Abstract
Purpose
The aim of this study was to demonstrate lipegfilgrastim superiority versus placebo in adults with non-small cell lung cancer receiving myelosuppressive chemotherapy.
Methods
This phase III, double-blind study randomized chemotherapy-naive patients to receive cisplatin and etoposide with either lipegfilgrastim 6 mg or placebo. Because of the placebo control, patients at individual high risk for febrile neutropenia (FN; ≥20%) were excluded. Study drug was administered on day 4 (24 h after chemotherapy) of a 21-day cycle for ≤4 cycles. Primary efficacy measure was FN incidence in cycle 1. Secondary assessments included duration of severe neutropenia (DSN), absolute neutrophil count (ANC) profile, and adverse events (AEs).
Results
The study included 375 patients (lipegfilgrastim, n = 250; placebo, n = 125). Lipegfilgrastim superiority for FN incidence in cycle 1 was not achieved but incidence was lower (2.4%) versus placebo (5.6%). Cycle 1 mean DSN was significantly shorter for lipegfilgrastim (0.6 ± 1.1 days) versus placebo (2.3 ± 0.5 days; p < 0.0001). Incidence of severe neutropenia was significantly lower for lipegfilgrastim versus placebo overall and in each cycle (all, p < 0.0001). Mean ANC nadir was lowest in cycle 1 but significantly higher for lipegfilgrastim (1.60 ± 1.64) than placebo (0.67 ± 0.85; p < 0.0001). Mean time to ANC recovery was shorter with lipegfilgrastim in each cycle. Treatment-emergent AEs were similar between treatment groups.
Conclusions
Lipegfilgrastim was not statistically superior to placebo for incidence of FN in cycle 1, but was more effective in reducing incidence of severe neutropenia, DSN, and time to ANC recovery, with an acceptable safety profile.
Controlled-trials.com identifier: ISRCTN55761467.
Keywords: Neutropenia, Non-small cell lung cancer, Recombinant granulocyte colony-stimulating factor, Lipegfilgrastim, Phase III clinical trial
Background
Neutropenia is a major dose-limiting toxicity in many myelosuppressive chemotherapy regimens (Holmes et al. 2002; Crawford et al. 2013). A patient’s risk of developing neutropenia or febrile neutropenia (FN) depends on several factors, including the type of cancer, chemotherapy regimen (standard-dose, dose-dense, or high-dose therapy), and patient-related and disease-related factors, such as age and comorbidities (Crawford et al. 2013).
Recombinant granulocyte colony-stimulating factors (G-CSFs) promote the proliferation and differentiation of neutrophils, alleviating the severity of chemotherapy-induced neutropenia and FN (Cooper et al. 2011). These agents are well established as primary prophylaxis for FN and are recommended in European and US guidelines for chemotherapy patients at high (≥20%) risk of FN (Crawford et al. 2010, 2013; Smith et al. 2006; Aapro et al. 2011). Filgrastim, the first recombinant human G-CSF, requires daily subcutaneous (SC) injections (Neupogen [package insert] 2013). With the attachment of polyethylene glycol (PEG) to the G-CSF molecule, the half-life of pegfilgrastim was extended compared with filgrastim, allowing once-per-chemotherapy cycle administration (Neulasta [package insert] 2012). Lipegfilgrastim is a glycoPEGylated, once-per-cycle recombinant human G-CSF expressed in Escherichia coli. It is approved by the European Medicines Agency for reducing the duration of neutropenia and the incidence of FN in adults treated with cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukemia and myelodysplastic syndromes) (Lonquex 2013).
A recent phase III trial (controlled-trials.com identifier ISRCTN56891934) demonstrated the clinical efficacy of lipegfilgrastim to be noninferior to pegfilgrastim in reducing neutropenia in breast cancer patients receiving myelosuppressive chemotherapy (Bondarenko et al. 2013). This trial was conducted to demonstrate superiority of once-per-cycle lipegfilgrastim versus placebo in patients with stage IIIb/IV non-small cell lung cancer (NSCLC) receiving up to four cycles of cisplatin and etoposide chemotherapy. Evaluations of efficacy, tolerability, and pharmacokinetic properties were secondary assessments.
Results
The study was conducted between May 2010 and April 2011 at 72 centers in eight countries. In total, 427 patients were screened and 376 were randomized (Belarus, n = 34; Bosnia-Herzegovina, n = 2; Bulgaria, n = 16; Poland, n = 4; Romania, n = 25; Russia, n = 160; Serbia, n = 20; Ukraine, n = 115). One patient in the lipegfilgrastim group who was randomized in error and received no chemotherapy or study medication was excluded from the efficacy and safety analyses. Thus, 250 patients in the lipegfilgrastim group and 125 in the placebo group were included in the intent-to-treat population (Figure 1). Of these, 169 (67.6%) patients in the lipegfilgrastim and 81 (64.8%) in the placebo group completed treatment. Two patients in the lipegfilgrastim group who died after randomization but did not receive study medication were included in the efficacy but not safety analyses.
Figure 1.
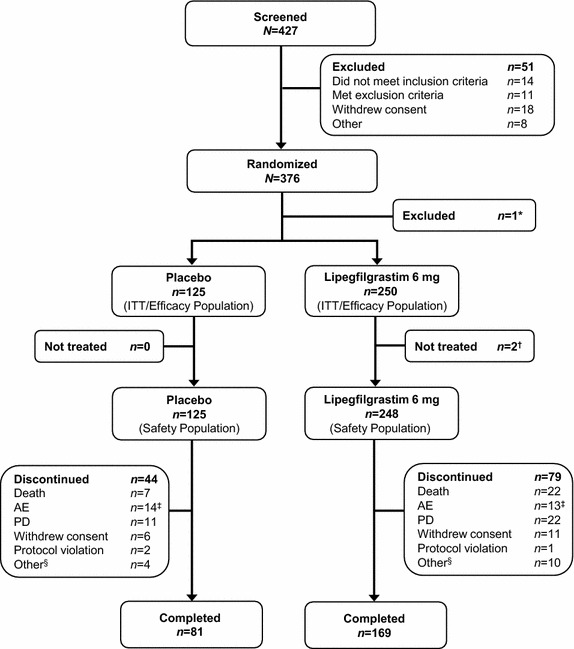
Patient disposition from randomization to study completion. *One patient randomized in error received no chemotherapy and no study medication and was excluded from all statistical analyses and populations. †Two patients who received chemotherapy but died after randomization, before study medication was administered, were included in the efficacy population but not in the safety population. ‡Adverse events listed as the primary reason for study discontinuation include placebo patients: two patients each with febrile neutropenia, cerebral infarction, and pneumonia; one patient each with back pain, general physical health deterioration, arterial thrombosis in a limb, pain in the extremities, inadequate control of diabetes mellitus, tumor lysis syndrome, and neutropenia; and one patient with anemia, thrombocytopenia, and neutropenia; lipegfilgrastim patients: two patients with anemia; one patient each with wound necrosis, syphilis, atrial fibrillation, pyothorax, fatigue, increased aspartate aminotransferase, gastric hemorrhage, dementia, pulmonary embolism, asthenia, and hemoptysis. §Includes patients lost to follow-up (n = 2), treatment failure (n = 3), and other (n = 5). AE adverse event, ITT intent to treat, PD progression of underlying disease.
