Abstract
P7C3 and its derivatives, 1-(3,6-dibromo-9H-carbazol-9-yl)-3-(p-tolylamino)propan-2-ol (1) and N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-hydroxypropyl)-N-(3-methoxyphenyl)-4-methylbenzenesulfonamide (2), were previously reported to increase neurogenesis in rat neural stem cells (NSCs). Although P7C3 is known to increase neurogenesis by protecting newborn neurons, it is not known whether its derivatives also have protective effects to increase neurogenesis. In the current study, we examined how 1 induces neurogenesis. The treatment of 1 in NSCs increased numbers of cells in the absence of epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2), while not affecting those in the presence of growth factors. Compound 1 did not induce astrocytogenesis during NSC differentiation. 5-Bromo-2′-deoxyuridine (BrdU) pulsing experiments showed that 1 significantly enhanced BrdU-positive neurons. Taken together, our data suggest that 1 promotes neurogenesis by the induction of final cell division during NSC differentiation.
Keywords: Neurodegenerative diseases, Neural stem cells, Neurogenesis, Final cell division, Aminopropyl carbazoles
INTRODUCTION
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, and amyotrophic lateral sclerosis (ALS) are characterized by progressive loss of neurons (Bossy-Wetzel et al., 2004). The number of AD patients in the United States in 2012 was approximately 5.4 million, while that of PD patients in 2010 was roughly 630,000 (Kowal et al., 2013). However, currently there is no pharmacological agent that cures neurodegenerative diseases.
Neural stem cells (NSCs) can self-renew and possess the ability to differentiate into neurons, astrocytes, and oligodendrocytes (Gage, 2000). NSCs are present not only in the developing central nervous system (CNS) but also in the adult CNS (Gage, 2000). NSCs in the adult CNS are known to produce new neurons throughout the subventricular zone and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Taupin and Gage, 2002). Thus, small molecules that can modulate the promotion of neurogenesis from endogenous NSCs may be considered as novel drug candidates for the treatment of these diseases.
Small molecules that increase neurogenesis from NSCs have recently been reported, however, the underlying mechanisms behind neuron generation have not yet been fully identified. A natural product, forskolin (FSK) was found to increase neuronal differentiation of NSCs (Lairson et al., 2013). FSK enhanced neurogenesis by stimulating adenylyl cyclase and resulted in an increase in the concentration of intracellular cyclic adenosine monophosphate. In addition, NSCs were synergistically differentiated into neurons upon treatment with FSK in combination with retinoic acid. Another synthetic molecule, neuropathiazol, has been reported to increase neuronal differentiation by inducing the mRNA expression of neuroD, while suppressing astrocytogenesis in NSCs (Warashina et al., 2006). Sertraline, an antidepressant drug, was found to increase neurogenesis through the glucocorticoid receptor in neural progenitor cells (NPCs) (Anacker et al., 2011; Peng et al., 2012). Sodium butyrate, a histone deacetylase inhibitor, was also shown to promote neurogenesis (Kim et al., 2009a). Sodium butyrate treatment resulted in the sprouting of dendrites, an increase in the number of synapses, and up-regulation of neuroD expression (Fischer et al., 2007). We have also recently reported that a phytochemical, kuwanon V, induced neurogenesis not only in the absence of but also in the presence of mitogens such as epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) (Kong et al., 2015).
Recently, a synthetic molecule, an aminopropyl carbazole, termed P7C3, was reported to increase neurogenesis in the mouse SGZ of the dentate gyrus, and improved the cognition of aged rats (Pieper et al., 2010). In addition, P7C3 derivatives had effects on PD and ALS in animal models (De Jesus-Cortes et al., 2012; Tesla et al., 2012). It has been reported that P7C3 exerted its proneurogenic activity by protecting newborn neurons from apoptosis but not by promoting the proliferation of mice hippocampal NPCs (MacMillan et al., 2011). The neuroprotection exerted by P7C3 is through the targeting of nicotinamide phosphoribosyltransferase (NAMPT) to produce nicotinamide adenine dinucleotide (NAD) (Wang et al., 2014). In a rodent model of blast-mediated traumatic brain injury (TBI), P7C3 treatment preserved axonal integrity after injury, before neuronal cell death occurred (Yin et al., 2014).
