Abstract
The effects of sorafenib for Chinese patients with metastatic renal cell cancer (mRCC) were evaluated to figure out the relationship between clinical variables and prognosis. The data were analyzed retrospectively from six comprehensive cancer centers in Northeast China. All cases were diagnosed as mRCC histopathologically without exception. Patients were taken 400 mg sorafenib orally twice daily until progression of disease or intolerable toxic reaction occurred. Overall survival (OS), progression-free survival (PFS), and the influence of clinical variables on survival were appointed as main outcome measures. Clinical data were analyzed using SPSS statistical software. P<0.05 was considered as statistically significant. A total of 131 patients were available for survival analysis. The median follow-up periods were 16.9 months, and the median OS and PFS were 16.1 months and 10.5 months, respectively. Univariate analysis showed that Eastern Cooperative Oncology Group performance status (ECOG PS), metastatic sites, and previous therapy were significantly associated with OS, whereas PFS was merely associated with ECOG PS and previous therapy. The multivariate analysis suggested that ECOG PS, metastatic sites, and previous therapy were the independent prognostic factors for OS, and ECOG PS and previous therapy as the independent prognostic factors for PFS. In the subgroup analysis for patients with visceral metastasis, the prognosis of patients with lung metastasis alone was better than those cases with liver metastasis alone or multiple organs metastasis. In our study, sorafenib shows a higher curative activity for patients with mRCC in Northeast China. ECOG PS, metastatic lesions, and previous therapy may be important parameters for OS and PFS prediction. Lung metastases alone may be a more sensitive indicator for sorafenib than other organ metastases.
Keywords: renal cell cancer, sorafenib, targeted therapy, prognosis
Introduction
Renal cell cancer/carcinoma (RCC) is considered to be one of the most treatment-resistant cancers and is the tenth cancer-related cause of death in USA, with an estimated 39,140 new cases and 8,900 deaths in 2014.1 In People’s Republic of China, the incidence of RCC is relatively low and accounts for 2% of adults’ malignant tumors. In nearly 20 years, the morbidity of RCC increases at an average of 6.5% per year. Its cancer-related mortality exceeds the bladder cancer and ranks the first in the genitourinary cancer.2 Due to the social, economic, and environmental factors, about half of the Chinese patients with RCC have been found in advanced stage at diagnosis and the curative effect is not satisfactory with traditional treatment strategies. In recent years, targeted therapies have dramatically improved prognosis of patients with metastatic renal cell cancer (mRCC). Sorafenib (Nexavar, Bayer AG, Leverkusen, Germany), a representative multikinase inhibitor of tumor-cell proliferation and angiogenesis, is a kind of novel multitargeting micromolecule compound aiming at vascular endothelial growth factor (VEGF). It is the first molecular-targeting agent approved by the FDA for patients with mRCC. Some international collaborative Phase III trials demonstrated that sorafenib could obviously improve progression-free survival (PFS) with tolerable toxicities in patients with mRCC.3–5 Until now, it still lacks the long-term follow-up data on overall survival (OS) and PFS from Asian patients. The objective of this study was to observe long-term efficacy of sorafenib and make an effort to explore the impact factors on prognosis in Chinese patients with mRCC.
