Abstract
Upregulation of matrix metalloproteinase MMP-14 (MT1-MMP) is associated with poor prognosis in cancer patients, but it is unclear how MMP-14 becomes elevated in tumors. Here we show that miR-181a-5p is downregulated in aggressive human breast and colon cancers where its levels correlate inversely with MMP-14 expression. In clinical specimens, enhanced expression of MMP-14 was observed in cancer cells located at the invasive front of tumors where miR-181a-5p was downregulated relative to adjacent normal cells. Bioinformatics analyses defined a potential miR-181a-5p response element within the 3' untranslated region (UTR) of MMP-14 that was validated in reporter gene experiments. Ectopic miR-181a-5p reduced MMP-14 expression, whereas miR-181a-5p attenuation elevated MMP-14 expression. In support of a critical relationship between these two genes, miR-181a-5p-mediated reduction of MMP-14 levels was sufficient to decrease cancer cell migration, invasion and activation of pro-MMP-2. Further, this reduction in MMP-14 levels was sufficient to reduce in vivo invasion and angiogenesis in chick chorioallantoic membrane assays. Taken together, our results establish the regulation of MMP-14 in cancers by miR-181a-5p through a post-transcriptional mechanism, and they further suggest strategies to elevate miR-181a-5p to prevent cancer metastasis.
Keywords: miR-181a-5p, MMP-14, Migration, Invasion, Angiogenesis, mRNA 3'UTR
Introduction
Metastasis, the dissemination of cancer cells from the primary tumor to a distant organ, accounts for 90% of human cancer-related deaths. Pathologically, metastasis is a multistep process consisting of a series of discrete biological processes. One of the key biological steps is cancer cell invasion, which is involved in almost every stage of the metastatic cascade from initiation of metastasis to establishment of a secondary tumor.
Matrix metalloproteinases(MMPs) are a family of twenty-five highly homologous Zn++-dependent endopeptidases(1). The primary functions of MMPs are traditionally considered to be the degradation of cell adhesion molecules and removal of extracellular matrix (ECM) to permit cancer cell invasion(1). MMPs have been recently recognized as cell migration enhancers, independent of their proteolytic activity(2). Matrix metalloproteinase-14 (MMP-14) is a membrane inserted MMP which has been found to play critical roles in cancer invasion and metastasis by cleaving ECM and basement membrane proteins, activating proMMP-2 and -13, inducing the activation of growth factors, and enhancing cell migration(3,4). Phenotypically, MMP-14 plays a critical role in converting epithelial cells to migratory mesenchymal-like cells (epithelial-to-mesenchymal transition, EMT)(5), which is considered an important mechanism for the initial step in the metastatic process. However, the regulatory mechanism of MMP-14 expression during cancer progression remains to be characterized.
It has been reported that MMP-14 expression is upregulated by transcription factors such as Sp1 and Egr-1(6,7). MMP-14 has also been demonstrated to be transcriptionally regulated by hypoxia inducible factor-2α(8). Not only can MMP-14 expression be positively regulated by these transcription factors, it can also be negatively regulated by microRNA, such as microRNA-9 and -133a(9,10).
microRNAs (miRNAs or miR) are small, highly conserved non-coding RNAs that have been reported to participate in the metastatic process by negatively or positively regulating gene expression of metastasis-associated genes through posttranscriptional repression, mRNA degradation, or promoter activation(11). Although miRNAs can positively affect gene expression by binding to the promoter region of a regulated gene, the majority of miRNAs are reported to negatively regulate target gene expression by repressing translation or inducing sequence-specific degradation of target mRNAs through interaction with the 3' untranslated regions (3'UTRs) of target mRNAs(12).
miRNAs are transcribed as ~70 nucleotide precursors and subsequently processed by the RNase-III type enzyme Dicer to give a ~22 nucleotide mature product(13). More than 1500 miRNA genes have been identified in the human genome that collectively control an estimated 30% of all human genes(13). Each miRNA appears to regulate the expression of tens to hundreds of genes to efficiently coordinate multiple cellular pathways. Interestingly, many miRNAs exist as a multi-member family, indicating their functional redundancy.
microRNA-181a-5p (miR-181a-5p) belongs to the miR-181s family, which includes four highly conserved mature miRNAs: miR-181a, b, c, and d. They are derived independently from six precursors located on three different chromosomes. This new class of genes has recently been shown to play a central role in malignant transformation(14,15). In contrast, miRNA-181s are downregulated in many tumors and thus appear to function as tumor suppressor genes(16–18).
In this study, we identified miR-181a-5p as a negative regulator of MMP-14 by directly targeting the 3'UTR of MMP-14 mRNA, resulting in decreased cell migration, invasion, and angiogenesis. Our data highlight a functional role of miR-181a-5p in cancer dissemination and uncover a potential prognostic biomarker and molecular target for the prevention of cancer metastasis.
Materials and Methods
Cell Culture and Transfection
All cell lines were purchased from ATCC (Manassas,VA) and were maintained in DMEM medium containing 10% FBS under a 5% CO2 atmosphere. Human breast cancer stem-like SK-3rd cells were maintained as previously described (19). Transfection of plasmid DNA to recipient cells was achieved using polyethylenimine (Polysciences) and the transfected cells were incubated for 48 h at 37 °C followed by biochemical and biological assays unless otherwise stated.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded (FFPE) tissue sections (5 µm) from Stony Brook University Research Histology Core Lab approved by the IRB of Stony Brook University were examined by a modified IHC method(20). Antigen retrieval was achieved by boiling tissue sections for 30 minutes in 0.01M sodium citrate, pH 4. Sections were blocked for one hour in 1% BSA at room temperature and incubated in rabbit anti-MMP-14 antibodies at 4°C overnight. After washing, samples were incubated with HRP-conjugated anti-rabbit IgG, and then by Biotin-XX-Tyramide amplification(Invitrogen), and streptavidin-HRP. Stained sections were visualized using 3,3’-diaminebenzidine tetrahydrochloride(DAB) and counterstained with hematoxylin. IHC staining without primary antibody was used as a negative control.
Dot-Based Cell Migration Assay
Transfected cells were mixed with an equal volume of neutralized type I collagen(3 mg/ml) on ice. The cell-collagen mixture(1 µl of 1×107cell/ml) was then dotted onto a 96-well plate. After solidification of cell-collagen dots, the cell-collagen hemispheres were covered with 100µl of complete media and incubated for 8 hours, followed by staining with nuclear dye Hoechst and counting of the migrated cells using Nikon NIS-Elements imaging software.
Macrodissection and RNA extraction
Tumor cells at the invasive front as well as tumor adjacent normal cells were macrodissected from four consecutive 12 µm-FFPE human colon cancer sections which were carefully aligned and marked with immunohistochemistry-staining performed by an experienced pathologist. miRNAs were then extracted using miRNeasy FFPE Kit(Qiagen).
Statistical Analysis
Data are expressed as the mean ± standard error of triplicates. Each experiment was repeated at least 3 times. Student’s t-test and analysis of variants(ANOVA) were used to assess differences with * P < 0.05, ** P < 0.01, *** P < 0.001.
All other methods are available as supplementary materials online.
