Tissue hypoperfusion and subsequent limited oxygen transport are critical features conducting to organ failure during shock states. Therefore, early identification of tissue hypoperfusion and adequate resuscitation are key for improving the probability of survival after septic shock.(1,2) However, how to identify organ perfusion abnormalities at the bedside and select the type and amount of fluids required to improve tissue hypoxia remain highly controversial. Traditionally, clinical signs, such as reduced blood pressure and urinary output, altered consciousness, and mottled skin, have been used to identify tissue perfusion abnormalities. Consequently, current hemodynamic monitoring during shock states mainly focuses on detection of pressure-derived hemodynamic variables related to systemic circulation. However, it has been largely recognized that monitoring these macro-hemodynamic variables is not sufficient to rule out persistent abnormalities of tissue oxygenation. Indeed, the usefulness of resuscitation targets, such as global oxygen-derived parameters, has been strongly questioned,(3) and recent data have failed to demonstrate beneficial effects of using central venous oxygen saturation as a goal of resuscitation.(4-6)
Fluid resuscitation therapy primarily aims to optimize cardiac output on the assumption that increasing macro blood flow can improve the convective transport of oxygen to the tissues and, therefore, maintain cellular respiration and support organ function.(7,8) Thus, fluid therapy targeting central venous pressure has been widely recommended to achieve adequate cardiac performance.(9) However, high positive fluid balances have also been associated with unfavorable clinical outcomes.(10) In this sense, dynamic approaches to assessing volume responsiveness to fluid administration seem to be superior to static variables.(11,12) Unfortunately, macro-hemodynamic optimization guided by either dynamic or static variables does not guarantee adequate tissue perfusion or adequate cellular respiration.
Oxygen transport to tissues is governed by convective and diffusive components. The convective component is determined by the microcirculatory blood flow itself, i.e., the number of red blood cells (RBCs) entering the microcirculation, and by oxygen content. In normal conditions, inflow and outflow pressures control the driving pressure at the microvascular level. Thus, the convective transport is regulated upstream at the arteriolar level through microcirculatory inflow changes with subsequent micro-hematocrit modifications and is limited downstream by venous pressure. Meanwhile, the diffusive component of oxygen transport is determined by the gradient between the capillary and mitochondrial oxygen partial pressures, the diffusional distance and the area available for gas exchange, according to Fick´s law.
Unfortunately, current resuscitation procedures are based on the assumption that defects in oxygen transport arise from a lack of perfusion. Thus, resuscitation efforts are mostly focused on the promotion of convective flow based on the assumption that hypovolemia is the main limiting factor of blood flow. However, a substantial contribution of oxygen tissue transport is determined by the capacity of diffusion of oxygen from RBCs to the cells, and this can be quantified by functional capillary density (FCD), i.e., the density of capillaries with flowing RBCs carrying oxygen. In normal conditions, the microvascular blood flow is carefully matched to the metabolic demands of tissues. However, septic shock is characterized by decreased FCD in addition to increased heterogeneity of blood flow, with zones of well-perfused vessels in close proximity to non-perfused capillaries.(13) The persistence of such alterations has been shown to be related to multiorgan dysfunction, even when global hemodynamics appear to be optimal.(14) Indeed, increases in cardiac output may be insufficient to correct tissue hypoxemia because microcirculation alterations might persist. Thus, global hemodynamic targets should be integrated with functional microcirculatory goals, as this would optimize both convective and diffusive components in order to maximize oxygen delivery to cells. Microcirculatory responsiveness should be defined as an increase in convective microvascular flow in response to fluid load in addition to a reduction of the heterogeneity of flow, resulting in a more balanced distribution of oxygen to the tissues (Figure 1). Nevertheless, efforts to increase blood flow by fluid administration could be counterbalanced by a decrease in FCD resulting in a limited oxygen diffusive capacity (Figure 2). Thus, excessive fluid administration could increase the distance between capillaries, reducing oxygen diffusion to the tissues and finally to the mitochondria.
