Abstract
Objective
To analyze the correlations of the blood flow/pump rotation ratio and the transmembrane pressure, CO2 and O2 transfer during the extracorporeal respiratory support.
Methods
Five animals were instrumented and submitted to extracorporeal membrane oxygenation in a five-step protocol, including abdominal sepsis and lung injury.
Results
This study showed that blood flow/pump rotations ratio variations are dependent on extracorporeal membrane oxygenation blood flow in a positive logarithmic fashion. Blood flow/pump rotation ratio variations are negatively associated with transmembrane pressure (R2 = 0.5 for blood flow = 1500mL/minute and R2 = 0.4 for blood flow = 3500mL/minute, both with p < 0.001) and positively associated with CO2 transfer variations (R2 = 0.2 for sweep gas flow ≤ 6L/minute, p < 0.001, and R2 = 0.1 for sweep gas flow > 6L/minute, p = 0.006), and the blood flow/pump rotation ratio is not associated with O2 transfer variations (R2 = 0.01 for blood flow = 1500mL/minute, p = 0.19, and R2 = - 0.01 for blood flow = 3500 mL/minute, p = 0.46).
Conclusion
Blood flow/pump rotation ratio variation is negatively associated with transmembrane pressure and positively associated with CO2 transfer in this animal model. According to the clinical situation, a decrease in the blood flow/pump rotation ratio can indicate artificial lung dysfunction without the occurrence of hypoxemia.
Keywords: Acute respiratory distress syndrome; Respiration, artificial; Extracorporeal membrane oxygenation; Multiple organ failure; Swine
Abstract
Objetivo
Analisar as correlações da taxa de fluxo sanguíneo e rotação da bomba com a pressão transmembrana e a transferência de CO2 e O2 durante o suporte respiratório extracorpóreo.
Métodos
Cinco animais foram instrumentalizados e submetidos à oxigenação extracorpórea de membrana em um protocolo de cinco fases, as quais incluíam sepse abdominal e lesão pulmonar.
Resultados
Este estudo demonstrou que as variações da taxa de fluxo sanguíneo e rotação da bomba dependem, de forma logarítmica positiva, do fluxo sanguíneo na membrana extracorpórea de oxigenação. As variações da taxa de fluxo sanguíneo e rotação da bomba têm associação negativa com a pressão transmembrana (R2 = 0,5 para o fluxo sanguíneo = 1.500mL/minuto e R2 = 0,4 para o fluxo sanguíneo = 3.500mL/minuto, ambos com p < 0,001) e associação positiva com as variações de transferência de CO2 (R2 = 0,2 para o fluxo do gás de varredura ≤ 6L/minuto, p < 0,001, e R2 = 0,1 para o fluxo de gás de varredura > 6L/minuto, p = 0,006). A taxa de fluxo sanguíneo com a rotação da bomba não se associa às variações na transferência de O2 (R2 = 0,01 para o fluxo sanguíneo = 1.500mL/minuto, p = 0,19, e R2 = -0,01 ao fluxo sanguíneo = 3.500mL/minuto, p = 0,46).
Conclusão
Neste modelo em animais, a variação da taxa de fluxo sanguíneo e rotação da bomba se associa negativamente com a pressão transmembrana e positivamente com a transferência de CO2. Conforme a situação clínica, uma diminuição na taxa do fluxo sanguíneo e rotação da bomba pode, na ausência de hipoxemia, indicar uma disfunção do pulmão artificial.
