Abstract
Functional dyspepsia (FD) is a prevalent idiopathic upper gastrointestinal (GI) disorder characterized by diverse symptomatology including epigastric pain or discomfort, postprandial fullness, and early satiety. Although its pathophysiological mechanisms have not yet been fully established, the available studies suggest that the etiology of FD is invariably multifactorial. Benachio-F® (BF) is a proprietary liquid formulation of 7 herbal extracts that has been proposed to address this multifactorial etiology using multi-drug phytotherapy. The pharmacological effects of BF, in comparison with those of two other herbal products (Whalmyungsu®; WM and Iberogast®; IB) were evaluated in rats. In a laparotomy-induced rat model of delayed GI transit, BF significantly accelerated the delayed gastric emptying caused by morphine, apomorphine, and cisplatin, and also significantly increased mean gastric transit, as compared to the control animals. BF markedly increased gastric accommodation in rats and produced higher gastric volume values than did the control treatment. The effects of BF were generally comparable or superior to those of WM and IB in these models. Furthermore, BF significantly stimulated biliary flow, as compared to the control treatment. These results indicated that BF might have great potential as an effective phytotherapeutic agent capable of reducing GI symptoms and increasing quality of life in FD patients.
Keywords: Functional dyspepsia, Benachio-F®, Gastric emptying, Gastric accommodation, Gastric transit, Bile secretion
INTRODUCTION
Functional dyspepsia (FD) is a clinical syndrome characterized by a diverse range of persistent or recurrent upper abdominal symptoms, with no demonstrable organic or structural lesions and no specific underlying etiology. This heterogeneous symptom complex includes epigastric pain/discomfort, bloating, early satiation, postprandial heaviness/fullness, anorexia, and belching. There are no specific diagnostic biomarkers for FD. It is diagnosed symptomatically after the exclusion of organic diseases like peptic ulcers, gastroesophageal reflux disease, and malignancies (Stanghellini et al., 2003; Halder and Talley, 2007; Piessevaux et al., 2009). The Rome criteria III, an international system that attempts to categorize functional gastrointestinal (GI) disorders, provided diagnostic criteria for FD whereby patients must have had one or more of the following symptoms for the past 3 months, with symptom onset at least 6 months prior to diagnosis: postprandial fullness, early satiety, epigastric pain or burning, and no evidence of structural disease that could explain the symptoms (including any condition detected by upper endoscopy) (Thompson, 2006). FD is further sub-classified as: (a) postprandial distress syndrome (PDS) characterized by meal-induced fullness and satiety; or (b) epigastric pain syndrome (EPS), characterized by epigastric pain or burning. These criteria, however, do not include nausea and vomiting as cardinal FD symptoms (Suzuki et al., 2006; Tack et al., 2006).
Several pathophysiological mechanisms have been proposed to contribute to FD, including abnormal gastroduodenal motility, central and autonomic nervous system dysregulation, neuro-hormonal dysfunction, infection by H. pylori and other GI organisms, psycho-somatic morbidities, visceral hypersensitivity, and genetic susceptibility, although the specific etiology remains to be elucidated (Mizuta et al., 2006). These mechanisms, either alone or in combination, might be responsible for heterogeneous and multifactorial dyspeptic symptoms, which has made it difficult to establish an optimum and uniform therapeutic strategy for FD. However, a major patient subgroup shows symptoms associated with GI sensori-motor abnormalities such as delayed gastric emptying, impaired gastric accommodation or gastric misdistribution of ingested material, and abnormal gastroduodenal motility and reflux. These findings have led to studies of the efficacy of prokinetic drugs such as D2 receptor antagonists, 5-HT4 agonists and CCK receptor antagonists in FD; in addition, acid suppressant drugs including H2 receptor antagonists and proton-pump inhibitors have been employed, along with anti-H. pylori agents, anticholinergics, and laxatives for symptom relief.
Although a plethora of agents have been evaluated, no medication is currently approved in the US, Canada, or the European Union for the treatment of FD. Prokinetic compounds and other drugs affecting GI motility have exhibited relatively modest success in several randomized controlled trials and are thus prescribed empirically. These prokinetic drugs improve FD symptoms by reducing gastroesophageal reflux, promoting fundus relaxation and gastric emptying, and improving gastric regulation. However, these agents come with their own disadvantages, mostly arising from their limited clinical effects and unwanted side-effects. For example, despite earlier promising results, itopride produced only slightly greater symptom alleviation than placebo treatment in a Phase III clinical trial (Holtmann et al., 2006). The 5-HT4 agonists, cisapride and tegaserod were withdrawn from the market due to their high risk of prolonging the QT interval and producing severe cardiovascular side effects (arrhythmias) (Wysowski et al., 2001). The D2 antagonist, metoclopramide, is associated with a high incidence of central nervous system toxicities, while domperidone caused hyperprolactinemia.
This has led to studies exploring whether multi-target phytotherapy could provide effective alleviation of FD symptoms. This could represent an alternative to monodrug therapy, which usually requires high doses. Multi-drug therapy has been claimed to confer the advantages of reducing or eliminating side-effects (probably due to the lower doses employed) and providing synergistic effects (Wagner, 2006). Indeed, the multi-extract preparation, Iberogast® (IB), has been shown to be effective in reducing the symptoms of FD, with minimal adverse events (Madisch et al., 2004).