We have previously synthesized and identified analogues of P7C3 that enhance in vitro neurogenesis (Yoon et al., 2013). Treatment of NSCs derived from the cortex of embryonic day (E) 14 rats with 5.0 μM of three derivatives (Fig. 1), 1-(3,6-dibromo-9H-carbazol-9-yl)-3-(p-tolylamino)propan-2-ol (1), N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-hydroxypropyl)-N-(3-methoxyphenyl)-4-methylbenzenesulfonamide (2), and 1-(3,6-dibromo-9H-carbazol-9-yl)-3-((3,4-dimethoxyphenyl)amino)propan-2-ol (3), significantly increased the percentage of TuJ1 (neuronal marker)-positive cells, compared to the vehicle-treated control group (Yoon et al., 2013). In the previous study, the mode of action of 2 was investigated, and it was reported that 2 boosts the survival of NSCs in the absence of EGF and FGF2, while inhibiting the proliferation of NSCs in the presence of EGF and FGF2 (Yoon et al., 2013). In the current study, we investigate the neurogenic mechanisms of 1 and found that 1 increased final cell division during differentiation of NSCs to enhance neurogenesis.
Fig. 1.

Chemical structures of P7C3 and 1–3.
MATERIALS AND METHODS
Cell culture
NSCs were cultured as previously described (Kim et al., 2013). Briefly, NSCs isolated from the cortex of E14 Sprague-Dawley rat embryos were dissociated, seeded onto tissue culture flasks (200,000 cells/ml), and expanded as neurospheres for 7 days in Dulbecco’s modified Eagle’s medium/F12 supplemented with 1% (v/v) PSA, 2% (v/v) B27 (all from Gibco, Grand Island, NY, USA), 20 ng/ml EGF and 20 ng/ml FGF2 (both from Chemicon, Temecula, CA, USA). The medium was replaced every 2 days. Neurospheres were dispersed into a single-cell suspension with accutase (Chemicon), and dissociated cells were seeded onto tissue culture plates pre-coated sequentially with 0.01% poly-D-lysine (Sigma-Aldrich, St. Louis, MO, USA) and 10 μg/ml laminin (Invitrogen, Grand Island, NY, USA). The cells were maintained at 37°C in 5% CO2.
Cell viability assay
Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay. NSCs were plated onto 48-well plates (Corning, Corning, NY, USA) at a density of 3×104 cells/well and treated with different concentrations of 1 or 0.1% dimethyl sulfoxide (DMSO; Sigma-Aldrich) as a vehicle for 2 days. The cells were incubated with MTT solution (1 mg/ml) for 2 hours at 37°C. Formazan crystals that formed in the NSCs were solubilized with 20% sodium dodecyl sulfate (Amresco, Solon, OH, USA) in 50% aqueous N,N-dimethyl-formamide (Sigma-Aldrich). Lysates were transferred to 96-well plates (Corning) and the absorbance was measured at 550 nm using the Synergy H1 Hybrid Multi-Mode Microplate Reader (Biotek, Winooski, VT, USA).
Immunocytochemistry and cell counting
Immunocytochemical examination was performed as previously described (Kim et al., 2007). Cell cultures were fixed with 4% paraformaldehyde (PFA; USB Products, Cleveland, OH, USA) for 30 minutes and washed with phosphate-buffered saline (PBS). Fixed cells were blocked with 5% normal goat serum (Millipore, Temecula, CA, USA) and 0.2% Triton X-100 (Amresco) in PBS for 30 minutes and incubated with primary antibodies against cleaved caspase-3 (rabbit monoclonal antibody, 1:400; Cell Signaling), glial fibrillary acidic protein (GFAP, rabbit polyclonal antibody, 1:1000; Dako, Denmark), and βIII Tubulin (TuJ1, mouse monoclonal antibody, 1:1000; Sigma-Aldrich) for 1 hour. After rinsing with PBS, the cells were incubated for 30 minutes with secondary antibodies conjugated to Cy3 (goat anti-rabbit immunoglobulin G [IgG], 1:1000; Jackson ImmunoResearch, West Grove, PA, USA) or Alexa Fluor 488 (goat anti-mouse IgG, 1:1000; Invitrogen) followed by 5 minutes in 4′,6-diamidino-2-phenylindole (DAPI, 1:10,000 in PBS; Sigma-Aldrich) to stain the nuclei. Images were obtained using an inverse fluorescence microscope (DMIL; Leica, Wetzlar, Hesse, Germany). Cleaved caspase-3-, GFAP-, TuJ1-, and DAPI-positive cells were counted and normalized to total DAPI-positive cell numbers. The value of the 1-treated group was divided by that of the DMSO-treated group to yield fold changes.
