Abstract
The oral taxanes are analogues of existing taxanes with a possible broad range of antitumor activity. They also have the potential advantages of ease of administration, better efficacy and lesser toxicity than currently available taxanes. These drugs have been used in several Phase I clinical trials, the methodology and results of which will be reviewed here.
Keywords: BMS-275183, DHP107, milataxel, ModraDoc001, oral taxanes, ortataxel, tesetaxel
The taxanes were among the first classes of chemotherapy agents identified, beginning with the discovery of paclitaxel in 1971. Today, paclitaxel and docetaxel are approved for use in a wide variety of malignancies including breast cancer, ovarian cancer, non-small-cell lung cancer, prostate cancer, head-and-neck cancer, gastric adenocarcinoma, and AIDS-related Kaposi’s sarcoma. A third drug in the class, cabazitaxel, has more recently been approved for use in advanced prostate cancer. The drugs are poorly water soluble and traditionally required vehicles, such as Cremophor EL® and Polysorbate 80®, for intravenous (iv.) drug delivery. These solvents may contribute to neurotoxicity as well as hypersensitivity reactions requiring pretreatment with antihistimines and corticosteroids to administer them safely [1,2]. Paclitaxel and docetaxel are also substrates for the P-glycoprotein transporter, which inhibits their gastrointestinal absorption and limits their effectiveness in tumors, expressing the multidrug resistance gene [2]. Novel taxane analogs have been developed that are poor substrates for P-glycoprotein, and are orally bioavailable, eliminating the need for complicated drug vehicles and iv. drug delivery. The oral taxanes have potential to lower cost by circumventing the need for iv. access and associated administration in an infusion center, which may also make them more convenient for patients. It is hoped that they may have less toxicity without the need for Cremophor EL or Polysorbate 80 as carriers, and may be more efficacious as they can overcome the multidrug-resistance gene. Phase I studies have been done for several orally available taxanes alone, while studies of combination regimens have began more recently. This paper will review those drugs and studies (Table 1).
Table 1.
Novel oral taxanes reviewed in this article.
| Taxane | Manufacturer (location) | Formulation | Major toxicity | Structure |
|---|---|---|---|---|
| DHP107 (Paclitaxel) | DAE HWA Pharmaceutical Co., Ltd (Seoul, Republic of Korea) | Oral | Diarrhea Neutropenia |
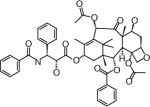
|
| ModraDoc001 (Docetaxel) | Slotervaart Hospital (Amsterdam, The Netherlands) | Oral | Diarrhea Fatigue Dehydration |
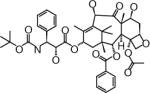
|
| Ortataxel | Spectrum Pharmaceuticals (CA, USA) | Intravenous and oral | Leukopenia Gastrointestinal |
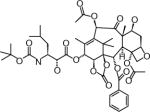
|
| Milataxel | Taxolog, Inc. (NJ, USA) | Intravenous and oral | Leukopenia |
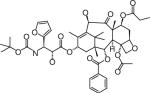
|
| BMS-275183 | Bristol-Myers Squibb (NY, USA) | Oral | Peripheral neuropathy Leukopenia |
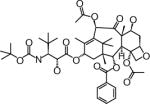
|
| Tesetaxel | Genta Inc. (NJ, USA) | Oral | Leukopenia Gastrointestinal |
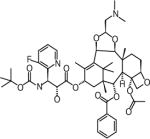
|
Structures are from the PubChem Substance database (Paclitaxel: CID36314; Docetaxel: CID148124; Ortataxel: CID 6918412; Milataxel: CID 6918589; BMS-275183: CID 6918594; Tesetaxel: CID 6918574).
