Abstract
Objective
Aptamers are oligonucleotides targeting protein/protein interactions with pharmacokinetic profiles and activity reversal options. Although P-selectin and von Willebrand factor (VWF) have been implicated in the development of venous thrombosis (VT), no studies have directly compared aptamer efficacy with standard of care in VT. In this study, ARC5692, an anti-P-selectin aptamer, and ARC15105, an anti- VWF aptamer, were compared to low molecular weight heparin, enoxaparin, to test the efficacy of P-selectin or VWF inhibition in promoting thrombus resolution and preventing vein wall fibrosis, in a baboon model of VT.
Approach and Results
Groups: Treatment arm: Animals received P-selectin or VWF aptamer inhibitors or enoxaparin (n=3 per group). Controls received no treatment (n=3). Prophylactic arm: Animals received P-selectin inhibitor (n=4) or VWF inhibitor (n=3). Treatment arm: P-selectin-inhibitor demonstrated a significant improvement in vein recanalization by MRV (73% at day 21), and significantly decreased vein wall collagen, compared to all groups. Anti-P-selectin equaled enoxaparin in maintaining valve competency by ultrasound. All control animals had compromised valve competency post-thrombosis. Prophylactic arm: animals receiving P-selectin and VWF inhibitors demonstrated improved vein recanalization by MRV versus controls (80% and 85% respectively at day 21). Anti-P-selectin protected iliac valve function better than anti-VWF, and both improved valve function versus controls. No adverse bleeding events were observed.
Conclusions
The P-selectin inhibitor aptamer promoted iliac vein recanalization, preserved valve competency and decreased vein wall fibrosis. The results of this work suggest that P-selectin inhibition maybe an ideal target in the treatment and prophylaxis of DVT, warranting clinical trials.
Keywords: Venous Thrombosis, Anti-P-selectin Aptamer, Anti-von Willebrand Factor Aptamer, Inflammation, Vein Wall Percent Collagen
Introduction
Deep vein thrombosis (VT) and pulmonary embolism (PE), known collectively as venous thromboembolism (VTE), affect an estimated 900,000 people in the U.S. each year, resulting in about 300,000 deaths1. Most of those deaths occurred within 1 month of the initial diagnosis2. Anticoagulation, is the current standard treatment for the prevention of VT, VT recurrence, PE and the progression of the thrombus. However, it does not lyse the thrombus or prevents the development of post-thrombotic syndrome. In addition, it carries with it significant bleeding risks such as intracranial hemorrhage, which occurs in 1.15% patients per year with a case fatality rate (major bleeding) of 13%3. Thus, new treatment options that limit bleeding risk, while decreasing thrombotic risk, should be investigated. Recently, it has been demonstrated that P-selectin and von Willebrand factor (VWF) can mediate events associated with VT4–7. Both are stored by platelets and endothelial cells (P-selectin is a component of the membrane and also the matrix of the alpha granule and the Weibel-Palade bodies), can be released from platelets and endothelial cells by pro-thrombotic and inflammatory factors generated upon cell activation and can be localized to the surface of the cells. The surface expressed P-selectin and VWF can promote accumulation of plasma derived, cell associated pro-coagulant factors and cells (platelets and leukocytes) that will promote vein wall injury and thrombus formation. Previous studies have demonstrated that P-selectin and VWF inhibition are attractive therapeutic approaches. In addition, increased levels of these molecules are being used clinically to direct treatment8–10. In this study we wanted to compare P-selectin and VWF inhibition by aptamers to standard of care low-molecular-weight heparin (LMWH) for VT to determine if one or both would inhibit VT and promote thrombus resolution better than LMWH in a baboon model of VT [Figure 1].
Figure 1. Nonhuman primate venous thrombosis model and experimental design.
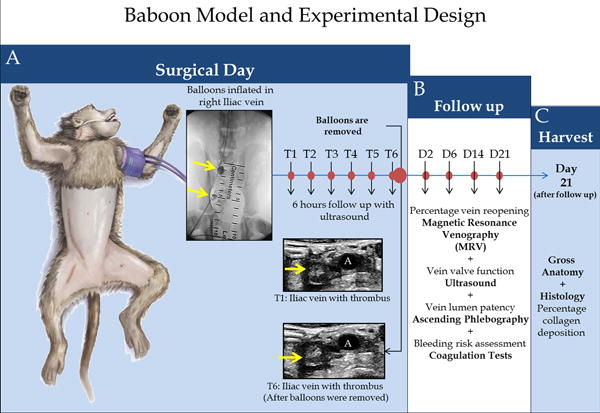
Schematic representation of the bi-balloon vein occlusion technique.
A) A balloon is placed and inflated in the iliac vein, just below the iliac bifurcation; another balloon is placed and inflated near the pelvic crest (yellow arrows); both balloons remain in initial position for 6 hours (T6) promoting thrombus formation. Baseline venogram; balloons inflated; hourly ultrasound follow-up; balloons removed; final ultrasound to confirm the presence of the thrombus at T6 and that a thrombus remains in a vein segment of approximately 3.5 cm in length. B) Percentage vein reopening magnetic resonance venography (MRV), Vein valve function ultrasound, vein lumen patency, ascending phlebography and bleeding risk assessment coagulation tests were evaluated at each time point: Days 2, 6, 14 and 21 after thrombosis. C) At day 21, after the follow up, a harvest of the tissue for gross anatomy and Histology (for leukocyte counts, quantification of percentage of thrombus fibrin and vein wall collagen deposition) were performed.
