Abstract
The clinical benefits of oncogenic BRAF inhibitor therapies are limited by the emergence of drug resistance. In this study, we investigated the role of a negative regulator of the MAPK pathway, Spry2, in acquired resistance using BRAF inhibitor-resistant derivatives of the BRAF-V600E melanoma (A375P/Mdr). Real-time RT-PCR analysis indicated that the expression of Spry2 was higher in A375P cells harboring the BRAF V600E mutation compared with wild-type BRAF-bearing cells (SK-MEL-2) that are resistant to BRAF inhibitors. This result suggests the ability of BRAF V600E to evade feedback suppression in cell lines with BRAF V600E mutations despite high Spry2 expression. Most interestingly, Spry2 exhibited strongly reduced expression in A375P/Mdr cells with acquired resistance to BRAF inhibitors. Furthermore, the overexpression of Spry2 partially restored sensitivity to the BRAF inhibitor PLX4720 in two BRAF inhibitor-resistant cells, indicating a positive role for Spry2 in the growth inhibition induced by BRAF inhibitors. On the other hand, long-term treatment with PLX4720 induced pERK reactivation following BRAF inhibition in A375P cells, indicating that negative feedback including Spry2 may be bypassed in BRAF mutant melanoma cells. In addition, the siRNA-mediated knockdown of Raf-1 attenuated the rebound activation of ERK stimulated by PLX4720 in A375P cells, strongly suggesting the positive role of Raf-1 kinase in ERK activation in response to BRAF inhibition. Taken together, these data suggest that RAF signaling may be released from negative feedback inhibition through interacting with Spry2, leading to ERK rebound and, consequently, the induction of acquired resistance to BRAF inhibitors.
Keywords: BRAF inhibitor, Spry2, Negative feedback, Melanoma, Acquired resistance
INTRODUCTION
Gain-of-function mutations in BRAF have been identified in various cancers (Davies et al., 2002). Notably, BRAF somatic mutations have been observed in 66% of malignant melanomas (Davies et al., 2002), and these exhibit a valine-to-glutamate substitution (V600E), which renders BRAF constitutively active. This prevalence of BRAF mutations has prompted efforts to develop inhibitors of the mutated BRAF (Sabbatino et al., 2013; Holderfield et al., 2014). Selective BRAF inhibitors including vemurafenib and dabrafenib displayed potent antineoplastic activities in tumor cells bearing BRAF V600E, whereas lacking their activity in cell lines that express wild-type (WT) BRAF. It has been demonstrated clinical benefits in patients with BRAF V600E melanoma (Flaherty et al., 2010; McGettigan, 2014). We also recently reported UAI-201 (also described as UI-152) as a potent ATP-competitive inhibitor of RAF proteins (Kim et al., 2012). UAI-201 was more than 1,000-fold more selective in inhibiting the proliferation of tumor cell lines bearing the BRAF-V600E mutation compared with cells bearing wild-type BRAF (Kim et al., 2012).
However, intrinsic and acquired resistance limits the therapeutic benefits of these oncogenic BRAF inhibitors (Flaherty et al., 2010; Rizos et al., 2014). The mechanisms that underlie the therapeutic resistance of melanoma include the overexpression of MAP kinase kinase kinase 8 (MAP3K8; COT) (Johannessen et al., 2010), mutations in N-Ras, and PDGFRβ overexpression (Nazarian et al., 2010). Our previous study also implicated the upregulation of N-Ras as a key mechanism of acquired resistance to oncogenic BRAF inhibitors (Ahn and Lee, 2014). In particular, the enhancement of RAF dimerization has been proposed as a principal mechanism of BRAF inhibitor resistance (Poulikakos et al., 2011).
In general, the feedback mechanism may be dysfunctional due to insensitivity of the mutant oncogene to normal regulation in some tumors. Actually, BRAF V600E is able to bypass the inhibitory effects of negative-feedback regulation induced by ERK. One potent inhibitor of the Raf/MAP kinase pathway is the Sprouty (Spry) protein (Yusoff et al., 2002), which was first identified in a genetic screen of Drosophila (Hacohen et al., 1998). Previous results have indicated that the MAPK pathway both transcriptionally upregulates Spry2 and post-transcriptionally attenuates its ability to inhibit MAPK signaling (Brady et al., 2009). In particular, relief of feedback after targeted therapy may be viewed as a key contributor to therapeutic resistance (Chandarlapaty, 2012). Consistent with this opinion, we previously showed that Raf-1 may be released from negative feedback inhibition by interacting with Spry2 in multi-drug-resistant Ras-NIH 3T3/Mdr cells (Ahn et al., 2011).