Results
MMP-14 is upregulated in various human cancers
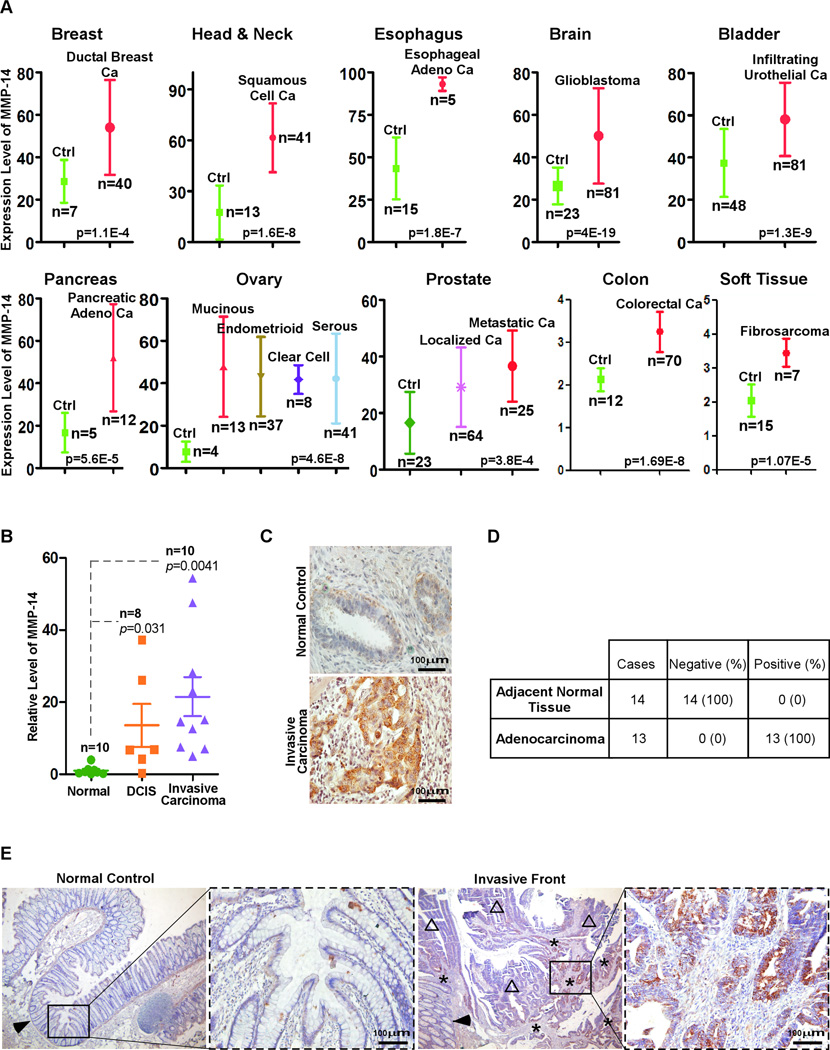
Upregulated MMP-14 expression in cancer cells has been shown to promote tumor growth and metastasis(4). Although MMP-14 has been reported to be upregulated in various human cancers(21), there is no systematic analysis of MMP-14 expression in different cancers from different organs. By mining DNA microarray databases at Oncomine(Cancer Profiling Database), expression of MMP-14 in human cancer specimens was assessed. When assigning a p<0.01 limit, a myriad of cancers displayed upregulation of MMP-14 as compared to respective non-malignant control tissues(22–31)(Fig.1A).
Figure 1. Upregulation of MMP-14 in invasive human cancers.
A) DNA microarray data mining was performed to analyze the expression of MMP-14 in various human cancers. B) Analysis of MMP-14 mRNA expression using real-time RT-PCR for total RNA isolated from archived FFPE tissue sections of DCIS, invasive ductal carcinoma, and normal breast epithelial cells using a LCM technique. C) Tissue distribution of MMP-14 in adjacent normal breast epithelium and invasive ductal carcinoma examined by IHC with an anti-MMP-14 antibody. D–E) Examination of MMP-14 expression in tumor adjacent normal tissue (arrowhead), adenocarcinoma (open triangles), and invasive adenocarcinoma (stars) of the colon by IHC with an anti-MMP-14 antibody. Representative images are shown (E).
To validate the data mining results, human breast cancer specimens were examined for the expression of MMP-14 by examining mRNA and protein levels. A laser capture microdissection(LCM) technique was employed to harvest breast carcinoma cells and tumor adjacent normal epithelial cells as we previously employed(32) followed by real-time RT-PCR for the mRNA of MMP-14. Our data shows that MMP-14 is selectively expressed in human breast ductal carcinoma in situ(DCIS) and in invasive ductal carcinoma, but not in normal epithelial cells. Expression of MMP-14 in invasive ductal carcinoma is elevated as compared to DCIS, although there is no statistical significance among them(Fig.1B). To confirm this observation, immunohistochemistry(IHC) was performed in the corresponding human breast cancer specimens using an anti-MMP-14 antibody. In agreement with the real-time RT-PCR data, MMP-14 protein was detected only in human breast cancer cells but not in adjacent normal epithelial cells(Fig.1C).
To further confirm our data mining results showing that the upregulation of MMP-14 is a general phenomenon in cancer, human colon cancer specimens were examined by IHC using the anti-MMP-14 antibody. We observed that MMP-14 is minimally expressed in normal colonic mucosal cells, but increased intensity of staining is found in cancer cells at the invasive front(Fig.1D–E). Thus, our study indicates that MMP-14 is upregulated in human cancers and the expression level of MMP-14 is correlated with the invasive status of human cancer.
Computational prediction of miRNA-181s as potential regulators of MMP-14
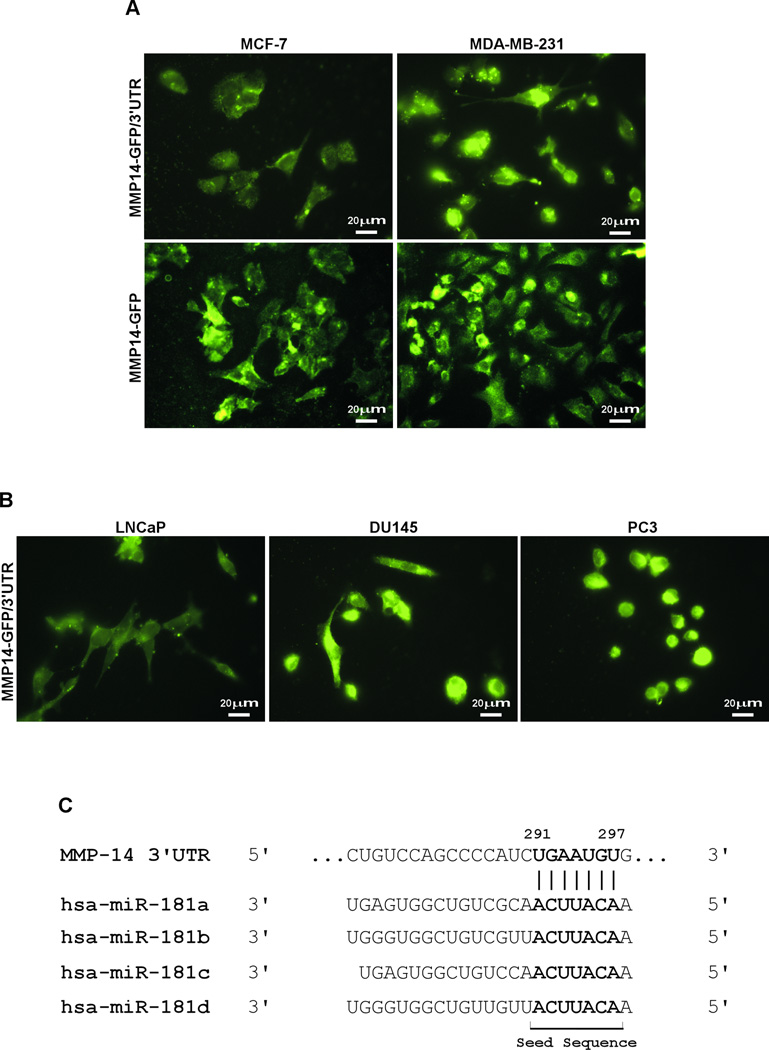
MMP-14 is upregulated in human cancers; however, the regulatory mechanism of MMP-14 remains to be fully characterized. When MMP14-GFP chimeric cDNA(MMP14-GFP) containing the 3'UTR of MMP-14(MMP14-GFP/3'UTR) was transfected into minimally invasive human breast cancer MCF-7 and highly invasive MDA-MB-231 cells, we noticed that the expression of MMP14-GFP in MCF-7 cells is relatively weak as compared to that in MDA-MB-231 cells(Fig. 2A, top panel). This difference, however, was not seen in these cells when transfected with the MMP14-GFP chimera that does not contain the 3'UTR(Fig.2A,bottom panel). This observation is reproduced in prostate cancer cell lines of LNCaP(minimally invasive) and DU145 and PC3 (highly invasive)(Fig.2B), suggesting that the 3’UTR of MMP-14 mRNA may contain a regulatory sequence(s) that is responsible for differential expression of MMP-14 in cancer lines.