Novel oral formulations of paclitaxel & docetaxel
DHP107 (paclitaxel)
One of the first attempts at oral taxane administration was using paclitaxel, which can be taken orally in its iv. solution, but has poor bioavailability. It was found that co-administration with Cyclosporine A actually improved bioavailability and made oral dosing of this formulation feasible. It is thought that Cyclosporine A inhibits both the P-glycoprotein efflux pump as well as CYP3A4-mediated metabolism of paclitaxel. Phase II trials of the oral ingestion of this formulation of paclitaxel with Cyclosporine A were completed in patients with advanced non-small-cell lung cancer and pretreated metastatic breast cancer [3,4]. They found similar efficacy and toxicity compared with patient populations treated with iv. paclitaxel, but further studies have not been carried out. More recently, a novel oral formulation of paclitaxel called DHP107 was developed that does not require co-administration of Cyclosporine A. DHP107 is a mucoadhesive formulation of paclitaxel, created by dissolving paclitaxel in a mixture of monoolein, tricarprylin and Tween® 80 [5]. One Phase I crossover study has been completed using DHP107 in patients with advanced solid tumor malignancies who had failed standard therapies [6]. In total, 33 patients were enrolled and given DHP107 once and then subsequently received iv. paclitaxel at a dose of 175 mg/m2 every 3 weeks for the remainder of the study. DHP107 was given to three patients each at 11 dosage levels from 60 to 600 mg/m2, and tolerated without dose-limiting toxicities. Major toxicities seen were grade 3 diarrhea (four patients), grade 3 neutropenia (two patients) and grade 3 fatigue (one patient). No objective responses were observed and 11 patients demonstrated stable disease. It should be noted that all patients only received one dose of the study drug and thus further studies are needed to better characterize toxicity for this medication. Pharmacokinetics showed that inter-patient variability increased greatly above doses of 250 mg/m2 and the authors of the study recommended this as the maximum dose for future studies. Another Phase I trial of DHP107 using a weekly dosing schedule is in progress [6]
ModraDoc001
ModraDoc001 is a novel formulation of docetaxel that increases its oral bioavailability and provides a practical oral form. It was developed as a solid dispersion of docetaxel with polyvinylpyrrolidone-K30 and sodium lauryl sulphate, which compared favorably to several other formulations that were tried [7]. It has been used in one dose escalation trial with ritonavir in 40 patients with advanced solid tumors [8]. The study designers coadministered ModraDoc001 with ritonavir to further increase its oral bioavailability as ritonavir is a known CYP3A4 inhibitor and thought to metabolize docetaxel in the liver and gut wall. A maximum tolerated dose of once weekly ModraDoc001 (60 mg) with ritonavir (200 mg) was determined. Dose-limiting toxicities were observed in the study were grade 3 diarrhea (four patients), elevated ASAT/ALAT (one patient), grade 4 dehydration and grade 3 mucositis (one patient) and grade 3 fatigue (two patients), all of which were seen collectively in a total of five patients [8]. A partial response was observed in four patients, one each with carcinoma of unknown primary, non-small-cell lung cancer, gastric cancer and breast cancer. In addition, 15 patients had sustained stable disease. The authors of the study plan to pursue further investigation of the drug in Phase II trials.
Orally bioavailable novel taxanes
Ortataxel
Ortataxel (IDN-5109, BAY 59-8862) is a novel analog of paclitaxel that has been shown to have comparable efficacy to paclitaxel in in vitro and in vivo tumor models. Oral ortataxel was shown to be as effective as iv. paclitaxel in mice transplanted with MX-1, a human breast carcinoma model [9]. It was also shown to be effective in mouse xenografts of human ovarian carcinoma when given via iv. and orally, including some activity in a paclitaxel-resistant xenograft MNB-PTX-1 [10]. It was also the first taxane shown to have oral bioavailability [9,10]. It is available in both iv. and oral formulations, and was used orally in one Phase I trial of 18 patients with treatment refractory solid tumors [11]. It was used in doses ranging from 10 to 70.1 mg/m2 given once daily for 5 days every 3 weeks. The major toxicities seen in this trial were grade 3 and 4 neutropenia (three patients each). Dose-limiting toxicities in this trial were grade 5 and 3 febrile neutropenia (one and two patients, respectively), grade 2 fatigue (one patient) and grade 2 neuropathy (one patient). One patient with carcinoma of unknown primary origin had a partial response after two cycles but then progressed after two further cycles, and two patients had stable disease. Ortataxel has not been used orally in further clinical trials.
Milataxel
Milataxel (MAC-321) is a novel analog of docetaxel that has been shown to compare favorably with paclitaxel in in vitro and in vivo tumor models [12]. It was shown to be more effective orally against mouse xenografts with the cell lines KB-8-5, MX-1W and HCT-15 than paclitaxel, all of which were known to express P-glycoprotein [12]. It is available in an oral and iv. formulation, and it has been used orally in one Phase I study of 18 patients with advanced solid malignancies [13]. Patients received miltaxael in escalating doses ranging from 25 to 75 mg/m2 once every 3 weeks. Grade 3 and 4 toxicities seen in this study were febrile neutropenia (two patients) and fatigue, infection, thrombocytopenia and neuropathy (one patient each). The one incidence of fatigue occurred at a dose of 25 mg/m2 but all other grade 3 and 4 toxicities were observed in patients who received the 75 mg/m2 dose. A dose of 60 mg/m2 once every 3 weeks was found to be well tolerated, but the study was stopped before completion and a maximum tolerated dose could be formally established, because of life-threatening toxicities seen in a concurrent trial of iv. milataxel [14]. That study was a Phase II trial of iv. milataxel in colorectal cancer patients. Of the 44 patients who received milataxel in that trial, six patients experienced neutropenic sepsis including two patients who died after receiving the first dose of the drug. Because of safety concerns, both milataxel studies were stopped. Of the 18 patients who received oral milataxel, none had an objective response. Disease stabilization was seen in four patients, in one patient each with mesothelioma, chondrosarcoma, small-cell carcinoma, and prostate carcinoma. Milataxel has not been used in further clinical trials since these trials were stopped.