A375P/Mdr cell lines with acquired resistance to BRAF inhibitors were generated by propagating parental A375P cells harboring BRAF-V600E in increasing concentrations of BRAF inhibitor to achieve chronic selection (Ahn and Lee, 2013). On the contrary, SK-MEL-2 cell line expressing WT BRAF has an intrinsic resistance to BRAF inhibition because BRAF inhibitor lacked activity in cell lines that express WT BRAF. To further identify potential mechanisms of resistance to BRAF inhibitors, we investigated the role of Spry2 in the resistance to BRAF inhibitors using A375P/Mdr and SK-MEL-2 cells. This manuscript provides the first evidence demonstrating that Spry2 exhibits strongly reduced expression in A375P/Mdr cells with acquired resistance to BRAF inhibitors. The present results demonstrated that long-term treatment with a BRAF inhibitor significantly downregulated Spry2 in BRAF-V600E-positive cell lines, which was concomitant with the rebound activation of the MAPK pathway.
MATERIALS AND METHODS
Antibodies and reagents
Rabbit polyclonal anti-Spry2 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-phospho-MEK and anti-phospho-ERK were purchased from Cell Signaling Technology (Danvers, MA, USA). SYBR Premix EX Taq II used for real-time PCR was obtained from Takara Korea Biomedical Inc. (Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum (FCS) and penicillin-streptomycin were purchased from GIBCO-Invitrogen (Carlsbad, CA, USA). Reagents for SDS-polyacrylamide gel electrophoresis were obtained from Bio-Rad (Hercules, CA, USA). PLX4720 was obtained from Selleck Chemicals (Houston, TX, USA). PLX4720 was dissolved in DMSO and freshly diluted for each experiment. The DMSO concentrations were less than 0.1% in all of the experiments.
Cell lines and cell culture
Melanoma cell lines (A375P and SK-MEL-2) were obtained from either the Korean Cell Line Bank (KCLB; Seoul, Korea) or YOUAI Co., Ltd. (Suwon-Si, Gyeonggi-Do, Korea). The development of BRAF inhibitor-resistant A375P melanoma cells (A375P/Mdr) was previously described (Ahn and Lee, 2013). All of the cell lines were maintained at 37°C in DMEM supplemented with 10% FCS, penicillin-streptomycin, and glutamine. The A375P/Mdr cells were further propagated in growth medium containing 1 μM PLX4720. Before their use in the experiments, the A375P/Mdr cells were maintained in PLX4720-free culture medium and subcultured at least three times. For experimental purposes, the cells were cultured in 60-mm tissue culture dishes until they reached ∼80% confluency.
Plasmid DNA and siRNA transfection
The pCMV6 vector encoding full-length Spry2 cDNA was obtained from OriGene Technologies, Inc. (Rockville, MD, USA). For Spry2 knockdown, a pool of three target-specific Spry2 siRNAs was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The targeted sequences were the following: CCAUCCGAAACACCAAUGAtt, GCAACCAACUAAACAGUUAtt, and CCUUUGGACUUCAUGUAUAtt. The three targeted sequences of the siRNA used for the knockdown of Raf-1 were GGAACACAAAGGUAAGAAAtt, CCCAUGCCUUCACUUUCAAtt and GUUGCAGUAAAGAUCCUAAtt. A non-targeting siRNA (Santa Cruz Biotechnology, CA, USA) was used as a control. The cells were transiently transfected with either the siRNAs or pCMV6-Spry2 as indicated using Lipofectamine 2000 in Opti-minimal essential medium I (Invitrogen).