Patients
Baseline demographics and clinical characteristics were similar between treatment groups (Table 1). Most patients were men, had stage IV NSCLC, an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 1, and were receiving chemotherapy for metastatic disease. The mean age was similar in both groups and was somewhat younger than is typically seen for patients with lung cancer. However, this was expected as one of the risk factors that would have contributed to the exclusion of patients at individual high risk for FN was age >65 years, leading to a relatively low percentage of patients aged >65 years (23.5%) being enrolled.
Table 1.
Patient demographics and baseline characteristics (intent-to-treat population)
| Characteristic | Placebo (n = 125) | Lipegfilgrastim 6 mg SC (n = 250) |
|---|---|---|
| Age, years | ||
| Mean ± SD | 58.7 ± 8.5 | 58.2 ± 8.5 |
| ≤64, n (%) | 94 (75.2) | 193 (77.2) |
| 65–74, n (%) | 29 (23.2) | 54 (21.6) |
| ≥75, n (%) | 2 (1.6) | 3 (1.2) |
| Weight, kg | ||
| Mean ± SD | 70.4 ± 13.4 | 69.0 ± 12.9 |
| ≤60, n (%) | 34 (27.2) | 70 (28.0) |
| >60 to ≤75, n (%) | 53 (42.4) | 106 (42.4) |
| >75, n (%) | 38 (30.4) | 74 (29.6) |
| Sex, n (%) | ||
| Female | 20 (16.0) | 30 (12.0) |
| Male | 105 (84.0) | 220 (88.0) |
| Region, n (%) | ||
| Russia | 54 (43.2) | 106 (42.4) |
| Ukraine | 38 (30.4) | 77 (30.8) |
| Rest of Europe | 33 (26.4) | 67 (26.8) |
| NSCLC stage at enrolment, n (%) | ||
| Stage IIIB | 49 (39.2) | 97 (38.8) |
| Stage IV | 76 (60.8) | 152 (60.8) |
| Unknown | 0 (0) | 1 (0.4) |
| Time since diagnosis, months | ||
| Mean ± SD | 3.4 ± 9.1 | 2.4 ± 6.2 |
| Median (range) | 1.0 (0–58.0) | 1.0 (0–52.0) |
| ECOG PS, n (%) | ||
| 0 | 19 (15.2) | 28 (11.2) |
| 1 | 96 (76.8) | 194 (77.6) |
| 2 | 10 (8.0) | 28 (11.2) |
| Reason for chemotherapy, n (%) | ||
| Adjuvant therapy | 21 (16.8) | 35 (14.0) |
| Treatment for metastatic disease | 104 (83.2) | 215 (86.0) |
| Lung cancer surgery | ||
| No | 98 (78.4) | 215 (86.0) |
| Yes | 27 (21.6) | 35 (14.0) |
ECOG PS Eastern Cooperative Oncology Group Performance Status, NSCLC non-small cell lung cancer, SC subcutaneously, SD standard deviation.
Most patients in both treatment groups received their planned chemotherapy dose in each cycle; ≤3.3% of patients receiving placebo and ≤2.3% of those receiving lipegfilgrastim had a dose reduction or omission. The total number of administered doses for both cisplatin and etoposide was similar between treatment groups (data not shown). The proportion of patients with chemotherapy dose delays was significantly lower in patients treated with lipegfilgrastim than in those receiving placebo (cycle 2, 28.5 vs. 65.1%; cycle 3, 42.1 vs. 66.3%; and cycle 4, 40.4 vs. 75.3%, respectively; all p ≤ 0.0001). The mean duration of chemotherapy delay across all cycles also was shorter for patients receiving lipegfilgrastim (6.4 ± 7.6 days) versus those receiving placebo (12.8 ± 10.2 days).
Efficacy
The primary efficacy measure, incidence of FN in cycle 1, was lower in the lipegfilgrastim group compared with the placebo group (Table 2), but the difference was not significant. Thus, the study failed to achieve its primary objective of demonstrating superiority of lipegfilgrastim versus placebo in these patients.
Table 2.
Febrile neutropenia in cycles 1, 2, 3, and 4 (intent-to-treat population)
| Cycle | Placebo | Lipegfilgrastim 6 mg SC | Lipegfilgrastim 6 mg SC vs. placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | FN | % | N | FN | % | OR | 95% CI | P value* | |
| 1 | 125 | 7 | 5.6 | 250 | 6 | 2.4 | 0.390 | 0.121–1.260 | 0.1151 |
| 2 | 105 | 0 | 0 | 214 | 1 | 0.5 | NE | NE | 0.9551 |
| 3 | 92 | 1 | 1.1 | 188 | 1 | 0.5 | 0.642 | 0.234–1.762 | 0.3883 |
| 4 | 81 | 2 | 2.5 | 171 | 2 | 1.2 | 0.421 | 0.119–1.489 | 0.1787 |
CI confidence interval, FN febrile neutropenia, NE not evaluable, OR odds ratio, SC subcutaneously.
* P values based on a null hypothesis of odds ratio = 1.