The proprietary liquid Benachio-F® (BF) formulation includes 7 herbal extracts from Corydalis Tuber, Foeniculi Fructus, Cinnamomi Cortex, Atractylodis Rhizoma, Zingiberis Rhizoma, Glycyrrhizae Radix and Citri Unshiu Pericarpium, which are used in traditional oriental medicines and have long been employed to treat functional GI disorders. BF is approved by the Korea Food and Drug Administration as an over-the-counter treatment for dyspeptic syndrome. Despite its long-standing use for dyspeptic symptoms, no animal studies have been performed to investigate the potential mechanisms underlying the beneficial effects of BF.
The present study aimed to examine the pharmacological effects of BF on GI motor functions relevant to FD therapy. In particular, the effects of BF on gastric emptying, GI transit, gastric accommodation, and bile secretion in rat models were compared with those of two other herbal products (Iberogast®; IB and Whalmyungsu®; WM).
MATERIALS AND METHODS
Materials
Cisplatin, morphine, apomorphine, phenol red, and hydroxypropylmethyl cellulose (HPMC) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fluorescein isothiocyanate (FITC) and dextran were purchased from Choongwae Pharma (Seoul, South Korea) and Fluka (Tokyo, Japan), respectively. IB (Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany; lot No. 431254; expiration date: 2016/09/07), BF (Dong-A Pharmaceutical Co. Ltd., Seoul, South Korea; lot No. 1403001; expiration date: 2016/03/02), and WM (Dong-Wha Pharmaceutical Co. Ltd., Seoul, South Korea; lot No. D067; expiration date: 2016/05/15) were used as received after vacuum concentration to the desired volume and concentration.
Animals and experimental procedure
Male Sprague-Dawley rats (200–220 g) were purchased from Central Laboratory Animal Inc. (Seoul, South Korea). The rats were individually housed in single, air-conditioned boxes under adequate temperature and humidity control with a 12-h light-dark cycle and access to rat food pellets. All animal care and experimental procedures were conducted according to the principles enunciated in the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources, National Research Council (http://www.nsa.edu/nrc), and approved by the Institutional Animal Ethical Committee, Yeungnam University, South Korea (approved protocol number: 2014-006). All rats were used after a week of acclimatization and were fasted for 12 h with free access to water prior to the experiments. Animals were orally administered 100 mg/kg body weight of BF, IB, or WM in a volume of 1 ml, or 3% (w/v) HPMC (control).
Gastric emptying
Gastric emptying was measured according to the method described by Ozaki and Sukamoto (Ozaki and Sukamoto, 1999) with some modifications. Each group of 6 rats were given 2 ml of a semi-solid meal 50 min after drug administration, and simultaneously injected subcutaneously with either morphine (0.05 mg/kg) or apomorphine (0.05 mg/kg). After 50 min, the animals were sacrificed, and the weights of their stomachs and stomach contents were measured in order to determine gastric emptying using the equation shown below.
Alternatively, the animals were given a 2 ml liquid meal containing 0.05% phenol red 60 min after drug administration, and simultaneously injected intraperitoneally with 10 mg/kg cisplatin. The amount of phenol red remaining in the stomach 20 min later was measured (optical density [OD] at 560 nm) and gastric emptying was calculated using the method described by Yoshida et al. (1989):
GI transit
Postoperative paralytic ileus (POI) was induced in the rats using a previously reported method (De Winter et al., 1998). The rats were fasted overnight with free access to water. They were then anesthetized with ether, and a laparotomy was performed using a 3-cm midline incision. The small intestine and caecum were gently pulled out of the abdominal cavity and furled like a fan on two moist gauzes covering the abdomen of the rat. The small intestine was gently manipulated (mechanical stimulation) with the fingers for 10 min. After manipulation, the small intestine and caecum were replaced in the abdominal cavity, and the surgical wound was sutured.
GI transit was measured using the method of Kalff et al. (1999), with some modification. Rats (n=6 in each group) were given a test meal (FITC-dextran) by oral gavage, 60 min after drug administration. After a further 15 min, the animals were sacrificed. The small intestine was carefully removed and divided into 10 equal segments. The FITC-dextran concentration in each segment was measured using a microplate reader (POLARstar OPTIMA, BMG Labtech), expressed as a fraction of the total tracer recovered, and presented as the mean geometric center (MGC) of distribution.
Gastric accommodation
Five male Sprague-Dawley rats (250 g) were anesthetized with urethane (3 mg/kg, i.p, Sigma) and a laparotomy was performed before drug administration. The trachea was cannulated with PE-90 tubing to maintain an open airway. A mid-line laparotomy was performed, and a deflated balloon (with attached tubing) was inserted into the stomach via a small incision into the fundus and secured with silk suture. A rigid, non-compliant polyethylene tubing connected the barostat to the balloon then tied to the polyethylene tubing with a silk suture (size 0 USP, Surgical Specialties Corporation, Reading, PA, USA).