BMS-275183
BMS-275183 was developed from an analog of paclitaxel [15]. It was shown to have comparable antitumor activity to paclitaxel in in vitro and in vivo tumor models [15,16]. In mice grafted with four different cell lines: M109 (lung carcinoma), Mam16/C (breast carcinoma), A2780 (ovarian carcinoma) and HCT/pk (colon carcinoma), BMS-275183 was found to have equivalent antitumor activity to iv. paclitaxel [16]. Subsequent Phase I doseescalation studies investigated dosing with several different dosing schedules with the rationale that different schedules would have different toxicity profiles. One trial occurred using BMS-275183 on a weekly schedule, with 48 patients enrolled all of whom had advanced solid-tumor malignancies that were not amenable to standard treatments [17]. Grade 3 and 4 toxicites seen in multiple patients were as follows: grade 3 and grade 5 neutropenia (three and five patients, respectively), grade 3 anemia (four patients), grade 3 sensory neuropathy (five patients), grade 3 motor neuropathy (two patients), grade 3 diarrhea (four patients) and grade 3 fatigue (six patients). Grade 4 fatigue, grade 3 abdominal pain, grade 3 arthralgia, grade 4 hypersensitivity, grade 3 constipation and grade 3 anorexia occurred in one patient each. In addition, 31 of the 48 patients in the study experienced new neuropathy or worsening of existing neuropathy. The maximum tolerated dose of BMS-275183 on this schedule was determined to be 200 mg/m2 [17]. Partial responses were found in nine patients; four had non-small-cell lung cancer, two with prostate carcinoma, one with primitive neuroectodermal tumor, one with cholangiocarcinoma, and one with undifferentiated sarcoma. Of the lung cancer patients that responded, three had been treated with prior taxane therapy.
Since neuropathy has been associated with short infusion times with paclitaxel, the authors then conducted a subsequent study of twice-weekly dosing of BMS-275183 with the rationale of spreading out the dose to potentially reduce neuropathy [18]. In this study, 38 patients with advanced solid tumors, not otherwise amenable to treatment, were given oral BMS-275183 twice weekly in escalating doses ranging from 60 to 140 mg/m2. Major toxicities observed in this trial were grade 4 neutropenia, grade 3 fatigue, and grade 3 diarrhea seen in four patients each. In addition, grade 3 and 4 sensory neuropathy, grade 3 hematuria and grade 3 increased urinary frequency were seen in one patient each. The maximum tolerated dose on this schedule was found to be 100 mg/ m2 twice weekly [18]. Compared with a weekly regimen, twice-weekly BMS-275183 was associated with less severe and less frequent peripheral neuropathy, while still showing antitumor activity. Four out of 30 patients with measurable disease were found to have partial responses: two with non-small-cell lung cancer, one with melanoma and one with prostate carcinoma. In addition, one patient, who was not included in the measurable disease population, had a drop in PSA from 670 to 6.1 ng/ml.
As the decrease in interval to twice- from once-weekly BMS-275183 led to a more favorable side-effect profile in respect to neurotoxicity, a Phase I study of BMS-275183 was undertaken using a daily schedule [19]. A total of 20 patients with advanced non-hematologic malignancies that had either failed or were not eligible for standard therapy were enrolled in this study using daily doses of BMS-275183 of 6–18 mg/m2. Alhough no peripheral neuropathy was observed on these regimens, there were other significant adverse effects. Of the 20 patients in the study, two patients died from leukopenia and subsequent sepsis. Other major toxicities were grade 3 (two patients) and 4 leukopenia, grade 4 neutropenia, grade 3 alkaline phosphatase elevation, grade 3 γ-gluatmyl transferase elevation, and grade 3 anorexia, all of which occurred in one patient each. Stable disease was the best response seen out of 16 patients with measurable disease as no patients who exhibited a partial response. Given the lack of clinical efficacy and two patient deaths, the authors of the study concluded there was no benefit to the daily dosing of BMS-275183.
In addition, a Phase I–II trial of pemetrexed (Alimta®) in combination with BMS-275183 in patients with recurrent non-small-cell lung cancer is designated as completed, but the results of this study are not available at this time [101]. There are currently no further clinical trials of BMS-274183.