Cell growth assay
The cells were plated in quadruplicates in 96-well microliter plates (Costar, Cambridge, MA, USA) at a density of 5×103 cells/well and then treated with PLX4720 at 37°C in a humidified 5% CO2/95% air incubator. On day 3, the cells were incubated with MTT at 37°C for 3 h. The absorbance of the samples against a background control (medium alone), which was used as a blank, was measured at 450 nm using a microliter plate (ELISA) reader (Molecular Devices, Sunnyvale, CA, USA).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA from BRAF inhibitor-sensitive and BRAF inhibitor-resistant melanoma cells was isolated with the RNeasy min kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Using a reverse transcriptase, cDNA was synthesized from 1 μg of total RNA. A 2-μl sample of cDNA was subjected to standard PCR amplification with PCR MasterMix (Bioneer, Daejeon, Korea) to determine the expression of Spry2. The human β-actin primers were used as control primers for amplification. The specificity of the PCR products was confirmed by 2% agarose gel electrophoresis. Real-time PCR was carried out with an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR Premix EX Taq II. Aliquots of cDNA were used as a template for real-time PCRs containing either primers for Spry2, Raf-1 or primers for β-actin. The primers were synthesized by Bioneer (Daejeon, Korea) with the following oligonucleotide sequences: Spry2, 5′-GAGTCGTCTCCAGCTCCGAAC-3′ and 5′-AGCTCTGGCCTCCATCAGG-3′; Raf-1, 5′-GCTGTGAAAGGAGGACGTGT-3′ and 5′-GGAGACACATGGGATTTTGG-3′. The temperature cycle profile for the real-time PCRs was 95°C for 10 min and 40 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 1 min. Melting curve analysis was also included using one cycle of 95°C for 1 min, 55°C for 30 s, and 95°C for 30 s to verify the specificity of the amplified PCR products. The real-time PCR data were normalized to differences in the β-actin levels through the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Preparation of cell lysates and immunoblot analysis
Whole-cell lysates were prepared as follows: The cells were washed twice with ice-cold PBS and harvested by scraping cells into RIPA lysis buffer. For immunoblotting, the whole-cell lysates were denatured in Laemmli sample buffer and resolved by SDS-polyacrylamide gel electrophoresis. The proteins were transferred to nitrocellulose, and immunoblot analysis was performed using the appropriate primary antibodies. The immune complexes on nitrocellulose were detected by the ECL-Plus chemiluminescent system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Fluorescent images were captured using KODAK Image Station 4000R (Carestream Health, Inc., Rochester, NY, USA). Band intensities were quantified using the Kodak Molecular Imaging software (version 4.5.0; Carestream Health, Inc.).
RESULTS
Downregulation of Spry2 in cells resistant to BRAF inhibitors
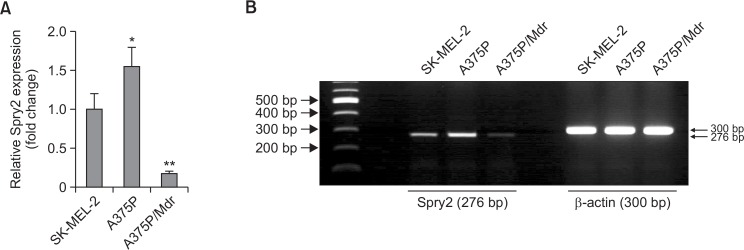
Spry2 has previously been suggested as a negative regulator of the MAPK pathway through interaction with Raf-1 (Yusoff et al., 2002). Our previous results also showed that Raf-1 may be released from negative feedback inhibition by interacting with Spry2 in multi-drug-resistant Ras-NIH 3T3/Mdr cells (Ahn et al., 2011). In this study, reverse-transcriptional real-time PCR was used for determination of the mRNA expression levels of Spry2 in BRAF inhibitor-sensitive and BRAF inhibitor-resistant melanoma cells. As shown in Fig. 1A, Spry2 expression was higher in A375P cells harboring the BRAF V600E mutation compared with wild-type BRAF-bearing cells (SKMEL-2) resistant to BRAF inhibitors. Most interestingly, Spry2 exhibited strongly reduced expression in A375P/Mdr cells with acquired resistance to BRAF inhibitors. Agarose gel separation ensured the product specificity (Fig. 1B).
Fig. 1.
Expression levels of the Spry2 proteins in BRAF inhibitor-sensitive and BRAF inhibitor-resistant melanoma cells. (A) Real-time RT-PCR was performed to measure the Spry2 mRNA levels. The comparative threshold cycle (Ct) method for relative quantification (2−ΔΔCt) was used for the quantitative analysis of gene expression. The expression of the target genes in each cell line was normalized to the β-actin expression level. The relative expression level in SK-MEL-2 cells was regarded as 1.0. The values represent the means ± SD of quadruplicate determinants from one of three representative experiments. **p<0.01 compared with SK-MEL-2 cells, as determined by Dunnett’s t-test. (B) The agarose gel separation results ensured the product specificity. The RNA extracted from each cell line was examined for the presence of Spry2 RNA transcripts by RT-PCR. Spry2 cDNA was subjected to PCR analysis with specific primers to determine the expression of Spry2 and the human β-actin gene. The PCR-amplified products were subjected to electrophoresis through a 1.2% agarose gel.