Figure 2. Reduced expression of MMP14-GFP with the 3' UTR in less invasive cancer cells.
A) MCF-7 and MDA-MB-231 cells transfected with MMP14-GFP/3'UTR (upper panels) and MMP14-GFP without 3'UTR (lower panels). Microscopic examination was performed. B) LNCaP, DU145, and PC3 cells transfected with MMP14-GFP/3'UTR were microscopically examined. C) Computational analysis of the miRNA response element within the MMP-14 3'UTR revealed a miR-181 binding site.
It is known that the 3'UTR often contains miRNA response elements(MREs), which are sequences to which miRNAs bind. To identify putative MREs within the 3’UTR of MMP-14 mRNA, we used TargetScan to search for potential MREs within the 3'UTR. This analysis revealed multiple MREs that potentially interact with miRNA-22, -24, -26, -133, -150, and -181s. To increase the probability of postulated MREs within the 3'UTR, another miRNA prediction algorithm, miRanda was employed. By analyzing predicted MREs from both TargetScan and miRanda, only miR-133 and -181s were found to overlap between the two computational analyses. The MRE of miR-181 within the 3'UTR between 291 nt and 297 nt received the highest prediction score for binding to miR-181s(Fig.2C), and hence miR-181s were further characterized.
Interference of ectopically expressed MMP-14 by miR-181a-5p
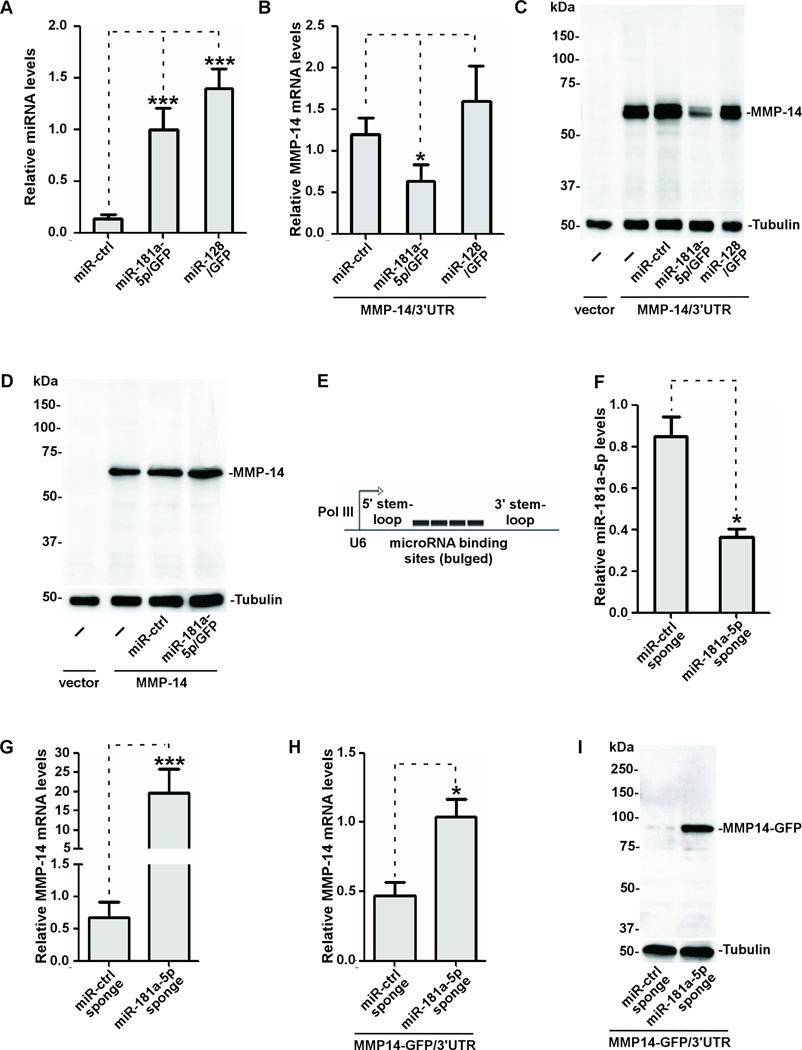
Since miR-181 isoforms contain identical seed sequence to the MMP-14 3'UTR(Fig.2C), miR-181a-2 along with the 140 bp flanking regions at both sides of miR-181a-2 was amplified from the chromosomal DNA and the resultant DNA was cloned into an MDH1-PGK-GFP2.0 retroviral vector that contains a GFP reporter(Addgene). Based on recent miRNA nomenclature guidelines (http://www.mirbase.org), we designated the mature sequence of hsa-miR-181a-2 in the vector as miR-181a-5p/GFP. Using a similar strategy, miR-128 that was not predicted to bind the MMP-14 3'UTR was also cloned in MDH1-PGK-GFP2.0 retroviral vector as a control(miR-128/GFP). Ectopic expression of miR-181a-5p was examined by transient transfection of the cDNAs encoding miR-181a-5p/GFP as well as control vectors into COS-1 cells, which do not express detectable endogenous MMP-14(33). Mature miR-181a-5p and -128 were detected in the transfected COS-1 cells by a real-time RT-PCR approach with 5- and 7-fold increases of miR-181a-5p and -128, respectively, as compared to vector control(Fig.3A), indicating mature miR-181a-5p and -128 are efficiently produced in the transfected cells.
Figure 3. Interference with ectopically expressed MMP-14 by miR-181a-5p.
A) Expression of miR-181a-5p and miR-128 in COS-1 cells transiently expressing the corresponding genes was examined by real-time RT-PCR. Levels of expression of miRNAs were normalized to U6 snRNA. B–C) Total RNAs and lysates isolated from COS-1 cells expressing cDNAs as indicated were examined for MMP-14 expression using real time RT-PCR (HPRT-1 as a normalization control) (B) and Western blotting (tubulin as a loading control) (C). D) No effect on MMP-14 expression by miR-181a-5p in COS-1 cells transfected with cDNAs encoding MMP-14 without the 3'UTR as examined by Western blotting. E) A schematic diagram of miR-181a-5p sponge. F) MCF-7 cells stably expressing the sponges as indicated were examined for miR-181a-5p expression using real-time RT-PCR. G) MCF-7 cells stably expressing the control and miR-181a-5p sponges were cultured under hypoxic conditions (1% O2) for 48 hours followed by real-time RT-PCR for MMP-14. H–I) MCF-7 cells stably expressing the sponges were transfected with MMP14-GFP/3'UTR followed by real-time RT-PCR for MMP-14 (H) and Western blotting using an anti-MMP-14 antibody (I).
We then examined whether miR-181a-5p can affect ectopically expressed MMP-14 in COS-1 cells by co-transfecting MMP-14/3'UTR with miR-control, miR-181a-5p/GFP, and miR-128/GFP, respectively. To increase the expression efficiency for both miRNA and MMP-14/3'UTR, COS-1 cells were first made to stably express each miRNA and was followed by transient transfection of MMP-14/3'UTR cDNA and vector control. Ectopic expression of miR-181a-5p, but not miR-control and miR-128, led to reduced MMP-14 mRNA and protein levels(Fig.3B–C), suggesting that MMP-14 mRNA is downregulated by miR-181a-5p.
To further determine whether the loss of MMP-14 expression in both mRNA and protein levels by miR-181a-5p is due to the existence of the MMP-14 3'UTR, we employed a previously generated plasmid DNA encoding only the open reading frame of MMP-14, and therefore lacks the 3'UTR of MMP-14 (34). Western blotting revealed that there was no distinct difference in ectopic MMP-14 expression between the cells that overexpressed either miR-181a-5p or miR-control (Fig.3D), confirming the existence of a miR-181 response element within the 3'UTR.