Seven investigator-assessed cases of FN were observed during cycles 2, 3, and 4 (Table 2), but no significant differences in the incidence of FN between treatment groups were observed (p > 0.05). None of the patients switched to open-label lipegfilgrastim experienced FN during chemotherapy cycles 2, 3, and 4. Patients receiving lipegfilgrastim experienced a significantly shorter duration of severe neutropenia (DSN) in cycle 1 compared with patients receiving placebo (p < 0.0001; Table 3). Similarly, the DSN in cycles 2, 3, and 4 was consistently and significantly shorter in the lipegfilgrastim group compared with the placebo group (p < 0.0001, all cycles). The incidence of severe neutropenia was significantly lower in the lipegfilgrastim group versus the placebo group overall (p < 0.0001) and in each cycle (p < 0.0001, each cycle; Table 4). Notably, in cycles 2, 3, and 4, approximately 80% of patients in the lipegfilgrastim group experienced no severe neutropenia compared with approximately 40% in the placebo group.
Table 3.
Duration of severe neutropenia in cycles 1, 2, 3, and 4 (ITT population)
| Cycle | Duration of severe neutropenia (days) | Placebo | Lipegfilgrastim 6 mg SC |
|---|---|---|---|
| 1 | N | 125 | 250 |
| Mean ± SD | 2.3 ± 2.5 | 0.6 ± 1.1 | |
| Median (range) | 2.0 (0–11.0) | 0 (0–5.0) | |
| LSM* | −1.661 | ||
| 95% CI* | −2.089 to −1.232 | ||
| P value* | <0.0001 | ||
| 2 | N | 122 | 244 |
| Mean ± SD | 2.2 ± 2.6 | 0.3 ± 0.7 | |
| Median (range) | 1.0 (0–11.0) | 0 (0–4.0) | |
| LSM* | −1.915 | ||
| 95% CI* | −2.317 to −1.512 | ||
| P value* | <0.0001 | ||
| 3 | N | 122 | 245 |
| Mean ± SD | 2.0 ± 2.4 | 0.4 ± 0.9 | |
| Median (range) | 1.0 (0–11.0) | 0 (0–5.0) | |
| LSM* | −1.640 | ||
| 95% CI* | −2.053 to −1.227 | ||
| P value* | <0.0001 | ||
| 4 | N | 123 | 246 |
| Mean ± SD | 2.3 ± 2.5 | 0.5 ± 1.1 | |
| Median (range) | 1.0 (0–11.0) | 0 (0–0.8) | |
| LSM* | −1.844 | ||
| 95% CI* | −2.281 to −1.407 | ||
| P value* | <0.0001 | ||
Includes patients from the ITT population who were withdrawn from the study.
CI confidence interval, ITT intent to treat, LSM least squares mean, SC subcutaneously, SD standard deviation.
*Least squares mean, 95% CI, and P value are for Poisson regression analysis lipegfilgrastim–placebo.
Table 4.
Incidence of severe and very severe neutropenia in cycles 1, 2, 3, and 4 (ITT population)
| Cycle | Placebo | Lipegfilgrastim 6 mg SC | Lipegfilgrastim 6 mg SC vs. placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | Odds ratio | 95% CI | P value* | |
| Severe neutropenia (grade 4, ANC <0.5 × 109/L) | |||||||||
| 1 | 125 | 74 | 59.2 | 249 | 80 | 32.1 | 0.325 | 0.206–0.512 | <0.0001 |
| 2 | 105 | 55 | 52.4 | 215 | 36 | 16.7 | 0.156 | 0.086–0.282 | <0.0001 |
| 3 | 92 | 47 | 51.1 | 188 | 26 | 13.8 | 0.115 | 0.057–0.229 | <0.0001 |
| 4 | 81 | 45 | 55.6 | 169 | 25 | 14.8 | 0.121 | 0.062–0.238 | <0.0001 |
| All | 125 | 100 | 80.0 | 249 | 103 | 41.4 | 0.176 | 0.105–0.294 | <0.0001 |
| Very severe neutropenia (ANC <0.1 × 109/L) | |||||||||
| 1 | 125 | 18 | 14.4 | 249 | 27 | 10.8 | 0.700 | 0.365–1.342 | NS |
| 2 | 105 | 10 | 9.5 | 215 | 8 | 3.7 | 0.298 | 0.099–0.895 | 0.031 |
| 3 | 92 | 9 | 9.8 | 188 | 9 | 4.8 | 0.421 | 0.156–1.138 | NS |
| 4 | 81 | 11 | 13.6 | 169 | 8 | 4.7 | 0.260 | 0.098–0.687 | 0.007 |
| All | 125 | 33 | 26.4 | 249 | 40 | 16.1 | 0.516 | 0.300–0.888 | 0.017 |
Based on data actually collected.
ANC absolute neutrophil count, CI confidence interval, ITT intent to treat, NS not significant, SC subcutaneously.
*P values based on a null hypothesis of odds ratio = 1.
Patients treated with lipegfilgrastim experienced a shorter mean duration of very severe neutropenia [absolute neutrophil count (ANC) <0.1 × 109/L] versus patients receiving placebo in cycle 1: 0.3 ± 0.9 days versus 0.2 ± 0.6 days for lipegfilgrastim and placebo, respectively. The mean duration of very severe neutropenia was also shorter for patients in the lipegfilgrastim group compared with the placebo group in cycles 2, 3, and 4. Patients receiving lipegfilgrastim had a significantly lower incidence of very severe neutropenia over all cycles versus those receiving placebo (p = 0.017). Differences between groups also were significant in cycles 2 (p = 0.031) and 4 (p = 0.007), with the lipegfilgrastim group having a lower incidence (Table 4).
The time course of median ANC during cycle 1 is shown in Figure 2. The mean depth of the ANC nadir in both treatment groups was lowest in cycle 1, but was significantly higher in patients treated with lipegfilgrastim (1.6 ± 1.6 × 109/L) versus patients receiving placebo (0.7 ± 0.9 × 109/L); p < 0.0001). In cycles 2, 3, and 4, the mean ANC nadir was greater than 2.5 × 109/L for the lipegfilgrastim group, but remained below 1.0 × 109/L for the placebo group (mean 2.8 vs. 0.8, 2.8 vs. 0.8, and 2.6 vs. 0.7 × 109/L, respectively; p < 0.0001 in each case).