BrdU assay
One day after plating, NSCs were treated with 5.0 μM compound 1 or DMSO for 4 days, and 5-bromo-2′-deoxyuridine (10.0 μM, BrdU; Sigma-Aldrich) was added to the media either during 0–12 hours or during 12–24 hours. The cells were fixed with 4% PFA for 30 minutes and then fixed with 70% ice-cold MeOH (Merck, Darmstadt, Hesse, Germany) for 10 minutes at room temperature. The cells were exposed to 2 M HCl (Sigma-Aldrich) for 20 minutes at 37°C and then incubated with 0.1 M sodium borate (Sigma-Aldrich) for 10 minutes at room temperature. Cells were washed with PBS containing 0.2% Triton X-100 and incubated with 5% normal goat serum and 0.2% Triton X-100 in PBS for 1 hour, followed by an overnight incubation at 4°C with an anti-BrdU antibody (mouse monoclonal antibody, 1:2000; Sigma-Aldrich). After rinsing, the cells were incubated with a Cy3-conjugated secondary antibody for 1 hour, followed by DAPI for 5 minutes. The images were acquired with an inverse fluorescence microscope. BrdU-positive cells were counted and normalized to the total cell number.
Neurosphere growth rate
Neurosphere growth was measured as previously described (Gamm et al., 2008; Kim et al., 2009b). Briefly, individual spheres (approximately 100–200 μm in diameter) were transferred to single wells of 96-well plates containing 200 μl growth media with 1 or DMSO (n≥4, for each treatment). The diameter of the spheres was measured daily using a lens-mounted microscope (Leica). The volume of the neurospheres was calculated by the equation V=4/3πr3, where r=1/2 diameter. Neurosphere volume on each day (day 0–3) was divided by the volume at day 0 and then multiplied by 100 to obtain the percentage increase in sphere volume.
Neurosphere formation assay
NSCs expanded as neurospheres in the presence of EGF and FGF2 for 1 week were dissociated and plated as single cells onto uncoated 48-well plates at clonal density (1 cell/μl) in 250 μl media in each well (Coles-Takabe et al., 2008). The cells were grown as floating cell aggregates (neurospheres) for 7 days in the presence of mitogens added with 5.0 μM compound 1 or DMSO. The number of neurospheres was counted from 3 independent fields photographed using the JULI-Digital Bio (NanoEnTek, Seoul, Korea).
Statistical analysis
Data were expressed as the mean ± standard error of the mean (S.E.M.) or the mean ± standard deviation (S.D.), and statistical significance was determined using Student’s t-test (*p<0.05, **p<0.01, and ***p<0.001).
RESULTS
Increased cell viability by compound 1 during NSC differentiation
NSCs proliferate in the presence of EGF and FGF2, and differentiate into neurons or glia in the absence of mitogens. It was previously reported that 5.0 μM of 1 increased neurogenesis compared to DMSO-treated control during differentiation (Yoon et al., 2013). To investigate the mode of action of 1, we first tested whether 1 affected the cell viability in the absence of growth factors. NSCs were isolated from the cortex of E14 rat embryos, dispersed into a single cell suspension, and expanded in tissue culture flasks as neurospheres for 6 days in the presence of EGF and FGF2. Neurospheres were dissociated into single cells, seeded onto 48-well plates, and treated with various concentrations (2.5, 5.0, 6.0, 7.5, and 10.0 μM) of 1 or DMSO as a vehicle for 2 days in the absence of mitogens. Subsequently, cell viability was assessed by an MTT assay. Treatment with 1 significantly increased the cell viability by 22.79, 44.44, 52.00, 30.64, and 53.63% at 2.5, 5.0, 6.0, 7.5, and 10.0 μM, respectively, compared with the DMSO-treated control (Fig. 2). These data suggest that 1 enhances cell viability/numbers during NSC differentiation.
Fig. 2.
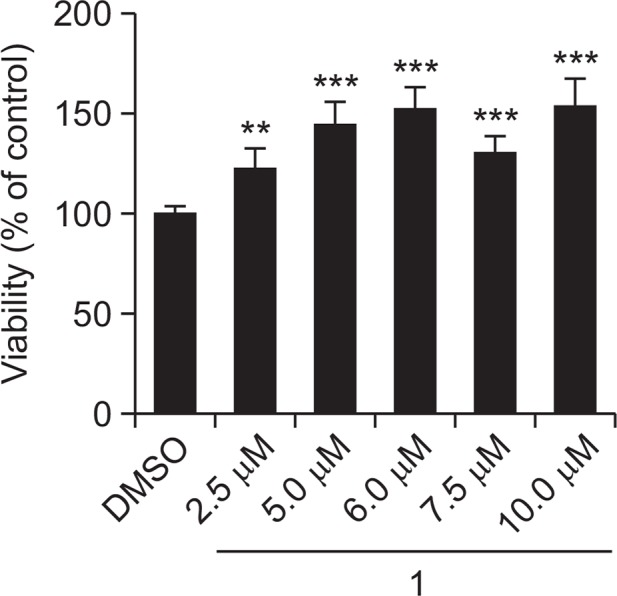
1 increases cell viability during NSC differentiation. NSCs were treated with various concentrations (2.5, 5.0, 6.0, 7.5, and 10.0 μM) of 1 or DMSO for 2 days in the absence of EGF and FGF2. Cell viability was assessed by an MTT assay. The values are presented as the mean ± S.D. (n=7, **p<0.01 and ***p<0.001). Statistical analysis was performed using Student’s t-test.