Patients and methods
Clinical data
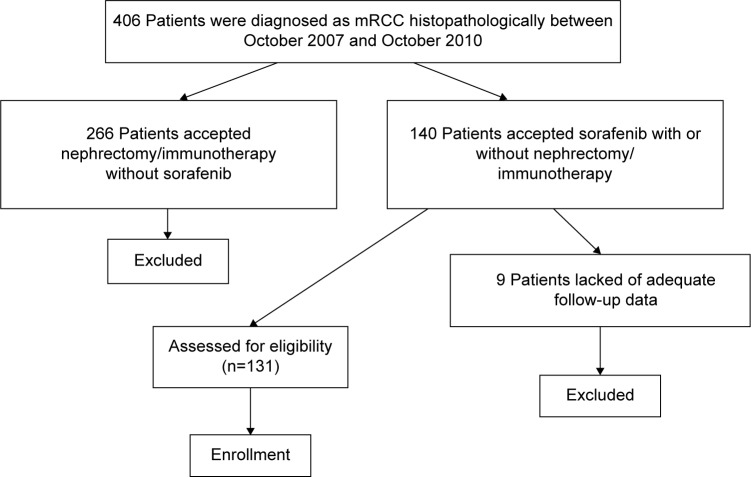
In this retrospective study, 406 consecutive patients were diagnosed as mRCC histopathologically between October 2007 and October 2010 from six comprehensive cancer centers in Northeast China. The primary metastatic sites included viscera (liver and lungs), bones, and lymph nodes. First, we excluded 266 patients who accepted nephrectomy or immunotherapy alone without sorafenib. Second, we excluded nine patients who were lack of adequate follow-up data. The Eastern Cooperative Oncology Group performance status (ECOG PS) of 131 patients enrolled in this study was 0–2 (Figure 1). The cutoff date for follow-up and data statistics was October 2013. All patients received 400 mg of sorafenib orally twice daily on a continuous basis until progression of disease or occurrence of intolerable toxicities. OS (definition as the time from initiation of sorafenib therapy to death) and PFS (definition as the time from initiation of sorafenib therapy to the first documentation of disease progression or to death from any cause, whichever occurred first) were appointed as main outcome measures. In addition, the influence of sex, ECOG PS, primary metastatic sites, and previous therapy on survival in patients were observed. The relationship between clinical variables and prognosis of patients was also evaluated.
Figure 1.
Patient disposition.
Note: This figure showed the screening process of patients.
Abbreviation: mRCC, metastatic renal cell cancer.
Statistical analyses
Clinical data were analyzed using the SPSS statistical software (version 17.0, SPSS Inc., Chicago, IL, USA). OS and PFS curves were drawn using the Kaplan–Meier method. Clinical variables were included on univariate and multivariate analysis to evaluate associations with OS and PFS by the log-rank test and Cox proportional hazard models, P<0.05 was considered as statistically significant.
Results
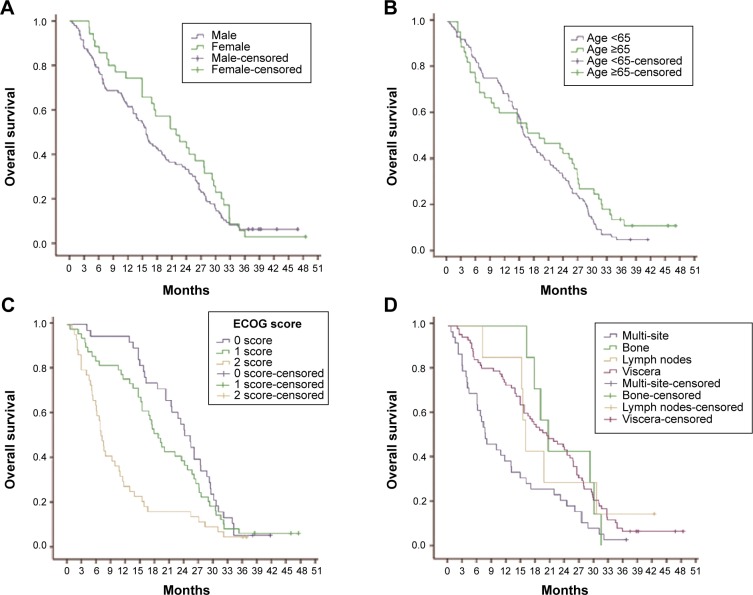