Figure 2.
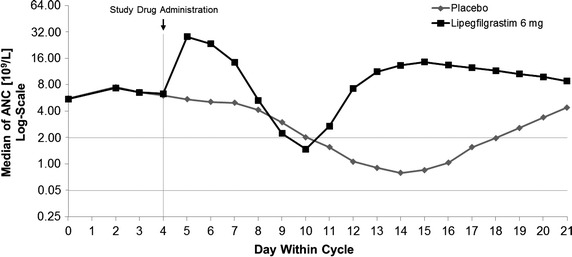
Time course of measured median absolute neutrophil count in cycle 1 (intent-to-treat population). ANC absolute neutrophil count.
The mean time to ANC nadir was consistently shorter in the lipegfilgrastim group compared with the placebo group in each cycle (cycle 1: 8.2 vs. 13.7; cycle 2: 9.6 vs. 13.6; cycle 3: 9.8 vs. 13.6; cycle 4: 9.6 vs. 14.2 days). In addition, the mean time to ANC recovery from ANC nadir was significantly shorter in the lipegfilgrastim versus placebo group in each cycle (cycle 1: 1.1 vs. 2.7; cycle 2: 0.6 vs. 2.6; cycle 3: 0.8 vs. 2.3; cycle 4: 0.7 vs. 2.6 days; p < 0.0001 between groups in each cycle). Patients treated with lipegfilgrastim also experienced a significantly shorter mean time to ANC recovery after chemotherapy in each chemotherapy cycle compared with patients receiving placebo (cycle 1: 6.8 vs. 13.0; cycle 2: 5.6 vs. 13.8; cycle 3: 6.0 vs. 13.7; cycle 4: 5.4 vs. 14.0 days, respectively; p < 0.0001 between groups in each cycle).
Safety
During the double-blind phase, 171 (69.0%) patients in the lipegfilgrastim group and 81 (64.8%) in the placebo group received four doses of study medication. The mean total amount of study medication administered was 19.9 ± 6.7 mg and 19.3 ± 7.0 mg in the lipegfilgrastim and placebo groups, respectively.
Adverse events (AEs) were similar between treatment groups. The most common AEs (total incidence of ≥10% in either treatment group) in the lipegfilgrastim and placebo groups were alopecia (40.7 and 33.6%), anemia (25.4 and 24.0%), nausea (23.8 and 21.6%), neutropenia (20.6 and 35.2%), thrombocytopenia (12.9 and 8.0%), asthenia (11.3 and 18.4%), vomiting (11.3 and 12.0%), and leukopenia (6.5 and 11.2%), respectively (Table 5). In total, 57 (23.0%) patients in the lipegfilgrastim group and 33 (26.4%) patients in the placebo group experienced an AE that lead to discontinuation from the study.
Table 5.
Most frequent TEAEs (≥5% of patients) in either treatment group across all cycles (safety population)
| AE by preferred term | Placebo (N = 125) | Lipegfilgrastim 6 mg SC (N = 248) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Alopecia | 42 | 33.6 | 101 | 40.7 |
| Anemia | 30 | 24.0 | 63 | 25.4 |
| Nausea | 27 | 21.6 | 59 | 23.8 |
| Neutropenia | 44 | 35.2 | 51 | 20.6 |
| Thrombocytopenia | 10 | 8.0 | 32 | 12.9 |
| Asthenia | 23 | 18.4 | 28 | 11.3 |
| Vomiting | 15 | 12.0 | 28 | 11.3 |
| Decreased appetite | 12 | 9.6 | 23 | 9.3 |
| Hypokalemia | 3 | 2.4 | 20 | 8.1 |
| Leukopenia | 14 | 11.2 | 16 | 6.5 |
| Fatigue | 6 | 4.8 | 16 | 6.5 |
| Disease progression | 5 | 4.0 | 16 | 6.5 |
| NSCLC | 4 | 3.2 | 16 | 6.5 |
| Chest pain | 8 | 6.4 | 14 | 5.6 |
| Febrile neutropenia | 10 | 8.0 | 11 | 4.4 |
| Dyspnea | 9 | 7.2 | 11 | 4.4 |
TEAEs include all AEs except those specifically rated by investigators as unrelated to study drug. Multiple mentions per patient are possible. TEAEs with onset after the start of prophylactic open-label lipegfilgrastim treatment are excluded.
AE adverse event, NSCLC non-small cell lung cancer, SC subcutaneously, TEAEs treatment-emergent adverse events.
Bone-pain-related symptoms (defined as arthralgia, back pain, bone pain, myalgia, noncardiac chest pain, and pain in extremity) were reported in 21 (8.5%) patients treated with lipegfilgrastim and 8 (6.4%) patients receiving placebo. These events were generally mild or moderate in severity and led to study discontinuation in only two patients (both receiving placebo).
Serious AEs in the lipegfilgrastim and placebo groups included anemia (3.2 and 1.6%), NSCLC (3.2 and 0.8%), disease progression (2.4 and 0%), FN (2.0 and 4.0%), neutropenia (1.6 and 0.8%), pulmonary embolism (1.2 and 1.6%), cardio-respiratory arrest (1.2% and 0), thrombocytopenia (1.2% and 0), pneumonia (0.8 and 2.4%), pulmonary hemorrhage (0.8% and 0), renal failure (0.8% and 0), and sudden death (0.8% and 0), respectively.
A total of 31 (12.5%) patients treated with lipegfilgrastim and nine (7.2%) patients receiving placebo died during the course of the study or up to 30 days after the last study drug injection. General disorders and administration site conditions accounted for eight deaths in the lipegfilgrastim group and two in the placebo group; benign, malignant, and unspecified neoplasms for eight in the lipegfilgrastim group and two in the placebo group; respiratory, thoracic, and mediastinal disorders for five in the lipegfilgrastim group and three in the placebo group; cardiac disorders for four in the lipegfilgrastim group and one in the placebo group; vascular disorders for two in the lipegfilgrastim group; renal and urinary disorders for two in the lipegfilgrastim group; nervous system disorders for one in the lipegfilgrastim group and one in the placebo group; and metabolism and nutrition disorders for one in the lipegfilgrastim group.
