Abstract
Betulinic acid, a pentacyclic triterpene isolated from Jujube tree (Zizyphus jujuba Mill), has been known for a wide range of biological and medicinal properties such as antibacterial, antimalarial, anti-inflammatory, antihelmintic, antinociceptive, and anticancer activities. In the study, we investigated the antiviral activity on influenza A/PR/8 virus infected A549 human lung adenocarcinoma epithelial cell line and C57BL/6 mice. Betulinic acid showed the anti-influenza viral activity at a concentration of 50 μM without a significant cytotoxicity in influenza A/PR/8 virus infected A549 cells. Also, betulinic acid significantly attenuated pulmonary pathology including increased necrosis, numbers of inflammatory cells and pulmonary edema induced by influenza A/PR/8 virus infection compared with vehicle- or oseltamivir-treated mice in vivo model. The down-regulation of IFN-γ level, which is critical for innate and adaptive immunity in viral infection, after treating of betulinic acid in mouse lung. Based on the obtained results, it is suggested that betulinic acid can be the potential therapeutic agent for virus infection via anti-inflammatory activity.
Keywords: Betulinic acid, Zizyphus jujuba, Influenza A/PR/8, A549, Inflammation
INTRODUCTION
Influenza virus is (−)-strand RNA virus containing viral genome consists of eight segments of single-strand RNA, and is a common cause of respiratory infection known as “the flu”. Influenza virus is included in Orthomyxoviridae family and known to have 3 different serotypes including A, B, and C. Among them, serotype B and C were known to infect only human, but serotype A shows broad-spectrum of infection in mammals and even in poultry (Slemons et al., 1974; Webster et al., 1992).
Influenza A virus have two surface proteins, hemagglutinin (HA) and nuraminidase (NA), and sub-classified by the antigenicity of HA and NA. HA was known to help virus to attach cells and NA is glycoside hydrolase enzyme that cleave the glycosidic linkages of neuraminic acids. Recently, several inhibitors targeting NA were introduced as anti-influenza drug, and known to efficiently prevent the spreading of virus, which includes oseltamivir and zanamivir. However, recently the occurrence of resistant virus against NA inhibitors was reported, and which make us to find other antiviral candidates against influenza virus (Ward et al., 2005).
Zizyphus jujuba Mill, (Jujube tree) is indigenous to China over 4000 years and is widely distributed in Europe, eastern Asia, and Australia (Huang et al., 2008). Dry fruits of Z. jujuba have been utilized as poplar food and tea additives or favor for a long time (Li et al., 2007). The extract of Z. jujuba is traditionally recognized as an outstanding source of anorexia, fatigue, and loose stools (Guo et al., 2010b). To date, it has been revealed that Z. jujuba contains the wide range of constituents including flavonoids (Pawlowska et al., 2009), triterepenic acids (Guo et al., 2010a), phenolic acids and amino acids (Choi et al., 2011).
Betulinic acid (BeA) is pentacyclic lupane-type triterpene that are widely distributed throughout the higher plant (Rastogi et al., 2015). The jujube tree (Zizyphus spp.) is known as one of the most extensively stated sources of BeA produced in considerable quantity (Dubey and Goel, 2013). In recent years, BeA has been reported to show a wide range of biological and medicinal properties such as of antibacterial, anti-malarial, anti-inflammatory, antihelmintic, antinociceptive, and anticancer activities of BeA (Yogeeswari and Sriram, 2005; Rastogi et al., 2015). Especially, its derivatives are a promising new therapeutic agent for the treatment of HIV infection (Dang et al., 2013). The interest in BeA with antiviral activities on a few viruses led to examine it against influenza A/PR/8 virus infected in A549 cells and C57BL/6 mice. The aim of the present study was compare the anti-influenza virus activity of BeA in vitro and in vivo models.
MATERIALS AND METHODS
Isolation of BeA from Z. jujuba
The pulverized dried roots (14.5 kg) of Z. jujuba were macerated with MeOH (2×60 L) for each one week at room temperature. The MeOH extract was concentrated in vacuo to give a crude extract (0.5 kg). The concentrated extract was suspended in H2O and acidified with 1N HCl to pH 3. The acidic solution was extracted with EtOAc to yield 186.2 g of EtOAc fraction. The aqueous residue was basified with NaOH to pH 9 and extracted with CHCl3 to provide an alkaloid fraction (1.7 g) which was not applied in this study. The EtOAc fraction was subjected to a normal silica gel CC with a mixture of CHCl3 and MeOH (100:1 to 1:1) and give 10 subfractions (EA-1-10). Among them, the EA-3 fraction (50.1 g) was fractioned by a normal silica gel CC with a mixture of CHCl3 and MeOH (100:1 to 5:1), giving seven fractions. Fraction 3 and 4 yielded BeA (betulinic acid, 5.4 g) by the re-crystallization with 100% MeOH.