The barostat (Distender Series IIR, G&J Electronics, Toronto, Canada) was used to confirm that the intragastric balloon was maintained at the specified operating pressure and gastric accommodation was defined as the mean volume during this ramp distention. intragastric balloon pressure was increased by 2-mmHg increments every 5 min, starting after an overnight fast, the gastric volume was recorded via the intragastric balloon for 5 min at a pressure of 1 mmHg (baseline). Data were acquired using a custom designed program (Protocol Plus, G&J Electronics, Toronto, Canada) onto a computer.
Bile secretion
Following a 15-min stabilization period, three different groups of rats (n=6–7/group) received drug (BF, WM, or IB) and a control group (n=7) received saline only, before being anesthetized. After 30 min, the bile duct was surgically exposed by a midline incision and cannulated with a polyethylene cannula inserted at the sphincter of Oddi. Bile secretions (combined bile-pancreatic secretions) were collected from each rat into pre-weighed vials at 15-min intervals for a period of 120 min.
Data analysis
Results were expressed as mean ± standard deviation (SD). Differences in the data were evaluated by Student’s t-test and p-values <0.05 were considered to indicate a statistically significant difference between the study groups.
RESULTS
Effects of BF on gastric emptying
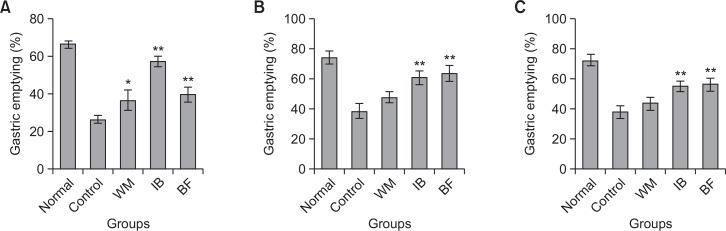
Opioids such as morphine delay gastric emptying via interacting with central or peripheral opiate receptors. Fig. 1A shows the effect of the tested herbal preparations on morphine-delayed gastric emptying in rats. Morphine significantly delayed gastric emptying observed in the untreated normal group (66.6 ± 1.8%) to 26.6 ± 2.2% in the control group. This delay was reversed by BF to 40.0 ± 4.0%. WM and IB also significantly improved gastric emptying to 36.9 ± 5.5% and 57.7 ± 2.8%, respectively. The effects of BF were comparable to those of WM, but lower than those of IB.
Fig. 1.
Gastric emptying in rats with (A) morphine-induced delay, (B) apomorphine-induced delay, and (C) cisplatin-induced delay. Each herbal drug (BF, Benachio-F®; WM, Whalmyungsu®; IB, Iberogast®) or control treatment (3% HPMC) was administered orally 50 min before meal administration. Morphine or apomorphine (0.05 mg/kg) was administered subcutaneously and cisplatin (10 mg/kg) was administered intraperitoneally immediately after meal administration. Gastric emptying was assessed over a 50 min period. Values are the mean ± SD; *p<0.05 or **p<0.01 vs. control, Student’s t-test.
Apomorphine is a non-selective dopamine agonist that can decrease gastric tone and delay gastric emptying by acting both centrally and peripherally. The effects of the tested herbal preparations on apomorphine-induced delay of gastric emptying are shown in Fig. 1B. Apomorphine significantly decreased gastric emptying in the normal group (74.3 ± 4.5%) to 38.5 ± 5.2% in the control group. BF and IB significantly ameliorated this delay (63.6 ± 5.2% and 60.9 ± 4.4%, respectively), but WM did not improve gastric emptying in this model (47.7 ± 3.8%).
The antineoplastic agent, cisplatin, was used in the third model of delayed gastric emptying. Cisplatin induces this delay via activation of 5-HT3 receptors in the GI tract and/or the central nervous system (Tyers, 1991). Cisplatin greatly delayed gastric emptying of a liquid meal (72.8 ± 4.0% vs. 38.2 ± 4.3%). Fig. 1C shows that gastric emptying following administration of BF, WM, and IB was 56.5 ± 4.4%, 43.9 ± 4.4%, and 55.5 ± 3.1%, respectively. BF and IB significantly ameliorated cisplatin-induced delay of gastric emptying, but WM did not improve gastric emptying in this model. Taken together, these results suggested that BF could attenuate gastric emptying deficits induced by these pharmacological agents.
Effects of BF on GI transit
Fig. 2 shows the effects of BF on laparotomy-induced delay of GI transit in rats. GI transit in the normal group (4.5 ± 0.1 cm) was significantly decreased to 2.8 ± 0.1 cm in control rats after laparotomy. BF treatment produced a considerable increase in the MGC, to 3.3 ± 0.1, after this operation. WM and IB also significantly improved the MGC to 3.4 ± 0.2 and 3.4 ± 0.2, respectively. The effect of BF was comparable to those of WM and IB. These results suggested that BF could accelerate GI transit in a laparotomy-induced model of delayed GI transit.
Fig. 2.