Facilitation of final cell division by compound 1 during NSC differentiation
To test whether the increased cell viability/number by 1 in the absence of EGF and FGF2 was due to increased cell survival, we examined the levels of cleaved caspase-3, an apoptosis marker, by immunocytochemistry. NSCs were treated with 5.0 μM compound 1 or DMSO for 4 days in the absence of growth factors, and apoptotic cells were immunostained with an anti-cleaved caspase-3 antibody. However, there was no significant difference in the number of cleaved caspase-3-positive cells between the 1- and DMSO-treated groups (Fig. 3). These data suggest that 1 does not affect cell survival during differentiation.
Fig. 3.
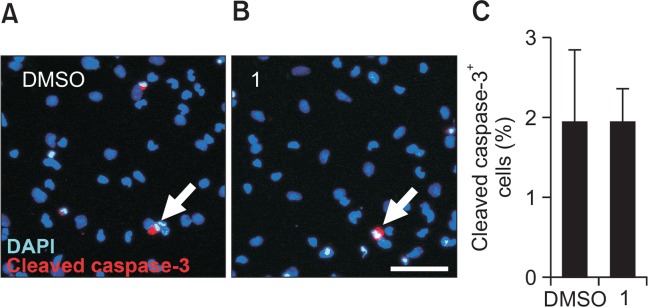
1 has no effect on cell survival during NSC differentiation. NSCs differentiated for 4 days in the presence of 1 (5.0 μM) or DMSO were immunostained with an anti-cleaved caspase-3 antibody. (A, B) Representative immunofluorescence images for apoptotic cells (red, cleaved caspase-3-positive) and nuclei (blue, DAPI-positive). Scale bar, 50 μm. (C) Quantification of cleaved caspase-3-positive apoptotic cells. The values are presented as the mean ± S.D. (n=3). Statistical analysis was performed using Student’s t-test.
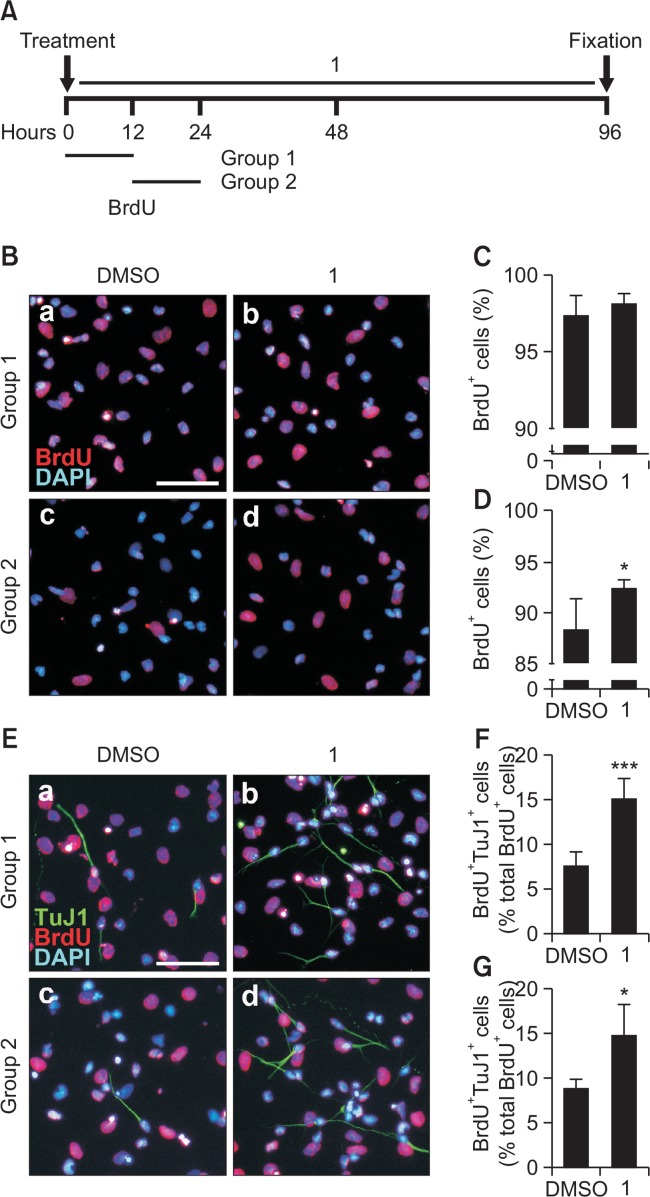
We then performed BrdU pulsing experiments to investigate whether the increased cell viability/number was a result of the stimulated proliferation during NSC differentiation. When BrdU, a thymidine analog that incorporates into the DNA of proliferating cells in the S-phase, was pulsed during the first 12 hours of differentiation, the number of BrdU-positive cells in the 1-treated group was not significantly different from the DMSO-treated control (Fig. 4B[a,b], 4C). However, when BrdU was added during the next 12 hours of differentiation, the number of BrdU-positive cells in the 1-treated group was significantly increased compared with that of cells treated with DMSO (Fig. 4B[c,d], 4D). Moreover, 1 markedly increased the number of BrdU/TuJ1 double-positive cells compared with the DMSO control when BrdU was added for both the first 12 hours and the next 12 hours of differentiation (Fig. 4E–4G). These data suggest that 1 promotes final cell division to generate neurons during NSC differentiation.