Material and Methods
Material and Methods are available in the online-only Data Supplement
Results
Anti-P-selectin aptamer improved vein recanalization over anti-VWF aptamer and enoxaparin
Treatment arm
Animals administered therapeutic anti-P-selectin aptamer (ARC5692) had a significant recanalization of 73 % by day 21, vs. controls. The animals that did receive therapeutic enoxaparin showed 0% on day 2 and a 42% vein recanalization by day 21. Of interest, the iliac veins of animals treated with the therapeutic anti-vWF aptamer (ARC15105) failed to recanalize at all by day 21. In comparison, the average percent vein recanalization of control animal iliac veins was 0% on day 2 and only 13% by day 21 post-thrombosis [Figure 2A].
Figure 2.
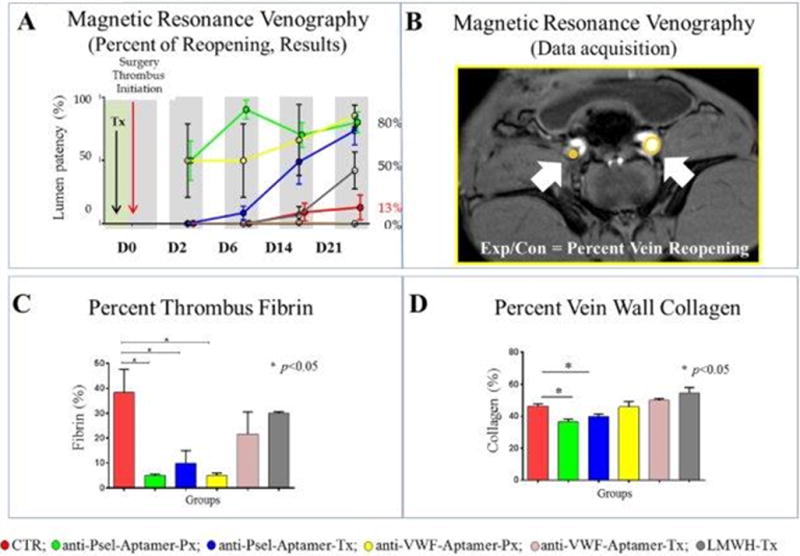
A) MRV percent reopening results: Magnetic resonance venography, time of flight, percent vein recanalization. Graph representing the percentage of reopening results from all groups at each time point. Circles represent the mean of the values obtained per animal per time point. D0: baseline; D2: two days after thrombosis; D6: six days after thrombosis; D14: fourteen days after thrombosis; D21: twenty-one days after thrombosis. B) MRV percent reopening: Representative picture showing the methodology to assess the percentage of recanalization. C) Percent of thrombus fibrin: Quantification of fibrin was calculated as percentage of fibrin-covered area in the thrombus at day 21 after thrombosis was initiated. Bars represent SEM. D) Percent of vein wall collagen: Masson trichrome analysis: Vein wall fibrosis was quantified by computerized analysis using Image J software (Scanalytics, Fairfax, VA). Three individual samples were analyzed per group for each time point. The mean fibrotic area was calculated from 10 to 12 regions of interest per vein wall section analyzed at 400× magnification. Bars represent SEM.
References: CTR: control group; anti-Psel-Aptamer-Px: P-selectin inhibitor prophylaxis group; anti-Psel-Aptamer-Tx: P-selectin inhibitor treatment group; LMWH-Tx: Low molecular weight heparin treatment group; anti-VWF-Aptamer-Px: von Willebrand factor inhibitor prophylaxis group; anti-VWF-Aptamer-Tx: von Willebrand factor inhibitor treatment group. Control group in red; anti-P-selectin aptamer prophylaxis in green; anti-P-selectin aptamer treatment in blue; anti-VWF aptamer prophylaxis in yellow; anti-P-selectin aptamer treatment in pink; LMWH treatment in grey.
Prophylactic arm
Animals receiving both aptamers in a prophylactic protocol showed the best vein lumen recanalization of 50% at day 2, for both groups, and 80%, 85% respectively, by 21 days post-thrombosis [Figure 2A]. Percentage of vein reopening was calculated using MRV as shown in Figure 2B.
Anti-P-selectin aptamer decreases fibrin thrombus content and vein wall collagen
Animals receiving anti-P-selectin aptamer, both in a prophylactic and treatment protocol, showed statistically significant decreases thrombus fibrin (P<0.05). The anti-VWF aptamer in a prophylactic protocol showed significantly lower thrombus fibrin content [Figure 2C]. Animals receiving anti-P-selectin aptamer, both in a prophylactic and treatment protocol, showed statistically significant decreases in vein wall collagen, versus control animals (P<0.05). Also, anti-P-selectin aptamer treatment primates showed significantly lower vein wall collagen compared to treatment animals given LMWH, and the anti-VWF aptamer (P<0.05) [Figure 2D].
Anti-P-selectin aptamer did not decrease circulating or leukocyte recruitment to the vein wall
Animals receiving anti-P-selectin aptamer, both in a prophylactic and treatment protocol, showed increases in vein wall leukocyte counts, versus control animals (PMN P<0.05 in the group receiving prophylaxis). Animals receiving anti-VWF aptamer in a prophylactic protocol and LMWH, showed decreases in vein wall monocyte counts, versus control animals (P<0.05) and increases in vein wall lymphocyte counts, versus control animals (P<0.05) [Figure 3A]. No differences were observed in circulating leukocyte counts [Figure 3B].