To further validate the role of miR-181a-5p in regulation of MMP-14 expression, we generated U6 promoter-driven sponge to downregulate miR-181a-5p (Fig.3E). When the sponges were stably introduced into MCF-7 cells which express a high level of endogenous miR-181a-5p, we found that the sponges efficiently reduced the miR-181a-5p level in the cells as compared to the control (Fig.3F).
To determine if reduced endogenous miR-181a-5p by the sponges leads to enhanced MMP-14 expression, endogenous and ectopically expressed MMP-14 were examined. It has been reported that hypoxia induces MMP-14 expression(8). When MCF-7 cells stably expressing the sponges were cultured under hypoxia(1% O2) for 48 hours, endogenous MMP-14 increased in cells expressing miR-181a-5p sponges as compared to control sponges (Fig.3G). To further validate the role of the miR-181a-5p in regulating MMP-14 expression, the effect of miR-181a-5p on ectopic MMP-14 was examined. MCF-7 cells stably expressing the sponges were transfected with MMP14-GFP/3'UTR and subsequently analyzed for the MMP14-GFP mRNA and protein expressions. Ectopic expression of miRNA-181a-5p sponge, but not the miR-control sponge, led to upregulated MMP14-GFP in both mRNA and protein levels(Fig.3H–I), further suggesting that MMP-14 is negatively regulated by miRNA-181a-5p.
Direct interaction of miR-181a-5p in the MMP-14 3'UTR
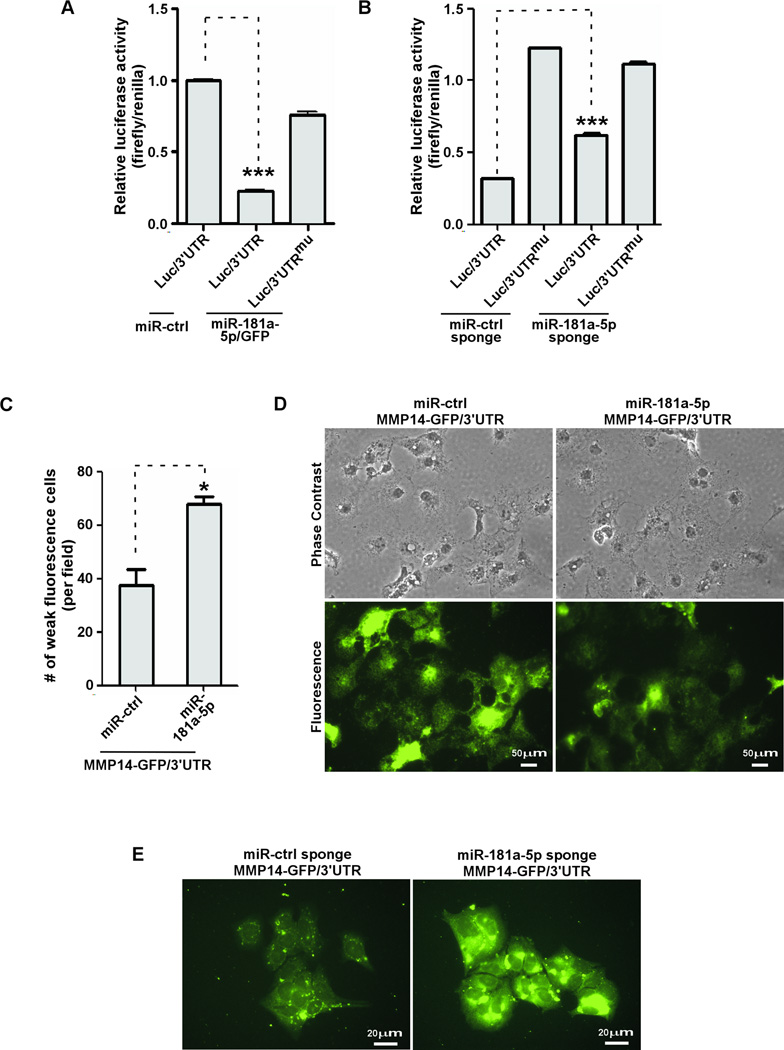
To determine if miR-181a-5p targets the MMP-14 3'UTR directly, we utilized a luciferase reporter gene fused to a sequence of the 3'UTR of MMP-14 that contains the predicted miR-181a-5p response element(Luc/3'UTR). As a control, the predicted miR-181a-5p response element (7 nt) was converted to the complementary sequence to eliminate potential miR-181a-5p binding by using a site-direct mutagenesis approach (Luc/3'UTRmu)(2). Expression of miR-181a-5p significantly reduced luciferase activity of Luc/3'UTR, whereas it had no effect on cells expressing miR-control or Luc/3'UTRmu (Fig.4A). When Luc/3'UTR reporter gene was transfected into MCF-7 cells stably expressing miR-181a-5p sponges, the luciferase activity significantly increased as compared to miR-control sponge infected cells, whereas luciferase activity from Luc/3'UTRmu was not affected by miR-181a-5p sponge (Fig.4B). Hence, the observed downregulation of MMP-14 by miR-181a-5p depends directly on a single cognate recognition site in the 3'UTR of MMP-14 mRNA.
Figure 4. Direct interaction of miRNA-181a-5p with the MMP-14 3'UTR.
A) A reporter gene assay was performed in HT1080 cells transfected with cDNAs as indicated. Renilla luciferase was used as a normalization control. B) MCF-7 cells stably expressing the sponges as indicated were transfected with reporter genes and followed by a dual luciferase assay. C–D) Microscopic examination of the fluorescence intensity of individual COS-1 cells co-transfected with MMP14-GFP/3'UTR cDNAs along with miR-control or miR-181a-5p. The number of cells displaying weak fluorescent signal was microscopically counted (C). Representative images are shown (D). E) Microscopic examination of fluorescence intensity of individual MCF-7 cells co-expressing MMP14-GFP/3'UTR with sponges.
Using an imaging-based assay, we observed that fluorescence intensity in COS-1 cells co-expressing MMP14-GFP/3'UTR with miR-181a-5p (lacking GFP) significantly decreased as compared to control(Fig.4C–D). Conversely, fluorescence intensity of MMP14-GFP/3'UTR in MCF-7 cells stably expressing the miR-181a-5p sponge significantly increased as compared to the control sponge (Fig.4E).