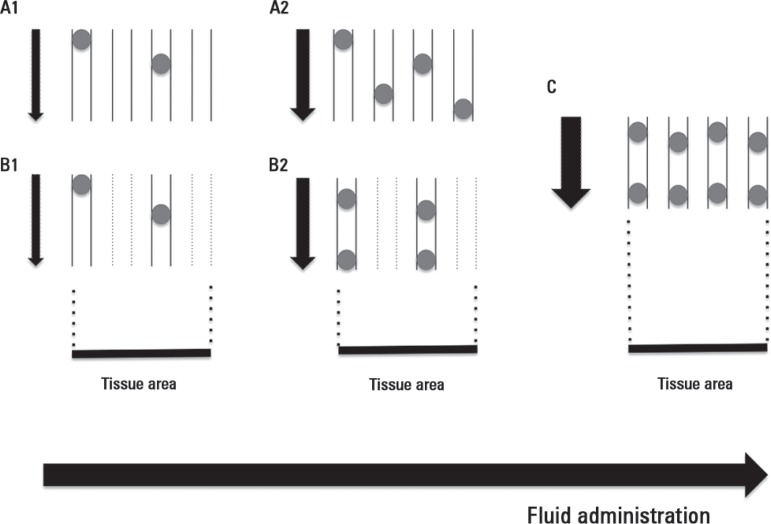
Figure 1.
Effects of adequate fluid administration on microvascular blood flow.
Progressive increasing of convective flow after fluid loading during microcirculatory conditions of pure convective flow derangements (A1, A2) and combined with increased heterogeneity of blood flow (B1, B2). An optimal fluid administration corrects convective and heterogeneity blood flow disturbances (C). Note that the number of vessels with adequate flow per tissue area represents the functional capillary density (FCD), i.e., the major determinant of the diffusive component of oxygen transport to tissues. Black arrows pointing down represent the magnitude of the blood flow.
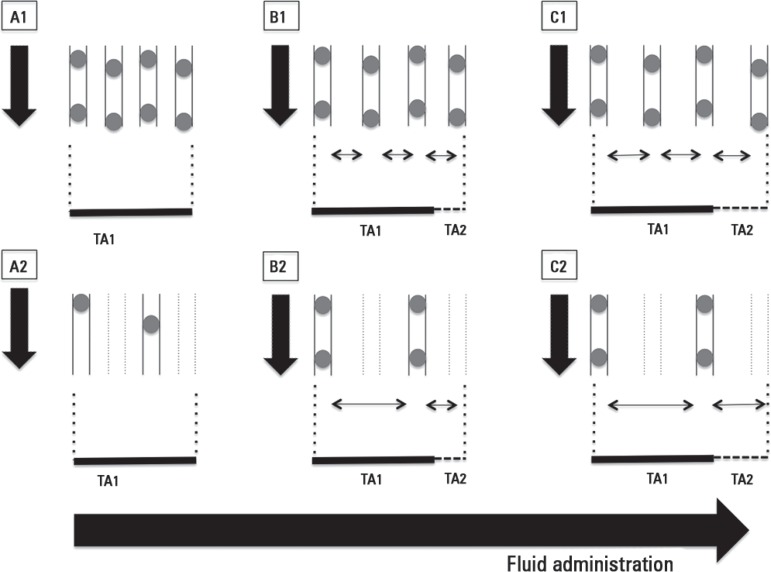
Figure 2.
Effects of fluid overload on microvascular blood flow.
Inadequate fluid overload conducts to progressive decrease in functional capillary density in cases of homogeneous (A1, B1, C1) or heterogeneous (A2, B2, C2) microcirculatory blood flow. Progressive increase in distance between capillaries impairs oxygen diffusive capacities to tissues despite apparently normal convective flow (A1, B1, C1) or in cases of apparent corrected convective component with persistence of increased heterogeneity (B2, C2). TA1 depicts the original tissue area. TA2 depicts the increased tissue area due to edema. Black arrows pointing down represent the magnitude of blood flow.
