Recurrent genomic abnormalities identified in acute myeloid leukemia (AML) improve prognostic stratification of patients with normal karyotype AML AML (NK-AML).1, 2 Retrospective studies show that mutations in FLT3, NPM1, and CEBPα are powerful tools to predict outcomes in younger and older patients with NK-AML.3-5 Limited data exists on the role of allogeneic hematopoietic cell transplant (HCT) in NK-AML patients with recurrent genomic abnormalities.6-8 In this retrospective cohort study, we evaluated the outcomes of patients with NK-AML transplanted at a single institution to determine if the widespread implementation of molecular testing in NK-AML patients at the time of diagnosis has impacted the outcomes of patients who ultimately undergo allogeneic HCT.
The Stanford University Division of Blood and Marrow Transplantation database was queried to compile all adult patients (age ≥ 18 years) with AML who received their first allogeneic HCT following full-dose or reduced intensity conditioning (RIC) at Stanford University between January 1, 2000 and September 30, 2011 (n=349). Patients were excluded if they had acute promyelocytic leukemia, prior autologous HCT, or a graft from a syngeneic, cord blood, or haploidentical donor, and if they lacked the source document diagnostic cytogenetic report. All patients were on Institutional Review Board protocols and signed written informed consent prior to transplant. We identified 143 consecutive patients with NK-AML who underwent first allogeneic HCT from matched or one-antigen mismatched related or unrelated donors. Patients underwent HCT in first complete remission (CR, n=88), in second CR or beyond (n=26), or not in remission (n=29). Beginning in 2006, results were available for molecular testing of FLT3, NPM1, and CEBPα mutations from the initial diagnostic bone marrow biopsy and/or peripheral blood analysis. Using the European LeukemiaNet (ELN) classification for patients with NK-AML, mutations in NPM1 (without concurrent FLT3-ITD) or mutations in CEBPα are considered favorable, while FLT3-ITD or wild-type NPM1 without FLT3-ITD are identified as intermediate-1.2 Three independent investigators (M.E.M.P, B.C.M., and R.L.) reviewed the medical records of adult patients with NK-AML who underwent HCT. The majority of patients (>95%) received standard induction chemotherapy (anthracycline plus cytarabine) followed by one or two consecutive cycles of high dose cytarabine (HiDAC) prior to allogeneic HCT. Patients underwent HCT using either full-dose or RIC regimens9-13 and received graft-versus-host-disease prophylaxis as previously described.11,15 Patients received unstimulated whole bone marrow or granulocyte-colony stimulating factor mobilized blood.
For statistical analysis, patient baseline characteristics were reported descriptively. The last follow up time point was any chart note prior to July 1, 2013. Remission status at the time of transplant was determined using standard definitions.2 Overall survival (OS) was defined as the time from hematopoietic cell infusion to death from any cause. Patients who were alive or lost to follow-up were censored at the date last seen alive. Event-free survival (EFS) was defined as the time from hematopoietic cell infusion to disease relapse or progression, or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the date last seen alive. Kaplan-Meier estimators were used for OS and EFS probabilities. The log-rank test was used for comparisons of survival probabilities. Cumulative incidence of relapse (CIR) was calculated by analyzing the time from allogeneic HCT to relapse (in patients in remission at the time of HCT) or progression (in patients not in remission at the time of HCT). A Cox regression analysis was used to make the same comparisons (OS, EFS, and CIR) adjusted for potential confounders including age, gender, disease status at the time of HCT, conditioning regimen, and time from transplant (as a continuous variable). P values are 2-sided with a significance level of 0.05. All statistical analyses were performed using IBM SPSS Statistics Data Editor 21.
The median age of the cohort was 50 (range 19 to 72). Molecular testing for FLT3, NPM1, 82 and/or CEBPα at the time of AML diagnosis was available in 37 patients (26%). The 83 remaining 106 patients (74%) did not have molecular testing performed. In the cohort with 84 molecular testing, the FLT3-ITD mutation was found in 22 patients (59%), NPM1 mutations 85 in 17 patients (46%), and a CEBPα mutation in one patient (3%). No significant differences were observed in the baseline characteristics (age, sex, disease status at the time of transplant, conditioning regimen, transplant product, and type of donor). Median follow-up was significantly longer for patients without diagnostic molecular testing (39.9 months vs. 22.8 months respectively, p = 0.005), as molecular testing was progressively adopted starting in 2006. By 2011, 60% of patients with NK-AML undergoing allogeneic HCT had molecular testing at diagnosis (Supplemental Figure 1). Patients without molecular testing underwent transplant throughout the study period, and 46 of 106 patients (43%) received an allogeneic HCT after July 2006.