Tesetaxel
Tesetaxel (DJ-927) is a novel taxane that has been shown to have more potent antitumor activity than paclitaxel and docetaxel in in vitro and in vivo studies [20]. It was active in mice xenografts with DLD-1 (colon cancer) and DU4475 (breast cancer) where both paclitaxel and docetaxel were ineffective [20]. It has also been shown to be effective against cell lines overexpressing P-glycoprotein, reaching higher concentrations than paclitaxel and docetaxel, possibly an indication that it would be effective against tumors resistant to these drugs [20]. In clinical trials, it has been most studied on a dosing schedule of once every 3 weeks with a maximum tolerated dose of 27–35 mg/ m2 [21–25]. The only study for which complete results are available in this dosing range of tesetaxel was a Phase I–II study done in patients with advanced or metastatic non-small-cell lung cancer who had previously failed treatment with a platinum-containing regimen, and who had not had prior taxane exposure [26]. In total, 36 patients were initially enrolled of whom 34 patients received tesetaxel starting at 27 mg/m2 every 3 weeks with doses adjusted up or down in a range from 18 to 35 mg/m2, depending on toxicity. Two patients did not receive any of the study medication. The grade 3–4 hematologic toxicities seen in this study were neutropenia (18 patients), leukopenia (14 patients), anemia (six patients) and febrile neutro-penia (one patient). Grade 3–4 nonhematologic toxicities were nausea (two patients), vomiting (one patient), fatigue (three patients), weight loss (one patient), dyspnea (two patients), anorexia (three patients) and transaminases elevation (three patients). Six patients were taken off the study due to toxicity. One complete and one partial response were seen with an overall response rate of 5.6%. In total, 17 patients had a best response of stable disease. Mean survival time for the study population was 4 months. According to the study authors, these results proved to be worse compared with studies of paclitaxel and docetaxel conducted in similar patient populations [26]. Further Phase II studies of tesetaxel at this dosing interval are ongoing in metastatic melanoma [102], previously treated bladder cancer [103], castration-resistant prostate cancer [104] and advanced gastric cancer [105].
Tesetaxel has also been studied on a weekly dosing schedule with the rationale that the toxicity profile may be better. A Phase I study in 26 advanced solid tumor patients was done with doses ranging from 8 to 17.5 mg/m2 once weekly for 3 weeks every 28 days [27]. The dose-limiting toxicities associated with this regimen were fatigue and anorexia, with only one out of 26 patients in the study experiencing a grade 3 and 4 myelotoxicity. The maximum tolerated dose at this schedule was 15 mg/m2 once weekly. One patient with metastatic breast cancer who had previously progressed on taxane therapy experienced a partial response, and one patient with prostate carcinoma experienced a prolonged reduction in PSA. This dosing schedule may allow for higher doses of tesetaxel to be given with a more favorable side-effect profile compared with once- every-third-week dosing. Phase II studies of tesetaxel in metastatic breast cancer patients using both weekly and every-third-week dosing are ongoing [106].
Tesetaxel is also undergoing study in combination therapy clinical trials. A Phase I dose-escalation study was carried out using an all oral combination of tesetaxel and capecitabine [28]. In this trial, 27 patients, all with advanced or metastatic solid tumors were enrolled. These patients had all undergone prior surgical treatment for their malignancies, and 21 of them had undergone prior systemic therapy with a median of two regimens. All patients received both tesetaxel and capecitabine. Tesetaxel was given once every 21 days at a starting dose of 18 mg/m2. Capecitabine was started at 1250 mg/m2/day given in equally divided doses every 12 h for 14 consecutive days followed by 7 days off to complete at 21-day cycle. The tesetaxel dose was escalated up to a maximum of 35 mg/m2, and the capecitabine dose was escalated up to a maximum of 2500 mg/m2/day. The grade 3 or 4 toxicities seen in multiple patients in this study were leukopenia (12 patients), neutropenia (11 patients), febrile neutropenia (four patients), anemia (four patients), thrombocytopenia (two patients), diarrhea (two patients), fatigue (two patients), hypokalemia (four patients) and palmar–plantar erythrodysaesthesia (four patients). Grade 3 or 4 peripheral neuropathy was not seen, but one patient was reported to have experienced grade 2 peroneal nerve palsy. A total of 18 patients (67%) experienced at least one grade 3 or 4 toxicity. The study determined the maximum tolerated dose of the combined regimen to be tesetaxel 27 mg/m2 with capecitabine 2500 mg/m2/day, as two of three patients experienced grade 4 myelotoxicities at a dose of tesetaxel 35 mg/m2 with capecitabine 1900 mg/m2/day. None of the eight patients treated at the maximum tolerated dose-experienced a dose limiting toxicity. The most frequent causes of study discontinuation were disease progression in 56% of patients, and adverse events in 26% of patients. The best tumor response in those patients that qualified for efficacy analysis (18 of 27 patients) was stable disease in 82% and progressive disease in 18%. Median time to tumor progression in this population was 3.3 months, with a median overall survival time estimated to be 13.1 months. Pharmacokinetics in the study were difficult to interpret due to high interpatient variability and low power, but the two drugs did not seem to have a significant effect on each other’s metabolism. Overall, this study supported the continued development of oral taxanes in combination regimens, and established a maximum tolerated dose for use in later studies of tesetaxel and capecitabine.