Fig. 4.
1 promotes final cell division during NSC differentiation. (A) NSCs were treated with 5.0 μM compound 1 or DMSO for 4 days in the absence of growth factors, and BrdU was added either during 0–12 hours (group 1) or 12–24 hours (group 2). After fixation, cells were immunostained with an anti-BrdU antibody. (B) Representative immunofluorescence images of BrdU-positive cells (red) and nuclei (blue). Scale bar, 50 μm. (C, D) Quantification of BrdU-positive cells in group 1 (C) and group 2 (D). (E) Representative immunofluorescence images of BrdU-positive cells (red), TuJ1-positive cells (green), and nuclei (blue). Scale bar, 50 μm. (F, G) Quantification of BrdU/TuJ1 double-positive cells in group 1 (F) and group 2 (G). The values are presented as the mean ± S.D. (n=4, *p<0.05 and ***p<0.001). Statistical analysis of all data was performed using Student’s t-test.
Compound 1 does not affect NSC proliferation in the presence of mitogens
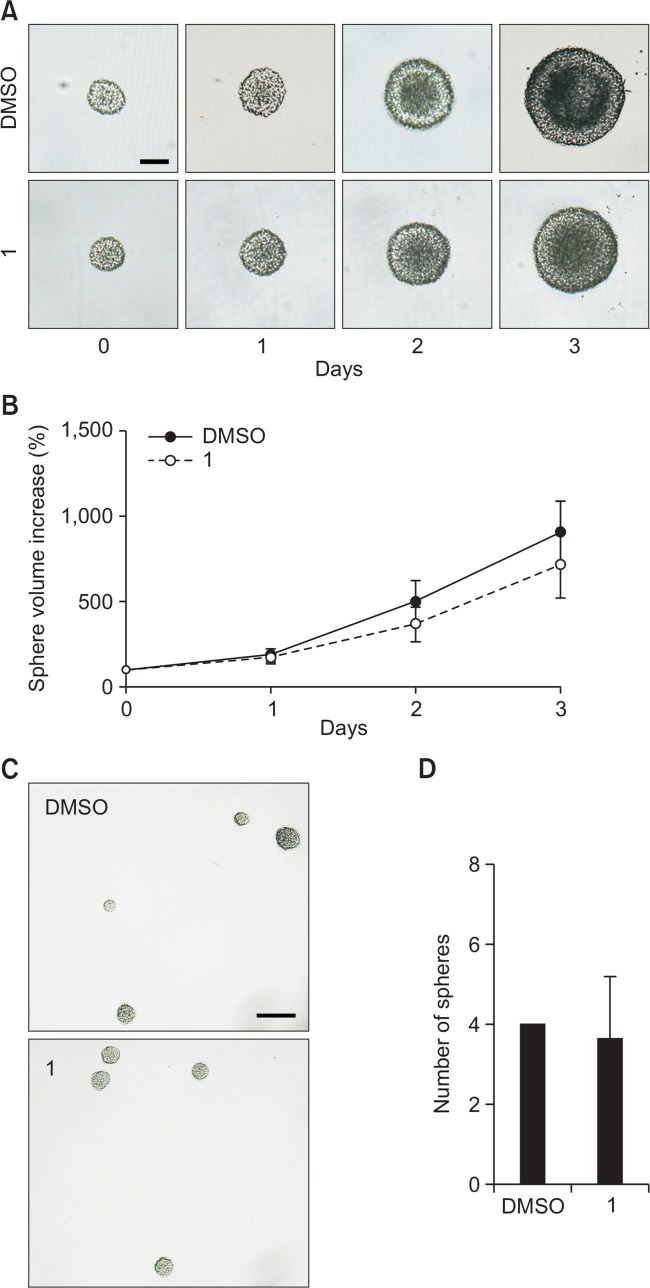
We next examined whether 1 affected NSC proliferation in the presence of EGF and FGF2. Firstly, we performed a neurosphere growth assay. The sizes of the neurospheres treated with 5.0 μM compound 1 or DMSO in the presence of growth factors were measured daily for 4 days (day 0–3). As shown in Fig. 5A, the sizes of the neurospheres became bigger in a time-dependent manner. To evaluate the effect of 1 on NSC proliferation, we measured the diameters of neurospheres and calculated the volumes of spheres using the equation V=4/3πr3, where r=1/2 diameter. Neurosphere volume on each day was divided by the volume on day 0 and then multiplied by 100 to obtain the percentage increase in sphere volume. However, there was no significant difference in the volume increase of the neurospheres between the two groups (Fig. 5B). Furthermore, when we dissociated, plated NSCs at clonal density (1 cell/μl), and cultured for 7 days in the presence of mitogens with 5.0 μM compound 1 or DMSO to determine neurosphere formation ability, we observed no significant difference between the two groups (Fig. 5C, 5D). Taken together, these data suggest that 1 does not change the proliferation or neurosphere formation ability of NSCs in the presence of growth factors.