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) support has been successfully used in selected patients with severe respiratory failure refractory to the conventional ventilation strategies.(1-4) The improving survival rates of ECMO-supported patients is ascribed to increased experience, improved technology and clinical monitoring.(5) In addition to patients’ physiological status, artificial lung performance monitoring during venous-venous ECMO support can indicate early interventions according to the clinical situation, precluding complications associated with hypoxemia, hypercapnia, hemolysis, and thrombocytopenia.(6,7)
Artificial lung performance is monitored by circuit blood gas analysis (oxygenator gas transfers) and/or pre- and post-membrane pressures in 47% and 82%, respectively, of ECMO centers registered in the Extracorporeal Life Support Organization (ELSO).(8) Despite the strong recommendation of timely circuit blood gas and pressure analysis,(6,7) some centers do not use these techniques.(8,9) The main reasons for not monitoring gas transfers and pressures are the increases of circuit complexity and costs.(10) However, the new generation of centrifugal pumps has pressure sensors integrated into the system, keeping the circuit simple and monitoring transmembrane pressure (TMP) in real time.(11)
For a given rotation, the centrifugal pump resulting blood flow depends on blood characteristics,(12) both pre andafter load.(13,14) Oxygenator failure mainly occurs due to clotting, resulting in reduced gas transfers and in a high resistance to blood passage, causing a high TMP. Moreover, an increased TMP increases the pump afterload and reduces the blood flow with constant blood characteristics and centrifugal pump rotations. We hypothesize that the blood flow/pump rotation ratio (BFRR) correlates with TMP and gas transfers and is therefore a potential practical approach to artificial lung performance monitoring.
The aim of this study was to explore whether BFRR variations are associated with the variation of artificial lung performance surrogates, such as TMP, CO2 and O2 gas transfers, in an animal model.
METHODS
This study was approved by the Institutional Animal Research Ethics Committee from the Hospital Sírio-Libanês in São Paulo - Brazil (CEUA-P-20143) and was performed according to the National Institutes of Health guidelines for the use of experimental animals.
Animal preparation and data collection
Five domestic female Agroceres pigs weighting 80 [79-81] kg were studied. Anesthesia was performed with thionembutal (10mg/kg, Tiopental, Abbott, Brazil) and pancuronium bromide (0.1mg/kg, Pavulon, AKZO Nobel, Brazil), and the pigs were connected to a mechanical ventilator (Evita XL Dräger, Dräger, Luebeck, Germany) with the following parameters: tidal volume of 8mL/kg, end-expiratory pressure of 5cmH2O, FiO2 initially set at 100% and subsequently adjusted to maintain arterial saturation between 94 - 96%, and respiratory rate titrated to maintain PaCO2 between 35 and 45mmHg or an end-tidal CO2 (NICO, Dixtal Biomedica Ind. Com., Sao Paulo, Brazil) between 30 and 40mmHg. The electrocardiogram, heart rate, oxygen saturation, and pressures of the animals were monitored with a multiparametric monitor (Infinity Delta XL, Dräger, Luebeck, Germany). Anesthesia was maintained during the study with midazolam (1 - 5mg/kg/h) and fentanyl (5 - 10mcg/kg/h) and muscular relaxation with pancuronium bromide (0.2mg/kg/h). Adequate depth of anesthesia during the surgical period was evaluated by maintenance of physiological variables (heart rate and arterial pressure) and the absence of reflexes (corneal and hind limb flexion response), as well as unresponsiveness to stimuli during manipulation. Supplementary boluses of 3 - 5mcg/kg fentanyl and 0.1 - 0.5mg/kg midazolam were administered as necessary.
The instrumentation, surgical preparation, pulmonary injury, induction of sepsis, and different clinical scenarios of data collection were performed as previously described.(12,15,16) Data were retrieved from a five-step protocol. Some of the data have already been published elsewhere: system pressures,(12,17) gases transfer analysis,(16) equilibrium analysis, PEEP titration, and multiple organ dysfunction phase. The ECMO system (Permanent life support system - PLS, Jostra - Quadrox D, Maquet Cardiopulmonary, Hirrlingen, Germany) was primed with a 37 degrees Celsius normal saline solution and was connected to a centrifugal pump (Rotaflow, Jostra, Maquet Cardiopulmonary, Hirrlingen, Germany). The venous loop port, pre-membrane and post-membrane ports were monitored using a pressure measurement system (DX 2020, Dixtal Biomedica Ind. Com., Sao Paulo, Brazil), through a stopcock, in which blood samples could be collected for gas analysis.