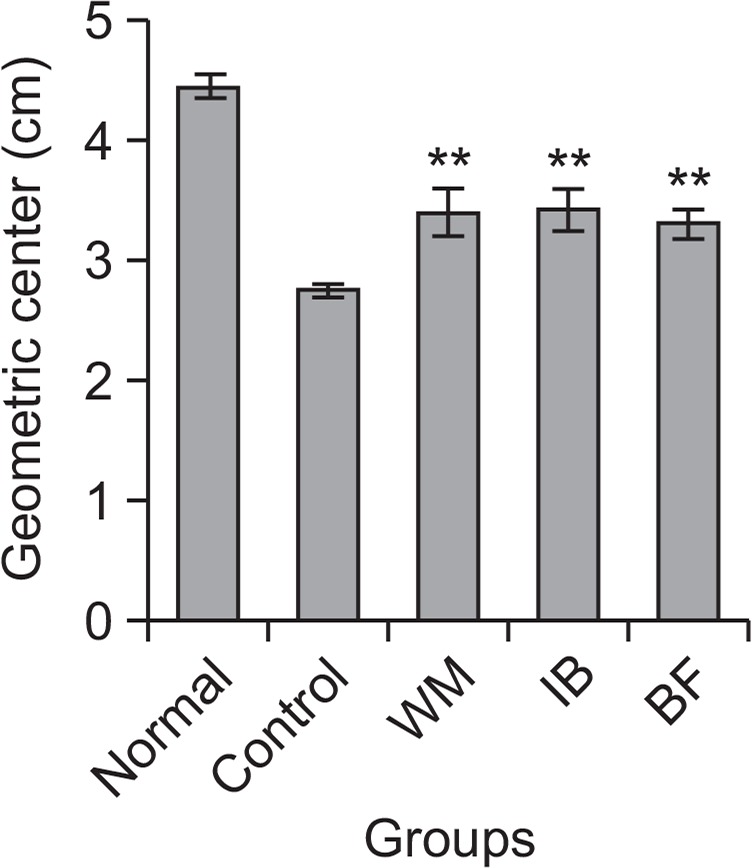
Gastrointestinal transit in a laparotomy-induced model of postoperative paralytic ileus. Each herbal preparation (BF, Benachio-F®; WM, Whalmyungsu®; IB, Iberogast®) or control treatment (3% HPMC) was administered orally 60 min before meal (FITC-dextran) administration. The mean geometric center of distribution was calculated as described in Materials and methods. Values shown are the mean ± SD; **p<0.01 vs. control, Student’s t-test.
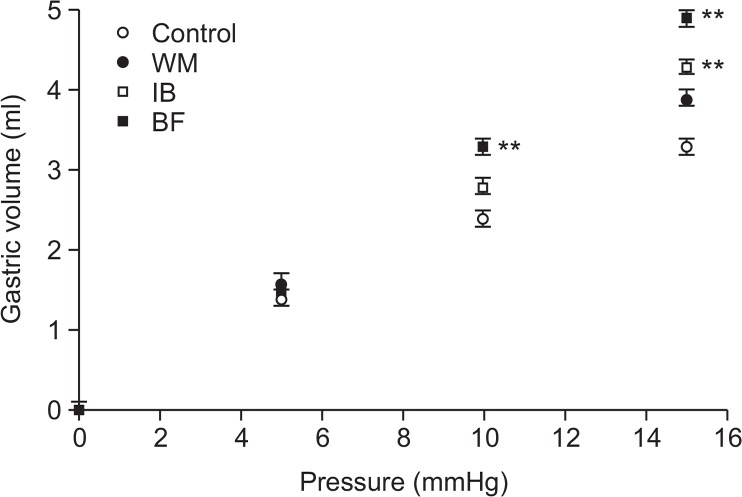
Effects of BF on gastric accommodation
As shown in Fig. 3, the baseline gastric volume of 0.00 ± 0.00 ml during ramp distention increased after administration of BF, WM, and IB. A consistent increase in gastric volume was noted at all pressures with all test drugs. However, BF provided significantly higher gastric volume values than those observed following WM and IB treatment. These results suggested that BF may induce gastric relaxation and thereby increase gastric compliance.
Fig. 3.
Gastric accommodation effects in rats. Each herbal preparation (BF, Benachio-F®; WM, Whalmyungsu®; IB, Iberogast®) or control treatment (3% HPMC) was administered orally before surgery. Intra-bag pressure was increased by 2-mmHg increments every 5 min in anesthetized rats. Starting after an overnight fast, the gastric volume was recorded via the intragastric balloon for 5 min at a pressure of 1 mmHg (baseline). Values are the mean ± SD. **p<0.01 vs. control, Student’s t-test.
Effects of BF on bile secretion
As shown in Fig. 4, control rats had a total bile flow of 12.78 ± 2.23 μl during the 120 min period of bile collection (n=7). Bile flow was significantly increased to 15.94 ± 1.20 (n=6) after a single dose of BF. The mean bile flows following administration of WM and IB were 10.42 ± 3.12 (n=6) and 13.05 ± 2.97 (n=6), respectively.
Fig. 4.