Reversal of BRAF inhibitor sensitivity by Spry2 overexpression in A375P/Mdr cells
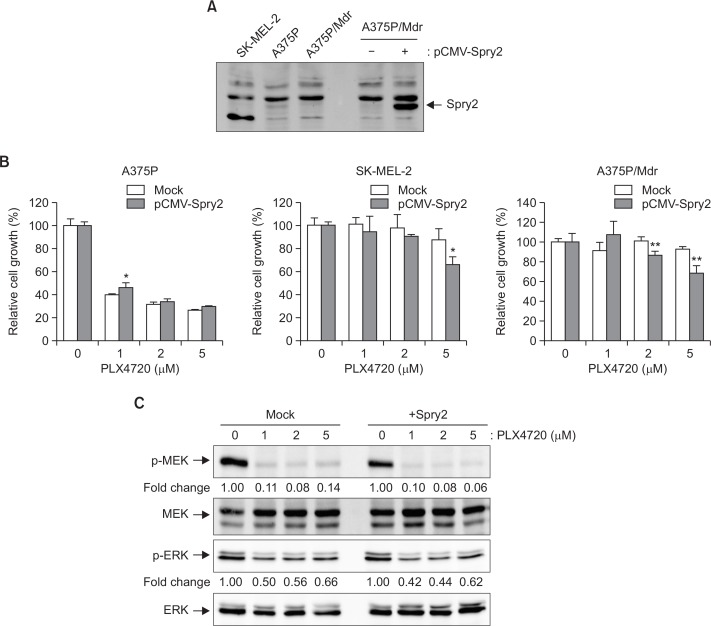
To test the hypothesis that Spry2 plays a critical role in the acquired resistance to BRAF inhibitors, we examined the difference in sensitivity to the BRAF inhibitor PLX4720 between mock- and Spry2-transfecetd cells. Three cell lines with differing resistance to BRAF inhibitors were grown on a 96-well plate, and the numbers of viable cells were quantified using a colorimetric assay. The overexpression of Spry2 was confirmed by immunoblotting analysis (Fig. 2A). Also, we confirmed that, although faint, a band for Spry2 is present in A375P cell lines. In A375P/Mdr and SK-MEL-2 cell lines, the endogenous protein level of Spry2 was too low to detect in immunoblot analysis. As shown in Fig. 2B, Spry2 overexpression partially restored sensitivity to the BRAF inhibitor PLX4720 in two BRAF inhibitor-resistant cell lines (SK-MEL-2 and A375P/Mdr), irrespective of their BRAF mutational status. Conversely, Spry2 overexpression had no effect on PLX4720 sensitivity in the BRAF inhibitor-sensitive A375P cells, which showed strong expression of endogenous Spry2. These results imply that Spry2 plays a positive role in the growth inhibition induced by BRAF inhibitors. However, Spry2 overexpression in A375P/Mdr cells resulted in no significant change in the levels of pERK or pMEK (Fig. 2C).
Fig. 2.
Effect of Spry2 overexpression on PLX4720-induced growth inhibition. BRAF inhibitor-sensitive (A375P) and BRAF inhibitor-resistant (A375P/Mdr and SK-MEL-2) melanoma cells were transiently transfected with the mock control pCMV vector or with the pCMV vector encoding Spry2 for 24 h. (A) The overexpression of Spry2 was assessed by immunoblotting. (B) The cell growth in response to the BRAF inhibitor PLX4720 was then evaluated using the MTT assay. The relative proliferation rate of the cells treated with vehicle alone was regarded as 100%. The values represent the means ± SD of quadruplicate determinants from one of three representative experiments. **p<0.01 and *p<0.05 compared with the mock control, as determined by two-tail Student’s t-test. (C) Cell lysates were prepared from A375P/Mdr cells transiently transfected with pCMV-Spry2. The phosphorylated forms of MEK and ERK were detected by immunoblotting using anti-phospho-MEK and anti-phospho-ERK antibodies. The same blots were reprobed with anti-MEK and anti-ERK antibodies to confirm that the expression levels of MEK and ERK proteins were similar in all of the lanes. Numbers listed below each band indicate the intensity value quantified by the Kodak Molecular Imaging software, expressed as fold change. The intensity value observed in vehicle control cells was defined as 1.0. The results presented are representative of at least three independent experiments.