Mathematical calculations
The formulas used for the mathematical calculations were the following: transmembrane pressure (mmHg) = pre-membrane pressure (mmHg) - post-membrane pressure (mmHg); barometric pressure = 690mmHg; blood oxygen content (mL/100mL of blood) = 0.0031 x PO2 + 1.36 x Hb x O2 saturation; CO2 transfer (mL/minute) = sweep gas flow (mL/minute) x (sweep gas out port CO2 partial pressure (EtCO2 from the gas oxygenator outlet)(mmHg)/Barometric pressure (mmHg)); O2 transfer (mL/minute) = (post-membrane blood oxygen content (mL/100mL of blood) - pre-membrane blood oxygen content (mL/100mL of blood)) x ECMO blood flow (mL/minute).
Data retrieval
The data were retrieved from the original database because the paired data analyzed were available. The samples are representative of all steps studied, with and without induced sepsis. All data were collected after the surgical instrumentation and one hour of resting to reach physiological equilibrium; therefore, there was still some residual post-operative inflammation. These clinical situations represent the same animals exposed to different phases of critical illness.
Statistical analysis
The variables tested for correlations have many other determinants, which are potential causes of errors in the final interpretation. BFRR and blood flow have an established correlation, which led to the categorized correlation analysis between BFRR and TMP according to more frequent blood flows (1500 and 3500mL/minute). During the experiment, these two blood flows were maintained to achieve a stable gas transfer; therefore, the pump rotations were adjusted to reach this steady blood flow. The same categorization was performed for the correlation between BFRR and O2 transfer because both variables are highly dependent on the blood flow.(16) For the correlation analysis between BFRR and CO2 transfer, the categorization was performed by the sweep gas flow (≤ 6L/minute and > 6L/minute, six was the median sweep gas flow used throughout the experiment), a known variable strongly associated with CO2 transfer.(16)
To analyze multiple correlations, a multiple linear regression model was used. A spider plot was used to show the amount of association between the TMP determinants and TMP. Scatter plots were used to graphically show the correlations, which were measured through the Nagelkerke adjusted determination coefficient (R2). The R statistical package and comprehensive-R archive network (CRAN)-specific libraries were used to create the graphics and to perform the statistical analyses.(18)