Effect of functional MMP-14 by miR-181a-5p
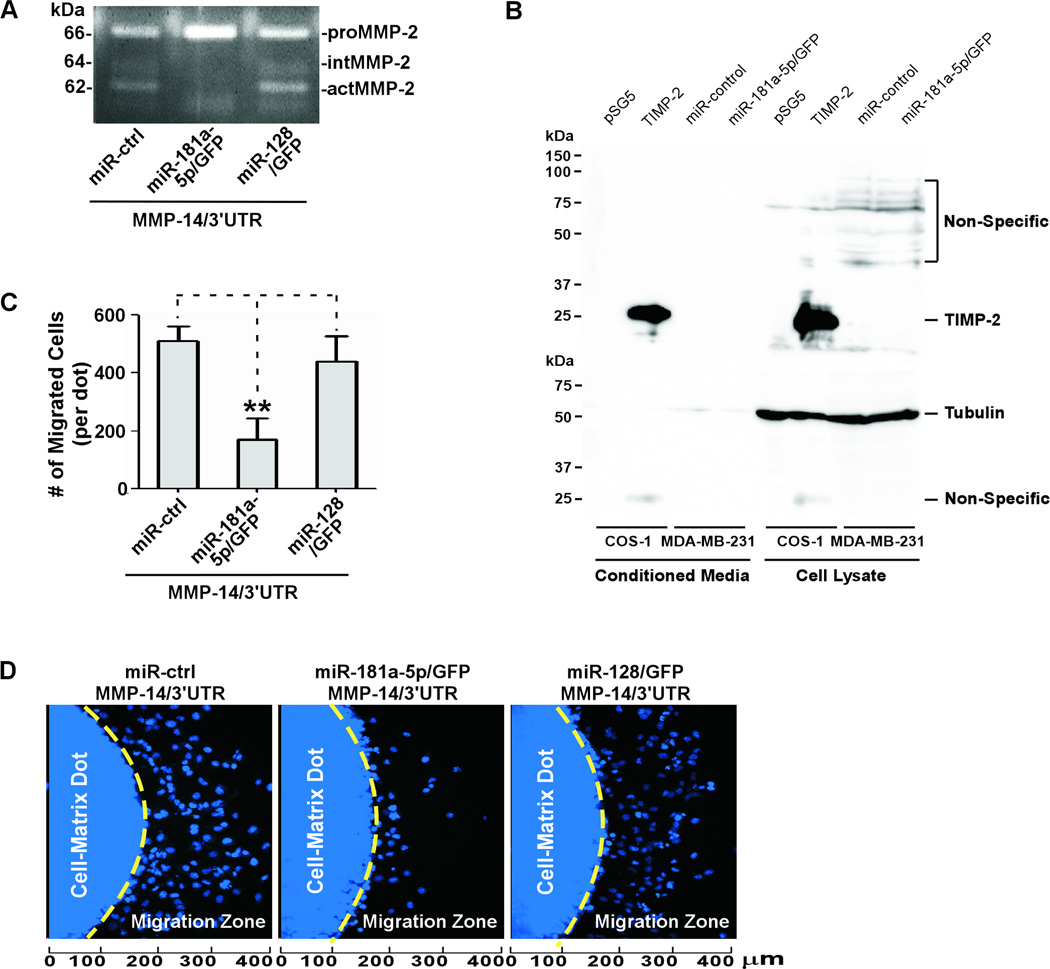
We previously reported that functional MMP-14 enhances proteolytic activity and cell migratory ability(3). MMP-2, a secretory MMP that promotes cancer invasion and metastasis, has been used as an indicator for functional MMP-14(35). We then examined whether reduced MMP-14 expression by miR-181a-5p results in decreased MMP-2 activation and cell migration. Co-expression of MMP-14/3'UTR with miR-181a-5p/GFP in COS-1 cells resulted in reduced proMMP-2 activation as demonstrated by decreased intermediate (intMMP-2) and fully activated (actMMP-2) MMP-2, along with increased latent MMP-2 (proMMP-2) as compared to cells expressing miR-control and miR-128 (Fig. 5A). This decrease correlates with reduced MMP-14 expression (Fig.3C). Since TIMP-2 is a natural inhibitor of MMP-14(4), we examined if the decreased activity of MMP-14 by miR-181a-5p is due to upregulated TIMP-2. Our Western blotting data rules out that possibility because miR-181a-5p does not enhance TIMP-2 expression in MDA-MB-231cells (Fig.5B).
Figure 5. Effect on functional MMP-14 by miR-181a-5p.
A) The conditioned medium from COS-1 cells transfected with a combination of DNAs as indicated was examined by gelatin zymography. B) Conditioned medium and cell lysates were harvested from COS-1cells transfected with vector control or TIMP-2 cDNA and MDA-MB-231 cells stably expressing miR-control or miR-181a-5p and followed by Western blotting using an anti-TIMP-2 antibody (top panel). The membrane was stripped and then probed with an anti-tubulin antibody as a loading control (bottom panel). C–D) COS-1 cells co-transfected with cDNAs as indicated were examined by a dot-based cell migration assay. Migrated cells within the Migration Zone were microscopically counted using the Nikon NIS Elements software (C). Representative images are shown (D).
Consistent with MMP-2 activation, ectopic expression of miR-181a-5p/GFP along with MMP-14/3'UTR in COS-1 cells led to a decrease in cell migration(Fig.5C–D). Importantly, miRNA-128 was unable to inhibit migration in the COS-1 cells expressing MMP-14/3'UTR (Fig.5C–D), suggesting that miR-181a-5p is a key factor in control of MMP-14 expression, whose downregulation results in decreased proteolytic potential and cell migratory ability.
Inverse correlation of miR-181a-5p and MMP-14 in human cancer cell lines and human cancer specimens
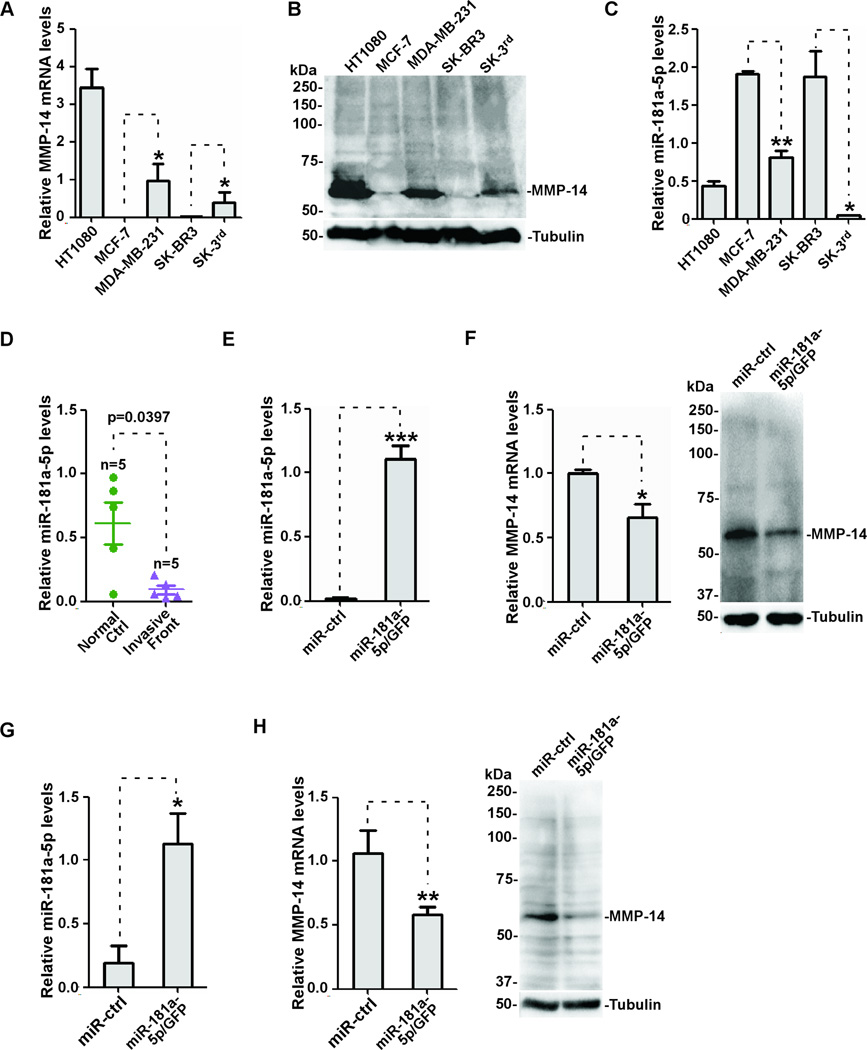
It has been reported that upregulated MMP-14 is often observed in invasive human cancers and correlates with aggressiveness of human cancer cell lines(21,36,37). To investigate the correlation between the endogenous miR-181a-5p level and MMP-14 expression in human cancer cell lines, we surveyed human cancer cell lines using real-time RT-PCR and Western blotting. As expected, MMP-14 expresses at a relatively high level in aggressive cancer lines, including HT1080, MDA-MB-231, and breast cancer stem SK-3rd cells(Fig.6A–B). Interestingly, miR-181a-5p expression is significantly lower in these cells as compared to less aggressive cancer cell lines, such as MCF-7 and SK-BR3 lines(Fig.6C). To determine clinical significance, we examined the correlation between miR-181a-5p and MMP-14 in human cancer specimens. Since MMP-14 is minimally expressed in normal primary colonic mucosal cells, but an increased intensity of staining is found in cancer cells located at the invasive front(Fig.1E), we harvested tumor cells at the invasive front of human colon cancer specimens as well as tumor adjacent normal epithelial cells using a macrodissection technique followed by real-time RT-PCR for miR-181a-5p. Consistent with the observation in cell lines(Fig.6C), miR-181a-5p is downregulated in invasive tumor cells as compared to tumor adjacent normal epithelial cells(Fig.6D). These data suggest that expression of MMP-14 in human invasive cancer is affected by miR-181a-5p levels. To further delineate the causal relationship between miR-181a-5p and MMP-14 expression in cancer progression, we utilized MDA-MB-231 cells because this cell line is highly invasive and expresses a high level of MMP-14 as compared to other cancer cell lines. When MDA-MB-231 cells were transiently transfected with miR-181a-5p/GFP or miR-control(Fig.6E), the endogenous MMP-14 expression at both mRNA and protein level was significantly reduced(Fig.6F). This correlation was also reproduced in HT1080 cells that express a high level of endogenous MMP-14(Fig. 6G–H).