Changes over time in laboratory assessments were consistent with the underlying disease and with the chemotherapy treatment received and, in the lipegfilgrastim group, with G-CSF therapy.
Discussion
In this study, patients with NSCLC and a low individual risk of FN who received lipegfilgrastim had a lower incidence of FN during cycle 1 (2.4%) compared with patients receiving placebo (5.6%). The incidence of FN was in line with previously published results for pegfilgrastim (Vogel et al. 2005). Because the difference between treatment groups was not statistically significant, the study did not achieve its primary efficacy measure of demonstrating the superiority of lipegfilgrastim versus placebo. Nevertheless, the reduction in the incidence of FN in cycle 1 of >50% in the lipegfilgrastim group versus the placebo group is clinically important, particularly when considering the potential serious effects of FN on the overall health of patients. The actual incidence of FN was lower than anticipated for the placebo group and higher than anticipated for the lipegfilgrastim group, affecting the statistical power of the study. The use of prophylactic G-CSFs is not recommended by treatment guidelines unless a patient’s risk for developing FN is high (≥20%); however, the current study used chemotherapy with a reported incidence of FN <20% and excluded patients with an individual high risk of developing FN (≥20% risk). Thus, the use of prophylactic G-CSF therapy in this study was experimental in nature and provides additional evidence for the recommendation that prophylactic G-CSFs should not be used in patients with a risk of FN <20% (Crawford et al. 2010, 2013; Smith et al. 2006; Aapro et al. 2011). The use of cisplatin and etoposide, along with the strict definition of FN, may have contributed to the overall rate of FN being lower than the rate reported for patients receiving placebo in previously published lung cancer studies using the same chemotherapy regimen (Bonomi et al. 2000; Cardenal et al. 1999; Eckardt et al. 2006; Hanna et al. 2006). In particular, excluding patients thought to be at high risk for FN led to a relatively low percentage of patients aged ≥65 years (24.8%). In a similar study of patients with NSCLC receiving myelosuppressive chemotherapy and adjuvant filgrastim therapy or placebo, 41.1% of all patients were aged ≥65 years. Of these patients, 71 and 43% experienced FN in the placebo and filgrastim groups, respectively (Crawford et al. 2005).
The study design and statistical assumptions of this phase III trial may have contributed to not achieving the primary measure of demonstrating superiority to placebo in the incidence of FN in cycle 1. The existence of effective G-CSF supportive care and the uniformity of treatment guidelines for the use of G-CSFs in patients at risk for developing chemotherapy-induced neutropenia mean that a placebo-controlled trial in this setting is now uncommon. In addition, the strict definition of FN used in this study may have excluded patients who otherwise may have been reported as having FN. Moreover, the incidence of FN in cycle 1 was used as the primary measure, whereas G-CSF studies often use DSN in cycle 1 as the primary measure.
At the time the study was conducted, cisplatin and etoposide chemotherapy at the doses used was recommended for patients with stage IIIb/IV NSCLC (Belani et al. 2005). The reported incidence of FN for this regimen ranged from 8 to 12% in a population with an average risk of FN (Bonomi et al. 2000; Cardenal et al. 1999; Eckardt et al. 2006; Hanna et al. 2006), permitting the placebo-controlled study design. After the completion of this study, a systematic review and meta-analysis of the use of G-CSFs for FN prophylaxis following chemotherapy was published (Cooper et al. 2011). Although a broad range of studies was included, no study of patients with NSCLC receiving cisplatin and etoposide chemotherapy was included. The meta-analysis reported a risk ratio for FN of 0.30 [95% confidence interval (CI), 0.14–0.65] for pegfilgrastim and 0.57 (95% CI, 0.48–0.69) for filgrastim. In the current study, the odds ratio (OR) for FN in cycle 1 of 0.39 (95% CI, 0.121–1.260) for the comparison of lipegfilgrastim with placebo is in line with these results.
Secondary objectives of this study included the duration and incidence of severe neutropenia, and ANC profile (including depth of and time to ANC nadir, and time to ANC recovery). For most of these secondary measures, treatment with lipegfilgrastim was superior to placebo. In each of the four chemotherapy cycles, DSN was significantly shorter in the lipegfilgrastim group versus the placebo group (p < 0.0001), and in cycles 2, 3, and 4; approximately 80% of the lipegfilgrastim group experienced no severe neutropenia compared with approximately 40% of the placebo group. The incidence of severe neutropenia also was significantly lower in the lipegfilgrastim group versus the placebo group (p < 0.0001). A commonly used primary measure in studies with G-CSFs, DSN in cycle 1 was used as a primary measure in a recently published study that found lipegfilgrastim to be noninferior to pegfilgrastim in patients with breast cancer (Bondarenko et al. 2013). In the current study, the depth of ANC nadir was significantly lower in patients treated with lipegfilgrastim versus patients receiving placebo (p < 0.0001), and time to ANC nadir was shorter with lipegfilgrastim treatment. Time to ANC recovery also was shorter in the lipegfilgrastim group compared with the placebo group. Furthermore, significantly fewer patients receiving lipegfilgrastim had chemotherapy dose delays compared with those receiving placebo. These results add further support to data indicating the clinical benefits of lipegfilgrastim (Bondarenko et al. 2013; Buchner et al. 2011).
The high incidence of AEs was anticipated in this population of patients with stage IIIb/IV NSCLC receiving chemotherapy, and AEs were consistent with the underlying disease and chemotherapy regimen administered. In addition to neutropenia, the most common AEs (≥10% in either group) were alopecia, anemia, nausea, thrombocytopenia, asthenia, vomiting, and leukopenia—all known to be associated with the chemotherapy agents used in this study. The incidence of study medication-related AEs was relatively low. Bone-pain-related symptoms, known AEs associated with G-CSF therapy, were generally mild or moderate in severity. The incidence of mortality in this study was low, considering that the study population had advanced-stage disease. Most deaths were related to NSCLC or to other underlying conditions and were not considered to be related to lipegfilgrastim treatment. Deaths occurred early in and did not increase during the study; no difference in mortality was observed at the end of the 12-month follow-up, suggesting that the difference in mortality between the lipegfilgrastim and placebo groups at the end of the study was likely a chance effect.
