: an updated patent review (2012–2014). Expert Opin Ther Pat 25: 5–15. 10.1517/13543776.2014.982532 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
BMDM were generated from Cre+Sirt2fl/fl mice or Cre-Sirt2fl/fl and left uninfected (NI) or infected with M. tuberculosis at a multiplicity of infection of 2 bacteria:1 cell for 24 hours. Supernatants were recovered and the amount of IL-6 quantified by immunoassay. The significance was determined by the Student’s t-test. ***p<0.001.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.