RESULTS
The paired variables from a total of 381 different timepoints were retrieved from the database: seventy nine from the pressures and gases transfer analysis, two hundred and thirteen from the equilibrium analysis, twenty four from PEEP titration, and sixty five from the multiple organ dysfunction phase. The results are presented according to the variable analyzed.
Analysis of BFRR and transmembrane pressure
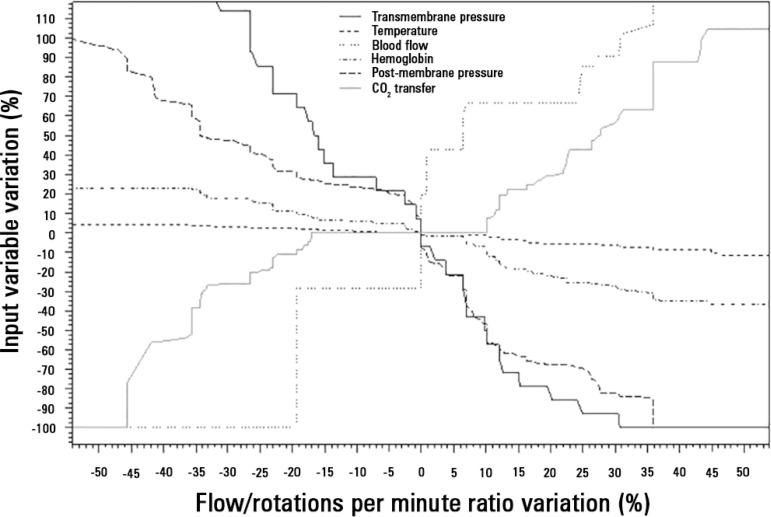
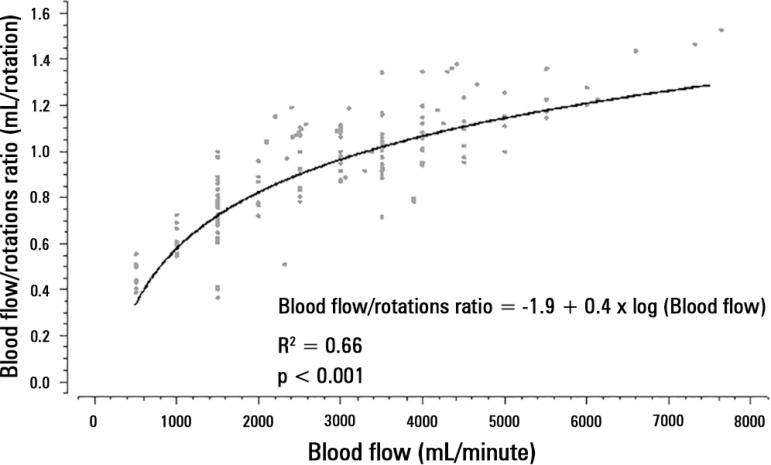
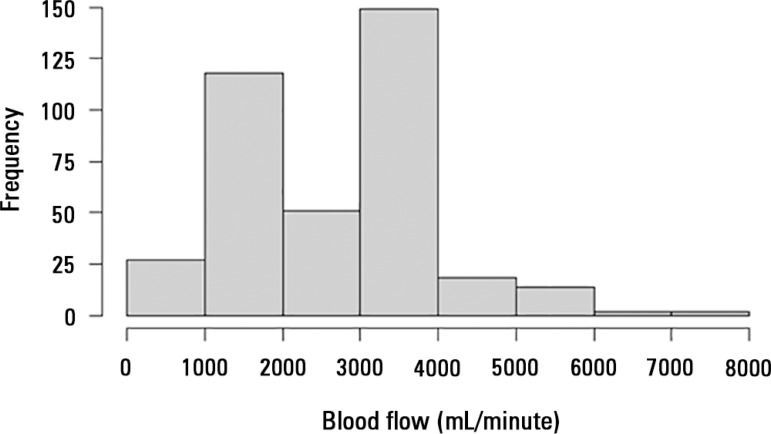
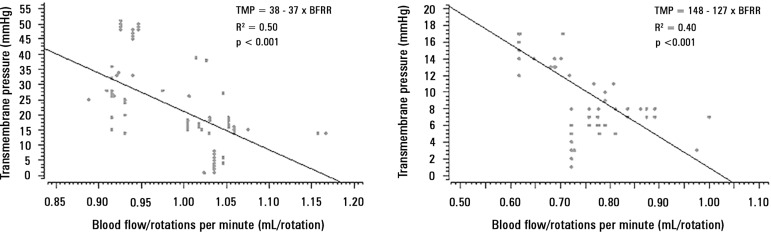
The mechanical determinants of the BFRR were as follows (with an R2 = 0.82): TPM (beta coefficient = -0.003; p < 0.001), blood temperature (beta coefficient = 0.02; p < 0.001), ECMO blood flow (beta coefficient = -0.0002; p < 0.001), hemoglobin (beta coefficient = - 0.006; p = 0.05), and post-membrane pressure (beta coefficient = -0.002; p < 0.001). The effect of BFRR variation according to the variation of each of the cited variables is shown in a spider plot (Figure 1). The BFRR varies according to the ECMO blood flow in a non-linear fashion, as shown in figure 2. Among the paired samples of BFRR and TPM, there are several during the blood flow of 1500 and 3500L/minute (Figure 3); therefore, the correlations between BFRR and TPM were measured using the two blood flows and are shown in the figure 4.
Figure 1.
Spider plot showing the relation between the variation of blood flow/rotations per minute ratio and the determinants of its variation.
Figure 2.
Non -linear relation between extracorporeal membrane oxygenation blood flow and the blood flow/pump rotation ratio.
Figure 3.
Distribution of the paired variables according to the blood flow distribution. The data are from blood flows of 1500 and 3500mL/minute.
Figure 4.
Correlation between the blood flow/rotations ratio and transmembrane pressure. Panel A) correlation of 107 pairs of data with a blood flow of 1500mL/minute, and Panel B) correlation of 127 pairs of data with a blood flow of 3500mL/minute.
TMP - transmembrane pressure.