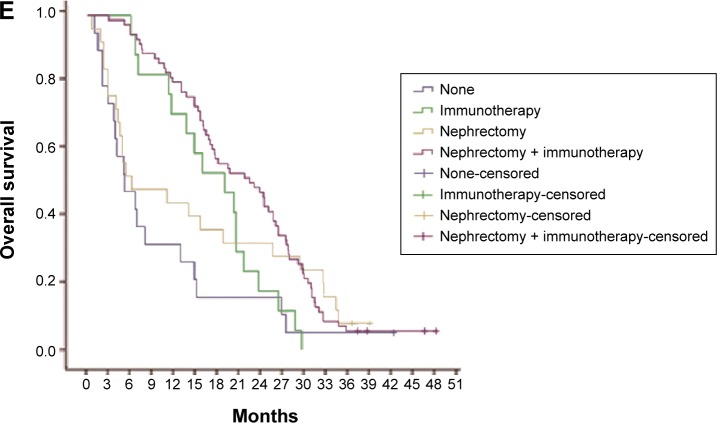
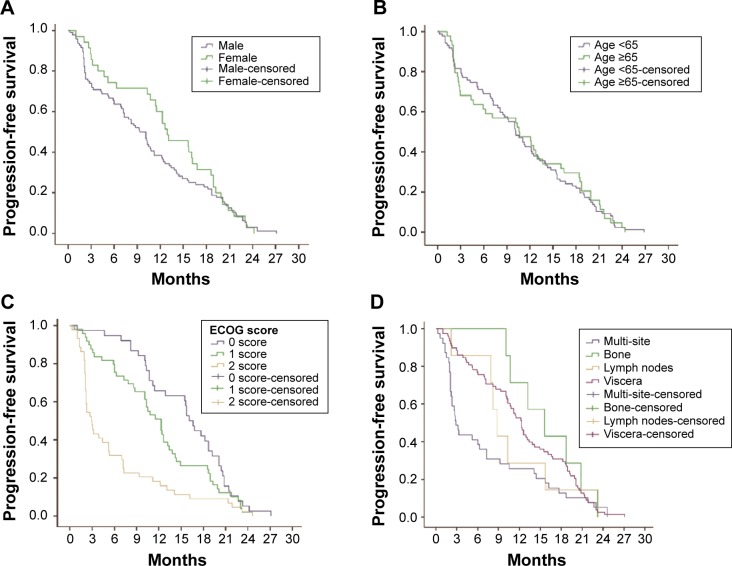
A total of 131 patients were available for survival analysis, including 96 males and 35 females. The median age was 60 years (33–79 years). By October 2013, the median follow-up periods were 16.9 months (0.6–48.5 months). The baseline characteristics of patients are shown in Table 1. According to the results of survival analysis, the median OS was 16.1 months and the median PFS was 10.5 months (Figure 2). OS of patients with ECOG PS 0/1 was 24.6 months and 19.1 months, respectively, which was significantly better than 7.1 months of those cases with PS 2. Compared with metastatic lesions such as viscera, lymph nodes, and bones, patients with multiple organs metastasis had the worst prognosis. According to the conditions of previous therapy, patients who undergo nephrectomy + immunotherapy or immunotherapy alone had a longer lifetime than those who only undergo surgery or untreated. Univariate analysis showed that PFS for patients with ECOG PS 0/1 was better than those cases with PS 2. In addition, patients who undergo nephrectomy + immunotherapy or immunotherapy alone had a longer PFS than those cases who only undergo surgery or untreated (Table 2). ECOG PS, metastatic lesions, and previous therapy were predictive factors for OS in Cox proportional hazard models (Figure 3). ECOG PS and previous therapy were predictive factors for PFS in Cox proportional hazard models (Figure 4). In the subgroup analysis for 78 patients with visceral metastasis, the prognosis of patients with lung metastasis alone was better than those cases with liver metastasis alone or multiple organs metastasis (Figure 5). Subsequently, the multivariate analysis suggested that ECOG PS (P=0.004), metastatic lesions (P=0.003), and previous therapy (P=0.019) were the independent prognostic factors for OS, and ECOG PS (P=0.000) and previous therapy (P=0.003) as the independent prognostic factors for PFS (Tables 3 and 4).