Figure 6. Inverse correlation between miR-181a-5p and MMP-14 in human cancer cell lines.
A–B) Elevated MMP-14 expression in invasive cancer cell lines examined by real-time RT-PCR (A) and Western blotting using an anti-MMP-14 antibody (B). C) Reduced miR-181a-5p was observed in invasive cancer cell lines as examined by real-time RT-PCR. D) miR-181a-5p levels in tumor adjacent normal cells and tumor cells at the invasive front isolated from FFPE sections by a macrodissection technique. Real time RT-PCR was then performed using the appropriate primers. E–F) MDA-MB-231 cells were transfected with miR-control and miR-181a-5p followed by real time RT-PCR to examine miR-181a-5p expression (E). The effect on MMP-14 expression in MDA-MB-231 cells expressing miR-181a-5p was examined by real time RT-PCR (F, left panel) or Western blotting (F, right panel). G–H) Expression of miR-181a-5p in HT1080 cells was examined by real time RT-PCR (G) and MMP-14 expression in the HT1080 cells were examined by real time RT-PCR and Western blotting (H).
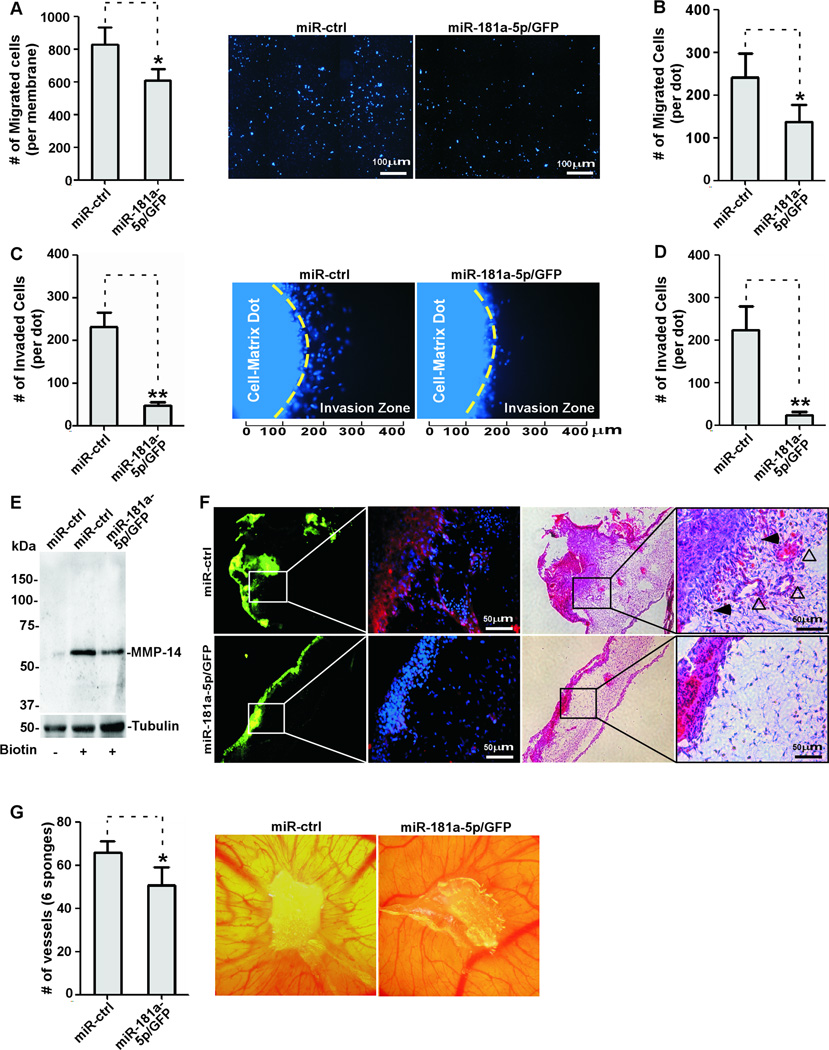
Specific downregulation of MMP-14 expression has been shown to significantly inhibit cancer cell migration and invasion, even though other MMPs continued to be expressed(38). Therefore, we asked whether miR-181a-5p can affect cancer cell migration. Expression of miR-181a-5p in MDA-MB-231 cells resulted in a significant decrease in cell migration as examined by a Transwell chamber migration assay(Fig.7A). The ability of miR-181a-5p to reduce cell migration was further confirmed in HT1080 cells ectopically expressing miR-181a-5p(Fig.7B).
Figure 7. Overexpression of miR-181a-5p attenuates in vivo angiogenesis and invasion by cancer cells.
A) A transwell chamber migration assay was performed with MDA-MB-231 cells stably expressing miR-181a-5p and miR-control. Migrated cells were automatically counted based on nuclear staining (Left panel). Representative images are presented (Right panel). B) A dot-based migration assay was performed with HT1080 cells stably infected with retrovirus expressing miRNA as indicated. C) A 3-D invasion assay using type I collagen gel was performed with MDA-MB-231 cells stably expressing miR-181a-5p. Invaded cells in the invasion zone were counted (Left panel). Representative images of cell invasion are shown (right panel). D) The effect of miR-181a-5p on HT1080 cell invasion was examined using the 3D invasion assay. E) A cell surface biotinylation assay was performed in HT1080 cells stably expressing miR-181a-5p and control and was followed by Western blotting using anti-MMP-14 antibody. F) The CAM invasion assay was performed with MDA-MB-231 cells stably expressing miR-181a-5p and control. Frozen sections were examined by IHC using an anti-MMP14 antibody (Left panel) and H&E staining (Right panel). Tumor cells can be visualized based on GFP in the miR-181a-5p or miR-control plasmid DNA. Open arrows used for blood vessels and solid arrows used for tumor cells at the invasive front. G) CAM angiogenesis was performed with HT1080 cells overexpressing miR-181a-5p. New blood vessels were counted under a dissecting microscope (left panel). Representative images of new blood vessel formation are shown (right panel).
Since cell migration is a critical determination of cancer invasion, a three-dimensional (3-D) invasion assay(39) was utilized to determine if overexpression of miR-181a-5p in MDA-MB-231 cells and HT1080 cells could inhibit cell invasion. As expected, when overexpressing miR-181a-5p, cell invasive ability was dramatically decreased in both cell lines(Fig. 7C–D).
Because MMP-14 is a transmembrane protease, we next asked if reduced cancer cell migration and invasion by miR-181a-5p were due to the loss of cell surface MMP-14. By cell surface biotinylation assay, we found that cell surface MMP-14 was markedly reduced in HT1080 cells ectopically expressing high levels of miR-181a-5p(Fig.7E).
Overexpression of miR-181a-5p attenuates in vivo invasion and angiogenesis
MMP-14 is linked to enhanced cancer invasion(40). To directly examine if miR-181a-5p is capable of inhibition of MMP-14-mediated cancer cell invasion through basement membrane in vivo, the chick chorioallantoic membrane (CAM) invasion assay was employed(40). The CAM consists of the chorionic epithelium and underlying allantoic membrane that is primarily made of type IV collagen(41), which simulates the basement membrane of human epithelium(42). Invasion of cancer cells through the epithelium and basement membrane of the upper CAM into connective tissue was examined by Hematoxylin and Eosin[H&E] staining. miR-control MDA-MB-231 cells that were loaded onto the CAM invaded into the connective tissues through the breached basement membrane(Fig.7F). In contrast, MDA-MB-231 cells stably expressing miR-181a-5p failed to cross through the basement membrane. In addition, more new blood vessels underneath the CAM can be found in miR-control cells as compared to miR-181a-5p cells. This reduction in invasion, along with the decrease in angiogenesis, suggests that miR-181a-5p is effective at interfering with the in vivo invasive ability of MDA-MB-231 cells, which normally express high levels of MMP-14.