Several other studies of tesetaxel in combination with other anticancer agents are in progress. A Phase IB study is underway using tesetaxel at 27 mg/m2 once every 3 weeks with capecitabine 1500–2000 mg/m2/day (in divided doses for 14 days) every 3 weeks in patients with advanced solid tumors [29]. Preliminarily, ten patients have been enrolled with the grade 3–4 toxicities being neutropenia (three patients) and palmar–plantar erthrodysaesthesia syndrome (four patients). In addition, a Phase II trial of capecitabine with tesetaxel versus capecitabine plus placebo as second-line therapy for patients with gastric cancer, is currently recruiting patients [107]. It is using capecitabine dosed at 1750 mg/m2/day (in divided doses for 14 days) every 3 weeks in all patients with tesetaxel 27 mg/m2 every 3 weeks or placebo. Furthermore, a Phase I–II trial of tesetaxel in combination with capecitabine and cisplatin is currently recruiting for patients with advanced gastric cancer [108]. It is using oral capecitabine dosed at 2000 mg/m2/day (in divided doses for 14 days) every 3 weeks with iv. cisplatin 60 mg/m2 on day 1 of each 3-week cycle. Tesetaxel is planned to be given in a dose escalation starting at 18 mg/m2 on day 1 of each cycle to a maximum dose of 27 mg/m2 to determine the dose used in the Phase II portion of the study. Completion of these studies will help to better characterize the efficacy and safety of tesetaxel.
Conclusion
Novel oral taxanes have been successfully used alone in Phase I studies and as part of combination regimens. In Phase I studies, it has been demonstrated that they can be administered successfully orally and with acceptable toxicity profiles. Ortataxel has been used orally at doses of up to 70.1 mg/m2 once daily for 5 days every 3 weeks without a formally established maximum tolerated dose. Milataxel has been associated with life threatening bone marrow toxicity in an iv.-formulation trial. The oral formulation was used safely at a dose of 60 mg/m2 once every 3 weeks but would warrant further study to establish this as a safe dose. The data for ortataxel and milataxel are both very limited, and a safe dosing regimen was not fully established for these drugs. BMS-275183 has been used in several dosing schedules with the most favorable toxicity profile at the dose of 100 mg/m2 twice weekly. However, none of these three drugs are undergoing further development at this time.
Tesetaxel has been studied in several Phase I trials with a dose of 27 mg/m2 once every 3 weeks established as a safe-dosing regimen. In the single completed Phase I–II study of tesetaxel in advanced non-small-cell lung cancer patients, efficacy was not found to be better than docetaxel and paclitaxel used in a similar population, but several other Phase II studies are in progress in several other tumor types. Tesetaxel is also being further explored in combination trials at this dose. The combination of tesetaxel and capecitabine is particularly promising as the two drugs are both administered orally and have non-overlapping mechanisms and toxicities. The combination of tesetaxel 27 mg/m2 once every 3 weeks with capecitabine 2500 mg/m2/day (in two equally divided doses on days 1–14 of a 3-week cycle) has been used safely, and Phase II studies are currently under way using similar regimens. An alternative dosing schedule of tesetaxel at 15 mg/m2 weekly for three doses every 4 weeks has been explored and offers the advantage of a better toxicity profile and increased dosing compared with a once-every-third week regimen. This regimen is currently under further study for use in single agent trials but could be a component of a combination therapy dosing regimen as well.
The novel oral taxanes have the potential advantages over iv. paclitaxel and docetaxel of a better toxicity profile and increased efficacy. Due to the oral route, it is easier to give them in smaller and more frequent doses, which may allow for an increase in the overall dose given, while optimizing toxicity. Since most of the available data are from dose-ranging studies, comparisons of toxicity profiles of the novel oral taxanes to iv. paclitaxel and docetaxel are difficult to make at this time. The available data on efficacy are also still insufficient to make a proper judgment. Thus far, they have largely been used only in populations that have been pretreated, or in patients with advanced cancers that have poor prognosis. In addition, many patients received what are likely subtherapeutic doses during the course of Phase I trials. However, there were patients in these trials that demonstrated a response to study drugs despite previously failing taxane-based regimens. Phase II studies are currently in progress, which will help determine in which populations these drugs may be most effective.