Figure 3.
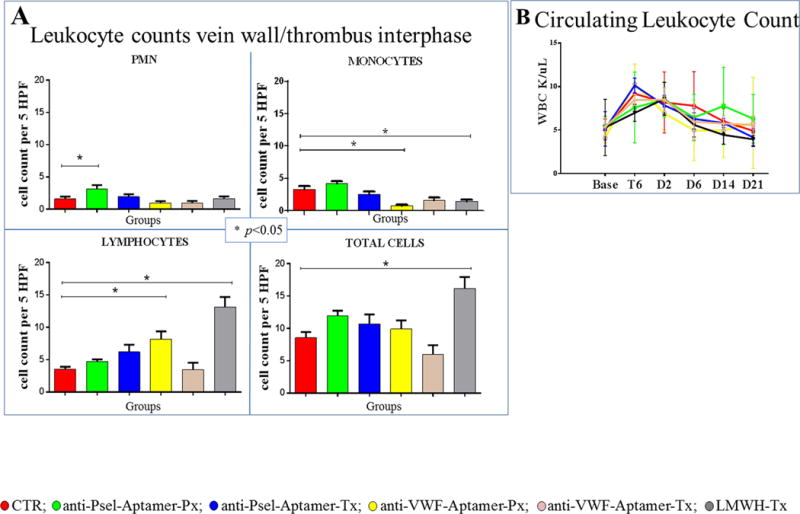
A) Leukocyte counts: Vein wall morphometrics were quantified at the vein wall/thrombus interphase (cells/5 high power fields). B) Circulating Leukocyte Count. Of note, P-selectin administration did not decrease circulating leukocyte counts. References: control group in red; anti-P-selectin aptamer prophylaxis in green; anti-P-selectin aptamer treatment in blue; anti-VWF aptamer prophylaxis in yellow; anti-P-selectin aptamer treatment in pink; LMWH treatment in grey. Base: Baseline; T6: 6 hours after thrombosis, D2: two days after thrombosis; D6: six days after thrombosis; D14: fourteen days after thrombosis; D21: twenty-one days after thrombosis.
Iliac valve function by ultrasound
Treatment arm
Both anti-P-selectin aptamer and LMWH given in a treatment protocol (n3 each group), demonstrated a 33% iliac venous valve competency. All control and anti-VWF aptamer treatment animals had 0% iliac valve competency.
Prophylactic arm
Ultrasound analysis demonstrated that animals given the anti P-selectin aptamer in the prophylactic protocol (n4), had 100% iliac valve competency, at day 21, and those given anti-VWF aptamer (ARC15105) in a prophylactic manner, had 67% valve competency (Figure 4).
Figure 4. Duplex Ultrasound Analysis: Iliac Vein Valve Function.
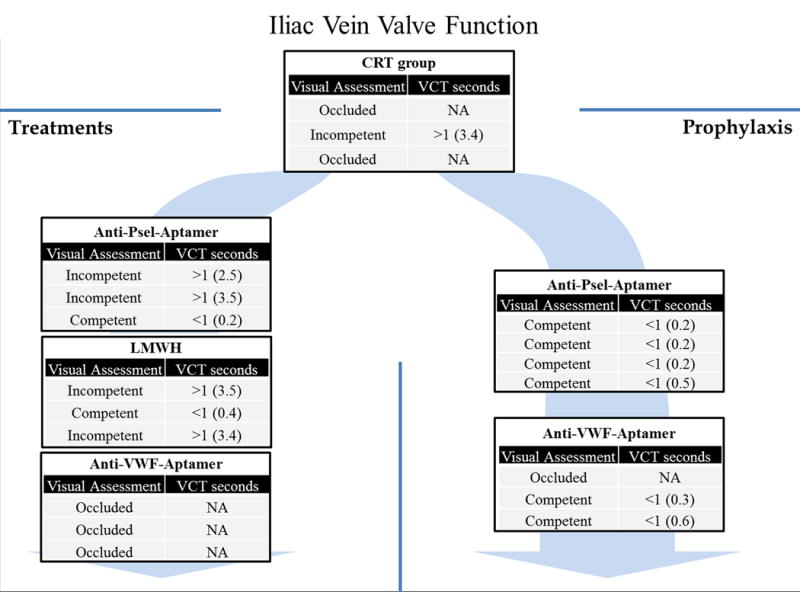
The evaluation of the iliac vein valve was assessed in all groups at each time point. The figure 4 shows our results at day 21 post-thrombosis. In the iliac veins that were occluded, the assessment was not possible (NA). Valve competency was defined as a valve closure time ≤1 second. VCT: valve closure time. CTR: control group; anti-Psel-Aptamer-Px: P-selectin inhibitor prophylaxis group; anti-Psel-Aptamer-Tx: P-selectin inhibitor treatment group; LMWH-Tx: Low molecular weight heparin treatment group; anti-VWF-Aptamer-Px: von Willebrand factor inhibitor prophylaxis group; anti-VWF-Aptamer-Tx: von Willebrand factor inhibitor treatment group; NA: not available.