Effect of RNAi targeting Spry2 on sensitivity to BRAF inhibitors
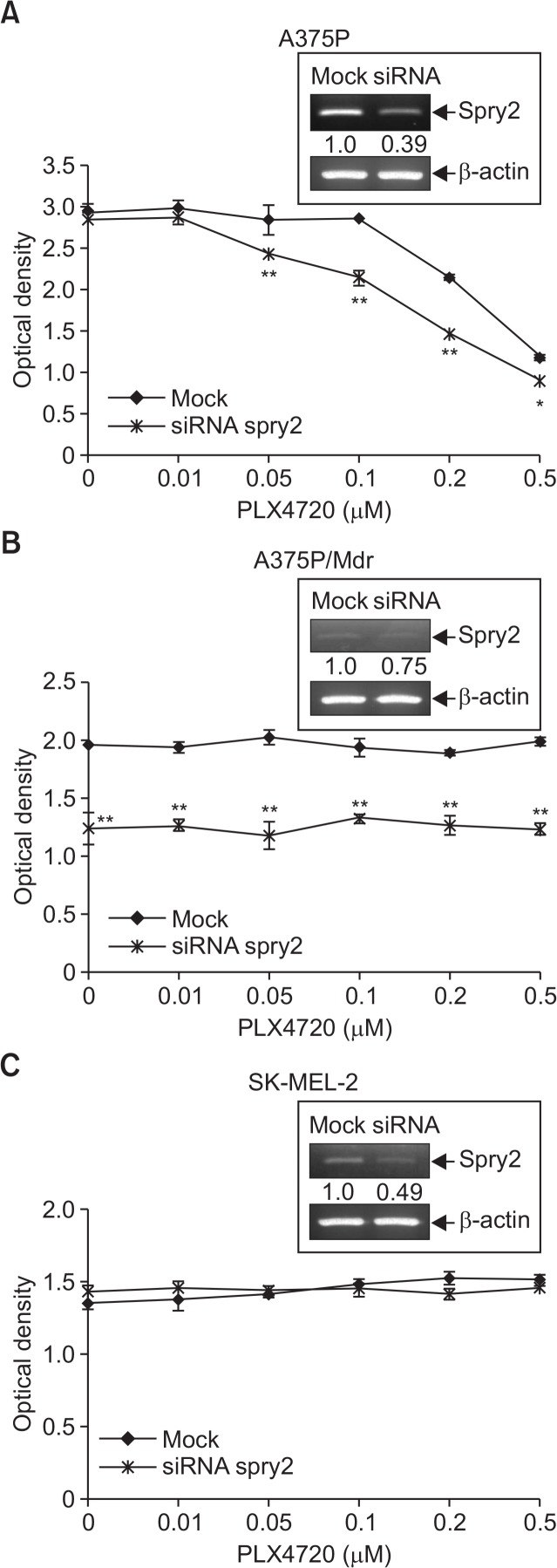
We then silenced Spry2 by siRNA-mediated knockdown to further assess a potential role of Spry2. The Spry2 siRNA transfection in A375P and SK-MEL-2 cells significantly decreased Spry2 expression compared with the controls. In case of A375P/Mdr cells with a very weak expression of Spry2, siRNA-mediated Spry2 knockdown was not efficient as much as in other two cell lines. We found that the siRNA-mediated Spry2 knockdown in A375P cells, which exhibit strong Spry2 expression, partially conferred PLX4720 sensitivity to the cells (Fig. 3A). Conversely, Spry2 knockdown had no effect on PLX4720 sensitivity in the BRAF inhibitor-resistant SK-MEL-2 and A375P/Mdr cells, which showed weak expression of endogenous Spry2 (Fig. 3B, 3C). Interestingly, the knockdown of Spry2 significantly inhibited the growth of only BRAF V600E mutant-harboring A375P/Mdr cells among two drug-resistant cell lines, regardless of drug treatment.
Fig. 3.
Effect of siRNA-mediated knockdown of Spry2 on PLX4720-induced growth inhibition. (A–C) BRAF inhibitor-sensitive (A375P) and BRAF inhibitor-resistant (A375P/Mdr and SK-MEL-2) melanoma cells were transfected with siRNA pools targeting Spry2 or scrambled oligonucleotides for 24 h. The cells were then treated with PLX4720 in 96-well plates for three days. The cell growth was then evaluated using the MTT assay. The values represent the means ± SD of quadruplicate determinants from one of three representative experiments. **p<0.01 and *p<0.05 compared with the vehicle control, as determined by two-tail Student’s t-test. In upper inset, siRNA-mediated knockdown of Spry2 was confirmed by RT-PCR analysis. Numbers listed below each band indicate the intensity value quantified by the Kodak Molecular Imaging software, expressed as fold change. The intensity value observed in mock control cells was defined as 1.0.