Table 1.
Baseline characteristics
| Characteristic | Patients, n (%) |
|---|---|
| Sex | |
| Male | 96 (73.3) |
| Female | 35 (26.7) |
| Age, years | |
| <65 | 87 (66.4) |
| ≥65 | 44 (33.6) |
| Metastatic sites | |
| Lung | 99 (75.6) |
| Liver | 55 (42.0) |
| Bone | 41 (31.3) |
| Lymph nodes | 17 (13.0) |
| Number of metastatic foci | |
| 1 | 75 (57.3) |
| 2 | 36 (27.5) |
| ≥3 | 20 (15.2) |
| ECOG PS | |
| 0 | 38 (29.0) |
| 1 | 49 (37.4) |
| 2 | 44 (33.6) |
| Prior nephrectomy | |
| Yes | 95 (72.5) |
| No | 36 (27.5) |
| Prior immunotherapy | |
| IL-2 + IFN | 46 (35.1) |
| IL-2 alone | 18 (13.7) |
| IFN alone | 23 (17.6) |
| None | 44 (33.6) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IFN, interferon; IL, interleukin.
Figure 2.
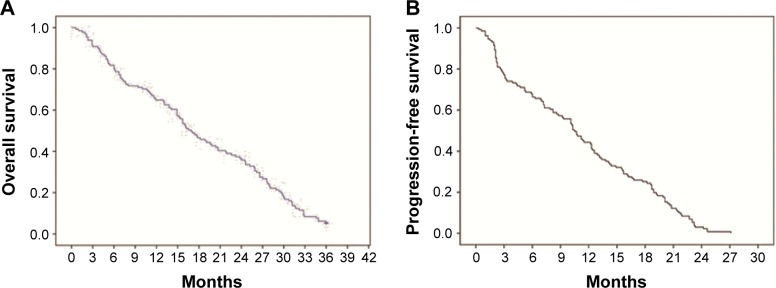
Survival curve for patients with mRCC.
Notes: (A) OS curve for all patients and the median OS was 16.1 months. (B) PFS curve for all patients and the median PFS was 10.5 months.
Abbreviations: mRCC, metastatic renal cell cancer; OS, overall survival; PFS, progression-free survival.
Table 2.
The univariate analysis to evaluate the relationship between clinical variables and OS/PFS
| Clinical variables | N | Median OS (months) | 95% CI | Log rank (P-value) | Median PFS (months) | 95% CI | Log rank (P-value) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 96 | 15.7 | 13.396–18.004 | 0.243 | 9.3 | 7.380–11.220 | 0.328 |
| Female | 35 | 21.9 | 14.598–29.202 | 12.9 | 8.940–16.860 | ||
| Age | |||||||
| <65 | 87 | 16.2 | 14.006–18.394 | 0.128 | 10.3 | 8.371–12.229 | 0.902 |
| ≥65 | 44 | 17.1 | 5.120–29.080 | 10.7 | 5.175–16.225 | ||
| ECOG PS | |||||||
| 0 | 38 | 24.6 | 21.428–27.772 | 0.000* | 16.1 | 14.650–17.550 | 0.000* |
| 1 | 49 | 19.1 | 16.219–21.981 | 12.3 | 9.843–14.757 | ||
| 2 | 44 | 7.1 | 6.017–8.813 | 2.9 | 1.971–3.829 | ||
| Metastatic sites | |||||||
| Multiple organs | 39 | 7.7 | 3.907–11.493 | 0.004* | 2.9 | 2.043–3.757 | 0.083 |
| Viscera# | 78 | 20.4 | 13.800–27.000 | 12.3 | 10.955–13.645 | ||
| Lymph nodes | 7 | 16 | 14.460–17.540 | 8.8 | 7.260–10.340 | ||
| Bones | 7 | 20.7 | 16.594–24.806 | 15.6 | 9.441–21.759 | ||
| Previous therapy | |||||||
| Nephrectomy + immunotherapy | 70 | 22.6 | 16.769–28.431 | 0.004* | 12.4 | 9.530–15.270 | 0.001* |
| Immunotherapy alone | 25 | 19.1 | 11.705–26.495 | 12.3 | 9.208–15.392 | ||
| Nephrectomy alone | 17 | 6.2 | 0.000–16.319 | 5.3 | 1.220–9.380 | ||
| None | 19 | 5.2 | 1.503–8.897 | 3.1 | 2.397–3.803 | ||
Notes:
P<0.05 was considered as statistically significant.