Contrast venography
Contrast venography showed complete occlusion of the iliac vein at day 2 in all animals with abundant collateral circulation. Contrast venography confirmed the MRV results and demonstrated that the collateral circulation disappeared by day 21 in those animals where recanalization occurred (Figure 5).
Figure 5. Contrast Venography.
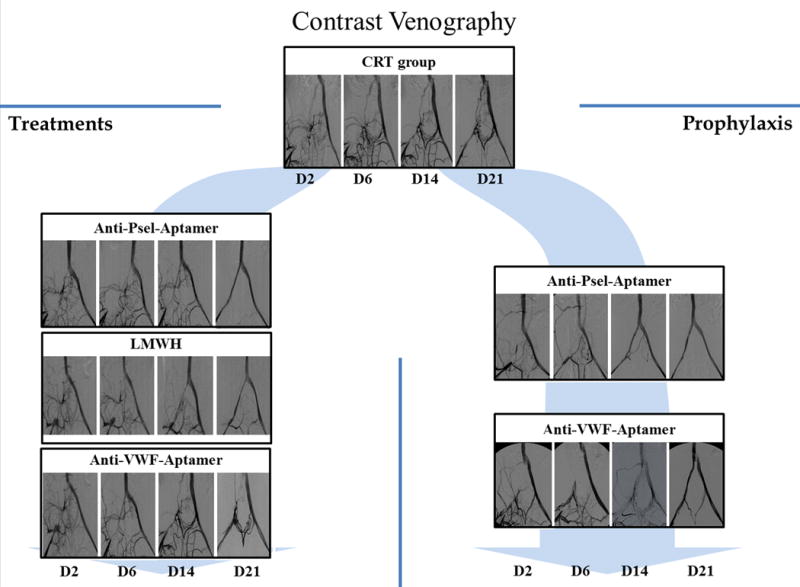
Selected pictures from contrast venography, performed at days 2, 6, 14 and 21 after thrombosis, that show the recanalization process. Occlusion of the iliac vein and collateral circulation was observed in all groups at day 2. Of note, recanalization was evident directly by the channel in the iliac vein and indirectly by the reduction of collateral circulation. CTR: control group; anti-Psel Aptamer-Px: P-selectin inhibitor prophylaxis group; anti-Psel-Aptamer-Tx: P-selectin inhibitor treatment group; LMWH-Tx: Low molecular weight heparin treatment group; anti-VWF-Aptamer-Px: von Willebrand factor inhibitor prophylaxis group; anti-VWF-Aptamer-Tx: von Willebrand factor inhibitor treatment group.
Histology, gross anatomy and venography
Representative corresponding vein wall histology and gross anatomy sections showed a strong correlation with the contrast venography at day 21 (Figure 6). The presence of thrombus was observed in all specimens except for the anti-P-selectin prophylaxis group, where only a small remnant of the thrombus was observed in the distal vein segment (Figure 6).
Figure 6. Hematoxylin and eosin, Fibrin Stain, Gross Anatomy, and Venography Evaluation at Day 21.
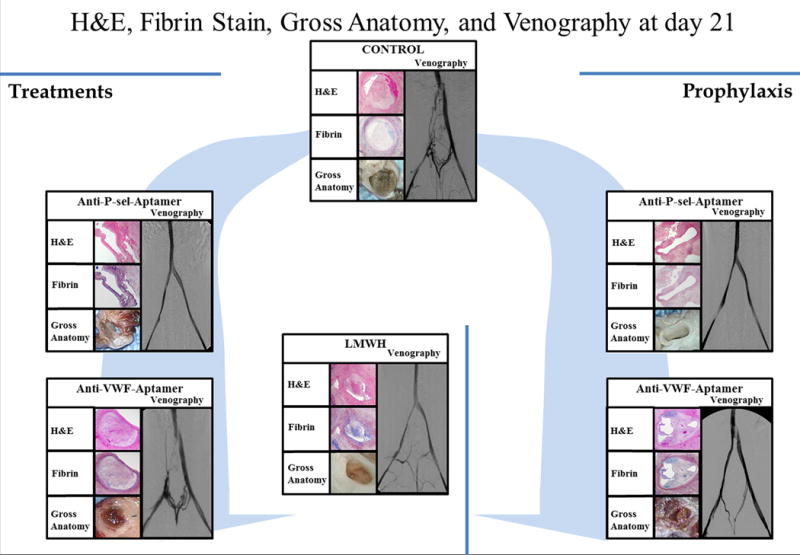
Representative pictures showing the gross anatomy, corresponding sections stained with hematoxylin and eosin (H&E), sections stained with Phosphotungstic acid haematoxylin (PTAH) which stains fibrin blue, and of all groups at harvest and its respective venogram, at day 21 post-thrombosis.
Aptamer effects on coagulation
Fibrinogen and aPTT followed the same non-significant trends for all groups, with levels of fibrinogen and aPTT peaking at two days post thrombosis. No adverse bleeding events occurred due to the anti-P-selectin or anti-VWF aptamers.
Treatment arm
Animals receiving the anti-P-selectin aptamer and controls were within normal reference ranges4, 5. Thrombin clotting times in animals treated with enoxaparin showed transient increases of 77% (day 2), 53% (day 6), and a 69% (day 14) above baseline levels. Coagulation values at peak activity time, along with anti-Xa levels between 0.5–1.01 IU/mL, confirm that these animals were anti-coagulated sufficiently during the study. Animals that received anti-VWF in a treatment protocol had also increased BT values of 24 min (T6), 11 min (day 2), 7 min (day 6) and 2.5 min (day 14) [Table 1].
