Summary
Spanning about 9 mm2 of the posterior cortex surface, the mouse’s small but organized visual cortex has recently gained attention for its surprising sophistication and experimental tractability [1–3]. Though it lacks the highly ordered orientation columns of primates [4], mouse visual cortex is organized retinotopically [5] and contains at least 10 extrastriate areas that likely integrate more complex visual features via dorsal and ventral streams of processing [6–14]. Extending our understanding of visual perception to the mouse model is justified by the evolving ability to interrogate specific neural circuits using genetic and molecular techniques [15, 16]. In order to probe the functional properties of the putative mouse dorsal stream, we used moving plaids, which demonstrate differences between cells that identify local motion (component cells) and those that integrate global motion of the plaid (pattern cells; Figure 1A; [17]). In primates, there are sparse pattern cell responses in primate V1 [18, 19], but many more in higher-order regions; 25–30% of cells in MT [17] and 40–60% in MST [20] are pattern direction selective. We present evidence that mice have small numbers of pattern cells in areas LM and RL, while V1, AL, and AM are largely component-like. Although the proportion of pattern cells is smaller in mouse visual cortex than in primate MT, this study provides evidence that the organization of the mouse visual system shares important similarities to that of primates, and opens the possibility of using mice to probe motion computation mechanisms.
Results
In an effort to extend our understanding of visual information processing in the rodent system so that we may capitalize on experimental advantages, we have used a common stimulus from primate research to probe motion processing in the mouse model. We used intrinsic signal imaging followed by two-photon calcium imaging in layer 2/3 of 2–4 month old anesthetized mice to record responses to grating and plaid stimuli in V1 and four extrastriate areas (LM, AL, RL, and AM).
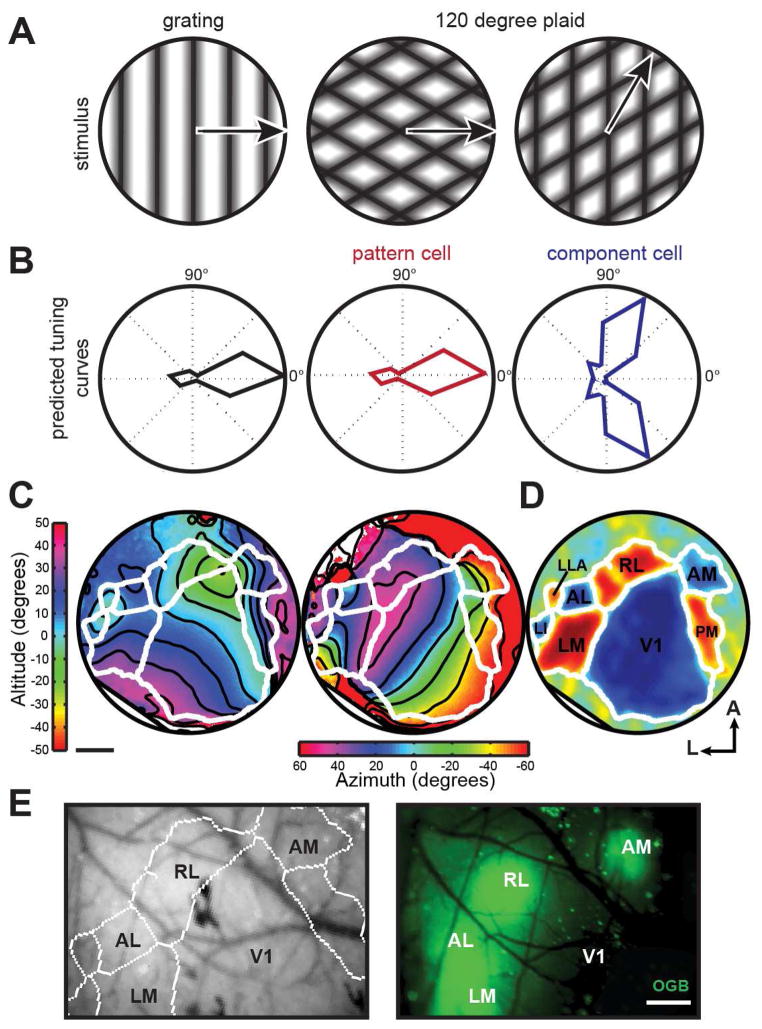
Although visual areas in the mouse are quite small, borders between areas can be functionally mapped using intrinsic signal optical imaging [21], ideally with a periodic stimulus [13, 22]. We therefore first used intrinsic signal optical imaging during the presentation of a full-field continuous contrasting-reversing checkerboard bar in altitude and azimuth directions to semi-automatically determine borders between visual areas (Figure 1C&D; [7, 13, 21, 22]). With this method, functional maps can be accurately computed for each mouse, allowing for individual identification of visual area borders, important due to small area size and slight differences between mice [13]. Using these functional maps overlaid on blood vessel patterns as a guide, we then loaded Oregon Green Bapta (OGB) into layer 2/3 of the targeted area (Figure 1E).