Fig. 5.
1 does not affect NSC proliferation in the presence of mitogens. (A) Individual neurospheres were treated with either 5.0 μM compound 1 or DMSO for 4 days in the presence of EGF and FGF2. Digital images of neurospheres on day 0–3 are shown. Scale bar, 100 μm. (B) Volumes of neurospheres were calculated using the measured diameters. The values are the mean ± S.D. (n≥4, for each treatment). (C) Passaged NSCs were dissociated, plated as single cells at clonal density (1 cell/μl), and grown as neurospheres for 7 days in the presence of growth factors added with 5.0 μM compound 1 or DMSO. Scale bar, 100 μm. (D) The number of spheres was counted from 3 independent randomly chosen fields. The values are the mean ± S.D. (n=3). Statistical analysis of all data was performed using Student’s t-test.
Induction of neurogenesis but no significant changes in astrogliogenesis by compound 1 during NSC differentiation
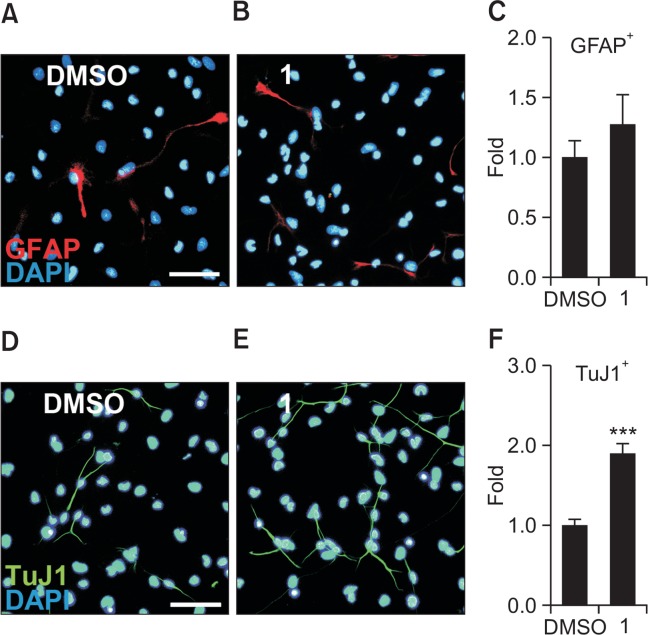
In order to investigate whether 1 has effects on the astroglial differentiation of NSCs, NSCs were treated with either 5.0 μM compound 1 or DMSO for 4 days in the absence of EGF and FGF2. Astroglia and neurons were detected using antibodies against GFAP and TuJ1, respectively. Immunocytochemistry results showed that there was no significant difference in the number of GFAP-expressing astrocytes between the 1-treated group and the DMSO-treated control group (Fig. 6A–6C). Conversely, we confirmed the neurogenic effects of 1 that had been reported in our previous study (Yoon et al., 2013). As shown in Fig. 6D–6F, 1 significantly increased the percentage of neurons by 1.90-fold, compared to the DMSO-treated control. These data suggest that 1 promotes neurogenesis but does not affect astroglial differentiation in NSCs.
Fig. 6.