Betulinic acid (BeA): whitish, amorphous powder, 1H NMR (400 MHz, C5D5N) : δ4.95 (1H, s, H-29a), 4.77 (1H, s, H-29b), 3.54 (1H, td, J=11.4, 5.0 Hz), 3.46 (1H, t, J=7.8 Hz, H-3), 2.74 (1H, td-like, H-13), 2.68 (1H, m, H-11), 2.63 (1H, dt, J=12.7, 3.0 Hz. H-16a), 2.26 (1H, m, H-22a), 2.24 (1H, m, H-21a), 1.93 (1H, m, H-12), 1.90 (1H, m, H-15a), 1.87 (1H, m, H-2), 1.79 (3H, s, H-30), 1.76 (1H, t, J=11.4, H-18), 1.66 (2H, dt, J=13.0 Hz, 3.0, H-1), 1.59 (1H, m, H-22b), 1.56 (2H, m, H-6a and H-16b), 1.52 (1H, m, H-21b), 1.42 (1H, m, H-7a), 1.41 (1H, m, H-6b), 1.40 (1H, m, H-7b), 1.39 (1H, m, H-9), 1.28 (1H, m, H-15b), 1.23 (3H, s, H-23), 1.07 (3H, s, H-27), 1.06 (3H, s, H-24), 1.01 (3H, s, H-26), 0.83 (3H, s, H-25), 0.82 (1H, m, H-5) and 13C NMR (100 MHz, C5D5N) : δ178.9 (C-28), 151.3 (C-20), 109.9 (C-29), 72.3 (C-3), 56.6 (C-17), 55.9 (C-5), 51.0 (C-9), 49.8 (C-18), 47.8 (C-19), 42.9 (C-14), 41.1 (C-8), 39.5 (C-1), 39.3 (C-4), 38.6 (C-13), 37.6 (C-22), 37.5 (C-10), 34.8 (C-7), 32.9 (C-16), 31.2 (C-21), 30.3 (C-15), 28.7 (C-23), 28.3 (C-2), 26.1 (C-12), 21.2 (C-11), 19.5 (C-30), 18.8 (C-6), 16.4 (C-24 and C-25), 16.3 (C-26), 14.9 (C-27)
Virus, cells, and reagents
Influenza A/PR/8 virus was obtained from provided by ATCC (American Type Culture Collection, Manassas, VA, USA). A549 cells were purchased from ATCC (Rockville, MD, USA) and maintained in Dulbacco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution. Antibiotic-antimycotic solution, FBS, and DMEM were supplied by Gibco BRL (Invitrogen Life Technologies, Karlsruhe, Germany). TPCK-Trypsin was purchased from Pierce (Thermo Fisher Scientific, Rockford, IL, USA). Both sulforhodamane B (SRB) and oseltamivir were purchased from Sigma-Aldrich (St. Louis, MO, USA). The tissue culture plates were purchased from Falcon (BD Biosiences, San Jose, CA, USA). All other chemicals were of reagent grade.
In vitro antiviral activity assay
Antiviral activity was evaluated by the SRB method using cytophathic effect (CPE) reduction, as previously reported (Song et al., 2014). A549 cells were seeded in a 96-well plate at a concentration of 2×104 cells per well and were incubated for 24 h. Next day, the diluted virus suspension containing TPCK-trypsin of 1 μg/mL was placed in each well and were added the selected concentration of BeA. Virus-infected non-compound-treated cells were used as viral controls, while non-infected non-compound-treated cells were used as cell controls. After incubation for 2 days, A549 cells were washed with PBS, and ice-cold 70% acetone was added and incubated for 30 min at −20°C. After removing the acetone, 96-well plates were dried in a dry oven for 30 min, after which we added 0.4% (w/v) SRB in 1% acetic acid solution to each well for 30 min at room temperature. SRB was then removed, and the plates were washed with 1% acetic acid before oven-drying. After drying for 1 day, SRB was then solubilized with 10 mM un-buffered Tris-based solution, and the absorbance was then read at 540 nm using a VERSAmax microplate reader (Molecular Devices, Palo Alto, CA, USA) with a reference absorbance at 620 nm. The antiviral activity of each test compound in influenza A/PR/8 virus-infected cells was calculated as a percentage of the corresponding untreated control.