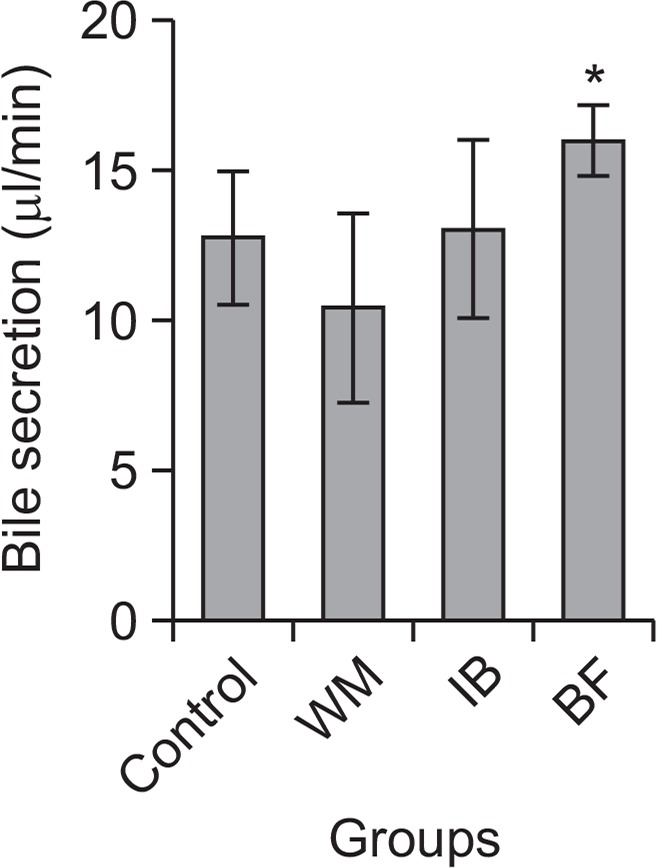
Bile flow in rats. Each herbal drug preparation (BF, Benachio-F®; WM, Whalmyungsu®; IB, Iberogast®) or control treatment (3% HPMC) was administered orally 30 min before meal administration. Results shown are means ± standard error of the mean (SEM). *p<0.05 vs. control, Student’s t-test.
DISCUSSION
Dyspepsia is one of the most routine presentations in many healthcare facilities, with an estimated prevalence of 20–40% in some countries; the majority of these cases are believed to have FD (Camilleri et al., 2005; Piessevaux et al., 2009). FD is known to significantly reduce the quality of life of the affected individuals and places a marked economic burden on healthcare facilities, because patients with FD can have complex treatment needs (Aro et al., 2011). Although the underlying pathophysiological mechanisms are not yet fully established, the available studies suggest interconnected and multifactorial causes that may include psychosocial factors, H. pylori infection, environment, diet, and genetics; these contribute to the manifestation of GI system changes including abnormal motility, visceral hypersensitivity, excess secretion of gastric acid, and duodenal acidity (Mizuta et al., 2006). Considering the large number of factors involved, no single medicine is likely to be effective for all patients with FD. In this context, multidrug and multi-targeted phytotherapy might prove advantageous.
Mono- and poly-herbal preparations have been used for the treatment of dyspeptic complaints since long before recorded history (Madsich et al., 2004; Allescher, 2006; Rösch et al., 2006). A herbal medicinal preparation derived from several plant extracts contains a large number of secondary phytochemical compounds such as essential oils; these are known to possess carminative, stomachic, spasmolytic, and local anesthetic actions. The Corydalis Tuber is used in Chinese traditional medicine for its analgesic and anti-ulcer effects. Extracts of tfhis root have been reported to have several biological activities including anti-spasmodic effects on the GI tract and analgesic activity (Ma et al., 2000). These preparations have been reported to enhance upper GI motility and GI transit in models of laparotomy-induced delay (Lee et al., 2008). Fruits of Foeniculum vulgare Miller (fennel) is used as a laxative and for the treatment of mild digestive disorders because it stimulates GI motility and shows anti-spasmodic activity at higher concentrations. Fennel seeds also act as a laxative, by providing roughage and stimulating peristaltic motion, thereby promoting production of gastric juices and bile and facilitating excretion (Klein et al., 1998). Fennel is also commonly found in medicines used to treat abdominal pain, diarrhea, irritable bowel syndrome, and other intestinal issues. Extracts of Cinnamomi Cortex are used to treat GI problems and to calm the stomach. Cinnamonum species (cinnamon) is a carminative agent, helping to reduce flatulence, and is traditionally used to combat diarrhea and morning sickness. Cinnamon was shown to reduce secretion of GI fluids in an animal model of diarrhea (Rao et al., 2008). The rhizomes of Atractylodes lancea DC (Compositae) are used to treat GI diseases including nausea, gastroparesis, and gastric atony in China and Japan (Chang, 2004). This extract may stimulate gastric emptying and increase small intestinal motility by inhibiting the dopamine D2 receptor and the 5-HT3 receptor (Kimura and Sumiyoshi, 2012). Powdered rhizomes of Zingiber officinale Roscoe (ginger) has long been used in traditional treatments for GI illnesses (Chopra et al., 1956). Recently, an acetone extract of ginger and its constituents were shown to enhance gastric emptying of a charcoal meal in mice. Ginger preparations also significantly reversed cisplatin-induced delayed gastric emptying (Yamahara et al., 1990). The roots and rhizomes of licorice (Glycyrrhiza glabra Linn) have been reported to possess anti-Helicobacter pylori activity, along with gastric mucus secretion enhancing activity (Raveendra et al., 2012). Aqueous extracts of Citri Unshius Pericarpium significantly increased the intestinal transit rate in normal mice and rats with GI motility dysfunctions (Lyu and Lee, 2013).