Novel oral formulations of paclitaxel and docetaxel such as DHP107 and ModraDoc001 represent an alternative approach to oral taxane therapy with the advantage that their iv. counterparts are already well studied. Phase I studies of these agents are currently under way, and it remains to be seen if they will be further explored.
One major disadvantage of the oral route is that it has less predictable pharmacokinetics, as there are more variables introduced with oral ingestion compared with an iv. administration. One limitation of the currently available pharmacokinetic data is that the data are only available on a small number of patients at any given dose and, thus, single outliers can have a large effect on variability (Table 2). In several of the trials reviewed, the authors noted that severe toxicity occurred in those patients with particularly high drug levels. Pharmacokinetic studies done on large populations at single doses will help to ascertain whether the interpatient variability of oral dosing proves to be a problem. Safe use of these drugs in the general population may require intermittent drug levels or step-wise increases in dose, to account for individual variability in drug pharmacokinetics. As the taxanes currently have a broad range of activity across a large number of tumor types, a viable oral alternative would potentially be very useful. Comparable efficacy and toxicity of these agents has yet to be proven, although the appropriate studies are under way.
Table 2.
Main pharmacokinetic parameters for the oral taxanes.
| Drug | Dose | Patients (n) | Tmax (h) | Cmax (ng/ml) | AUC (ng h/ml) | Half-life (h) | Ref. |
|---|---|---|---|---|---|---|---|
| DHP107 | 250 mg/m2 | 3 | 3.6 (2.9–4.0)† | 409,700 (116,100)† | 2149.7 (253.5)† | 15.3 (2.4)† | [6] |
| Modradoc001 | 30 mg | 6 | 1.9 (0.85)† | 105 (53)† | 513 (219)† | NR | [7]‡ |
| Ortataxel | 60 mg/kg | 4 | NR | 7,300 (700)† | 35 (7)† | 6.9 (NR) | [10]§ |
| Milataxel | 60 mg/m2 | 3 | 1.0 (0)† | 85 (41)† | 1,683 (1399)† | 178 (NR)† | [13] |
| BMS-275183 | 100 mg/m2 | 17 | 1.0 (0.5–3.0)¶ | 233 (78–1700)¶ | 1,437 (707–7640)¶ | 26 (19–76)¶ | [18] |
| Tesetaxel | 27 mg/m2 | 34 | 2 (1–8)† | 42.72 (34.2)† | 1752 (1355)† | 167 (77)† | [26] |
In cases where pharmacokinetic data was available for multiple doses, data for the recommended study dose are shown.
Data shown in parenthesis represent the standard deviation (±) from the mean.
Data reflect coadministration with ritonavir 100 mg.
Study conducted in nude mice. Human data not available.
Data shown in parenthesis represent the range.
NR: Not reported.
Future perspective
Three of the novel oral taxanes are no longer being developed, but several Phase II studies of tesetaxel are in progress, both as a single agent and in combination studies. At the completion of these studies, much more will be known about tesetaxel’s toxicity profile and efficacy, and appropriate comparisons to iv. paclitaxel and docetaxel will be possible. If the results are promising, larger scale Phase III trials can be conducted in target populations in which the drug was most effective. In addition, the novel formulations ModraDoc001 and DHP107 are also undergoing further study and may eventually prove to be effective alternative ways to administer paclitaxel and docetaxel.
Executive summary.
Background
-
■
The taxanes are a widely used class of chemotherapy agents with a broad spectrum of activity across many tumor types.
-
■
The limitations of current taxanes include intravenous formulations requiring vehicles, such as Cremophor EL® and Polysorbate 80®, which potentially contribute to their toxicity.
-
■
Orally available taxanes have been developed that have the potential advantages of patient convenience, a comparably better toxicity profile and efficacy in tumors expressing the multidrug-resistance gene.
Novel oral formulations of docetaxel & paclitaxel
-
■
DHP107 and ModraDoc001 are novel oral formulations of paclitaxel and docetaxel, respectively, which may provide an alternative route of dosing those medications.
Orally bioavailable novel taxanes
-
■
Ortataxel and milataxel have been used in Phase I studies but oral dosing is not yet well established.
-
■
BMS-275183 has been used in several Phase I studies at different doses with established oral dosing.
-
■
Tesetaxel is the most widely studied of the novel oral taxanes and is currently being studied in Phase II trials both alone, as well as in combination regimens with capecitabine.
Conclusion
-
■
Further study is needed to determine the role that the oral taxanes will have in cancer treatment, specifically if toxicity can be safely predicted given interpatient variability in absorption.