Mice and virus infection
C57BL/6 mice between 6 and 7 weeks of age were purchased from SPL laboratory animal company (KOATCH Bio, Pyeongtaek, Korea). Mice were infected intranasally with 5×103 pfu/30 μl of influenza A/PR/8 virus. Mice were maintained in animal facility at the Kangwon National University All experiments were approved by the Institutional Animal Care and Use Committees of the Kangwon National University.
Histology and scoring
Lung tissue was washed with PBS containing and fixed in 4% formaldehyde for 1 hour at 4°C. The tissues were dehydrated by gradually soaking them in alcohol and xylene and then embedded in paraffin. The paraffin-embedded specimens were cut into 10 μm sections, stained with hematoxylin and eosin (H&E) and viewed with a digital light microscope (Olympus, Tokyo, Japan). As previously described (Shim et al., 2007), we used a scoring system to evaluate the level of lung tissue destruction, epithelial cell layer damage, polymorphonuclear cell infiltration into the site, and alveolitis.
Cytokine analysis
The levels of interferon gamma (IFN-γ), interleukin-1b (IL-1b), and tumor necrosis factor-α (TNF-α) were measured by mouse ELISA Ready-SET-GO kit (eBioscience), according to the manufacturer’s instructions.
Statistics
The Kaplan-Meier method was used to determine the statistical significance of differences in survival time. We performed the Log-Rank test (Mantel-Cox), using SPSS 12.0K for Windows. To compare the differences between two groups, Student’s t-test was used. To compare multiple groups, we carried out one-way ANOVA, followed by the Tukey-HSD post hoc test.
RESULTS
Isolation and Determination of BeA from Z. jujuba
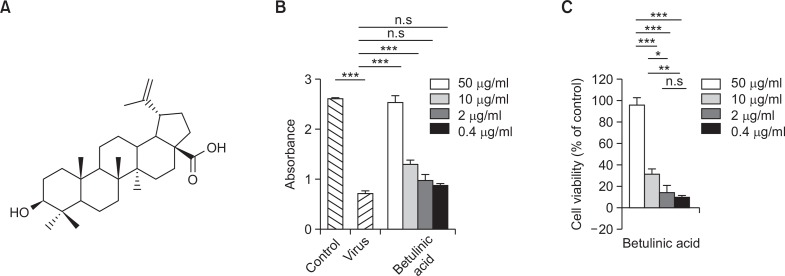
BeA were isolated from the methanolic extract of Z. jujuba using a series of column chromatography followed by the recrystallization. The structure of BeA was determined on the basis of NMR spectroscopic data (Fig. 1A). In 13C NMR spectrum, the pattern of 30 carbon signals including the characteristic peaks at δC 151.3 (C-20) and 109.9 (C-29) exhibited the presence of the lupane-type triterpene having an olefinic bond. Additionally, the signals at δC178.9 (C-28) and 78.1 (C-3) showed that this compound bear a carboxylic and hydroxyl moieties, respectively. After confirming the location of characteristic groups by HMBC correlation, it was determined as betulinic acid (BeA) (Yili et al., 2009).
Fig. 1.
Antiviral activity BeA against influenza A/PR/8 virus in A549 cells. Antiviral activity of BeA was assessed against influenza A/PR/8 virus in A549 cells. The indicated concentrations of BeA was determined using a concentration series of 0.4, 2, 10, and 50 μM. After 48 h of incubation, the antiviral activity was investigated by CPE reduction assay using SRB. (A) The structure of betulinic acid. (B) Absorbance in each well was measured using microplate reader. ***p<0.0001 and n.s. for not significant. (C) The percent value was calculated by absorbance value as mentioned in Materials and Methods. Results are presented as the mean ± SD of the absorbance and percentage values obtained from three independent experiments carried out in triplicate. *p<0.001; **p<0.03; ***p<0.0001 and n.s. for not significant.
Antiviral activity of BeA against influenza A/PR/8/34 virus
The antiviral activities of BeA against influenza A/PR/8/34 were assessed using the SRB method, which monitors the alteration of CPE induced by virus infection. The antiviral assays demonstrated that BeA possessed strong antiviral activity of about 98% against influenza A/PR/8/34 virus at the concentration of 50 μM and antiviral activity of about 30% at the same virus at the concentration 10 μM (Fig. 1B, 1C). BeA was not toxic to A549 cells with cell viability of about 100% at the concentration of 50 μM (Data not shown).