Figure 1. Classifying pattern and component-like responses to plaid stimuli in multiple visual areas.
(A) Schematic of sinusoidal gratings and plaids. Left plaid has same pattern motion as grating, right plaid has a different pattern motion but contains the rightward-moving grating component. (B) Left - hypothetical response to grating, center and right - generated predictions for pattern and component tuning curves in response to plaids. The pattern response is identical to the DS cell response to the grating, whereas the component response has two lobes to account for the two directions of the plaid (one direction shown in (A)) that contain the preferred component. (C) Sample azimuth and altitude ISI data from one animal with 5 repeats of the stimulus. Contour lines are overlaid in black, area borders as determined by semi-automatic border analysis are overlaid in white. (D) Visual field sign computed as the sine of the difference in the angle between the horizontal and vertical map gradients. Regions with a red visual field sign have a non-mirror representation of visual space, whereas areas in blue have a mirror representation. Regions that are not clearly red or blue lack retinotopic structure. Identified visual areas are labeled. (E) Left - visual area borders generated from (C and D) overlaid on blood vessel picture. Right - subsequent OGB loading into targeted areas. Scale bar represents 500 μm.
Moving plaids consist of two drifting gratings combined additively and offset by an angle (Figure 1A; [23]). In primates, visual area MT/V5 contains cells that respond to the global motion of the plaid, termed “pattern” or “pattern direction selective (PDS)” cells (Figure 1B; [17]). Other cells, present in both V1 and MT, encode the individual gratings of the plaid and are termed “component” or “CDS” cells (Figure 1B). Thus, after OGB loading, we investigated the responses of cells to full screen 100% contrast drifting gratings and 120° plaids (50% contrast for each grating) moving in 12 different directions to identify cells that responded to either the individual, component motions of the plaid or the global, perceived motion of the plaid (Supplemental Methods; [17]).
We imaged thousands of cells in V1, LM, AL, RL, AM in 34 different animals (Table S1). Of these cells, 15–25% (depending on visual area) were responsive (ΔF/F > 6%) and reliable (determined by a D-prime metric; [7]; Supplemental Methods) to at least one type of stimulus [LM: 12.8% (588 out of 4577), AL: 13.4% (508 out of 3970), RL: 17.6% (921 out of 5232), V1: 25% (1192 out of 4743); Table S1], consistent with previous studies investigating visual responses in these areas in both awake [8] and anesthetized [7] mice. Only cells meeting the responsive and reliable criteria for at least one stimulus were included in further analysis to determine stimulus preferences.