Orthogonal polarization spectral and sidestream dark field imaging techniques have helped us gain a better understanding of microcirculatory derangements during severe sepsis and septic shock at the bedside. In fact, deeper microvascular alterations, such as a reduced percentage of small perfused vessels (PPV), decreased FCD and increased heterogeneity of flow, have been shown to be related to more severe organ dysfunctions and unfavorable outcomes.(13,15,16) Interestingly, reductions in FCD in sepsis are completely explained by decreased PPV, while RBC velocities are similar in both survivors and non-survivors.(16) These findings suggest that variables that describe the diffusional component of oxygen transport, i.e., FCD and heterogeneity of microvascular blood flow, are more closely related to clinical outcomes than pure convective components, such as RBC velocity.(16) Remarkably, these derangements are amenable to correction over time and are dissociated from global hemodynamics. Consequently, micro-hemodynamics cannot be predicted by the typical systemic hemodynamic parameters, although more severe microcirculatory alterations coexist with high lactate levels and the requirement of higher doses of vasopressors.(17)
In a recent study, Ospina-Tascón et al.(18) explored the effect of fluids on microcirculatory blood flow using the sidestream dark field imaging technique in severe sepsis and septic shock. They found that early, but not late fluid challenge can increase the PPV, with subsequent improvement in FCD and decreased heterogeneity of blood flow. Analogous to macro-hemodynamic fluid responsiveness predictors, the prior status of the microcirculation is strongly related to the response to volume expansion.(18,19) Indeed, a recent study(20) demonstrated that the effects of fluid load on the microcirculation are dependent on both basal microvascular perfusion and the magnitude of the increase in cardiac output.
Theoretically, direct evaluation of microcirculation at the bedside would be a more physiological-based approach to fluid administration that may predict fluid responsiveness at the microvascular level and avoid unnecessary and unfavorable fluid overload. However, current data regarding the use of devices to evaluate microcirculation at the bedside only support pathophysiological concepts and require confirmation through clinical trials.
Footnotes
Conflicts of interest: None.
Responsible editor: Jorge Ibrain Figueira Salluh
REFERENCES
- 1.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Reade MC, Warrillow SJ. The pursuit of a high central venous oxygen saturation in sepsis: growing concerns. Crit Care. 2008;12(2):130. doi: 10.1186/cc6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ProCESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peake SL, Bailey M, Bellomo R, Cameron PA, Cross A, Delaney A, Finfer S, Higgins A, Jones DA, Myburgh JA, Syres GA, Webb SA, Williams P, ARISE Investigators, for the Australian. New Zealand Intensive Care Society Clinical Trials Group Australasian resuscitation of sepsis evaluation (ARISE): A multi-centre, prospective, inception cohort study. Resuscitation. 2009;80(7):811–818. doi: 10.1016/j.resuscitation.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM, ProMISe Trial Investigators Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 7.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33(3):229–241. doi: 10.1097/SHK.0b013e3181c30f0c. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006;34(5):1333–1337. doi: 10.1097/01.CCM.0000214677.76535.A5. [DOI] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 11.Pinsky MR. Functional hemodynamic monitoring. Crit Care. 2005;9(6):566–572. doi: 10.1186/cc3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teboul JL, Monnet X. Detecting volume responsiveness and unresponsiveness in intensive care unit patients: two different problems, only one solution. Crit Care. 2009;13(4):175. doi: 10.1186/cc7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Backer D, Ospina-Tascon G, Salgado D, Favory R, Creteur J, Vincent JL. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med. 2010;36(11):1813–1825. doi: 10.1007/s00134-010-2005-3. [DOI] [PubMed] [Google Scholar]
- 14.Doerschug KC, Delsing AS, Schmidt GA, Haynes WG. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol. 2007;293(2):H1065–H1071. doi: 10.1152/ajpheart.01237.2006. [DOI] [PubMed] [Google Scholar]
- 15.Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM, Microcirculatory Alterations in Resuscitation and Shock Investigators Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49(1):88-98, 98.e1-2. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. 2012;40(5):1443–1448. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care. 2013;28(4):538.e9-14. doi: 10.1016/j.jcrc.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 19.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39(4):612–619. doi: 10.1007/s00134-012-2793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edul VS, Ince C, Navarro N, Previgliano L, Risso-Vazquez A, Rubatto PN, et al. Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann Intensive Care. 2014;4:39. doi: 10.1186/s13613-014-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]