The incidence of FN in patients with NSCLC receiving lipegfilgrastim or placebo in this study is similar to those reported in two placebo-controlled studies of pegfilgrastim in patients with advanced or metastatic colorectal cancer receiving chemotherapy regimens reported to have a low risk of FN (allowing the use of a placebo group). Again, the use of prophylactic G-CSF therapy in this setting is experimental, as the main chemotherapy regimens administered (oxaliplatin, fluorouracil, and leucovorin [FOLFOX4] or irinotecan, fluorouracil [infusion], and leucovorin [FOLFIRI]) have been reported to have a 6 and 9% incidence of FN, respectively, in patients with advanced colorectal cancer (Smith et al. 2006). In the large phase III trial (N = 845), grade 3/4 FN was reported in 2.4% of patients receiving pegfilgrastim and in 5.7% of patients receiving placebo after four cycles of chemotherapy (p = 0.014) (Pinter et al. 2013). In the phase II study (n = 252), grade 3/4 FN was reported in 2% of pegfilgrastim recipients and 8% of placebo recipients after four cycles of chemotherapy (p = 0.04) (Hecht et al. 2010).
In this study in patients with stage IIIb/IV NSCLC, treatment with lipegfilgrastim did not demonstrate superiority to placebo in terms of the incidence of FN in cycle 1. Use of a G-CSF in this setting was investigational in that it was administered to patients contrary to current treatment guidelines (Crawford et al. 2010, 2013; Smith et al. 2006; Aapro et al. 2011), yet treatment with lipegfilgrastim was consistently more effective than placebo in reducing the duration and incidence of severe neutropenia and time to ANC recovery, with an acceptable safety and tolerability profile in this patient population.
Methods
This phase III, multinational, multicenter, double-blind, randomized, placebo-controlled study (controlled-trials.com identifier ISRCTN55761467) was designed to demonstrate superiority of a fixed 6-mg dose of lipegfilgrastim (XM22, Lonquex; Teva Pharmaceuticals Ltd, Petach Tikva, Israel) versus placebo in patients with NSCLC. The study was conducted in eight European countries (Belarus, Bosnia-Herzegovina, Bulgaria, Poland, Romania, Russia, Serbia, Ukraine) and included four phases: screening and randomization, double-blind treatment (chemotherapy cycles 1–4), end-of-study (or withdrawal) visit, and antibody follow-up. The study design followed the European Medicines Agency’s guidelines for a confirmatory study (EMEA 2007). Everyone involved in the conduct of the study was blinded to study medications.
Patients
Eligible patients were men and women aged ≥18 years of any ethnic origin with a diagnosis of stage IIIb/IV NSCLC, documented histologically or cytologically, and a life expectancy of at least 4 months. Patients had to be chemotherapy naive, eligible to receive four cycles of cisplatin and etoposide as myelosuppressive chemotherapy (i.e., baseline ANC ≥1.5 × 109/L and platelets ≥100 × 109/L), have an ECOG PS ≤2, and have adequate hepatic, cardiac, and renal function. Women of childbearing potential had to use an effective method of contraception.
Because this was a placebo-controlled study, patients with an individual high risk of developing FN (i.e., ≥20%) with regard to the cisplatin and etoposide chemotherapy were excluded from the study. Potential individual high risk factors were patient age >65 years, low ECOG PS, poor nutritional status, and liver, kidney, or cardiovascular disease. Risk factors were considered together to determine a patient’s risk of developing FN, and therefore having only one risk factor did not automatically result in exclusion from the study. Other exclusion criteria included previous exposure to any G-CSF within 6 months before randomization, treatment with systemically active antibiotics within 72 h before chemotherapy, chronic use of oral corticosteroids, prior radiation therapy within 4 weeks of randomization, prior bone marrow or stem cell transplantation, or concomitant malignancy (other than in situ melanoma, skin cancer, or cervical carcinoma) within the preceding 5 years. Women who were pregnant or breastfeeding were excluded.
Study design and treatment
All patients received chemotherapy with cisplatin and etoposide and were randomized (2:1) to receive lipegfilgrastim or placebo. Randomization was performed by Biostatistics Merckle GmbH through an interactive voice response system, using a block size of 2 and stratified by country. Patients received up to four 21-day chemotherapy cycles. On day 1 of each cycle, patients received cisplatin 80 mg/m2 intravenously (IV), with etoposide 120 mg/m2 IV administered on days 1, 2, and 3. Patients had to have an ANC ≥1.5 × 109/L and platelet count of ≥100 × 109/L to begin the next full-dose cycle. A dose delay of up to 2 weeks was acceptable. Absolute neutrophil counts were determined at local or regional laboratories rather than a central laboratory, for logistical reasons; all other laboratory measures were determined by two central laboratories. In addition, the patient’s overall condition had to allow further chemotherapy treatment as determined by the treating investigator. Generally, any chemotherapy-related toxicity had to have resolved to at least grade 1 toxicity prior to continuation of chemotherapy.
Patients received one dose of lipegfilgrastim 6 mg or placebo SC on day 4 of each chemotherapy cycle, approximately 24 h after the last chemotherapy infusion and after blood sampling to determine ANC and body temperature. The lipegfilgrastim 6-mg dose was chosen based on findings from a phase II dose-finding study in breast cancer patients that demonstrated neutrophil support that was at least equivalent to the standard 6.0-mg fixed dose of pegfilgrastim. Patients who developed FN in any cycle were not discontinued except by investigator decision; instead, they received open-label prophylactic treatment with lipegfilgrastim in subsequent cycles, regardless of treatment group.
Efficacy assessments
The primary efficacy measure was the incidence of FN in cycle 1. Febrile neutropenia was defined as an oral body temperature >38.5°C for at least 1 h (two consecutive same-day measurements, ≥60 min apart) with severe neutropenia (ANC <0.5 × 109/L) on the day before, same day, or day after the elevated temperature readings; neutropenic sepsis (sepsis with ANC <0.5 × 109/L); or serious or life-threatening neutropenic infection (infection with ANC <0.5 × 109/L).