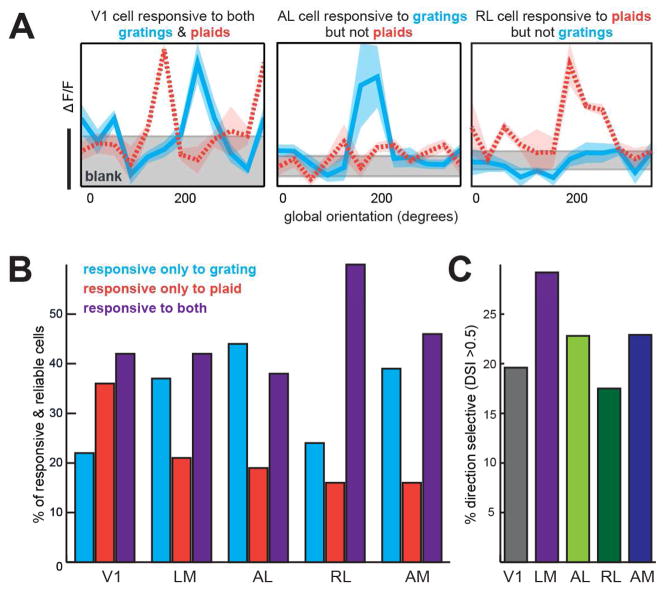
We then looked to see whether these cells responded to gratings, plaids, or both. While some cells were responsive and reliable to both stimuli, certain cells responded only to the simple drifting gratings, and another subset responded solely to plaids (Figure 2A). Across areas, there were differences in the proportions of cells that were responsive to each stimulus (Figure 2B); while 38–46% of responsive and reliable cells in V1, LM, AL, and AM responded to both gratings and plaids, 60% (553/921) of cells in RL responded to both. AL had the highest proportion of cells responsive only to gratings (43%; 218/508), while V1 and RL had the lowest (22% and 24%, respectively). A relatively high proportion (37%; 441/1192) of cells in V1 responded exclusively to plaids and not gratings (Figure S1).
Figure 2. Sample tuning curves and distribution of responses to gratings and plaids.
(A) Example tuning curves from V1, AL, and RL, demonstrating diverse visual responses to grating or plaid stimuli. Left - V1 cell responds above baseline (gray) to both gratings (cyan) and plaids (orange dashed line), center - AL cell responds to only gratings, and right - RL cell responds only to plaids. Shaded area around curves represents S.E.M; gray baseline shaded area is the mean ΔF/F +/− S.E.M. Scale bar corresponds to 5% ΔF/F. (B) Percent of responsive and reliable cells in each area that responded to only drifting gratings, only 120° plaids, or both. (C) Percent of cells that were direction selective (DSI>0.5), taken out of the total number of responsive & reliable cells. See also Figure S1.
Only cells that respond to both gratings and plaids can be assessed for their preference for pattern or component motion [17]. Furthermore, only direction selective cells can be pattern or component motion direction selective (by the standard definition). Therefore, the subset of cells that were responsive and reliable to both gratings and plaids were then tested for direction selectivity. In V1, about 19.6% of these cells were direction selective (DS; determined by standard metrics where DSI > .5), whereas 22.8–29.2% of cells in LM, AL, and AM were DS, consistent with previous reports (Figure 2C; [7]). We found a relatively low percentage of DS cells in RL (17.5%), possibly because the stimulus was not optimized for the high-temporal and low-spatial frequency preferences of this area [7]. The cells that were responsive and reliable to both gratings and plaids and were also DS were included in the subsequent component/pattern correlation analysis.
In order to characterize cells as pattern, component, or unclassified, we generated predicted tuning curves for pattern and component cells from the grating responses for each cell, as previously described (Figure 1B; [17]). The two predicted tuning curves were then correlated with the responses to the plaid stimulus to give two correlation values for each cell, Rc and Rp. These correlation values were then normalized with a Fisher r-to-Z transformation to permit the calculation of a difference between correlation values, generating Z-pattern (Zp) and Z-component (Zc; [24]). A significantly high Zp value classifies the cell as pattern-direction selective (PDS), whereas a high Zc value classifies it as component-direction selective (CDS). Cells with correlation values that were not significantly different from each other or zero were deemed unclassified.