Table 1. Coagulation Test.
TCT, Fibrinogen, aPTT and bleeding time was assessed in all groups at baseline, T6, and days 2, 6, 14 and 21. CTR: control group; anti-Psel-Aptamer-Px: P-selectin inhibitor prophylaxis group; anti-Psel-Aptamer-Tx: P-selectin inhibitor treatment group; LMWH-Tx: Low molecular weight heparin treatment group; anti-VWF-Aptamer-Px: von Willebrand factor prophylaxis group; anti-VWF-Aptamer-Tx: von Willebrand factor inhibitor treatment group. Base: Baseline; T6: 6 hours after thrombosis, D2: two days after thrombosis; D6: six days after thrombosis; D14: fourteen days after thrombosis; D21: twenty-one days after thrombosis. In bold: values out of range.
| TCT (seconds) | ||||||
|---|---|---|---|---|---|---|
| CONTROL | P-sel PX | P-sel TX | VWF PX | VWF TX | LMWH | |
| BASELINE | 17.1 | 17.2 | 16.8 | 15.2 | 17.8 | 17.7 |
| T6 | 15.7 | 17.2 | 15.3 | 15.2 | 16.4 | 16.2 |
| D2 | 18.3 | 20.6 | 19.1 | 20.6 | 20.1 | 78.0 |
| D6 | 20.6 | 19.4 | 19.0 | 19.0 | 19.0 | 42.9 |
| D14 | 18.5 | 18.4 | 18.2 | 17.7 | 20.5 | 58.7 |
| D21 | 17.0 | 17.1 | 17.6 | 17.0 | 18.2 | 17.7 |
| Fibrinogen (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| CONTROL | P-sel PX | P-sel TX | VWF PX | VWF TX | LMWH | |
| BASELINE | 186.7 | 138.8 | 193.2 | 147.5 | 193.5 | 210.0 |
| T6 | 133.5 | 120.8 | 134.3 | 153.8 | 128.5 | 160.4 |
| D2 | 321.7 | 352.5 | 349.7 | 450.0 | 333.3 | 632.5 |
| D6 | 298.3 | 265.0 | 247.2 | 300.0 | 246.7 | 463.3 |
| D14 | 185.0 | 163.0 | 180.0 | 177.5 | 118.0 | 232.3 |
| D21 | 163.3 | 148.8 | 158.5 | 165.0 | 144.7 | 193.3 |
| aPTT (seconds) | ||||||
|---|---|---|---|---|---|---|
| CONTROL | P-sel PX | P-sel TX | VWF PX | VWF TX | LMWH | |
| BASELINE | 30.0 | 31.3 | 31.8 | 33.7 | 22.5 | 29.8 |
| T6 | 35.8 | 34.7 | 36.1 | 34.6 | 36.0 | 37.1 |
| D2 | 40.4 | 42.0 | 42.7 | 34.5 | 40.7 | 55.8 |
| D6 | 29.7 | 28.7 | 30.8 | 25.8 | 27.9 | 32.7 |
| D14 | 33.1 | 31.0 | 31.6 | 27.4 | 30.3 | 33.3 |
| D21 | 30.5 | 31.9 | 32.9 | 29.0 | 29.1 | 29.4 |
| Bleeding Time (minutes) | ||||||
|---|---|---|---|---|---|---|
| CONTROL | P-sel PX | P-sel TX | VWF PX | VWF TX | LMWH | |
| BASELINE | 3.0 | 2.1 | 2.3 | 2.5 | 2.5 | 1.8 |
| T6 | 3.5 | 3.6 | 3.1 | 24.3 | 4.8 | 2.8 |
| D2 | 4.5 | 3.9 | 4.4 | 11.0 | 20.0 | 7.5 |
| D6 | 4.8 | 2.6 | 3.4 | 6.7 | 15.7 | 4.3 |
| D14 | 4.2 | 2.3 | 3.1 | 2.5 | 6.7 | 2.2 |
| D21 | 2.8 | 2.4 | 3.4 | 2.5 | 2.5 | 2.2 |
Prophylactic arm
Animals receiving the anti-P-selectin aptamer, in the prophylactic protocol, were within normal reference ranges for this thrombosis model4, 5. Animals receiving anti-VWF aptamer in a prophylactic manner had significantly increased BT values over baseline (2.5 – 4.5 min) of 20 min (day 2), 16 min (day 6), and 7 min (day 14) [Table 1].
Anti-VWF aptamer modulates platelet aggregation
Anti-VWF aptamer significantly decreased platelet aggregation compared to baseline (non-treated), 60 minutes post administration (212.0±10.63 vs. 92.0±12.7, Closure Time Col/ADP seconds, P=0.00008). Platelet aggregation was significantly inhibited at days 2, 6 (in both treatment and prophylactic groups), 10, and 14 (only in the treatment group) compared to baseline (P<0.05) thus confirming the inhibitory effects of ARC15105 on platelet aggregation.