Secondary efficacy measures included the incidence of FN in cycles 2, 3, and 4 and across all cycles; incidence and DSN (defined as grade 4 neutropenia with ANC <0.5 × 109/L); incidence and duration of very severe neutropenia (ANC <0.1 × 109/L); depth of ANC nadir (lowest ANC in each cycle); time to ANC nadir (defined as time from chemotherapy administration until occurrence of ANC nadir); time to ANC recovery (defined as time from chemotherapy administration until ANC increased to ≥2.0 × 109/L after nadir); and time to ANC recovery from ANC nadir (defined as the difference in days between day of ANC nadir to first day with ANC ≥1.5 × 109/L).
Blood samples to determine ANC were obtained daily until day 15, or longer, of each cycle, until ANC ≥2.0 × 109/L was reached. A blood sample was taken on day 4 of each cycle, before administration of study medication. Body temperature was measured orally at least twice daily (morning and evening) until day 15, or longer, of each cycle, until ANC ≥2.0 × 109/L was reached.
Other secondary efficacy measures, including hospitalizations, use of IV antibiotics, delivered versus scheduled chemotherapy, chemotherapy dose modifications (reductions, omissions, delays), quality of life, and the incidence of patients requiring prophylactic open-label treatment, as well as pharmacokinetic properties were assessed but are not reported here.
Safety assessments
Safety was assessed using reported treatment-emergent AEs data, including intercurrent illnesses and clinically abnormal laboratory values; AEs were recorded until 3 weeks after the last injection of study medication. Adverse events were summarized by seriousness, severity, and investigator-assessed relationship to study medication. A serious AE was one that was life-threatening or resulted in death, required hospitalization or prolongation of hospitalization, or resulted in a persistent or significant disability or incapacity that required medical or surgical intervention. Investigators assessed AEs as probably, possibly, unlikely, or not related to the study medication or chemotherapy regimen, or as not classifiable. For laboratory values, all AEs of grade 3 and higher were documented. The AEs were assessed on days 1 and 7 of each chemotherapy cycle. Safety samples (hematology and clinical chemistry) were taken on day 15. Other safety assessments, including physical examination and vital signs, were performed within 24 h before chemotherapy administration on day 1 and on days 7 and 15 of each chemotherapy cycle.
Statistical analysis
The planned sample size was 375 patients from approximately 90 centers in nine countries, based on the assumption that the incidence of FN would be in the range of 7% to 10% in the placebo group and, at most, 1% in the lipegfilgrastim group. For a statistical test with a two-sided significance level α of 5%, a required power of at least 80%, and a sampling rate of 2:1 (lipegfilgrastim:placebo), sample size requirements were 250 patients in the lipegfilgrastim group and 125 patients in the placebo group. As the actual incidence of FN in the placebo group was expected to be closer to 10%, a power of at least 90% was expected.
For the primary efficacy measure (incidence of FN in cycle 1), a 95% CI for the OR (placebo/lipegfilgrastim) was calculated to assess the relative efficacy of lipegfilgrastim versus placebo. For secondary efficacy measures, no adjustment for Type I error was applied, so all secondary analyses should be interpreted as exploratory. When applicable, for secondary efficacy measures for which regression analyses were planned, statistical models with the same explanatory variables as in the analysis of the primary measures were estimated. Demographic and baseline characteristics, AEs, and other safety assessments were presented as descriptive statistics (continuous variables) or frequency tables (categorical variables).
The intent-to-treat population, which included all patients randomized at baseline, was also the efficacy population. The safety population included all randomized patients who received at least one dose or partial dose of study medication.
Endnote
Results from this study were presented in part at the Multinational Association of Supportive Care in Cancer International Symposium, New York, NY, June 28–30, 2012.
Authors’ contributions
Study design: RE, AB, PB, UM. Study investigator: CV, IMB, OAG. Enrolled patients: CV, IMB, OAG. Collection and assembly of data: IMB, RE, PB. Data analysis and interpretation: RE, AB, PB, UM. Manuscript preparation: All authors. Manuscript review and revisions: All authors. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the research nurses, study coordinators, operations staff, and biostatisticians as well as all patients who participated in this study. They also acknowledge Yvonne E. Yarker, PhD, CMPP, of Peloton Advantage (Parsippany, NJ) for medical writing and editorial support, which were funded by Teva Pharmaceuticals, Petach Tikva, Israel. This study was funded by Teva Pharmaceuticals, Petach Tikva, Israel.
The following lead investigators, listed alphabetically by country, also participated in this study:
Belarus: Vasily Belyakovsky (Gomel); Evgenia Karchmit (Vitebsk); Anton Lysov (Mogilev); Alexander Prokharau (Minsk); Konstantin Shelepen (Brest); Edward Zhavrid (Minsk Region).
Bosnia-Herzegovina: Ljubica Miljanovic (Trebinje); Mirko Turic (Banja Luka).
Bulgaria: Blaga Baeva (Gabrovo); Ivan Ivanov (Ruse); Tatyana Koynova (Sofia); Hristina Markova (Shumen); Velko Minchev (Plovdiv).
Poland: Jan Cieslicki (Wodzislaw Slaski); Krzysztof Marzewski (Gorzów Wielkopolski); Wojciech Jarosz (Pilchowice).
Romania: Dumitru Filip (Baia Mare); Carmen-Elena Floares (Cluj-Napoca); Cristina-Marinela (Oprean Timisoara); Daniela Smeianu (Ploiesti); Cristina-Lucia Taflan (Brasov); Constantin Volovat (Iasi).
Russia: Andrey Akopov (Saint Petersburg); Svetlana Averyanova (Saratov); Dmitry Bentsion (Ekaterinburg); Mikhail Biakhov (Moscow); Olga Burdaeva (Arkhangelsk); Nina Chekha (Magnitogorsk); Sergey Cheporov (Yaroslavl); Irina Davidenko (Krasnodar); Yuriy Dykhno (Krasnoyarsk); Alexander Filippov (Novosibirsk); Andrey Gavrilenko (Cherepovets); Oleg Gladkov (Chelyabinsk Region); Evgeny Gotovkin (Ivanovo); Nina Karaseva (Saint Petersburg); Igor Kiselev (Kursk); Nadezhda Kovalenko (Stavropol); Mikhail Kulaev (Saransk); Evgeny Levchenko (St. Petersburg); Oleg Lipatov (Ufa); Elena Matyushina (Syktyvkar); Valentin Pavlov (Ufa); Alexander Pecheny (Orel); Igor Pimenov (Tula); Vadim Popov (Voronezh); Nailya Sherman (Kirov); Sergey Shinkarev (Lipetsk); Vadim Shirinkin (Orenburg); Andrey Sinyakov (Tyumen); Yuriy Skripchak (Murmansk); Vladimir Solovyev (Smolensk); Tatiana Vinogradova (Bryansk); Vladimir Vladimirov (Pyatigorsk); Lyubov Vladimirova (Rostov-On-Don); Dmitry Udovitsa (Sochi).