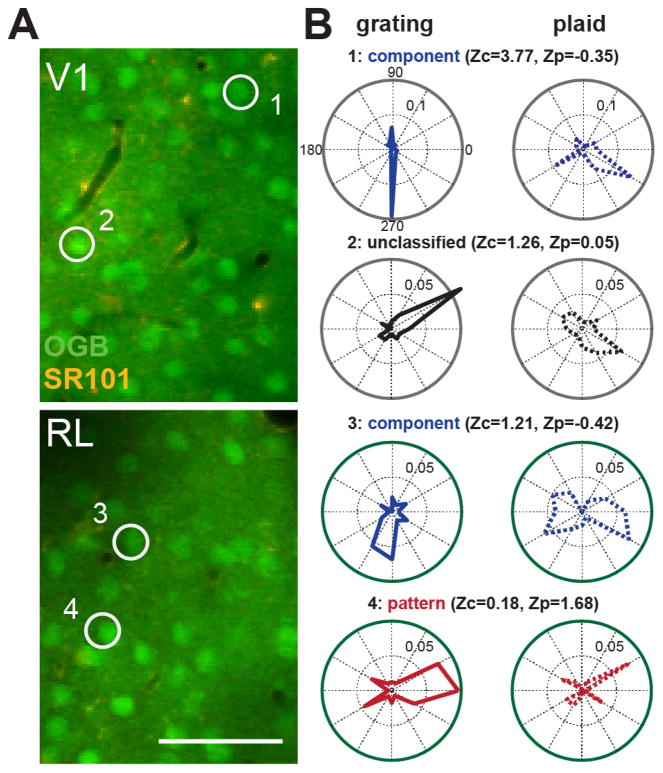
Cell responses to the stimulus set of grating and plaids varied on a continuum from PDS to CDS responses. Some cells were CDS and had a very clear bi-lobed tuning curve in response to plaids because a plaid moving in two different directions contained the grating component that the cell preferred (Figure 3B). Alternatively, several cells responded to just one plaid with the same global motion as its preferred grating (Figure 3B), and were therefore PDS.
Figure 3. OGB and SR101 loading in V1 and RL with cell examples.
(A) Example two-photon data from V1 (top) and RL (bottom) with OGB (neurons) and SR101 (glia) loading. Scale bar represents 50 μm. (B) Sample tuning curves from component, unclassified, and pattern cells. Z-scored component (Zc) and pattern (Zp) values are given for each cell. Numbered circles in the images (A) indicate neurons that correspond to the numbered tuning curves (B). Values within polar plots indicate ΔF/F scale to the inner dotted ring of each plot.
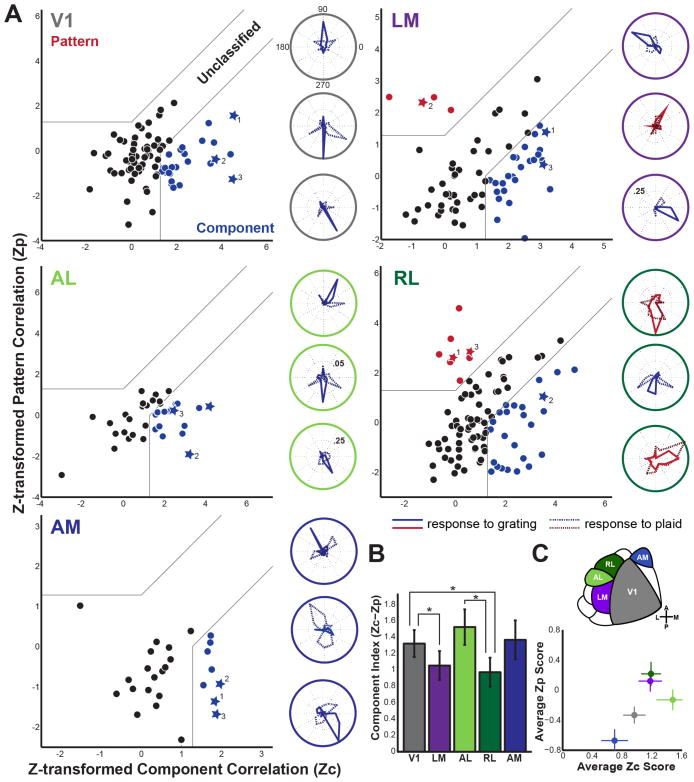
Across areas, the proportion of PDS, CDS, and unclassified cells differed: LM and RL were the only areas containing cells exhibiting pattern-direction selectivity (Figure 4A). Approximately 5.8% of cells in LM were PDS (4/69), while 8.3% (8/96) of cells in RL were PDS. V1 had no PDS cells but 30.1% (25/83) of the cells included in the analysis (as described above) were classified as CDS. Area AL was marked by the highest percentage of CDS cells (39.5%, 15/38), with many cells that had well-tuned responses to both gratings and plaids. Lastly, AM did not have any PDS cells, but 30.8% (8/26) were clearly CDS. In addition, we conducted a subset of experiments with awake-behaving mice, but this did not drastically change the proportion of pattern cells in RL (Figure S1). Each area had a set of cells that did not significantly correlate with a CDS or PDS prediction, though often these were qualitatively component- or pattern-like (see cell example 2 in Figure 3B).