Compound 1 induces neurogenesis while not affecting astrocytogenesis. NSCs treated with 5.0 μM compound 1 or DMSO for 4 days during differentiation were immunostained with a TuJ1 or anti-GFAP antibody. (A, B) Representative immunofluorescence images for astrocytes (red, GFAP-positive) and nuclei (blue). Scale bar, 50 μm. (C) Quantification of GFAP-positive astrocytes. (D, E) Representative immunofluorescence images for neurons (green, TuJ1-positive) and nuclei (blue). Scale bar, 50 μm. (F) Quantification of TuJ1-positive neurons. The values are presented as the mean ± S.E.M. (n=3, ***p<0.001). Statistical analysis of all data was performed using Student’s t-test.
DISCUSSION
NSCs can self-renew and differentiate into neurons, astrocytes, and oligodendrocytes (Gage, 2000). Since neurodegenerative diseases are characterized by a loss of neurons (Bossy-Wetzel et al., 2004), several studies have attempted to identify small molecules that increase the neuronal differentiation of NSCs to facilitate regeneration (Kim and Jin, 2012).
Recently, P7C3 has been discovered to increase neurogenesis (Pieper et al., 2010) and compound 1, a derivative of P7C3, has been identified as a neurogenesis enhancer in our previous study (Yoon et al., 2013). P7C3 functions as a neuroprotective compound by activating NAMPT and increasing NAD levels in cells (Wang et al., 2014). P7C3 and its derivative, P7C3-S243 not only protect neurons from death but also preserve axonal integrity, learning and memory, and motor coordination in a rodent TBI model (Yin et al., 2014). In the present study, we investigated whether a P7C3 derivative, compound 1, functions in a similar manner to P7C3 during the neurogenesis of NSCs.
When NSCs were treated with 1 or DMSO in the absence of growth factors, 1 significantly increased not only neuronal differentiation but also overall cell viability compared with the DMSO control. Due to the fact that P7C3 and its derivatives including compound 2 are known to protect newly generated neurons from death and block axonal degeneration (Pieper et al., 2010; Yoon et al., 2013; Yin et al., 2014), we first hypothesized that 1 would also enhance cell survival like other aminopropyl carbazoles. However, treatment with 1 did not change the number of cleaved caspase-3-positive cells compared with the DMSO-treated control, suggesting that 1 may act differently to P7C3.
Interestingly, it was shown that 1 promoted cell division during NSC differentiation. When BrdU was pulsed during 12–24 hours of differentiation, 1 significantly increased the number of proliferating cells compared with the DMSO-treated control. Moreover, 1 considerably increased the number of BrdU/TuJ1 double-positive cells within 24 hours of differentiation. During neurogenesis, neural progenitor cells undergo symmetric or asymmetric divisions (Noctor et al., 2004). A time-lapse and immunocytochemistry study revealed that just before neurogenesis NSCs do not transform to neurons but undergo final cell division (Kim et al., 2007). Thus, data obtained in the current study suggest that compound 1 may facilitate final cell division to produce daughter neurons during NSC differentiation. Similar to compound 1, kuwanon V, a phenolic compound isolated from the Morus bombycis bark, increased neurogenesis and final cell division in early NSC differentiation (Kong et al., 2015). Insulin-like growth factor-1 is also known to induce neurogenesis by stimulating cell division of granule cell precursors (Lin and Bulleit, 1997).
Although the detailed mechanisms involved in increased neurogenesis by aminopropyl carbazole derivatives should be investigated in further studies, our results provide another underlying proneurogenic mechanism of aminopropyl carbazole derivatives that is different from those reported with P7C3 and its other derivatives (Yoon et al., 2013; Wang et al., 2014; Yin et al., 2014). P7C3 was found to increase neurogenesis by increasing survival of newborn neurons but not by promoting proliferation of neural precursor cells, and 2 was also shown to enhance neurogenesis by boosting cell survival during NSC differentiation (MacMillan et al., 2011; Yoon et al., 2013). In contrast, 1 did not show protective effects but increased neurogenesis by the facilitation of final cell division during NSC differentiation. Identification of P7C3 derivatives or variants may provide not only neuroprotective but also neuroproducing agents for neurodegenerative diseases.
Acknowledgments
This work was supported by the Chung-Ang University Excellent Student Scholarship, the National Research Foundation (NRF-2014R1A1A1002607) and the Ministry of Knowledge Economy [No. 10041913, Development of stem cell culture system based on surface-nano-structure control].