Several mono- and poly-herbal medicinal products have undergone randomized controlled clinical trials, with varying degree of success. For example, IB contains extracts from 9 plants and has shown promising results in clinical and observational studies (Heinle et al., 2006; Rösch et al., 2006). IB exhibited dual motility-modulating effects; relaxing spastic intestine (Wegenera and Wagnerb, 2006), and improving atonic intestine (Ammon et al., 2006). Its effects were also region-specific; relaxing the gastric corpus and fundus and increasing tone in the antrum (Schemann et al., 2006). This region-specific effect has also been confirmed by clinical pharmacological data (Thompson and Ernst, 2002; Pilichiewicz et al., 2006). The mode of action of IB and its individual components on gastric motility include relaxation of the proximal stomach and increased antral motility (Sharma and Gupta, 1998).
Some components of BF have been linked to adverse effects when administered at very high doses for prolonged periods. The most prominent effects were reported for Corydalis Tuber (alkaloid: bulbocapnine) and Glycyrrhizae Radix (triterpenoid saponin: glycyrrhizine). Bulbocapnine has been reported to induce dose dependent catalepsis and convulsions, while glycyrrhizine has been associated with hypertension, hypokalemia, hypernatremia and some very rare cases of pseudoaldosteronism (Loizzo et al., 1971; Robles et al., 2103). The most commonly reported side-effect was allergic reaction in sensitive patients. However, the doses of these components used in BF therapy are much lower than the levels reported to cause these adverse reactions. For example, the single oral ethanolic extract dose that caused 50% mortality (LD50) in mice was reported to be 100 ± 4.58 g/kg, which is more than 250 times the content found in BF (Koo et al., 2010). Glycyrrhizae Radix is Generally Recognized as Safe (GRAS) for use in foods by the U.S. FDA (21 CFR 184.1408).
After the ingestion of a solid or liquid meal, bolus passage through the esophagus normally causes an initial receptive relaxation, followed by adaptive relaxation of the proximal stomach to provide a reservoir for the food (gastric accommodation). The meal is then transferred to the antrum of the distal stomach where it is ground and mixed with the gastric contents prior to delivery to the duodenum via the pylorus (gastric emptying). In healthy humans, more than 90% of ingested food is emptied from the stomach within 4 h (Tougas et al., 2000). In this study, we observed that BF had beneficial effects on GI motor activity, which included acceleration of gastric emptying and meal transit, as well as enhancement of gastric accommodation. Morphine, apomorphine, and cisplatin inhibit gastric emptying via effects on opiate, dopamine, and 5-HT3 receptors, respectively (Blancquaert et al., 1982; Tyers, 1991; Ozaki and Sukamoto, 1999). BF significantly ameliorated the delayed gastric emptying induced by these agents, suggesting that it could accelerate gastric emptying under these conditions.
Delayed gastric emptying is also a feature of gastroparesis and impaired gastric accommodation. Gastric accommodation is a motor function reflex of the gastric corpus-fundus which enables it to house a high volume (of food) with a minimal rise in intragastric pressure, reducing discomfort. It has been reported that gastric accommodation is impaired in approximately 40% of FD patients (Mizuta et al., 2006). It has been suggested that the pre- and post-prandial bloating and sensation of fullness experienced by dyspeptic patients could result from impairments of gastric accommodation and intragastric distribution of the meal between the proximal and distal parts of the stomach. This has also been associated with early satiety and weight loss. In a subset of dyspeptic patients with visceral hypersensitivity to gastric distention, impaired gastric accommodation may lead to post-prandial pain, belching, and weight loss. It was found that the intragastric pressure-related symptom cluster in dyspeptic patients closely resembled that of vagotomized patients, suggesting that impaired gastric accommodation and antral hypomotolity may be due to vagal dysfunction. Efferent vagal dysfunction was also implicated in patients with abnormal motility-related FD. In the present study, a miniature barostat attached to a small balloon via a polyethylene cannula was used to examine gastric relaxation in rats administered the test preparations. BF induced superior gastric relaxation.
POI often occurs after abdominal surgery and is characterized by a transient hypomotility of the GI tract; this prolongs hospitalization, raises medical costs, and increases morbidity (Holte and Kehlet, 2000). Inflammatory responses and inhibited neuronal reflex pathways have been implicated in the pathogenesis of POI (Zittel et al., 2001; Kreiss et al., 2003). Surgical stress stimulates the release of endogenous opioids, which can impair GI function after surgery. In the present study, a laparotomy-induced delay in GI transit was significantly reduced by BF treatment. Based on these results, BF may prove to be superior to conventional therapeutics, particularly for the improvement of GI transit and gastric accommodation.
We also found that BF significantly stimulated biliary flow in rats. Bile production represents an important function of the liver and changes in biliary flow rates or alterations in biliary constituents have important impacts on health (Hofmann, 1999). Based on these results, BF would be predicted to enhance digestion and GI function by stimulating bile secretion.
Taken together, the findings of the present study indicated that BF enhanced gastric emptying and intestinal transit, increased gastric accommodation by inducing gastric relaxation, and thus increased gastric compliance in rats. BF also stimulated bile flow, which may further enhance digestion.