Figure 4. Pattern and component correlation plots by visual area.
(A) Z-transformed pattern (Zp) vs. component (Zc) correlation for V1, LM, AL, RL, and AM. Cells with tuning curves plotted to the right are denoted as stars in the scatterplots. All cells are colored according to classification (red-pattern, black-unclassified, blue-component). Gray lines divide plots into areas of significantly Pattern Direction Selective (PDS), unclassified, or Component Direction Selective (CDS). Outside ring of polar plots is color coded for each visual area. Values within polar plots indicate ΔF/F scale to the inner dotted ring of each plot; inner ring is 10% ΔF/F unless otherwise noted. (B) Mean component index (Zc-Zp) by visual area, error bars show S.E.M. *p < .05 (Fisher Exact Test). (C) Top – schematic of mouse visual areas. Bottom -Average Zc score plotted against the average Zp score for each visual area, error bars show S.E.M. The visual area corresponding to each point is indicated by colors in top area schematic. For further characterization of this data, see Figure S2.
In addition to cells that are clearly classified as CDS or PDS, the unclassified cells have biases in their responses that can be observed as the difference between Zc and Zp. We therefore also computed a Component Index for each cell by subtracting Zc-Zp to obtain a more graded measure of how the cell responses differed across areas (Figure 4B). The distributions of Zc-Zp values as well as their means differed between the populations of cells sampled in each area with AL being the most component-like and RL the most pattern-like. Specifically, the mean Zc-Zp value was highest (most component-like) for area AL (1.53 +/− 0.22, mean +/− SEM) and was lowest (most pattern-like) for LM (1.05+/−0.18) and RL (0.97+/−0.18). The mean values for areas V1 and AM were intermediate (1.32+/−0.17 and 1.37+/−0.24 respectively).
When proportions of CDS, unclassified, and PDS cells were compared across areas, there were clear significant differences. The number of PDS, unclassified, and CDS was significantly different between V1 and LM (p<.05), V1 and RL (p<.01), and AL and RL (p<.05) as determined by a Fisher Exact Test (Figure 4B; these differences remain significant when corrected for multiple comparisons with a Benjamini-Hochberg procedure, FDR = 0.2). While the number of PDS cells was significantly different in RL and LM when compared to V1, AL and AM were not different from V1 (Figure 4B).
Discussion
While mice have been shown to have multiple visual cortical areas with functional preferences, it is unknown whether these areas generate higher-order functional specializations like those in the primate visual system. In particular, it is unknown whether mice compute complex visual movement akin to primates. To further assess the potential of mouse visual system for elucidating circuit mechanisms of complex behaviors, we turned to the plaid stimulus, which has proved useful for visual neuroscience in cats and monkeys for the past 30 years [17].