Discussion
Deep venous thrombosis remains a significant health care problem today. Even with the most effective current therapies, there remains a significant risk of recurrence, extension of a primary DVT and the development of pain and swelling leading to post-thrombotic syndrome. A recurrence rate of 29% to 47% is observed with iliofemoral DVT without anticoagulation, 5% to 7% with full heparin anticoagulation, 4% to 5% with LMWH anticoagulation, and 3% to 9% with direct thrombin inhibitors11–13. The incidence of the post-thrombotic syndrome (PTS) is approximately 29% after 8 years in treated patients, with the development of ipsilateral recurrent DVT strongly associated with an increased risk of this syndrome14. In this study, we hypothesized that inhibition of P-selectin or of VWF would be at least equal to, if not an improvement, over the most commonly used standard-of care agent clinically used for VT prophylaxis and treatment, enoxaparin sodium, a low-molecular-weight heparin. Non-human primate thrombosis models have flow hemodynamics and hematologic parameters that closely resemble human vasculature making them valuable models to determine the clinical usefulness of therapies for humans15, 16, 17–19.
The anti-P-selectin aptamer ARC5692 was effective in accelerating vein recanalization and decreasing vein wall collagen deposition, without adverse bleeding events post thrombosis, compared to animals receiving treatment with anti-von Willebrand factor or LMWH, the standard of care therapy. Animals receiving anti-P-selectin aptamer therapy had vast improvements in vein recanalization that was significantly better than enoxaparin and controls. In addition, we observed a decreased effect after 6 days in animals receiving prophylactic P-selectin aptamer. Day 6 was the final day of P-selectin inhibitor administration in the prophylactic protocol. We believe that during the first 6 days, the continuous administration of the P-selectin inhibitor favors vein reopening. After its administration to the animals was stopped, a small rebound in thrombus deposition was observed.
There is supporting evidence for the probable mechanism that anti- P-selectin therapy decreases vein wall and thrombus fibrin deposition. Platelet – leukocyte interactions, mediated by P-selectin, have been implicated in thrombus amplification and stabilization. We observed a significantly decreased fibrin content in the thrombi in animals receiving anti-P-selectin in both prophylactic and treatment protocols. Our results are supported by Palabrica et al. that demonstrated that leukocyte accumulation and fibrin deposition in thrombi are P-selectin dependent20. Also, the adhesion of monocytes and platelets through P-selectin lead to tissue factor release from monocytes which initiates further coagulation and conversion of fibrinogen to fibrin21. In addition, the localization of fibrin, platelets, and leukocytes in a developing thrombus supports this mechanism of fibrin deposition during VT22. Our laboratory and others have previously shown, in rodent models of venous thrombosis, that inhibition of P-selectin or its receptor P-selectin glycoprotein ligand (PSGL)-1 significantly decreases pro-fibrotic mediators, such as interleukin-13 which selectively stimulates TGF-β and subsequent collagen deposition, and monocyte chemotactic protein (MCP)-1. MCP-1 directs monocytes to areas of inflammation and initiates a pro-fibrotic environment23, 24. Anti P-selectin therapy decreased vein wall collagen, fibrosis, and or intra-thrombus fibrin deposition, while conferring either decreased thrombosis or enhanced thrombus resolution5, 25, 26 In the present study, fibrin deposition and vein wall collagen was significantly decreased in animals treated with the anti- P-selectin aptamer, and these animals had significant vein re-opening and retention of venous valve competency. In addition, the anti-P-selectin aptamer does not impair inflammatory recruitment to the vein wall. Paradoxically, we observed increased numbers of PMN, monocytes and lymphocytes in the thrombotic area, in animals receiving anti-P-selectin aptamer. These results are consistent with previous work on VT using the same species4, 27. Interestingly, we didn’t find significant differences between groups on circulating leukocyte counts. Six hours after the thrombotic process was initiated, we observed an increase of circulating leukocytes that returned to baseline levels by day 21 in all groups.
Post thrombotic syndrome has two underlying mechanisms which promote venous pathology, persistent venous obstruction and valvular reflux28. Ultrasound analysis demonstrated non-functioning valves in controls and animals treated with anti-VWF aptamer, while animals treated with anti-P-selectin aptamer had an equal rate of valve competency to enoxaparin. When agents were on board at the time of thrombus formation, both anti-P-selectin and anti-VWF aptamers demonstrated improved iliac vein valve competency, with the best results in the anti-P-selectin treated animals. In addition, we believe that it is more likely that the efficiency of the agent to rapidly remove (or promote quick reduction in) the thrombus from the valve area was critical in improving valve function.
VWF mediates the initial contact of platelets with the injured vessel wall and is necessary for normal hemostasis and thrombosis. Its role in arterial disease has been well characterized. However, the role of VWF during venous thrombosis and resolution is still under investigation. In this study, anti-VWF aptamer treatment failed to promote vein re-opening or limit the proliferation of vein wall collagen, 21 days post-venous thrombosis. The therapeutic levels of anti-VWF significantly inhibited platelet aggregation, as determined by the PFA-100 analysis, while it increased template bleeding times by 43–80% over control animals, yet did not promote vein recanalization. In arterial thrombosis, one of the most important differences is that platelet thrombus formation in vivo is initiated by endothelial injury, such as that occurring after atheroma rupture29. Brill et al. recently evaluated the role of VWF in two mouse models of venous thrombosis7. The authors found that VWF inhibition protected mice from venous thrombosis more effectively in the presence of disturbed blood flow in one of the IVC stenosis model30, 31. In our non-human primate 6-hour balloon occlusion model of venous thrombosis, all animals had confirmed occlusive thrombosis 2 days post induction.