Viscera only consider the lungs and liver, except for other organs.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; OS, overall survival; PFS, progression-free survival.
Figure 3.
Relationship between clinical variables and OS.
Notes: (A) There was no significant difference between male group and female group for OS in patients with mRCC (P>0.05). (B) Both groups with age <65 years and age ≥65 years had no difference in OS (P>0.05). (C) OS for patients with ECOG PS 0/1 was significantly better than those cases with PS 2 (24.6 months vs 7.1 months, P<0.05; 19.1 months vs 7.1 months, P<0.05). (D) Compared with other metastatic lesions such as viscera, lymph nodes, and bones, patients with multiple organs metastasis had the worst prognosis (20.4 months vs 7.7 months, P<0.05; 16.0 months vs 7.7 months, P<0.05; 20.7 months vs 7.7 months, P<0.05). (E) According to the conditions of previous therapy, patients who undergo nephrectomy + immunotherapy or immunotherapy alone had a longer lifetime than those cases who only undergo surgery or untreated (22.6 months vs 6.2 months, P<0.05; 22.6 months vs 5.2 months, P<0.05; 19.1 months vs 6.2 months, P<0.05; 19.1 months vs 5.2 months, P<0.05).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; mRCC, metastatic renal cell cancer; OS, overall survival.
Figure 4.
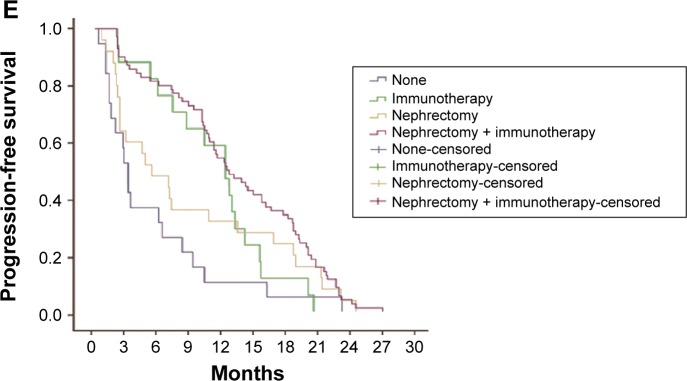
Relationship between clinical variables and PFS.
Notes: (A) There was no significant difference between male group and female group for PFS in patients with mRCC (P>0.05). (B) Both groups with age <65 years and age ≥65 years had no difference in PFS (P>0.05). (C) PFS for patients with ECOG PS 0/1 was better than those cases with PS 2 (16.1 months vs 2.9 months, P<0.05; 12.3 months vs 2.9 months, P<0.05). (D) Metastatic sites had no obvious correlation with PFS in patients with mRCC (P>0.05). (E) Patients who undergo nephrectomy + immunotherapy or immunotherapy alone had a longer lifetime than those cases who only undergo surgery or untreated (12.4 months vs 5.3 months, P<0.05; 12.4 months vs 3.1 months, P<0.05; 12.3 months vs 5.3 months, P<0.05; 12.3 months vs 3.1 months, P<0.05).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; mRCC, metastatic renal cell cancer; PFS, progression-free survival.
Figure 5.