Here we present evidence that mice have cells that can compute pattern motion, and that in the five areas that were tested, these cells are found only in visual areas LM and RL. Meanwhile, mouse V1, AL, and AM do not have any evidence of PDS responses. We found CDS responses in all of the visual areas we tested, suggesting that this is a more fundamental computation that each area can complete. It should be noted that our experiments were restricted to layers 2/3 of cortex, and it is possible that there are laminar differences in responses to plaids. In essence, V1, AL, and AM appear to be “blind” to the global motion of the stimulus, even though many cells in these regions responded in some way to the plaid stimulus. On the contrary, proper processing of moving plaids to provide accurate information about the global movement of the stimulus is effectively completed in specific cells of areas LM and RL, which may constitute a dorsal, movement-sensitive pathway in the mouse [7, 8]. This integration is essential for correctly computing optic flow and effectively initiating movement.
Although RL contains a much lower percentage of pattern cells than seen in primate MT, it is worth noting that it shares other important similarities with MT. Anatomical studies have suggested that RL is a node of the mouse dorsal stream [10]. Like MT, RL receives direct input from V1 as well as V2/LM [9, 10], and both MT and RL have a bias towards the lower visual field in their retinotopic organization [13, 25]. RL projects to barrel and whisker motor cortex as well as deep layers of the superior colliculus [11], suggesting it is involved in navigation and visually-guided orienting. In addition, RL exhibits multisensory enhancement for visual and tactile stimuli [26].
Despite these similarities, there is a marked difference in the direction selectivity of MT and RL – almost every cell in MT is direction selective [27, 28] whereas about 18–27% of RL cells are direction selective [7]. Shown here, RL is the most pattern-selective area in the mouse, with 8% of direction selective cells responding to pattern motion. While this is a small proportion compared to primate MT, it is unlikely these were recorded by chance in light of the differences between RL and V1. In addition, because the stimulus was not optimized for each individual neuron as in single-cell electrophysiology, it is likely that we have undersampled the number of responsive, and potentially pattern-selective cells. Future studies will need to address the known anatomical and functional markers of MT such as surround suppression, binocular disparity, and direction selective V1 inputs [34] in order to fully test the validity of the comparison between RL and MT.
Previous studies have shown that mice can compute the global motion of a stimulus, but have not explored the mechanistic basis for this behavior [29]. The presence of PDS cells in the mouse suggests that they achieve this computation in a similar way to primates, but with fewer cells overall. It is possible that the downstream consequences of pattern integration, such as motor output for head or body orienting, are achieved with fewer cells that can compute such motion, or that these computations are completed in networks rather than individual cells. Our observation that many cells respond to plaid stimuli (Figure 2), often in ways that did not conform to a CDS or PDS prediction (Figure 4), suggests that mice might employ a novel computation to perform pattern motion integration and inform downstream behavioral output. This speculation is further supported by the fact that many cells, even in V1, responded significantly to plaids but not gratings (Figure S1). Such cells might support sensation of global motion differently than in primates, obviating the need for large numbers of PDS cells. Alternatively, these cells might prefer spatial frequencies that were present in the plaids but not the gratings.
Evidence for pattern direction selectivity in LM and RL – but not V1, AL, or AM – can build on current anatomical frameworks to inform proper parsing of dorsal and ventral streams in the mouse. While most of the focus has been on pattern selectivity, we are intrigued by the high proportion of component cells in AL. Previous studies have suggested that AL is a gateway to the dorsal stream [7, 9], yet the present work suggests that it is not involved in plaid motion integration, a prominent characteristic of dorsal stream function. On the other hand, anatomical data has led other researchers to position LM as part of the ventral stream [9, 10], although it clearly projects to both dorsal and ventral targets. Indeed, the rationale used to place LM in the ventral stream places V1 there as well [10]. Our past investigation into the spatial and temporal frequency preferences of LM [7] and present data for plaid motion processing suggests that LM is involved in the dorsal stream as well, and may be akin to primate V2 in this regard [22, 35]. Further functional studies of these areas with more diverse and complex visual stimuli – including objects, figure/ground separation, colors, etc. – will be necessary to explicate functional differences and draw a clear hierarchy between these regions.