Acknowledgments
Financial disclosure:MW Saif has received grant funding for DJ-927.
No writing assistance was utilized in the production of this manuscript.
Footnotes
competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Websites
101 ClinicalTrials Database: NCT00103831. www.clinicaltrials.gov/show/NCT00103831
102 ClinicalTrials Database: NCT01064713. www.clinicaltrials.gov/show/NCT01064713
103 ClinicalTrials Database: NCT01215877. www.clinicaltrials.gov/show/NCT01215877
104 ClinicalTrials Database: NCT01296243. www.clinicaltrials.gov/show/NCT01296243
105 ClinicalTrials Database: NCT01095120. www.clinicaltrials.gov/show/NCT01095120
106 ClinicalTrials Database: NCT01221870. www.clinicaltrials.gov/show/NCT01221870
107 ClinicalTrials Database: NCT01573468. www.clinicaltrials.gov/show/NCT01573468
108 ClinicalTrials Database: NCT01348009. www.clinicaltrials.gov/show/NCT01348009
References
Papers of special note have been highlighted as:
■ of interest
- 1.Rowinsky EK, Donehower RC. Paclitaxel (Taxol) N Engl J Med. 1995;332(15):1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 2■.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17(5):735–749. doi: 10.1093/annonc/mdj100. Excellent review of the limitations of paclitaxel and docetaxel and some of the novel formulations and drugs that were developed to address these limitations. [DOI] [PubMed] [Google Scholar]
- 3.Kruijtzer CM, Schellens JH, Mezger J, et al. Phase II and pharmacologic study of weekly oral paclitaxel plus cyclosporine in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2002;20(23):2508–2516. doi: 10.1200/JCO.2002.04.058. [DOI] [PubMed] [Google Scholar]
- 4.Helgason HH, Kruijtzer CM, Huitema AD, et al. Phase II and pharmacological study of oral paclitaxel (paxoral) plus ciclosporin in anthracycline-pretreated metastatic breast cancer. Br J Cancer. 2006;95(7):794–800. doi: 10.1038/sj.bjc.6603332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong JW, Lee IH, Kwak YH, et al. Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther. 2007;6(12 Pt 1):3239–3247. doi: 10.1158/1535-7163.MCT-07-0261. [DOI] [PubMed] [Google Scholar]
- 6.Hong YS, Kim KP, Lim HS, et al. A Phase I study of DHP107, a mucoadhesive lipid form of oral paclitaxel, in patients with advanced solid tumors: crossover comparisons with intravenous paclitaxel. Invest New Drugs. 2012 doi: 10.1007/s10637-012-9841-7. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Moes JJ, Koolen SL, Huitema AD, et al. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001) Int J Pharm. 2011;420(2):244–250. doi: 10.1016/j.ijpharm.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti S, Stuurman F, Koolen S, et al. Phase I study of weekly oral docetaxel (ModraDoc001) plus ritonavir in patients with advanced solid tumors. J Clin Oncol. 2012;30 Abstract 2550. [Google Scholar]
- 9.Polizzi D, Pratesi G, Monestiroli S, et al. Oral efficacy and bioavailability of a novel taxane. Clin Cancer Res. 2000;6(5):2070–2074. [PubMed] [Google Scholar]
- 10.Nicoletti MI, Colombo T, Rossi C, et al. IDN5109, a taxane with oral bioavailability and potent antitumor activity. Cancer Res. 2000;60(4):842–846. [PubMed] [Google Scholar]
- 11.Tonkin K, Herbert H, Lathia C, et al. A Phase I and pharmacokinetic study of a novel oral taxane BAY 59–8862 in solid tumors. Proc Am Soc Clin Oncol. 2003;22 Abstract 965. [Google Scholar]
- 12.Sampath D, Discafani CM, Loganzo F, et al. MAC-321, a novel taxane with greater efficacy than paclitaxel and docetaxel in vitro and in vivo. Mol Cancer Ther. 2003;2:873–884. [PubMed] [Google Scholar]
- 13.Lockhart AC, Bukowski R, Rothenberg ML, et al. Phase I trial of oral MAC-321 in subjects with advanced malignant solid tumors. Cancer Chemother Pharmacol. 2007;60(2):203–209. doi: 10.1007/s00280-006-0362-y. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan RK, Picus J, Raftopoulos H, et al. A Phase II study of milataxel: a novel taxane analogue in previously treated patients with advanced colorectal cancer. Cancer Chemother Pharmacol. 2008;61(3):453–458. doi: 10.1007/s00280-007-0489-5. [DOI] [PubMed] [Google Scholar]