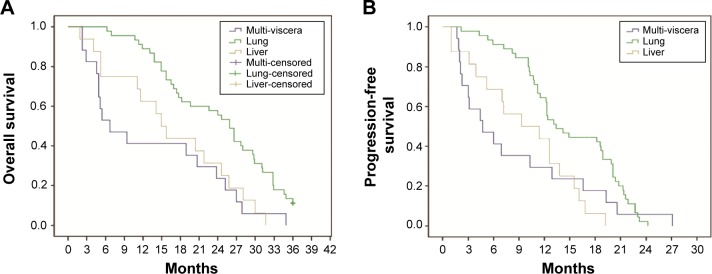
Relationship between metastatic sites and OS or PFS in the subgroup of patients with visceral metastasis.
Notes: (A) Patients with lung metastasis alone had better OS than those cases with liver metastasis alone or multiple organs metastasis (25.9 months vs 14.9 months, P<0.05; 25.9 months vs 5.4 months, P<0.05). (B) Patients with lung metastasis alone had better PFS than those cases with liver metastasis alone or multiple organs metastasis (13.1 months vs 9.3 months, P<0.05; 13.1 months vs 4.4 months, P<0.05).
Abbreviations: OS, overall survival; PFS, progression-free survival.
Table 3.
Multivariate analysis for OS
| Clinical variables | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| ECOG PS | 1.423 | 1.120–1.808 | 0.004 |
| Metastatic sites | 0.816 | 0.713–0.933 | 0.003 |
| Previous therapy | 0.820 | 0.694–0.968 | 0.019 |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; OS, overall survival.
Table 4.
Multivariate analysis for PFS
| Clinical variables | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| ECOG PS | 1.561 | 1.233–1.977 | 0.000 |
| Previous therapy | 0.775 | 0.654–0.918 | 0.003 |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival.
Discussion
RCC is one of the most common malignant tumors in Caucasian and one-third of cases with localized RCC eventually develop metastatic disease despite surgical resection of the primary tumor.6,7 In People’s Republic of China, 20%–30% of patients with RCC have metastases at diagnosis and 20%–40% of postnephrectomy patients will subsequently develop metastases.8,9 Metastasis is the leading cause of death in Chinese patients with RCC, and previously we lack effective treatment strategy for mRCC.
During the past few years, strategies targeted at VEGF, platelet-derived growth factor, and the mammalian target of rapamycin pathway dominate in RCC treatment. As a representative drug, sorafenib brings great benefit to Asian patients.10 It is a novel signal transduction inhibitor that can inhibit RAF-1 and B-RAF serine/threonine kinase activities as well as VEGF-2, VEGF-3, platelet-derived growth factor-β, KIT, and FLT-3 tyrosine kinases.11 It also has double-antitumor activity that directly inhibits tumor-cell proliferation by blocking the RAF/MEK/ERK pathways or inhibits neovascularization by cutting off nutritional supply to tumor cells.12–14 However, it still lacks data about sorafenib from Asian patients with mRCC in a large sampling and long-term follow-up profile.
This study was the first major clinical retrospective investigation for mRCC in Northeast China. After more than 3 years follow-up for all cases, we found that the median OS and PFS were 16.1 months and 10.5 months, respectively. The median OS in this study was similar to the data from some European and American research, but there were differences in median PFS.15–17 In the global Phase III study TARGET, the final OS and PFS of Caucasian patients receiving sorafenib were 17.8 months and 5.5 months, respectively.5 Compared with the TARGET study, the median PFS of our study was much longer (10.5 months vs 5.5 months). The results of our study were consistent with reported data from a small sampling of Chinese patients, which were little different from other Asian non-Chinese data.9,18 In a Japanese study, they found that the median PFS was 7.4 months, the median OS was 25.3 months, and these results were also longer than that in the TARGET study.19 Some results from another Japanese study showed that the median PFS was 9.0 months and sorafenib was effective in Japanese patients with advanced renal cell cancer in general clinical practice.20 In the study of Korean patients, OS and PFS in subgroup of patients receiving sorafenib were 25.7 months and 8.6 months, respectively.21 Compared with Asian non-Chinese data, we found that OS in our study was worse. The reason for this phenomenon was not clear, but it might be related to some patients who occurred to progression of disease in the early stage of sorafenib therapy. However, from the above data, Asian patients may acquire more benefit from sorafenib treatment.