Antiviral activity of BeA against influenza A/PR/8 virus infected mice
To confirm the anti-influenza activity of BeA in vivo, BeA or oseltamivir were administered to mice as follows: BeA (10 mg/kg/dose) or oseltamivir (30 mg/kg/dose), dissolved in PBS, was intraperitoneally administered daily for 7 days after influenza virus infection. The mice were infected intranasally with 30 μL of influenza A/PR/8 virus suspension 5×103 plaque forming unit (PFU).
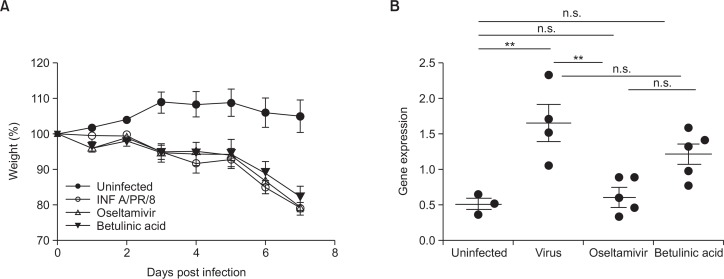
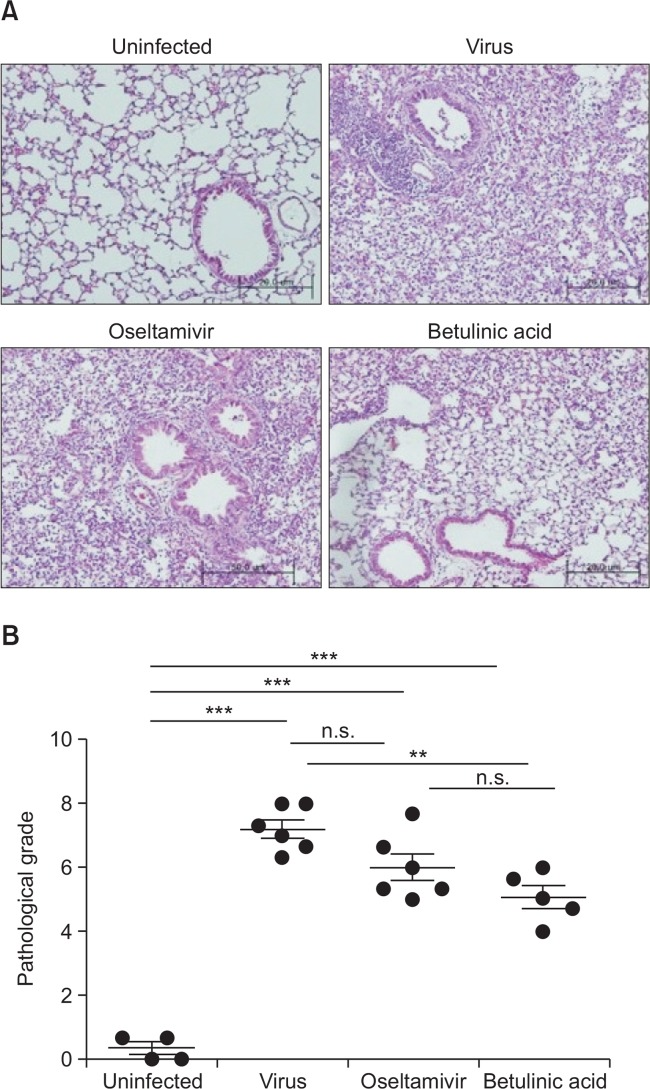
We monitored daily the body weight of influenza A/PR/8 virus-infected mice for all experiments to compare anti-influenza virus effect of BeA with oseltamivir treatment. However, BeA and oseltamivir did not attenuated body weight loss induced by infection of influenza A/PR/8 virus (Fig. 2A). Further evidence of the anti-influenza effects of BeA on influenza A/PR/8 virus-infected mice lung tissue was provided viral gene expression by real-time PCR analysis. However, BeA did not influence on influenza A/PR/8 virus replication (Fig. 2B). Interestingly, however, influenza infected mice treated with BeA significantly attenuated pulmonary pathology including increased necrosis, numbers of inflammatory cells and pulmonary edema induced by influenza A/PR/8 virus infection, assessed at 7 days post infection, as compared with vehicle- or oseltamivir-treated mice (Fig. 3A). In addition, there are significant difference in scoring system to evaluate the level of lung tissue destruction, epithelial cell layer damage, polymorphonuclear cell infiltration into the site, and alveolitis as compared with influenza A/PR/8 virus infected group (Fig. 3B).