In summary, the pharmacological effects of BF, a proprietary liquid formulation containing 7 herbal extracts that could provide multi-target phytotherapy for FD, were evaluated in rat models relating to the symptoms of this condition. BF significantly reversed the delayed gastric emptying caused by morphine, apomorphine, and cisplatin. In addition, it markedly increased gastric accommodation and showed higher gastric volumes, as compared with control rats. Furthermore, it exhibited significant stimulatory effects on bile flow. Its effects on these GI sensorimotor mechanisms were comparable to, or even better than, other similar herbal preparations. Taken together, BF might have great potential as an effective prokinetic agent capable of reducing GI symptoms and increasing quality of life in FD patients.
Acknowledgments
This study was supported by a grant from the Medical Cluster R&D Support Project of Daegu Gyeongbuk Medical Innovation Foundation, Republic of Korea (2013) (No. HT13C0011).
Footnotes
DISCLOSURES
The authors report no conflicts of interest about this work.
REFERENCES
- Allescher HD. Functional dyspepsia-a multicausal disease and its therapy. Phytomedicine. 2006;13:2–11. doi: 10.1016/j.phymed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Ammon HP, Kelber O, Okpanyi SN. Spasmolytic and tonic effect of lberogast (STWS) in intestinal smooth muscle. Phytomedicine. 2006;13:67–74. doi: 10.1016/j.phymed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Aro P, Talley NJ, Agréus L, Johansson SE, Bolling-Sternevald E, Storskrubb T, Ronkainen J. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215–1224. doi: 10.1111/j.1365-2036.2011.04640.x. [DOI] [PubMed] [Google Scholar]
- Blancquaert JP, Lefebvre RA, Willems JL. Gastric relaxation by intravenous and intracerebroventricular administration of apomorphine, morphine and fentanyl in the conscious dog. Arch Int Pharmacodyn Ther. 1982;256:153–154. [PubMed] [Google Scholar]
- Camilleri M, Dubois D, Coulie B, Jones M, Kahrilas PJ, Rentz AM, Sonnenberg A, Stanghellini V, Stewart WF, Tack J, Talley NJ, Whitehead W, Revicki DA. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543–552. doi: 10.1016/S1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- Chang L. Epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:31–39. doi: 10.1111/j.1365-2036.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research; New Delhi: 1956. [Google Scholar]
- De Winter BY, Boeckxstaens GE, De Man JG, Moreels TG, Herman AG, Pelckmans PA. Differential effect of indomethacin and ketorolac on postoperative ileus in rats. Eur J Pharmacol. 1998;344:71–76. doi: 10.1016/S0014-2999(97)01563-X. [DOI] [PubMed] [Google Scholar]
- Halder SL, Talley NJ. Functional dyspepsia: a new rome III paradigm. Curr Treat Options Gastroenterol. 2007;10:259–272. doi: 10.1007/s11938-007-0069-0. [DOI] [PubMed] [Google Scholar]
- Heinle H, Hagelauer D, Pascht U, Kelber O, Weiser D. Intestinal spasmolytic effects of STW 5 (Iberogast) and its components. Phytomedicine. 2006;13:75–79. doi: 10.1016/j.phymed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Sur. 2000;87:1480–1493. doi: 10.1046/j.1365-2168.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006;354:832–840. doi: 10.1056/NEJMoa052639. [DOI] [PubMed] [Google Scholar]
- Kalff JC, Buchholz BM, Eskandari MK, Hierholzer C, Schraut WH, Simmons RL, Bauer AJ. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126:498–509. doi: 10.1016/S0039-6060(99)70091-7. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M. Effects of an Atractylodes lancea rhizome extract and a volatile component β-eudesmol on gastro-intestinal motility in mice. J Ethnopharmacol. 2012;141:530–536. doi: 10.1016/j.jep.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Klein S, Rister R, Riggins C. The complete German commission E monographs: therapeutic guide to herbal medicines. American Botanical Council; Austin: 1998. [Google Scholar]
- Koo J, Lee S, Seo B. A philological study on poisoning of herbal medicines used to activate blood flow and remove blood stasis. Kor J Herbology. 2010;25:21–39. [Google Scholar]
- Kreiss C, Birder LA, Kiss S, VanBibber MM, Bauer AJ. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52:527–534. doi: 10.1136/gut.52.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Choi JJ, Kim DH, Choi S, Lee KR, Son M, Jin M. Gastroprokinetic effects of DA-9701, a new prokinetic agent formulated with Pharbitis Semen and Corydalis Tuber. Phytomedicine. 2008;15:836–843. doi: 10.1016/j.phymed.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Loizzo A, De Carolis AS, Longo VG. Studies on the central effects of bulbocapnine. Psychopharmacologia. 1971;22:234–249. doi: 10.1007/BF00401786. [DOI] [PubMed] [Google Scholar]
- Lyu JH, Lee HT. Effects of dried Citrus unshiu peels on gastrointestinal motility in rodents. Arch Pharm Res. 2013;36:641–648. doi: 10.1007/s12272-013-0080-z. [DOI] [PubMed] [Google Scholar]