REFERENCES
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry. 2011;16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(Suppl):S2–9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- Coles-Takabe BL, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM, van der Kooy D. Don’t look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26:2938–2944. doi: 10.1634/stemcells.2008-0558. [DOI] [PubMed] [Google Scholar]
- De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, Britt J, Tesla R, Morlock L, Naidoo J, Melito LM, Wang G, Williams NS, Ready JM, McKnight SL, Pieper AA. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci USA. 2012;109:17010–17015. doi: 10.1073/pnas.1213956109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gamm DM, Wright LS, Capowski EE, Shearer RL, Meyer JS, Kim HJ, Schneider BL, Melvan JN, Svendsen CN. Regulation of prenatal human retinal neurosphere growth and cell fate potential by retinal pigment epithelium and Mash1. Stem Cells. 2008;26:3182–3193. doi: 10.1634/stemcells.2008-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Jin CY. Stem cells in drug screening for neurodegenerative disease. Korean J Physiol Pharmacol. 2012;16:1–9. doi: 10.4196/kjpp.2012.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009a;110:1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, McMillan E, Han F, Svendsen CN. Regionally specified human neural progenitor cells derived from the mesencephalon and forebrain undergo increased neurogenesis following overexpression of ASCL1. Stem Cells. 2009b;27:390–398. doi: 10.1634/stemcells.2007-1047. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Sugimori M, Nakafuku M, Svendsen CN. Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol. 2007;203:394–405. doi: 10.1016/j.expneurol.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim JH, Kong SY, Park MH, Sohn UD, Kim HJ. Comparison of ectopic gene expression methods in rat neural stem cells. Korean J Physiol Pharmacol. 2013;17:23–30. doi: 10.4196/kjpp.2013.17.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SY, Park MH, Lee M, Kim JO, Lee HR, Han BW, Svendsen CN, Sung SH, Kim HJ. Kuwanon V inhibits proliferation, promotes cell survival and increases neurogenesis of neural stem cells. PloS one. 2015;10:e0118188. doi: 10.1371/journal.pone.0118188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- Lairson LL, Lyssiotis CA, Zhu S, Schultz PG. Small molecule-based approaches to adult stem cell therapies. Annu Rev Pharmacol Toxicol. 2013;53:107–125. doi: 10.1146/annurev-pharmtox-011112-140300. [DOI] [PubMed] [Google Scholar]
- Lin X, Bulleit RF. Insulin-like growth factor I (IGF-I) is a critical trophic factor for developing cerebellar granule cells. Brain Res Dev Brain Res. 1997;99:234–242. doi: 10.1016/S0165-3806(97)00015-1. [DOI] [PubMed] [Google Scholar]
- MacMillan KS, Naidoo J, Liang J, Melito L, Williams NS, Morlock L, Huntington PJ, Estill SJ, Longgood J, Becker GL, McKnight SL, Pieper AA, De Brabander JK, Ready JM. Development of proneurogenic, neuroprotective small molecules. J Am Chem Soc. 2011;133:1428–1437. doi: 10.1021/ja108211m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Peng ZW, Xue YY, Wang HN, Wang HH, Xue F, Kuang F, Wang BR, Chen YC, Zhang LY, Tan QR. Sertraline promotes hippocampus-derived neural stem cells differentiating into neurons but not glia and attenuates LPS-induced cellular damage. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;36:183–188. doi: 10.1016/j.pnpbp.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, Kotti T, Ure K, Brat DJ, Williams NS, MacMillan KS, Naidoo J, Melito L, Hsieh J, De Brabander J, Ready JM, McKnight SL. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Tesla R, Wolf HP, Xu P, Drawbridge J, Estill SJ, Huntington P, McDaniel L, Knobbe W, Burket A, Tran S, Starwalt R, Morlock L, Naidoo J, Williams NS, Ready JM, McKnight SL, Pieper AA. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2012;109:17016–17021. doi: 10.1073/pnas.1213960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warashina M, Min KH, Kuwabara T, Huynh A, Gage FH, Schultz PG, Ding S. A synthetic small molecule that induces neuronal differentiation of adult hippocampal neural progenitor cells. Angew Chem, Int Ed Engl. 2006;45:591–593. doi: 10.1002/anie.200503089. [DOI] [PubMed] [Google Scholar]
- Yin TC, Britt JK, De Jesus-Cortes H, Lu Y, Genova RM, Khan MZ, Voorhees JR, Shao J, Katzman AC, Huntington PJ, Wassink C, McDaniel L, Newell EA, Dutca LM, Naidoo J, Cui H, Bassuk AG, Harper MM, McKnight SL, Ready JM, Pieper AA. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 2014;8:1731–1740. doi: 10.1016/j.celrep.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HJ, Kong SY, Park MH, Cho Y, Kim SE, Shin JY, Jung S, Lee J, Farhanullah, Kim HJ. Aminopropyl carbazole analogues as potent enhancers of neurogenesis. Bioorg Med Chem. 2013;21:7165–7174. doi: 10.1016/j.bmc.2013.08.066. [DOI] [PubMed] [Google Scholar]