Fig. 2.
Active concentration of BeA was determined. (A) 5×103 pfu/30 μl of influenza A/PR/8 virus was inoculated. The indicated treatment was started after infection. BeA and oseltamivir were orally administerd daily for 7 days and the survival rate of mice were measured. The body weights of mice were measured. (B) Influenza A/PR/8 virus gene expressions in the lung tissue were detected by real-time PCR 7 days after influenza A/PR/8 virus infection. **p<0.05 and n.s. for not significant.
Fig. 3.
Anti-influenza virus activity of BeA and oseltamivir in the mice. Mice were infected with 5×103 pfu/30 μl of influenza A/PR/8 virus, orally administered with BeA and oseltamivir for 7 days after virus infection. Mice were sacrificed at 7 days post infection, and lung sections were prepared as described in Materials and Methods. (A) Representative H&E stained samples of lung section from uninfected or influenza A/PR/8 virus-infected mice were shown at 200× magnification. Infected mice were treated with BeA or oseltamivir. (B) Pathological grade of mice which were administrated orally for 7 days after infection. **p<0.01; ***p<0.001, and n.s. for not significant.
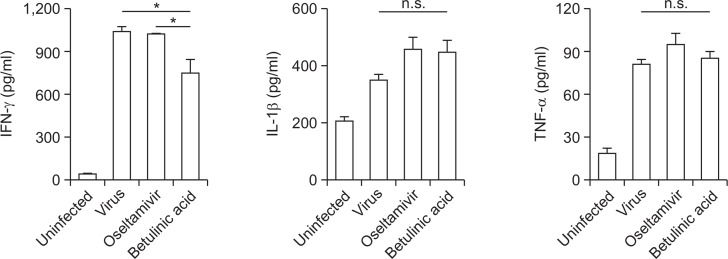
Alteration of pulmonary cytokine signature in PR8-infeced mice after the treatment of BeA and oseltamivir
Virus-induced cytokines conduct a major role in recruiting leukocytes to the site of infection and activating innate immune responses to induce inflammation. Despite their protective roles, severe inflammation induced by cytokine storm was known to be associated with influenza-induced pulmonary pathology. To evaluate cytokines production at protein levels, mice were infected and treated by the same scheme as before and 6 hrs after final administration of BeA and oseltamivir, lungs from mice were obtained. The levels of cytokines including IFN-γ, IL-1β and TNF-α in lungs were measured with ELISA. The intranasal infection of influenza A/PR/8 virus increased the levels of IFN-γ, IL-1β and TNF-α at day 7 post infection as compared with PBS-treated control mice. Oseltamivir did not induce significant changes in the IFN-γ, IL-1β and TNF-α levels over vehicle treatment in influenza A/PR/8 virus-infected mice. Interestingly, at day 7 post infection, although BeA did not reduce the level of IL-1β and TNF-α, it significantly decreased the levels of IFN-γ as compared with that of oseltamivir treatment (Fig. 4).
Fig. 4.
Cytokine quantitation changed from BeA or oseltamivir in mice. Lung inflammation cytokine levels were evaluated according to ELISA. C57BL/6 mice were intranasally infected with 5×103 pfu/30 μl of influenza A/PR/8 virus, and orally administered with BeA (10 mg/kg) and oseltamivir (5 mg/kg). Orally administration daily for 7 days and sacrificed in mice after 6 hrs. Proinflammatory cytokines and chemokine were measured in lung by ELISA. *p<0.02 and n.s. for not significant.
DISCUSSION
The present study demonstrated that BeA inhibits the proliferation of influenza A/PR/8 virus with a dose dependent manner (0.4–50 μM) in A549 cells without significant cytotoxicity. After confirming antiviral activity of BeA in vitro model, we further studied whether BeA exerts sufficient therapeutic efficacy in influenza virus infected mouse model. Although BeA did not restore the body weight loss after the infection of influenza A/PR/8 virus similar to oseltamivir used as the positive control, the histochemical staining result showed BeA significantly reduced the inflammation and pulmonary edema induced by influenza virus.