- Ma WG, Fukushi Y, Tahara S, Osawa T. Fungitoxic alkaloids from Hokkaido Papaveraceae. Fitoterapia. 2000;71:527–534. doi: 10.1016/S0367-326X(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Madisch A, Holtmann G, Mayr G, Vinson B, Hotz J. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion. 2004;69:45–52. doi: 10.1159/000076546. [DOI] [PubMed] [Google Scholar]
- Mizuta Y, Shikuwa S, Isomoto H, Mishima R, Akazawa Y, Masuda J, Omagari K, Takeshima F, Kohno S. Recent insights into digestive motility in functional dyspepsia. J Gastroenterol. 2006;41:1025–1040. doi: 10.1007/s00535-006-1966-z. [DOI] [PubMed] [Google Scholar]
- Ozaki A, Sukamoto T. Improvement of cisplatin induced emesis and delayed gastric emptying by KB-R6933, a novel 5-HT3 receptor antagonist. Gen Pharmacol. 1999;33:283–288. doi: 10.1016/S0306-3623(98)00286-9. [DOI] [PubMed] [Google Scholar]
- Piessevaux H, De Winter B, Louis E, Muls V, De Looze D, Pelckmans P, Deltenre M, Urbain D, Tack J. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterol Motil. 2009;21:378–388. doi: 10.1111/j.1365-2982.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- Pilichiewicz AN, Horowitz M, Russo A, Maddox AF, Jones KL, Schemann M, Holtmann G, Feinle-Bisset C. Effects of Iberogasts on proximal gastric volume, antropyloroduodenal (APD) motility and gastric emptying in healthy men. Am J Gastroenterol. 2006;102:1276–1283. doi: 10.1111/j.1572-0241.2007.01142.x. [DOI] [PubMed] [Google Scholar]
- Rao CV, Vijayakumar M, Sairam K, Kumar V. Antidiarrhoeal activity of the standardised extract of Cinnamomum tamala in experimental rats. J Nat Med. 2008;62:396–402. doi: 10.1007/s11418-008-0258-8. [DOI] [PubMed] [Google Scholar]
- Raveendra KR, Jayachandra, Srinivasa V, Sushma KR, Allan JJ, Goudar KS, Shivaprasad HN, Venkateshwarlu K, Geetharani P, Sushma G, Agarwal A. An extract of glycyrrhiza glabra (GutGard) alleviates symptoms of functional dyspepsia: a randomized, double-blind, placebo-controlled study. Evid Based Complement Alternat Med. 2012;2012:216970. doi: 10.1155/2012/216970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles BJ, Sandoval AR, Dardon JD, Blas CA. Lethal liquorice lollies (liquorice abuse causing pseudohyperaldosteronism) BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösch W, Liebregts T, Gundermann KJ, Vinson B, Holtmann G. Phytotherapy for functional dyspepsia: a review of the clinical evidence for the herbal preparation STW 5. Phytomedicine. 2006;13:114–121. doi: 10.1016/j.phymed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Schemann M, Michel K, Hohenester B, Rühl A. Region-specific effects of STW 5 and its components in gastric fundus, corpus and antrum. Phytomedicine. 2006;13:90–99. doi: 10.1016/j.phymed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Sharma SS, Gupta YK. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale) J Ethnopharmacol. 1998;62:49–55. doi: 10.1016/S0378-8741(98)00053-1. [DOI] [PubMed] [Google Scholar]
- Stanghellini V, De Ponti F, De Giorgio R, Barbara G, Tosetti C, Corinaldesi R. New developments in the treatment of functional dyspepsia. Drugs. 2003;63:869–892. doi: 10.2165/00003495-200363090-00003. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Nishizawa T, Hibi T. Therapeutic strategies for functional dyspepsia and the introduction of the Rome III classification. J Gastroenterol. 2006;41:513–523. doi: 10.1007/s00535-006-1847-5. [DOI] [PubMed] [Google Scholar]
- Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Ernst E. Systematic review: herbal medicinal products for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2002;16:1689–1699. doi: 10.1046/j.1365-2036.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- Thompson WG. The road to Rome. Gastroenterology. 2006;130:1552–1556. doi: 10.1053/j.gastro.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Ryden J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- Tyers MB. 5-HT3 receptors and the therapeutic potential of 5-HT3 receptor antagonists. Therapie (Paris) 1991;46:431–435. [PubMed] [Google Scholar]
- Wagner H. Multitarget therapy-the future of treatment for more than just functional dyspepsia. Phytomedicine. 2006;13(Suppl 5):122–129. doi: 10.1016/j.phymed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Wegenera T, Wagnerb H. The active components and the pharmacological multi-target principle of STW 5 (Iberogasts) Phytomedicine. 2006;13:20–35. doi: 10.1016/j.phymed.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698–1703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- Yamahara J, Huang Q, Li Y, Xu L, Fujimura H. Gastrointestinal motility enhancing effect of ginger and its constituents. Chem Pharm Bull. 1990;38:430–431. doi: 10.1248/cpb.38.430. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Omoya H, Oka M, Furukawa K, Ito T, Karasawa T. AS-4370, a novel gastrokinetic agent free of dopamine D2 receptor antagonist properties. Arch Int Pharmacodyn Ther. 1989;300:51–67. [PubMed] [Google Scholar]
- Zittel TT, Meile T, Jehle EC, Becker HD. Intraperitoneal capsaicin treatment reduces postoperative gastric ileus in awake rats. Langenbecks Arch Surg. 2001;386:204–211. doi: 10.1007/s004230100228. [DOI] [PubMed] [Google Scholar]