At the same time, we surveyed some clinical factors that might affect the prognosis of Chinese patients with mRCC. Previous studies showed that tumor burden should be an independent prognostic factor in mRCC and c-KIT might be a potential predictive factor for the efficacy of sorafenib in mRCC with sarcomatoid feature.22,23 In this study, we discussed the relationship between clinical factors and prognosis. We used some statistical methods to certify that ECOG PS, metastatic lesions, and previous therapy were the independent prognostic factors for OS, and ECOG PS and previous therapy as the independent prognostic factors for PFS. Furthermore, in a retrospective study, authors found that metastatic sites were associated with PFS and OS in patients with RCC treated with targeted therapies and patients with multi-metastatic sites had shorter PFS and OS.24 The subgroup analysis showed that patients with lung metastases were more sensitive to sorafenib than those with other organ metastases, which was similar to Kondo’s study.25 In addition, there were some limitations in our study. Retrospective data were collected from local area of the Northeast China and patient sampling was not large enough to revise the treatment guideline for mRCC. Due to a larger proportion of the enrolled patients with poor PS, the OS data were lower than that of the Asian population.
Taken together, according to the present data from European/American/Asian patients with mRCC, Asian patients may be more benefit from sorafenib treatment. This long-term follow-up study also demonstrated that Chinese patients with lung metastases were more sensitive to sorafenib than those with other organ metastases. ECOG PS, metastatic lesions, and previous therapy may be important parameters for OS and PFS prediction. Further researches on selecting the candidate patients with mRCC for sorafenib treatment should be done to confirm these findings.
Acknowledgments
We thank Prof Li Xin for expert technical assistance with statistics. We thank Dr Yongye Liu and Dr Long Xu for secretarial and organizational support in this study. We thank Prof Jia Li and Dr Qingqing Sun for critical revision of the manuscript. This work was supported by grants from the National Research Key Project of The Twelfth Five-Year Plan of People’s Republic of China (No 2012ZX09303016-002) and Science and Technology key programs of Liaoning Province (No 2012225019). No additional external funding received for this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. Some results in this study had been presented at the 2013 ASCO Annual Meeting and abstract number is e15582. We confirm that the actual paper has never been published.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Expert Committee for Kidney Cancer in CSCO . Chinese Clinical Practice Guidelines in Kidney Cancer v2013. Vol. 9. Beijing: People’s Medical Publishing House; 2013. pp. 3–5. [Google Scholar]
- 3.Ramakrishnan V, Timm M, Haug JL, et al. Sorafenib, a dual Raf kinase/vascular endothelial growth factor receptor inhibitor has significant anti-myeloma activity and synergizes with common anti-myeloma drugs. Oncogene. 2010;29(8):1190–1202. doi: 10.1038/onc.2009.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28(6):1054–1060. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the Phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. J Clin Oncol. 2009;27(20):3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Cheng S, Qu AQ, Pastorek J, Atkins MB, Signoretti S. Carbonic anhydrase IX as a potential biomarker of efficacy in metastatic clear-cell renal cell carcinoma patients receiving sorafenib or placebo: analysis from the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) Urol Oncol. 2013;31(8):1788–1793. doi: 10.1016/j.urolonc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicken V, Bornemann L, Moltz JH, Peitgen HO, Zaim S, Scheuring U. Comparison of volumetric and linear serial CT assessments of lung metastases in renal cell carcinoma patients in a clinical phase IIB study. Acad Radiol. 2015;22(5):619–625. doi: 10.1016/j.acra.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Xie XD, Piao Y, Liu ZZ, Qu SX. Clinical observation of 21 cases of metastatic renal cell carcinoma treated with sorafenib. Chin J Oncol. 2009;31(9):714–715. [PubMed] [Google Scholar]