Also, the change of IFN-γ level after treating of BeA in mouse lung supported that BeA can be the potential therapeutic agent for the inflammation by virus infection. IFN-γ has been known to have critical for innate and adaptive immunity in some viral, bacterial and protozoal infections. Collectively, these results suggested that BeA decrease inflammatory cytokine levels, especially IFN-γ, and help influenza A/PR/8 virus-infected mice to rapidly recover from severe pulmonary inflammation. Although further studies are necessary to clarify the detailed anti-influenza mechanisms, it is suggested that BeA can be the potential therapeutic agent for treating of influenza viral infection via anti-inflammation.
Acknowledgments
This work was carried out with the support of Forest Science and Technology Projects (S121214L120100) provided by Korea Forest Service. This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2014R1A1A2003820).
REFERENCES
- Choi SH, Ahn JB, Kozukue N, Levin CE, Friedman M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of Jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J Agr Food Chem. 2011;59:6594–6604. doi: 10.1021/jf200371r. [DOI] [PubMed] [Google Scholar]
- Dang Z, Ho P, Zhu L, Qian K, Lee KH, Huang L, Chen CH. New betulinic acid derivatives for bevirimat-resistant human immunodeficiency virus type-1. J Med Chem. 2013;56:2029–2037. doi: 10.1021/jm3016969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey KK, Goel N. Evaluation and optimization of downstream process parameters for extraction of betulinic acid from the bark of Ziziphus jujuba L. Scientific Wolrd Journal. 2013;2013:469674. doi: 10.1155/2013/469674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Duan JA, Tang YP, Yang NY, Qian DW, Su SL, Shang EX. Characterization of triterpenic acids in fruits of ziziphus species by HPLC-ELSD-MS. J Agr Food Chem. 2010a;58:6285–6289. doi: 10.1021/jf101022p. [DOI] [PubMed] [Google Scholar]
- Guo S, Duan JA, Tang YP, Zhu ZH, Qian YF, Yang NY, Shang EX, Qian DW. Characterization of nucleosides and nucleobases in fruits of Ziziphus jujuba by UPLC-DAD-MS. J Agr Food Chem. 2010b;58:10774–10780. doi: 10.1021/jf102648q. [DOI] [PubMed] [Google Scholar]
- Huang YL, Yen GC, Sheu F, Chau CF. Effects of water-soluble carbohydrate concentrate from Chinese jujube on different intestinal and fecal indices. J Agr Food Chem. 2008;56:1734–1739. doi: 10.1021/jf072664z. [DOI] [PubMed] [Google Scholar]
- Li JW, Fan LP, Ding SD, Ding XL. Nutritional composition of five cultivars of chinese jujube. Food Chem. 2007;103:454–460. doi: 10.1016/j.foodchem.2006.08.016. [DOI] [Google Scholar]
- Pawlowska AM, Camangi F, Bader A, Braca A. Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chem. 2009;112:858–862. doi: 10.1016/j.foodchem.2008.06.053. [DOI] [Google Scholar]
- Rastogi S, Pandey MM, Kumar Singh Rawat A. Medicinal plants of the genus Betula-Traditional uses and a phytochemical-pharmacological review. J Ethnopharmacol. 2015;159:62–83. doi: 10.1016/j.jep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim DH, Chang SY, Park SM, Jang H, Carbis R, Czerkinsky C, Uematsu S, Akira S, Kweon MN. Immunogenicity and protective efficacy offered by a ribosomal-based vaccine from Shigella flexneri 2a. Vaccine. 2007;25:4828–4836. doi: 10.1016/j.vaccine.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Slemons RD, Johnson DC, Osborn JS, Hayes F. Type-A influenza viruses isolated from wild free-flying ducks in California. Avian Dis. 1974;18:119–124. doi: 10.2307/1589250. [DOI] [PubMed] [Google Scholar]
- Song J, Yeo SG, Hong EH, Lee BR, Kim JW, Kim J, Jeong H, Kwon Y, Kim H, Lee S, Park JH, Ko HJ. Antiviral activity of hederasaponin B from Hedera helix against enterovirus 71 Subgenotypes C3 and C4a. Biomol Ther. 2014;22:41–46. doi: 10.4062/biomolther.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J. Antimicrob. Chemother. 2005;55(Suppl 1):i5–i21. doi: 10.1093/jac/dki018. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yili A, Mutalipu, Aisa HA, Isaev MI. Betulinic acid and sterols from Astragalus altaicus. Chem Nat Compd. 2009;45:592–594. doi: 10.1007/s10600-009-9377-z. [DOI] [Google Scholar]
- Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]