This investigation indicated that the therapeutic dosing regimen for anti-P-selectin aptamer in our thrombosis model did not elevate any coagulation test versus the non-treated controls. Also, enoxaparin treated animals, while having anti-Xa activity within the reported target range of 0.5 U/ml – 1 U/ml32, had increases in thrombin clotting time, indicating the bleeding potential of this compound. Animals receiving the anti-VWF aptamer had significantly inhibited platelet aggregation and elevated bleeding times. In addition, animals receiving anti-VWF showed increased bleeding times for both prophylaxis and treatment protocols4, 5, 26, 33.
Limitations
The number of animals per group in this work is small (n3–4); however we have found in our previous studies a valid sample size to discriminate statistical significances between those animals not given selectin inhibitors and those given the inhibitors using the same animal species26.
Our data bring insights on venous thrombus physiopathology
P-selectin inhibition was effective in both prophylactic and treatment applications. This suggests that the inflammatory and pro-coagulant factors involved with thrombus initiation and resolution are associated with P-selectin localization on platelets and endothelial cells. VWF inhibition was effective only in prophylactic application. This suggests VWF has a greater participation in the early stages of thrombogenesis and plays a less important role in the later pathophysiology events of VT. In addition, the prolongation of bleeding times with VWF inhibition, make bleeding a higher potential side effect for use in VT, than P-selectin inhibition.
Conclusions
The P-selectin inhibitor treatment promoted iliac vein recanalization better than enoxaparin and the VWF inhibitor treatment. The P-selectin inhibitor preserved valve competency equal to enoxaparin and better than the VWF inhibitor. Only the P-selectin inhibitor decreased vein wall fibrosis, and exclusively did not cause any increase in bleeding parameters. The results of this work suggest that P-selectin inhibition maybe an ideal target in the treatment and prophylaxis of DVT, warranting a clinical trial.
Supplementary Material
Significance.
New treatment options for VT are needed because the current standard of care only prevents: recurrence, PE and the progression of the primary thrombus. Anti-coagulation options do not prevent the development of post-thrombotic syndrome, and unfortunately carry with them significant bleeding risks3. It is clear an improved clinical approach is necessary. In an effort to gain new treatment options, our group has been studying P-selectin biology for the last 20 years. The results of this work, in the most translational animal model of VT, support the benefits of P-selectin inhibition and the necessity for clinical trials.
Acknowledgments
We would like to thank Dr. Robert E. Sigler for his timely pathology consultation.
Source of Funding
Funding for this project was provided by the National Institute of Health RFA-HLBI HL095091.
Abbreviations
- ARC5692
anti-P-selectin Aptamer
- ARC15105
anti-von Willebrand factor Aptamer
- LMWH
low-molecular weight heparin
- DVT
deep venous thrombosis
- PE
pulmonary embolism
- MRV
magnetic resonance venography
- TOF
time of flight
- Gd
gadolinium
- ROI
region of interest
- BT
bleeding time
- aPTT
activated partial thromboplastin time
- TCT
thrombin clotting time
- CT
closure time
- SQ
subcutaneous
- IV
intravenous
Footnotes
This work was presented, in part, at the American Venous Forum 26th Annual Meeting, New Orleans, Friday, February 21st, 2014.
Authorship
Conception and design: TWW-RGS-DDM-SKW
Analysis and interpretation: JAD-DDM-RGS-TWW
Data collection: JAD-SKW-CMA-AEH-JEG-KJR-DDM
Writing the article: JAD-SKW-PKH-DDM-TWW
Critical revision of the article: JAD-SKW-PKH-DDM-RGS-TWW
Final approval of the article: JAD-SKW-AEH-PKH-DDM- RGS-TWW
Statistical analysis: JAD-DDM
Obtained funding: TWW
Overall responsibility: JAD-TWW-DDM
References
- 1.Wakefield TW, McLafferty RB, Lohr JM, Caprini JA, Gillespie DL, Passman MA, Executive Committee of the American Venous F Call to action to prevent venous thromboembolism. Journal of vascular surgery. 2009;49:1620–1623. doi: 10.1016/j.jvs.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Deep vein thrombosis (dvt) / pulmonary embolism (pe) — blood clot forming in a vein. 2012. [Google Scholar]
- 3.Linkins L-A, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: A meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 4.Myers DD, Jr, Schaub R, Wrobleski SK, Londy FJ, 3rd, Fex BA, Chapman AM, Greenfield LJ, Wakefield TW. P-selectin antagonism causes dose-dependent venous thrombosis inhibition. Thromb Haemost. 2001;85:423–429. [PubMed] [Google Scholar]
- 5.Myers DD, Jr, Wrobleski SK, Longo C, et al. Resolution of venous thrombosis using a novel oral small-molecule inhibitor of p-selectin (psi-697) without anticoagulation. Thromb Haemost. 2007;97:400–407. [PubMed] [Google Scholar]