To further characterize the anti-angiogenic activity of miR-181a-5p through downregulation of MMP-14 expression, HT1080 cells were employed. Since minimal miR-181a-5p is present in HT1080 cells, we stably expressed miR-181a-5p in HT1080 cells and applied the cells over chorioallantoic membranes via sponges as previously described (19,38). miR-181a-5p, but not miR-control, statistically impaired new blood vessel formation induced by HT1080 cells (Fig.7G).
Taken together, we, for the first time, demonstrate that miR-181a-5p is a critical regulator for MMP-14 expression and can affect MMP-14-mediated cancer cell migration, invasion, and angiogenesis.
Discussion
In this study, we first validated that MMP-14 is highly upregulated in human breast and colon cancers. We then demonstrated that miR-181a-5p is inversely correlated with MMP-14 expression and the invasive capacity of cancer cell lines. We also identified the miR-181a-5p target sequence within the MMP-14 3'UTR that is responsible for the stability of MMP-14 mRNA. Ectopic expression of miR-181a-5p resulted in downregulation of both endogenous and exogenous MMP-14 expression, leading to decreased cell migration, invasion, and angiogenesis. Although MMP-14 is transcriptionally regulated by activating the gene’s promoter (7), the effect of the stability of MMP-14 mRNA is another key regulatory mechanism in controlling MMP-14 expression. Hence, our observations unravel the post-transcriptional regulatory mechanism for MMP-14 expression.
miRNAs can induce gene expression by binding to the 5’UTR of the promoter region of targeted genes or reduce gene expression by binding to the 3’UTR of the target gene and allowing for mRNA degradation or preventing mRNA from being translated. Our data indicates that miR-181a-5p negatively affects MMP-14 expression through binding to the 3’UTR of MMP-14. Because MMP-14 mRNA is reduced by miR-181a-5p, it is assumed that miR-181a-5p induces MMP-14 mRNA degradation, rather than blocking MMP-14 protein translation. Since upregulation of MMP-14 directly associates with cancer aggressiveness, induction of endogenous miR-181a-5p provides a potential approach to prevent cancer invasion and metastasis. However, it should be pointed out that the role of miR-181s in cancer is still controversial depending on the tumor type.
Several studies have indicated that miR-181s serve as tumor-promoting genes, but the function of miR-181a-5p is tumor-type specific. miR-181a-5p was found to be upregulated in gastric and liver cancers (43,44) and in two separate studies, miR-181a-5p overexpression was reported to enhance ovarian, liver, and breast cancer progression through different mechanisms (14,15,45). However, miRNA181s have also been suggested as tumor suppressors in several types of human cancers including leukemia, glioma, and oral squamous cell carcinoma (16–18). These controversial observations suggest the complexity of miRNAs and the function of specific miRNAs can differ markedly depending on tumor types. It is worth noting that most of these studies use the bulk of human tumor tissues which contain not only tumor cells, but also various stromal cell types and adjacent normal epithelial cells. This may not faithfully represent the expression level of specific miRNAs in cancer. Indeed, our data support this notion by showing that miR-181a-5p and MMP-14 are differentially expressed in invasive tumor cells and tumor adjacent normal epithelial cells.
Although this study focuses on miR-181a-5p because it received the highest probability score by computational analysis, other miRNAs may also affect MMP-14 expression. Akanuma et al. reported that miR-133 reduces MMP-14 expression leading to decreased cancer cell migration and invasion (10). Since we also identified a miR-133 response element within the 3'UTR of MMP-14 mRNA by miRNA prediction algorithms, Akanuma's report supports our bioinformatics analysis. Another miRNA, miR-9, was recently reported to downregulate MMP-14 expression by targeting the 3'UTR resulting in decreased cellular invasion and metastasis in neuroblastoma cells (9). Although two separate miRNA prediction algorithms were employed to identify miRNA response elements within the MMP-14 3'UTR in our study, we did not observe the miR-9 response element within the MMP-14 3'UTR. Interestingly, miR-9 was also reported to directly target CDH1, the E-cadherin-encoding mRNA, leading to increased cell motility and invasiveness, which ultimately contributes to metastasis (46). Since mounting evidence indicates that MMP-14 cleaves E-cadherin at the cell surface leading to enhanced cancer aggressiveness (5), both reports seems contradictory under pathological conditions. Questions remain as to whether these miRNAs synergistically or individually affect MMP-14 gene expression leading to reduced cell invasion.
Upregulation of MMP-14 in cancer has been recognized as one of the critical mechanisms of cancer dissemination. The expression of MMP-14 has been detected in tumor cells and adjacent stromal cells in a variety of human tumors (21). Using in situ hybridization techniques, several reports have demonstrated that MMP-14 is mostly expressed in stromal cells within tumors or expressed in both cancer cells and surrounding fibroblasts and macrophages (47,48). In our IHC, we found MMP-14 exclusively expressed in cancer cells. Our real-time RT-PCR data examining laser microdissected cancer cells supports our IHC data. Interestingly, we found that MMP-14 expresses at a low level in primary colon cancer cells, whereas expression of MMP-14 dramatically increases in colon cancer cells located at the invasive front or those that have already invaded into the submucosa. Since MMP-14 plays a critical role in cancer cell EMT (5), our data support the notion that tumor cells at the invasive front gradually change their phenotype to facilitate invasion into surrounding tissues.
In light of the pivotal role of MMP-14 in cancer progression, the current report provides scientific evidence for inducing endogenous miR-181a-5p expression to suppress MMP-14-mediated cancer cell migration, invasion, and angiogenesis. However, because miR-181a-5p can potentially target over 500 different mRNAs (as analyzed by PicTar prediction algorithm) and miR-181a-5p-targeted genes may exert differential or even opposing effects in different cellular contexts, the challenge ahead is to resolve inconsistent observations that exist and unify the current data into a coherent mechanism for miR-181a-5p function within particular tumor types. Interestingly, recent studies using a DNA microarray or quantitative mass spectrometry approach indicate that the repression of mRNAs and proteins by a miRNA is often relatively small (less than 2 fold and rarely exceeds 4 fold) (49,50). Given the technical limitation, the only way to validate the effects of reduced gene expression by a miRNA on cellular phenotypic changes is to characterize the miRNA-targeted gene individually. Nevertheless, our findings identify an additional post-transcriptional mechanism for regulating MMP-14 expression and indicate that downregulated expression of miR-181a-5p may contribute to cancer invasion and angiogenesis in certain tumors.
Supplementary Material
Footnotes
This work is supported in part by the Baldwin Breast Cancer Foundation and the National Cancer Institute (1R01CA166936 to J.C.), by The Simons Foundation (to E.L.),by the National Natural Science Foundation of China (81272507/H1610 to L.H.C.) and by the Excellent Young Teachers Program of Higher Education of Guangdong Province (Yq2013040 to X.X.Z.).
The authors have no conflict of interest associated with this manuscript.