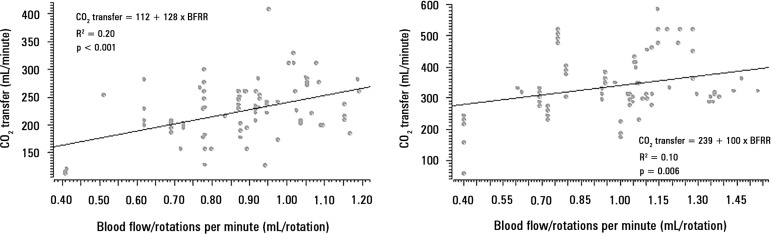
Analysis of BFRR and CO2 transfer
The correlations between CO2 transfer and BFRR are presented in figure 5 (Panels A and B), stratified according to the sweep gas flow ≤ 6L/minute or > 6L/minute, respectively.
Figure 5.
Correlation between the blood flow/rotations ratio and CO2 transfer. Panel A) correlation of 98 pairs of data with a sweep gas flow ≤ 6L/minute, and Panel B) correlation of 94 pairs of data with a sweep gas flow > 6L/minute.
BFRR - blood flow/rotations ratio.
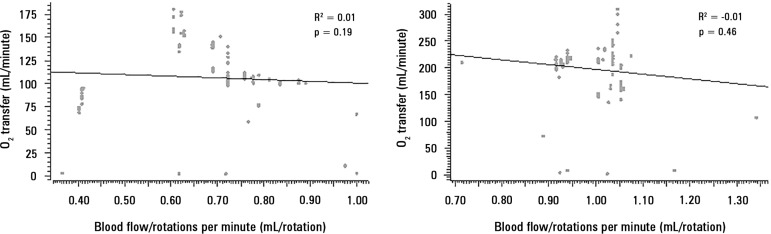
Analysis between BFRR and O2 transfer
The correlations between O2 transfer and BFRR are presented in figure 6 (Panels A and B), stratified according to blood flows of 1500 and 3500mL/minute, respectively.
Figure 6.
Correlation between the blood flow/rotations ratio and O2 transfer. Panel A) correlation of 98 pairs of data with a blood flow = 1500mL/minute, and Panel B) correlation of 114 pairs of data with a blood flow = 3500mL/minute.
DISCUSSION
This study showed that BFRR variations are dependent on ECMO blood flow in a positive logarithmic fashion. BFRR variations are negatively associated with the TMP (R2 = 0.5 for blood flow = 1500mL/minute and R2 = 0.4 for blood flow = 3500 mL/minute, both with p < 0.001), positively associated with CO2 transfer variations (R2 = 0.2 for sweep gas flow ≤ 6L/minute, p < 0.001, and R2 = 0.1 for sweep gas flow > 6L/minute, p = 0.006), and not associated with O2 transfer variations (R2 = 0.01 for blood flow = 1500mL/minute, p = 0.19, and R2 = - 0.01 for blood flow = 3500mL/minute, p = 0.46).
The centrifugal pumps suck the fluid from the venous line using the Venturi effect, driving the fluid forward through the resulting positive pressure inside the pump head.(19) The impeller inside the pump head is not occlusive; therefore, when the outlet circuit is obstructed, the blood can settle inside the pump head, without risk of circuit rupture. However, hemolysis can occur, and the blood flow is reduced, increasing the BFRR.
The pre- and post-membrane pressure monitoring, specifically their difference, also known as transmembrane pressure drop or TMP, is commonly used as an indicator of artificial lung performance.(6-8) High TMP pressures (> 50 - 60mmHg in polymethylpentene oxygenators) indicate a high resistance to blood passage through the respiratory membrane.(7,12) Furthermore, this high resistance most commonly is secondary to oxygenator clotting. Other variables, such as the blood flow rate and temperature, are also determinants of the TMP.(12) In this study, we found that BFRR variation is negatively associated with TMP variation; therefore, a decrease in the BFRR for a given pump rotation and no or slight variation in the blood temperature can be associated with and increased TMP, indicating oxygenator clotting. The oxygenator failure alone does not result in oxygenator substitution, but must be evaluated in addition to other clinical variables, such as hemolysis, thrombocytopenia, hypoxemia, and hypercapnia. Another intuitive finding was the correlation between hemoglobin and BFRR, highlighting the importance of hemoglobin/hematocrit verification when temporally analyzing and interpreting the BFRR in the same patient.