- 9.Yang L, Shi L, Fu Q, Xiong H, Zhang M, Yu S. Efficacy and safety of sorafenib in advanced renal cell carcinoma patients: results from a long-term study. Oncol Lett. 2012;3(4):935–939. doi: 10.3892/ol.2012.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Dong B, Lu JJ, et al. Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer. 2009;9:249–256. doi: 10.1186/1471-2407-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreicer R, Li H, Stein M, et al. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115(18):4090–4095. doi: 10.1002/cncr.24467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 13.Bose D, Meric-Bernstam F, Hofstetter W, Reardon DA, Flaherty KT, Ellis LM. Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. Lancet Oncol. 2010;11(4):373–382. doi: 10.1016/S1470-2045(09)70341-9. [DOI] [PubMed] [Google Scholar]
- 14.Keefe SM, Cohen MA, Brose MS. Targeting vascular endothelial growth factor receptor in thyroid cancer: the intracellular and extracellular implications. Clin Cancer Res. 2010;16(3):957–966. doi: 10.1158/1078-0432.CCR-08-2743. [DOI] [PubMed] [Google Scholar]
- 15.Beck J, Procopio G, Bajetta E, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol. 2011;22(8):1812–1823. doi: 10.1093/annonc/mdq651. [DOI] [PubMed] [Google Scholar]
- 16.Procopio G, Verzoni E, Bracarda S, et al. Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. Br J Cancer. 2011;104(8):1256–1261. doi: 10.1038/bjc.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukowski RM, Stadler WM, McDermott DF, et al. Safety and efficacy of sorafenib in elderly patients treated in the North American advanced renal cell carcinoma sorafenib expanded access program. Oncology. 2010;78(5–6):340–347. doi: 10.1159/000320223. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Zhu Y, Dong D, et al. Clinical outcome of advanced and metastatic renal cell carcinoma treated with targeted therapy: is there a difference between young and old patients? Onco Targets Ther. 2014;7:2043–2052. doi: 10.2147/OTT.S70012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito S, Tsukamoto T, Murai M, Fukino K, Akaza H. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int. 2011;108(11):1813–1819. doi: 10.1111/j.1464-410X.2011.10281.x. [DOI] [PubMed] [Google Scholar]
- 20.Tanigawa G1, Kawashima A, Yamaguchi S, et al. Clinical outcome and prognostic factors of sorafenib in Japanese patients with advanced renal cell carcinoma in general clinical practice. Jpn J Clin Oncol. 2011;41(11):1265–1270. doi: 10.1093/jjco/hyr137. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Lee JL, Park I, Park K, Ahn Y, Ahn JH. Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma. Chemotherapy. 2012;58(6):468–474. doi: 10.1159/000346484. [DOI] [PubMed] [Google Scholar]
- 22.Iacovelli R, Lanoy E, Albiges L, Escudier B. Tumour burden is an independent prognostic factor in metastatic renal cell carcinoma. BJU Int. 2012;110(11):1747–1753. doi: 10.1111/j.1464-410X.2012.11518.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HL, Zhu Y, Qin XJ, et al. c-KIT: potential predictive factor for the efficacy of sorafenib in metastatic renal cell carcinoma with sarcomatoid feature. Clin Genitourin Cancer. 2013;11(2):134–140. doi: 10.1016/j.clgc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Grassi P, Verzoni E, Porcu L, et al. Targeted therapies in advanced renal cell carcinoma: the role of metastatic sites as a prognostic factor. Future Oncol. 2014;10(8):1361–1372. doi: 10.2217/fon.14.69. [DOI] [PubMed] [Google Scholar]
- 25.Kondo T, Nakazawa H, Oya M, et al. Clinical efficacy and prognostic factors of tumor progression in Japanese patients with advanced renal cell carcinoma treated with sorafenib. Jpn J Clin Oncol. 2015;45(3):274–280. doi: 10.1093/jjco/hyu200. [DOI] [PubMed] [Google Scholar]