Reference
- 1.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. JCell Physiol. 2008;217(3):643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao J, Kozarekar P, Pavlaki M, Chiarelli C, Bahou WF, Zucker S. Distinct roles for the catalytic and hemopexin domains of membrane type 1-matrix metalloproteinase in substrate degradation and cell migration. JBiolChem. 2004;279(14):14129–14139. doi: 10.1074/jbc.M312120200. [DOI] [PubMed] [Google Scholar]
- 4.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer letters. 2003;194(1):1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 5.Cao J, Chiarelli C, Richman O, Zarrabi K, Kozarekar P, Zucker S. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. JBiolChem. 2008;283(10):6232–6240. doi: 10.1074/jbc.M705759200. [DOI] [PubMed] [Google Scholar]
- 6.Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242(1–2):75–86. doi: 10.1016/s0378-1119(99)00549-1. [DOI] [PubMed] [Google Scholar]
- 7.Haas TL, Stitelman D, Davis SJ, Apte SS, Madri JA. Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. The Journal of biological chemistry. 1999;274(32):22679–2285. doi: 10.1074/jbc.274.32.22679. [DOI] [PubMed] [Google Scholar]
- 8.Petrella BL, Lohi J, Brinckerhoff CE. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene. 2005;24(6):1043–1052. doi: 10.1038/sj.onc.1208305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Qi M, Li S, Qi T, Mei H, Huang K, et al. microRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Molecular cancer therapeutics. 2012;11(7):1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 10.Akanuma N, Hoshino I, Akutsu Y, Murakami K, Isozaki Y, Maruyama T, et al. MicroRNA-133a regulates the mRNAs of two invadopodia-related proteins, FSCN1 and MMP14, in esophageal cancer. British journal of cancer. 2014;110(1):189–198. doi: 10.1038/bjc.2013.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic acids research. 2006;34(Database issue):D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh A, Lee C, Peronne J, Marchini S, Baccarini A, Kolev V, et al. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nature communications. 2014;5:2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor MA, Sossey-Alaoui K, Thompson CL, Danielpour D, Schiemann WP. TGF-beta upregulates miR-181a expression to promote breast cancer metastasis. The Journal of clinical investigation. 2013;123(1):150–163. doi: 10.1172/JCI64946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin KH, Bae SD, Hong HS, Kim RH, Kang MK, Park NH. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochemical and biophysical research communications. 2011;404(4):896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 17.Fei J, Li Y, Zhu X, Luo X. miR-181a post-transcriptionally downregulates oncogenic RalA and contributes to growth inhibition and apoptosis in chronic myelogenous leukemia (CML) PloS one. 2012;7(3):e32834. doi: 10.1371/journal.pone.0032834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, et al. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain research. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zucker S, Pulkoski-Gross A, Kuscu C, Karaayvaz M, Ju J, et al. Conversion of stationary to invasive tumor initiating cells (TICs): role of hypoxia in membrane type 1-matrix metalloproteinase (MT1-MMP) trafficking. PloS one. 2012;7(6):e38403. doi: 10.1371/journal.pone.0038403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evensen NA, Kuscu C, Nguyen HL, Zarrabi K, Dufour A, Kadam P, et al. Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer cell migration. Journal of the National Cancer Institute. 2013;105(18):1402–1416. doi: 10.1093/jnci/djt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP) CurrTopDevBiol. 2003;54:1–74. doi: 10.1016/s0070-2153(03)54004-2. [DOI] [PubMed] [Google Scholar]
- 22.Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer research. 2004;64(1):55–63. doi: 10.1158/0008-5472.can-03-2144. [DOI] [PubMed] [Google Scholar]
- 23.Hao Y, Triadafilopoulos G, Sahbaie P, Young HS, Omary MB, Lowe AW. Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology. 2006;131(3):925–933. doi: 10.1053/j.gastro.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9(4):287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(5):778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 27.Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer research. 2006;66(3):1354–1362. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 28.Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A 'metastasis-prone' signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clinical & experimental metastasis. 2010;27(2):83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 29.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(14):2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 30.Detwiller KY, Fernando NT, Segal NH, Ryeom SW, D'Amore PA, Yoon SS. Analysis of hypoxia-related gene expression in sarcomas and effect of hypoxia on RNA interference of vascular endothelial cell growth factor A. Cancer research. 2005;65(13):5881–5889. doi: 10.1158/0008-5472.CAN-04-4078. [DOI] [PubMed] [Google Scholar]
- 31.Iacobuzio-Donahue CA, Maitra A, Olsen M, Lowe AW, van Heek NT, Rosty C, et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. The American journal of pathology. 2003;162(4):1151–1162. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuscu C, Evensen N, Kim D, Hu YJ, Zucker S, Cao J. Transcriptional and epigenetic regulation of KIAA1199 gene expression in human breast cancer. PloS one. 2012;7(9):e44661. doi: 10.1371/journal.pone.0044661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J, Drews M, Lee HM, Conner C, Bahou WF, Zucker S. The propeptide domain of membrane type 1 matrix metalloproteinase is required for binding of tissue inhibitor of metalloproteinases and for activation of pro-gelatinase A. JBiolChem. 1998;273(52):34745–34752. doi: 10.1074/jbc.273.52.34745. [DOI] [PubMed] [Google Scholar]
- 34.Zucker S, Conner C, DiMassmo BI, Ende H, Drews M, Seiki M, et al. Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. The Journal of biological chemistry. 1995;270(40):23730–23738. doi: 10.1074/jbc.270.40.23730. [DOI] [PubMed] [Google Scholar]
- 35.Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996;393(1):101–104. doi: 10.1016/0014-5793(96)00861-7. [DOI] [PubMed] [Google Scholar]
- 36.Cao J, Chiarelli C, Kozarekar P, Adler HL. Membrane type 1-matrix metalloproteinase promotes human prostate cancer invasion and metastasis. ThrombHaemost. 2005;93(4):770–778. doi: 10.1160/TH04-08-0555. [DOI] [PubMed] [Google Scholar]
- 37.Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mason MD, et al. Expression of membrane type-1 matrix metalloproteinase, MT1-MMP in human breast cancer and its impact on invasiveness of breast cancer cells. Int J Mol Med. 2006;17(4):583–590. [PubMed] [Google Scholar]
- 38.Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, et al. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. The Journal of biological chemistry. 2011;286(38):33167–33177. doi: 10.1074/jbc.M111.256644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evensen NA, Li J, Yang J, Yu X, Sampson NS, Zucker S, et al. Development of a high-throughput three-dimensional invasion assay for anti-cancer drug discovery. PloS one. 2013;8(12):e82811. doi: 10.1371/journal.pone.0082811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu C, Li XY, Hu Y, Rowe RG, Weiss SJ. MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood. 2010;115(2):221–229. doi: 10.1182/blood-2009-06-228494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribatti D, Nico B, Vacca A, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nature protocols. 2006;1(1):85–91. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- 42.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Experimental biology and medicine. 2007;232(9):1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Nie Y, Li X, Wu G, Huang Q, Cao J, et al. MicroRNA-181a Functions as an Oncomir in Gastric Cancer by Targeting the Tumour Suppressor Gene ATM. Pathology oncology research : POR. 2014 doi: 10.1007/s12253-013-9707-0. [DOI] [PubMed] [Google Scholar]
- 44.Ji D, Chen Z, Li M, Zhan T, Yao Y, Zhang Z, et al. MicroRNA-181a promotes tumor growth and liver metastasis in colorectal cancer by targeting the tumor suppressor WIF-1. Molecular cancer. 2014;13:86. doi: 10.1186/1476-4598-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korhan P, Erdal E, Atabey N. MiR-181a-5p is downregulated in hepatocellular carcinoma and suppresses motility, invasion and branching-morphogenesis by directly targeting c-Met. Biochemical and biophysical research communications. 2014;450(4):1304–1312. doi: 10.1016/j.bbrc.2014.06.142. [DOI] [PubMed] [Google Scholar]
- 46.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afzal S, Lalani EN, Poulsom R, Stubbs A, Rowlinson G, Sato H, et al. MT1-MMP and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol. 1998;29(2):155–165. doi: 10.1016/s0046-8177(98)90226-x. [DOI] [PubMed] [Google Scholar]
- 48.Ohtani H, Motohashi H, Sato H, Seiki M, Nagura H. Dual over-expression pattern of membrane-type metalloproteinase-1 in cancer and stromal cells in human gastrointestinal carcinoma revealed by in situ hybridization and immunoelectron microscopy. International journal of cancer Journal international du cancer. 1996;68(5):565–570. doi: 10.1002/(SICI)1097-0215(19961127)68:5<565::AID-IJC2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.