- 6.Diaz JA, Fuchs TA, Jackson TO, Kremer Hovinga JA, Lammle B, Henke PK, Myers DD, Jr, Wagner DD, Wakefield TW, for the Michigan Research Venous G Plasma DNA is elevated in patients with deep vein thrombosis. J Vasc Surg: Venous and Lym Dis. 2013;1:341–348. doi: 10.1016/j.jvsv.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. Von willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, Pabinger I. High plasma levels of soluble p-selectin are predictive of venous thromboembolism in cancer patients: Results from the vienna cancer and thrombosis study (cats) Blood. 2008;112:2703–2708. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Lowe GD, Rumley A, Dunn FG. Increased markers of thrombogenesis in chronic atrial fibrillation: Effects of warfarin treatment. British heart journal. 1995;73:527–533. doi: 10.1136/hrt.73.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Body R, Pemberton P, Ali F, McDowell G, Carley S, Smith A, Mackway-Jones K. Low soluble p-selectin may facilitate early exclusion of acute myocardial infarction. Clinica chimica acta; international journal of clinical chemistry. 2011;412:614–618. doi: 10.1016/j.cca.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: The seventh accp conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 12.Hirsh J. Heparin. N Engl J Med. 1991;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 13.Becker RC. Antithrombotic therapy after myocardial infarction. N Engl J Med. 2002;347:1019–1022. doi: 10.1056/NEJMe020097. [DOI] [PubMed] [Google Scholar]
- 14.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Myers DD., Jr Nonhuman primate models of thrombosis. Thrombosis research. 2012;129(Suppl 2):S65–69. doi: 10.1016/j.thromres.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Melaragno AJ, Abdu W, Katchis R, Doty A, Valeri CR. Liquid and freeze preservation of baboon platelets. Cryobiology. 1981;18:445–452. doi: 10.1016/0011-2240(81)90202-9. [DOI] [PubMed] [Google Scholar]
- 17.Vecchione JJ, Melaragno AJ, Weiblen BJ, Halkett JA, Callow AD, Valeri CR. Repeated intravenous administrations of human albumin and human fibrinogen in the baboon: Survival measurements. Journal of medical primatology. 1982;11:91–105. doi: 10.1159/000460040. [DOI] [PubMed] [Google Scholar]
- 18.Feingold HM, Pivacek LE, Melaragno AJ, Valeri CR. Coagulation assays and platelet aggregation patterns in human, baboon, and canine blood. American journal of veterinary research. 1986;47:2197–2199. [PubMed] [Google Scholar]
- 19.Kelly CA, Gleiser CA. Selected coagulation reference values for adult and juvenile baboons. Laboratory animal science. 1986;36:173–175. [PubMed] [Google Scholar]
- 20.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, Sajer SA, Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by p-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 21.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B. P-selectin induces the expression of tissue factor on monocytes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furie B, Furie BC, Flaumenhaft R. A journey with platelet p-selectin: The molecular basis of granule secretion, signalling and cell adhesion. Thromb Haemost. 2001;86:214–221. [PubMed] [Google Scholar]
- 23.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) The Journal of experimental medicine. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr, Diaz JA. Interleukin-6: A potential target for post-thrombotic syndrome. Annals of vascular surgery. 2011;25:229–239. doi: 10.1016/j.avsg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan VV, Hawley AE, Farris DM, Knipp BS, Varga AJ, Wrobleski SK, Thanapron P, Eagleton MJ, Myers DD, Fowlkes JB, Wakefield TW. Decrease in fibrin content of venous thrombi in selectin-deficient mice. J Surg Res. 2003;109:1–7. doi: 10.1016/s0022-4804(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 26.Meier TR, Myers DD, Jr, Wrobleski SK, Zajkowski PJ, Hawley AE, Bedard PW, Ballard NE, Londy FJ, Kaila N, Vlasuk GP, Schaub RG, Wakefield TW. Prophylactic p-selectin inhibition with psi-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99:343–351. doi: 10.1160/TH07-10-0608. [DOI] [PubMed] [Google Scholar]
- 27.Wakefield TW, Strieter RM, Schaub R, Myers DD, Prince MR, Wrobleski SK, Londy FJ, Kadell AM, Brown SL, Henke PK, Greenfield LJ. Venous thrombosis prophylaxis by inflammatory inhibition without anticoagulation therapy. Journal of vascular surgery. 2000;31:309–324. doi: 10.1016/s0741-5214(00)90162-9. [DOI] [PubMed] [Google Scholar]
- 28.Goldhaber SZ. Venous thromboembolism: Epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235–242. doi: 10.1016/j.beha.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Goto S. Understanding the mechanism and prevention of arterial occlusive thrombus formation by anti-platelet agents. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:149–156. doi: 10.2174/1568016043477233. [DOI] [PubMed] [Google Scholar]
- 30.Greer IA. Prevention of venous thromboembolism in pregnancy. Best Pract Res Clin Haematol. 2003;16:261–278. doi: 10.1016/s1521-6926(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 31.Motto D. Clues to dvt pathogenesis. Blood. 2011;117:1106–1107. doi: 10.1182/blood-2010-11-315879. [DOI] [PubMed] [Google Scholar]
- 32.Sule AA, Tay JC, Arul E. Effect of enoxaparin on peak and trough levels of antifactor xa in patients with a creatinine clearance of less than 30 ml/min. Int J Angiol. 2009;18:184–186. doi: 10.1055/s-0031-1278351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers D, Wrobleski S, Londy F, Fex B, Hawley A, Schaub R, Greenfield L, Wakefield T. New and effective treatment of experimentally induced venous thrombosis with anti-inflammatory rpsgl-ig. Thromb Haemost. 2002;87:374–382. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