Role of Raf-1 kinase in the rebound activation of ERK signaling in A375P/Mdr cells
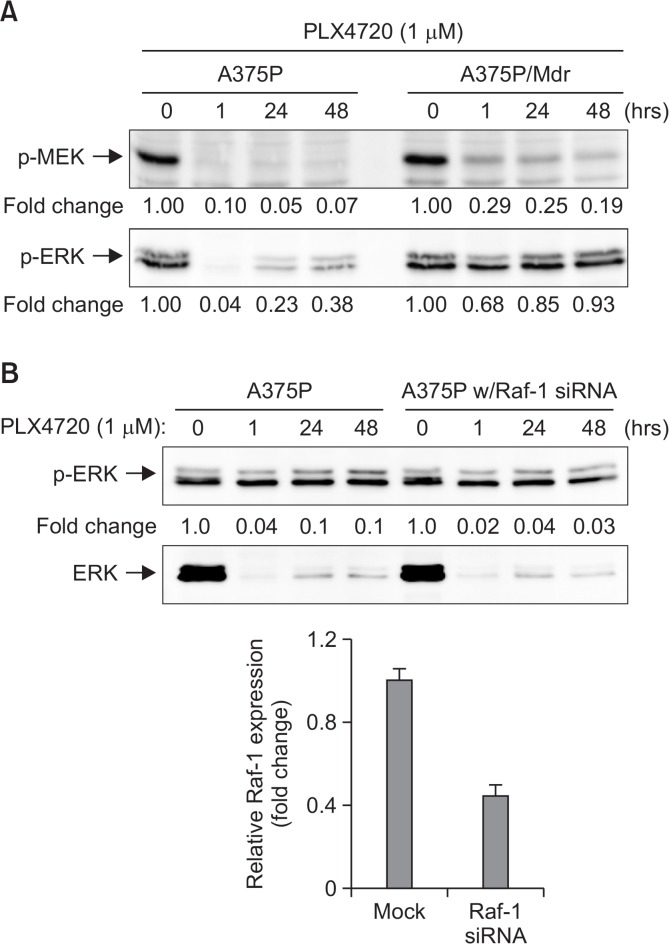
Re-activation of the RAF/MEK/ERK pathway has been observed in mutant BRAF melanoma cell lines with acquired resistance to BRAF inhibitors after continuous culture in the presence of a BRAF inhibitor (Aplin et al., 2011). As shown in Fig. 4A, long-term treatment with PLX4720 induced pERK reactivation following BRAF inhibition in A375P cells, indicating that the negative feedback induced by Spry2 may be bypassed in BRAF mutant-harboring melanoma cells due to the presence of the BRAF mutation. It has been reported that BRAF inhibition induces BRAF binding to Raf-1, leading to Raf-1 hyperactivation and consequently elevated MEK and ERK signaling (Heidorn et al., 2010). Thus, siRNA-mediated Raf-1 knockdown was used to further test the hypothesis that Raf-1 kinase plays a positive role in the BRAF inhibitor-induced reactivation of MEK-ERK signaling. As observed in Fig. 4B, the siRNA-mediated knockdown of Raf-1 attenuated the rebound activation of ERK stimulated by PLX4720 in A375P cells, strongly suggesting the positive role of Raf-1 kinase in ERK activation in response to BRAF inhibition.
Fig. 4.
Rebound activation of pERK signaling after long-term treatment with PLX4720. (A) Cell lysates were prepared from BRAF inhibitor-sensitive (A375P) and BRAF inhibitor-resistant (A375P/Mdr) melanoma cells treated with PLX4720 (1 μM) for the indicated times. (B) BRAF inhibitor-sensitive A375P cells were transfected with siRNA pools targeting Raf-1 or scrambled oligonucleotides for 24 h. The cells were then treated with PLX4720 for the indicated times. (A, B) The phosphorylated forms of MEK and ERK were detected by immunoblotting using anti-phospho-MEK and anti-phospho-ERK antibodies. The same blots were reprobed with anti-MEK and anti-ERK antibodies to confirm that the expression levels of MEK and ERK proteins were similar in all of the lanes. Numbers listed below each band indicate the intensity value quantified by the Kodak Molecular Imaging software, expressed as fold change. The intensity value observed in vehicle control cells was defined as 1.0. The data are representative of at least two independent experiments. In lower inset of (B), siRNA-mediated knockdown of Raf-1 was confirmed by real-time RT-PCR analysis. The Raf-1 expression was normalized to the β-actin expression level. The relative expression level in mock control was regarded as 1.0.
DISCUSSION
BRAF inhibitors have recently shown therapeutic promise for the treatment of metastatic melanoma harboring BRAF mutations (Holderfield et al., 2014). However, the therapeutic efficacy of BRAF inhibitor therapy is short in many patients due to the onset of acquired resistance to oncogenic BRAF inhibitors (Flaherty et al., 2010). Re-activation of the MAPK pathway has been accepted as a mechanism that contributes to the mechanism of acquired resistance to BRAF inhibitors after continuous culture in the presence of a BRAF inhibitor (Aplin et al., 2011). Sprouty proteins have previously been suggested as negative regulators of the MAPK pathway through interaction with Raf-1 (Gross et al., 2001). Our previous study showed evidence that enhancement of Raf-1 kinase activity, which occurs in parallel with a decrease in the interaction with Spry2, may contribute to the development of acquired resistance in Ras-NIH 3T3 cells (Ahn et al., 2011). Our findings also suggested that Spry2 did not directly regulate Raf-1 kinase activity but rather acted as a scaffolding protein that assists interactions between Raf-1 kinase and its direct regulators (Ahn et al., 2010). In this study, we found that Spry2 expression was higher in cells harboring the BRAF V600E mutation compared with cells with WT BRAF. This result is consistent with the results from other laboratories (Tsavachidou et al., 2004; Xu et al., 2010; Pratilas et al., 2012) and show that oncogenic BRAF mutations in melanoma lead to elevated Sprouty levels.