- 15.Mastalerz J, Cook D, Fairchild CR, et al. The discovery of BMS-275183: an orally efficacious novel taxane. Bioorg Med Chem. 2003;11(20):4315–4323. doi: 10.1016/s0968-0896(03)00495-4. [DOI] [PubMed] [Google Scholar]
- 16.Rose WC, Long BH, Fairchild CR, Lee FYF, Kadow JF. Preclinical pharmacology of BMS-275183, an orally active taxane. Clin Cancer Res. 2001;7(7):2016–2021. [PubMed] [Google Scholar]
- 17.Broker LE, de Vos FYFL, van Groeningen CJ, et al. Phase I trial with BMS-275183, a novel oral taxane with promising antitumor activity. Clin Cancer Res. 2006;12(6):1760–1767. doi: 10.1158/1078-0432.CCR-05-2093. [DOI] [PubMed] [Google Scholar]
- 18.Broker LE, Veltkamp SA, Heath EI, et al. A Phase I safety and pharmacologic study of a twice weekly dosing regimen of the oral taxane BMS-275183. Clin Cancer Res. 2007;13(13):3906–3912. doi: 10.1158/1078-0432.CCR-06-2875. [DOI] [PubMed] [Google Scholar]
- 19.Heath EI, LoRusso P, Ramalingam SS, et al. A Phase 1 study of BMS-275183, a novel oral analogue of paclitaxel given on a daily schedule to patients with advanced malignancies. Invest New Drugs. 2011;29:1436–1431. doi: 10.1007/s10637-010-9498-z. [DOI] [PubMed] [Google Scholar]
- 20.Shionoya M, Jimbo T, Kitagawa M, Soga T, Tohgo A. DJ-927, a novel oral taxane, overcomes P-glycoprotein-mediated multidrug resistance in vitro and in vivo. Cancer Sci. 2003;94(5):459–466. doi: 10.1111/j.1349-7006.2003.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syed SK, Beeram M, Takimoto CH, et al. Phase I and pharmacokinetics (PK) of DJ-927, an oral taxane, in patients (Pts) with advanced cancers. J Clin Oncol. 2012;22(14S) Abstract 2028. [Google Scholar]
- 22.Szczesna A, Milanowski E, Juhász E, et al. A Phase II study of DJ-927 administered orally once every three weeks as second line therapy to subjects with locally advanced or metastatic non small cell lung cancer (NSCLC) after failure of platinum-based non-taxane regimen. J Clin Oncol. 2006;24(Suppl. 18) Abstract 17006. [Google Scholar]
- 23.Evans T, Dobrila R, Berardi K, et al. A Phase II study of DJ-927 as second-line therapy in patients (Pts) with advanced gastric cancer (GC) who have failed a 5-FU non taxane based regimen. J Clin Oncol. 2006;24(Suppl. 18) Abstract 4081. [Google Scholar]
- 24.Moore MR, Jones C, Harker G, et al. Phase II trial of DJ-927, an oral tubulin depolymerization inhibitor, in the treatment of metastatic colorectal cancer. J Clin Oncol. 2006;24(Suppl. 18) Abstract 3591. [Google Scholar]
- 25.Seidman AD, Schwartzberg LS, O’Shaughnessy A, et al. Tesetaxel: activity of an oral taxane as first-line treatment in metastatic breast cancer. J Clin Oncol. 2012;30 Abstract 1016. [Google Scholar]
- 26.Baas P, Szczesna A, Albert I, et al. Phase I/II study of a 3 weekly oral taxane (DJ-927) in patients with recurrent, advanced non-small-cell lung cancer. J Thorac Oncol. 2008;7:745–750. doi: 10.1097/JTO.0b013e31817c73ff. [DOI] [PubMed] [Google Scholar]
- 27■.Lang A, Beeram M, Tolcher AW, et al. Phase I development of a weekly dosing schedule for the oral taxane tesetaxel. J Clin Oncol. 2012;30 Abstract 2555. A Phase I trial studying tesetaxel on a weekly schedule that may allow higher doses and less toxicity than previously studied regimens. [Google Scholar]
- 28■.Saif MW, Sarantopoulos J, Patnaik A, et al. Tesetaxel, a new oral taxane, in combination with capecitabine: a Phase I, dose-escalation study in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:1565–1573. doi: 10.1007/s00280-011-1639-3. The only completed and published Phase I study of an oral taxane in combination therapy. [DOI] [PubMed] [Google Scholar]
- 29.Martin MG, Fryar BB, Danesi H, Schwartznerg LS. Phase IB study of an all-oral chemotherapy regimen, tesetaxel plus capecitabine, in patients with advanced solid tumors. J Clin Oncol. 2012;30 Abstract 3085. [Google Scholar]