Serbia: Branislav Perin (Sremska Kamenica); Gordana Radosavljevic Asic (Belgrade).
Ukraine: Orest Andrusenko (Lutsk); Volodymyr Bondar (Donetsk); Prof. Ihor Bondarenko (Dnipropetrovsk); Anatolii Hontsa (Chernivtsi); Natalya Martsynkovska (Odesa); Prof. Stanislav Myasoyedov (Kyiv); Serhii Polenkov (Chernihiv); Olexandr Popovych (Donetsk-92); Prof. Andrii Rusyn (Uzhhorod); Anatolii Shevchenko (Zaporizhia); Yaroslav Shparyk (Lviv); Oksana Tarasova (Kharkiv); Ihor Vynnychenko (Sumy); Yrii Vinnyk (Kharkiv).
Compliance with ethical guidelines
Competing interests CV, IMB, and OAG disclose no relevant conflicts of interest. RE, AB, PB, and UM are employees of Teva Pharmaceuticals, Inc.
Consent for publicationIndependent ethics committees approved the protocol, and all patients provided written informed consent prior to participation. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Abbreviations
- AEs
adverse events
- ANC
absolute neutrophil count
- CI
confidence interval
- DSN
duration of severe neutropenia
- ECOG PS
Eastern Cooperative Group Performance Status
- FN
febrile neutropenia
- G-CSFs
granulocyte colony-stimulating factors
- IV
intravenous
- NSCLC
non-small cell lung cancer
- PEG
polyethylene glycol
- SC
subcutaneous
Contributor Information
Constantin Volovat, Email: cvolovat@yahoo.com.
Igor M Bondarenko, Email: igor.bondarenko@rdp-ukraine.com.
Oleg A Gladkov, Email: gladkov_o@rambler.ru.
Reiner Elsässer, Email: reiner.elsaesser@ratiopharm.de.
Anton Buchner, Email: anton.buchner@ratiopharm.de.
Peter Bias, Email: peter.bias@ratiopharm.de.
Udo Müller, Email: udo.mueller@teva.de.
References
- Aapro MS, Bohlius J, Cameron DA, Dal LL, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Belani CP, Lee JS, Socinski MA, Robert F, Waterhouse D, Rowland K, et al. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005;16:1069–1075. doi: 10.1093/annonc/mdi216. [DOI] [PubMed] [Google Scholar]
- Bondarenko I, Gladkov OA, Elaesser R, Buchner A, Bias P. Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer. 2013;13:386–398. doi: 10.1186/1471-2407-13-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- Buchner A, Bias P, Kaufmann M. A randomized, double-blind, active control, multicenter, dose-finding study of XM22, glycopegfilgrastim, in patients with breast cancer receiving myelosuppressive therapy [abstract 9080] J Clin Oncol. 2011;29:9080. [Google Scholar]
- Cardenal F, Lopez-Cabrerizo MP, Anton A, Alberola V, Massuti B, Carrato A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17:12–18. doi: 10.1200/JCO.1999.17.1.12. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. doi: 10.1186/1471-2407-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, Glaspy JA, Stoller RG, Tomita DK, Vincent ME, McGuire BW, et al. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther. 2005;3:36–46. doi: 10.3816/SCT.2005.n.023. [DOI] [PubMed] [Google Scholar]
- Crawford J, Caserta C, Roila F. Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010;21(Suppl 5):v248–v251. doi: 10.1093/annonc/mdq195. [DOI] [PubMed] [Google Scholar]
- Crawford J, Armitage J, Balducci L, Becker PS, Blayney DW, Cataland SR, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2013;11:1266–1290. doi: 10.6004/jnccn.2013.0148. [DOI] [PubMed] [Google Scholar]
- Eckardt JR, Von Pawel J, Papai Z, Tomova A, Tzekova V, Crofts TE, et al. Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol. 2006;24:2044–2051. doi: 10.1200/JCO.2005.03.3332. [DOI] [PubMed] [Google Scholar]
- European Medicine Agency (2007) Guideline on clinical trials with haematopoietic growth factors for the prophylaxis of infection following myelosuppressive or myeloablative therapy (EMEA/CPMP/555/95 Rev. 1). London, UK: European Medicine Agency. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/12/WC500017738.pdf. Accessed 31 March 2015
- Hanna N, Bunn PA, Jr, Langer C, Einhorn L, Guthrie T, Jr, Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- Hecht JR, Pillai M, Gollard R, Heim W, Swan F, Patel R, et al. A randomized, placebo-controlled phase II study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer. 2010;9:95–101. doi: 10.3816/CCC.2010.n.013. [DOI] [PubMed] [Google Scholar]
- Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–731. doi: 10.1200/JCO.20.3.727. [DOI] [PubMed] [Google Scholar]
- Lonquex . Summary of product characteristics. London: European Medicines Agency; 2013. [Google Scholar]
- Neulasta (2012) Highlights of prescribing information. Amgen Inc, Thousand Oaks
- Neupogen (2013) Full prescribing information. Amgen Inc, Thousand Oaks
- Pinter T, Abella E, Cesas A, Croitoru A, Decaestecker J, Gibbs P, et al. Results of a phase III, randomized, double-blind, placebo-controlled trial of pegfilgrastim (PEG) in patients (pts) receiving first-line FOLFOX or FOLFIRI and bevacizumab (B) for colorectal cancer (CRC) [abstract] J Clin Oncol. 2013;31:3575. [Google Scholar]
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23:1178–1184. doi: 10.1200/JCO.2005.09.102. [DOI] [PubMed] [Google Scholar]