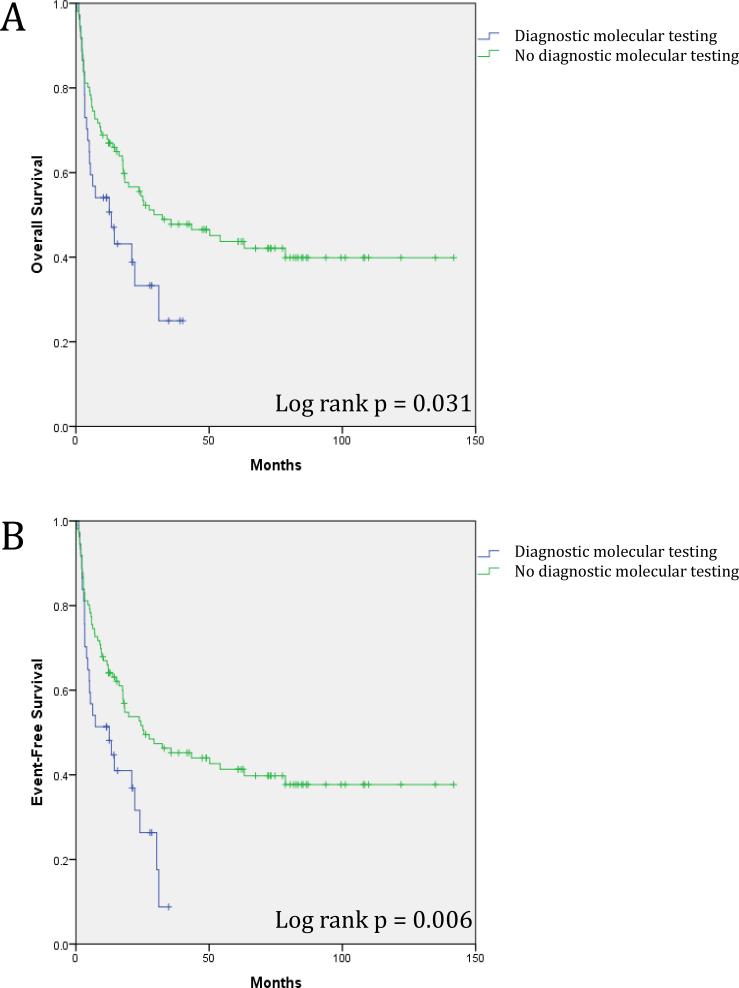
Patients with molecular testing at diagnosis had significantly inferior OS. Median OS was 17.9 months in those with molecular testing compared to 67.0 months in those without (Figure 1A; Table 1). The EFS was 14.8 months in patients with molecular testing compared to 63.9 months in those without (Figure 1B; Table 1). A Cox regression multivariate analysis confirmed that presence of molecular testing was negatively associated with OS (Table 1 and Supplemental Table 1).
Figure 1.
Survival for entire cohort of 143 NK-AML patients according to diagnostic molecular testing. (A) Overall survival for the entire cohort of NK-AML patients. (B) Event-free survival for the entire cohort of NK-AML patients.
Table 1.
Median overall and event-free survival according to availability of diagnostic molecular testing in all patients.
| All Patients (n = 143) | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| Molecular testing performed (95% CI) | Molecular testing not performed (95% CI) | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| OS (months) | 17.9 (12.53, 23.24) | 67.0 (54.32, 79.71) | 1.72 (1.04, 2.82) | 0.033 | 1.48 (1.07, 2.05) | 0.019 |
| EFS (months) | 14.8 (10.73, 19.03) | 63.9 (51.40, 76.41) | 1.92 (1.20, 3.09) | 0.007 | 1.48 (1.09, 2.01) | 0.013 |
| Patients in Complete Remission at Time of Hematopoietic Cell Transplant (n = 114) | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| Molecular testing performed (95% CI) | Molecular testing not performed (95% CI) | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| OS (months) | 19.4 (13.35, 25.52) | 78.6 (64.27, 92.91) | 2.06 (1.14, 3.71) | 0.016 | 2.55 (1.19, 5.49) | 0.017 |
| EFS (months) | 15.9 (11.22, 20.60) | 74.6 (60.34, 88.78) | 2.37 (1.36, 4.12) | 0.002 | 2.44 (1.19, 4.97) | 0.014 |
Abbreviations: OS- Overall survival, EFS- Event free survival, HR – Hazard ratio, CI – Confidence Interval.
Disease in remission at HCT was also independently associated with OS in the multivariate analysis (p < 0.001; HR 1.89, 95%CI=[1.43, 2.50]). Therefore, we examined the outcomes for patients in CR at the time of HCT (n = 114; 77% of these were in first CR). In this cohort of patients in CR at the time of HCT, presence of molecular testing was also associated with a statistically significant decrease in OS and EFS in both univariate and multivariate analysis (Table 1). We saw similarly decreased OS and EFS in the subset of patients in first CR (n = 88; data not shown).