The post-membrane pressure is another determinant of the BFRR. An elevation of post-membrane pressure can lead to a fall in BFRR with a preserved TMP. Therefore, with a BFRR reduction, it is important to verify factors associated with elevations of post-membrane pressure, such as arterial line kinking. Furthermore, at this time, the TMP measurement could be performed if clinically indicated.
A progressive difficulty in CO2 removal with a high post-membrane PCO2 indicates a reduction in CO2 transfer and can be a marker of oxygenator failure.(7) The ECMO blood flow and sweep gas flow are the most important determinants of CO2 transfer.(16) Therefore, for a given blood and sweep gas flow, the high CO2 diffusibility promotes a total CO2 equilibrium between the gas and blood inside the oxygenator.(20) Therefore, a reduction in the lung membrane exchange surface due to clotting or water deposition can reduce CO2 transfer. When clotting is the mechanism of CO2 transfer reduction, an increased resistance to blood passage through the artificial lung is expected, resulting in a high centrifugal pump afterload, which will result in a lower BFRR. Our findings are compatible with this idea; furthermore, a high BFRR is associated with a high CO2 transfer, with a weak R2 due to the variation of other determinants of CO2 transfer and BFRR.
The O2 transfer impairment and the resulting hypoxemia are also related to oxygenator failure.(6,7) Out of oxygenator and native lung residual function, O2 transfer is mainly modulated by the ECMO blood flow.(16) Moreover, our analysis was categorized into two constant blood flows (1500 and 3500mL/minute). We stress that pump rotation was adjusted to maintain blood flow throughout the experiment. Our findings did not show a correlation between BFRR and O2 transfer. One possible explanation is the absence of BFRR sensitivity to O2 transfer impairment; however, a BFRR decreases caused an elevation of TMP and a reduced CO2 transfer. The former finding was interesting because CO2 is 16 - 20 times more diffusible than O2,(21,22) so it is intuitively expected that a decrease in CO2 transfer would be an earlier marker of oxygenator failure than a decrease in O2 transfer.(7) Our findings indicated that BFRR is an earlier marker of oxygenator dysfunction than progressive hypoxemia due to O2 transfer impairment. The clinical situation is imperative in the interpretation of the data presented in this manuscript.
The 381 analyzed timepoints were retrieved from a four-step experiment using the same animals. In brief, seventy nine timepoints were from the pressure and gas transfer analysis, in which gas variations pre- and post-membrane were analyzed with different combinations of gas and blood flows;(16) two hundred and thirteen were from the equilibrium analysis step, in which the time to systemic blood CO2 partial pressure equilibrium was measured with different gas and blood flow combinations (data not published); twenty four were from PEEP titration, in which after lung injury induction, an open lung approach ventilation was compared to a lower tidal volume ventilation in ECMO support (data not published); and sixty five were from the multiple organ dysfunction phase.(12) These steps represent many of the clinical conditions experienced by critically ill patients.
For a given ECMO-supported patient in a steady clinical situation, using a pump rotation = 3500 RPM, resulting in an ECMO blood flow = 4000mL/minute, the BFRR is 1.14. If during the evolution, the clinical situation is still stable, but at 3500 RPM, the blood flow decreases to 3800mL/minute (a fall of 5%) and the BFRR will decrease to 1.08. In this situation, a TMP elevation of approximately 20% is expected. Clinical stability precludes any other intervention modulating arterial blood gases; however, anticoagulation deserves more attention. If this same patient presents severe hemolysis and/or severe thrombocytopenia and/or hypercapnia that are difficult to control, but not hypoxemia, the oxygenator function must be questioned and, if necessary, investigated with in-line blood sample collection and pressure measurement.
CONCLUSION
Blood flow/pump rotations ratio variation is negatively associated with transmembrane pressure and positively associated with CO2 transfer in this experimental model. According to the clinical situation, a decrease in the blood flow/pump rotation ratio can indicate artificial lung dysfunction without the occurrence of hypoxemia.
Footnotes
Conflicts of interest: None.