This paradoxical overexpression of the feedback regulator Spry2 in BRAF V600E-harboring tumors can be attributed to the inability of Spry2 to inhibit BRAF V600E activity (Brady et al., 2009). In fact, it has been found that Raf signaling is insusceptible to negative feedback in BRAF mutant-harboring tumors (Pratilas et al., 2009). In transformed cells, increased MAPK/ERK activity is associated with increased output of the pathway, leading to enhanced expression of transcription factors and negative feedback regulators, such as Spry2 (Pratilas et al., 2009). Constitutive activation of MAPK/ERK signaling by activated oncoproteins, such as BRAF V600E, is likely to result in constitutive negative feedback (Pratilas et al., 2009). These data suggest that Spry2 may be bypassed in melanoma cells by the presence of BRAF mutations. In particular, oncogenic BRAF mutants, such as BRAF V600E, is believed to induce active kinase conformations and thereby prevent Sprouty2 binding (Brady et al., 2009).
More interestingly, we found that Spry2 exhibits strongly reduced expression in A375P/Mdr cells with acquired resistance to BRAF inhibitors. Several researchers have reported that the Spry2 levels are markedly diminished after exposure to BRAF inhibitors in BRAF V600E cells (Joseph et al., 2010; Lito et al., 2012). In addition, Spry2 overexpression partially restored sensitivity to the BRAF inhibitor PLX4720 in two BRAF inhibitor-resistant cell lines (SK-MEL-2 and A375P/Mdr), irrespective of their BRAF mutational status. However, we found that the effect of Spry2 overexpression on cell proliferation was negligible in A375P cells. Although Spry2 overexpression caused a very weak but statistically significant increase in cell proliferation of A375P cells at 1 μM of PLX4720, the actual difference in cell growth rate between mock- and Spry2 overexpressing A375P cells was just 6%. In contrast, in A375P/Mdr and SK-MEL-2 cell lines, the actual difference in cell growth rate was greater than 20%. This result implies that the downregulation of Spry2 plays a critical role in the acquired resistance to the BRAF inhibitor. However, in our study, Spry2 overexpression in A375P/Mdr cells resulted in no significant change in the levels of pERK or pMEK. Lito et al. (Lito et al., 2012) also reported that the knockdown of Spry proteins does not affect EGFR-induced pERK. Unexpectedly, Spry2-silenced A375P cells were found to be more sensitive to BRAF inhibitors than control cells. Notably, a finding consistent with our results was reported by another research group that examined Spry2 feedback dysregulation in BRAF-mutant thyroid cancer (Dultz et al., 2013). In their study, the BRAF mutant-harboring cells were found to be significantly more sensitive to MAPK/ERK inhibition after Spry2 was knocked down by siRNA.
BRAF inhibition has been known to induce BRAF binding to Raf-1 in the presence of activated Ras, leading to Raf-1 hyperactivation and consequently paradoxical activation of MEK/ERK signaling (Heidorn et al., 2010). Our previous finding also showed that a BRAF inhibitor markedly induced the interaction between BRAF and Raf-1 proteins (Kim et al., 2012). In this study, we also observed a marginal rebound after initial inhibition of ERK phosphorylation in A375P cells treated with PLX4720. It has been reported that ERK rebound is dependent on the expression of Raf-1 containing a dimer that is resistant to Raf inhibition (Lito et al., 2012). In fact, we found that pERK rebound was significantly reduced after Raf-1 expression was reduced by siRNA in A375P cells treated with RAF inhibitors, strongly suggesting the positive role of Raf-1 kinase in ERK reactivation in response to BRAF inhibition.
Our data suggest that the induction of resistance to a BRAF inhibitor in BRAF V600E-harboring cells can be attributed to the inability of Spry2 to inhibit BRAF V600E activity. In summary, in BRAF inhibitor-resistant BRAF V600E-harboring cells, RAF signaling may be released from negative feedback inhibition through interaction with Spry2, leading to ERK rebound and, consequently, the induction of acquired resistance to BRAF inhibitors.