To determine if the decreased OS and EFS were related to relapse, we evaluated the CIR in patients with and without molecular testing. The difference in CIR showed a trend toward statistical significance in univariate analysis [p = 0.06; HR = 1.72, 95% CI=(0.98, 3.03)], and was statistically significant when analysis was limited to the subset of patients in CR at the time of transplant [n = 114, p = 0.009; HR 2.42, 95%CI=(1.25, 4.70)]. In the entire cohort (n = 143), active disease (compared to any CR) at HCT was also independently associated with worse CIR [p = 0.003; HR = 1.73, 95%CI=(1.20, 2.48)]. To confirm that date of transplant was not a significant factor, we also repeated the analysis on patients transplanted from 2006 onwards who were in CR at the time of HCT and confirmed significant differences in OS and EFS (n = 70, data not shown).
In this study, we observed the effect of a rising trend in testing for molecular abnormalities 122 in the FLT3-ITD, NPM1, and CEBPα genes in NK-AML patients who received an allogeneic HCT. The molecular testing included here is part of the ELN standardized reporting system previously shown to improve prognostic stratification following treatment with chemotherapy alone.2 In our cohort, the OS and EFS were significantly inferior in NK-AML patients who had molecular testing performed, and who could therefore be classified in an ELN risk group. These inferior outcomes reflect a higher CIR. These data support the contention that molecular testing at diagnosis has led to a selection bias in NK-AML allogeneic HCT recipients that has negatively impacted survival outcomes.
We speculate that the inferior outcomes for patients with knowledge of diagnostic molecular testing reflect a bias to transplant patients with higher risk molecular features. Our cohort of patients with diagnostic molecular testing had a high percentage of FLT3-ITD mutations (59%), and 73% were classified as intermediate-1 group by ELN (compared to approximately 30% and 20% respectively in large analyses of unselected NK-AML patients). 3, 6-8 We also found similar survival outcomes for patients transplanted before 2006 compared to the ELN not assigned patients transplanted after 2006, which suggests that the referral patterns for NK-AML without diagnostic molecular testing have not significantly evolved over the past decade.
In summary, we observed worse outcomes in NK-AML patients with diagnostic molecular testing who received an allogeneic HCT, suggesting a selection towards preferentially transplanting patients with an adverse molecular profile. Our study is limited in that it is retrospective and that we describe a relatively small number of consecutive patients at a single center. However, the potential selection bias we identify may make comparisons between past and future studies difficult, especially if molecular testing is not uniformly performed.14 Additionally, allogeneic HCT decreases relapse rate and improves EFS in most patients with intermediate risk AML in first CR, including those without well-established adverse molecular markers.2, 6 Future studies should evaluate a larger cohort to further assess the impact of the rising trend in molecular typing of NK-AML patients on survival outcomes after allogeneic HCT.
Supplementary Material
Acknowledgements
This work was supported by a National Institute of Health grant P01 CA049605-22. This work was additionally supported by a Conquer Cancer Foundation of ASCO Young Investigator Award (M.-E.M.P.) and a KL2 Mentored Career Development Award (M.-E.M.P.) of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083). Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the American Society of Clinical Oncology® or the Conquer Cancer Foundation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Information
Supplementary information is available at BMT's website.
References
- 1.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 3.Mrozek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–23. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rollig C, Bornhauser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758–65. doi: 10.1200/JCO.2010.32.8500. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros BC, Tian L, Robenson S, Laport GG, Johnston LJ, Shizuru JA, et al. European LeukemiaNet classification intermediate risk-1 cohort is associated with poor outcomes in adults with acute myeloid leukemia undergoing allogeneic hematopoietic cell transplantation. Blood cancer journal. 2014;4:e216. doi: 10.1038/bcj.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 7.Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–41. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 8.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–65. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 9.Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100(4):1201–7. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 10.Naik S, Wong R, Arai S, Brown J, Laport G, Lowsky R, et al. Long-term outcomes in patients with high-risk myeloid malignancies following matched related donor hematopoietic cell transplantation with myeloablative conditioning of BU, etoposide and CY. Bone Marrow Transplant. 2011;46(2):192–9. doi: 10.1038/bmt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–9. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 12.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–31. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 13.Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A, et al. Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: particular activity against advanced hematologic malignancies. Blood. 2008;112(2):415–25. doi: 10.1182/blood-2007-08-104745. [DOI] [PubMed] [Google Scholar]
- 14.Appelbaum FR. Indications for allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the genomic era. American Society of Clinical Oncology educational book / ASCO. American Society of Clinical Oncology. Meeting. 2014:e327–33. doi: 10.14694/EdBook_AM.2014.34.e327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.