Responsible editor: Rui Moreno
REFERENCES
- 1.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 2.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 3.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306(15):1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 4.Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, Clavel M, Constan A, Marie Richard JC, Brun-Buisson C, Brochard L, REVA Research Network Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187(3):276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 5.Zampieri FG, Mendes PV, Ranzani OT, Taniguchi LU, Pontes Azevedo LC, Vieira Costa EL, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care. 2013;28(6):998–1005. doi: 10.1016/j.jcrc.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 6.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth. 2010;24(1):164–172. doi: 10.1053/j.jvca.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sidebotham D. Troubleshooting adult ECMO. J Extra Corpor Technol. 2011;43(1):P27–P32. [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton RG, Salatich A, Jegier B, Chabot D. A 2007 survey of extracorporeal life support members: personnel and equipment. J Extra Corpor Technol. 2009;41(3):172–179. [PMC free article] [PubMed] [Google Scholar]
- 9.Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo) 2012;67(10):1157–1163. doi: 10.6061/clinics/2012(10)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park M, Mendes PV, Zampieri FG, Azevedo LC, Costa EL, Antoniali F, Ribeiro GC, Caneo LF, da Cruz LM, Neto, Carvalho CR, Trindade EM, ERICC research group. ECMO group Hospital Sírio Libanês and Hospital das Clínicas de São Paulo The economic effect of extracorporeal membrane oxygenation to support adults with severe respiratory failure in Brazil: a hypothetical analysis. Rev Bras Ter Intensiva. 2014;26(3):253–262. doi: 10.5935/0103-507X.20140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maquet Getinge Group . Cardiohelp system. The world’s smallest portable heart-lung support system. 2015. Available in: http://www.maquet.com/int/product/CARDIOHELP-System. [Google Scholar]
- 12.Park M, Costa EL, Maciel AT, Barbosa EV, Hirota AS, Schettino GP, et al. Effect of flow rate and temperature on transmembrane blood pressure drop in an extracorporeal artificial lung. Perfusion. 2014;29(6):517–525. doi: 10.1177/0267659114525986. [DOI] [PubMed] [Google Scholar]
- 13.Naganuma S, Yambe T, Sonobe T, Kobayashi S, Nitta S. Development of a novel centrifugal pump: magnetic rotary pump. Artif Organs. 1997;21(7):746–750. doi: 10.1111/j.1525-1594.1997.tb03734.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishida H, Akazawa T, Nishinaka T, Aomi S, Endo M, Koyanagi H. Afterload-dependent flow fluctuation of centrifugal pump: should it be actively fixed? Artif Organs. 1998;22(5):362–365. doi: 10.1046/j.1525-1594.1998.06140_22_5.x. [DOI] [PubMed] [Google Scholar]
- 15.Park M, Costa EL, Maciel AT, Hirota AS, Vasconcelos E, Azevedo LC. Acute hemodynamic, respiratory and metabolic alterations after blood contact with a volume priming and extracorporeal life support circuit: an experimental study. Rev Bras Ter Intensiva. 2012;24(2):137–142. [PubMed] [Google Scholar]
- 16.Park M, Costa EL, Maciel AT, Silva DP, Friedrich N, Barbosa EV, et al. Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS One. 2013;8(1): doi: 10.1371/journal.pone.0054954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassi E, Azevedo LC, Costa EL, Maciel AT, Vasconcelos E, Ferreira CB, et al. Hemodynamic and respiratory support using venoarterial extracorporeal membrane oxygenation (ECMO) in a polytrauma patient. Rev Bras Ter Intensiva. 2011;23(3):374–379. [PubMed] [Google Scholar]
- 18.R Development Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 19.Moazami N, Fukamachi K, Kobayashi M, Smedira NG, Hoercher KJ, Massiello A, et al. Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J Heart Lung Transplant. 2013;32(1):1–11. doi: 10.1016/j.healun.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Kolobow T, Gattinoni L, Tomlinson T, White D, Pierce J, Iapichino G. The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs. 1977;23:17–21. doi: 10.1097/00002480-197700230-00005. [DOI] [PubMed] [Google Scholar]
- 21.Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care. 2010;14(2):220. doi: 10.1186/cc8926. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson CT, Breen PH. Carbon dioxide kinetics and capnography during critical care. Crit Care. 2000;4(4):207–215. doi: 10.1186/cc696. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]