Acknowledgments
This work was supported by the Incheon National University Research Grant in 2014.
REFERENCES
- Ahn JH, Eum KH, Lee M. Spry2 does not directly modulate Raf-1 kinase activity in v-Ha-ras-transformed NIH 3T3 fibroblasts. BMB Rep. 2010;43:205–211. doi: 10.5483/BMBRep.2010.43.3.205. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Kim YK, Lee M. Decreased interaction of Raf-1 with its negative regulator Spry2 as a mechanism for acquired drug resistance. Biolmol Ther. 2011;19:174–180. doi: 10.4062/biomolther.2011.19.2.174. [DOI] [Google Scholar]
- Ahn JH, Lee M. Autophagy-dependent survival of mutant B-Raf melanoma cells selected for resistance to apoptosis induced by inhibitors against oncogenic B-Raf. Biomol Ther. 2013;21:114–120. doi: 10.4062/biomolther.2013.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Lee M. The siRNA-mediated downregulation of N-Ras sensitizes human melanoma cells to apoptosis induced by selective BRAF inhibitors. Mol Cell Biochem. 2014;392:239–247. doi: 10.1007/s11010-014-2034-2. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Kaplan FM, Shao Y. Mechanisms of resistance to RAF inhibitors in melanoma. J Invest Dermatol. 2011;131:1817–1820. doi: 10.1038/jid.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SC, Coleman ML, Munro J, Feller SM, Morrice NA, Olson MF. Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation. Cancer Res. 2009;69:6773–6781. doi: 10.1158/0008-5472.CAN-08-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2012;2:311–319. doi: 10.1158/2159-8290.CD-12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell G, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the B-Raf gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dultz LA, Dhar S, Ogilvie JB, Heller KS, Bar-Sagi D, Patel KN. Clinical and therapeutic implications of Sprouty2 feedback dysregulation in BRAF V600E-mutation-positive papillary thyroid cancer. Surgery. 2013;154:1239–1244. doi: 10.1016/j.surg.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross I, Bassit B, Benezra M, Licht JD. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J Biol Chem. 2001;276:46460–46468. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/S0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer. 2014;14:455–467. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, Halilovic E, Persaud Y, Xing F, Viale A, Tsai J, Chapman PB, Bollag G, Solit DB, Rosen N. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci USA. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Ahn SK, Lee M. Differential sensitivity of melanoma cell lines with differing B-Raf mutational status to the new oncogenic B-Raf kinase inhibitor UI-152. Cancer Lett. 2012;320:215–224. doi: 10.1016/j.canlet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, Huang A, Wong WL, Callahan MK, Merghoub T, Wolchok JD, de Stanchina E, Chandarlapaty S, Poulikakos PI, Fagin JA, Rosen N. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McGettigan S. Dabrafenib: a new therapy for use in BRAF-mutated metastatic melanoma. J Adv Pract Oncol. 2014;5:211–215. [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, Salton M, Dahlman KB, Tadi M, Wargo JA, Flaherty KT, Kelley MC, Misteli T, Chapman PB, Sosman JA, Graeber TG, Ribas A, Lo RS, Rosen N, Solit DB. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, Rosen N. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA. 2009;106:4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Xing F, Solit DB. Targeting oncogenic BRAF in human cancer. Curr Top Microbiol Immunol. 2012;355:83–98. doi: 10.1007/82_2011_162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, Haydu LE, Mijatov B, Becker TM, Boyd SC, Howle J, Saw R, Thompson JF, Kefford RF, Scolyer RA, Long GV. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- Sabbatino F, Wang Y, Wang X, Ferrone S, Ferrone CR. Emerging BRAF inhibitors for melanoma. Expert Opin. Emerg. Drugs. 2013;18:431–443. doi: 10.1517/14728214.2013.842975. [DOI] [PubMed] [Google Scholar]
- Tsavachidou D, Coleman ML, Athanasiadis G, Li S, Licht JD, Olson MF, Weber BL. SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Res. 2004;64:5556–5559. doi: 10.1158/0008-5472.CAN-04-1669. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhou JL, Cohen M, Bar-Sagi D, Patel KN. Spry2 expression correlates with BRAF mutation in thyroid cancer. Surgery. 2010;148:1282–1287. doi: 10.1016/j.surg.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Yusoff P, Lao DH, Ong SH, Wong ESM, Lim J, Lo TL, Leong F, Fong CW, Guy GR. Sprouty2 inhibits the Ras/MAP Kinase pathway by inhibiting the activation of Raf. J Biol Chem. 2002;277:3195–3201. doi: 10.1074/jbc.M108368200. [DOI] [PubMed] [Google Scholar]