As there is a significant gain in response intensity with movement [36, 37], and other researchers have posited that plaid motion integration may change with brain state ([38], but see [39]), we ran a subset of experiments in awake animals but did not see a striking difference in the proportion of pattern cells (Figure S1). Although preliminary, this suggests that plaid motion integration does not depend on the state of the animal [39].
Our work here provides a basis to test the nuances of complex motion perception in mammalian visual systems and further unravels the function of higher-order mouse cortex. The demonstration of pattern motion selective cells in genetically tractable mice, where specific cell types can be selectively manipulated [15], opens the door to studies probing the neural circuit mechanisms that underlie the production of pattern motion cells in higher-order visual areas from their component motion selective V1 inputs. The use of single-cell monosynaptic tracing with the rabies virus [40, 41] in conjunction with genetically-encoded calcium indicators could be a fruitful way to understand which cells provide inputs to pattern cells, and how and when these inputs are combined [42, 43]. Already, various groups have capitalized on the methodological advantages afforded by the mouse model to address circuit-level questions of visual perception [44–46] – our work provides a necessary basis for similar future studies. The presence of pattern cells in mouse visual cortex suggests that questions regarding the cell types and connectivity motifs that underlie pattern motion computation can indeed be investigated in the mouse model.
Supplementary Material
Highlights.
Five different visual areas in the mouse were investigated for responses to plaids
Mouse visual cortex cells respond in diverse ways to gratings and plaids
Areas LM and RL contain small proportions of pattern cells; V1, AL, and AM do not
LM and RL may constitute a dorsal, motion-sensitive stream in the mouse
Acknowledgments
We wish to thank Drs. Tony Movshon and Marina Garrett for insightful feedback on the experimental design and manuscript, Drs. Jim Marshel and Ian Nauhaus for MATLAB assistance, as well as the Callaway lab members for helpful discussions. This work was supported by the NIH (EY022577) and the Gatsby Charitable Foundation (E.M.C.), and the NSF and Martinet Foundation (A.L.J.).
Footnotes
Author Contributions
A.L.J. and E.M.C. designed experiments, A.L.J. conducted experiments and analyzed data, A.L.J. and E.M.C. wrote the manuscript.
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glickfeld LL, Reid RC, Andermann ML. A mouse model of higher visual cortical function. Current Opinion in Neurobiology. 2014;24:28–33. doi: 10.1016/j.conb.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carandini M, Churchland AK. Probing perceptual decisions in rodents. Nat Neurosci. 2013;16:824–831. doi: 10.1038/nn.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hübener M. Mouse visual cortex. Current Opinion in Neurobiology. 2003;13:413–420. doi: 10.1016/s0959-4388(03)00102-8. [DOI] [PubMed] [Google Scholar]
- 4.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 5.Wagor E, Mangini NJ, Pearlman AL. Retinotopic organization of striate and extrastriate visual cortex in the mouse. Journal of Comparative Neurology. 1980;193:187–202. doi: 10.1002/cne.901930113. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- 7.Marshel JH, Garrett ME, Nauhaus I, Callaway EM. Functional Specialization of Seven Mouse Visual Cortical Areas. Neuron. 2011;72:1040–1054. doi: 10.1016/j.neuron.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. Functional Specialization of Mouse Higher Visual Cortical Areas. Neuron. 2011;72:1025–1039. doi: 10.1016/j.neuron.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Gao E, Burkhalter A. Gateways of Ventral and Dorsal Streams in Mouse Visual Cortex. Journal of Neuroscience. 2011;31:1905–1918. doi: 10.1523/JNEUROSCI.3488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Sporns O, Burkhalter A. Network Analysis of Corticocortical Connections Reveals Ventral and Dorsal Processing Streams in Mouse Visual Cortex. Journal of Neuroscience. 2012;32:4386–4399. doi: 10.1523/JNEUROSCI.6063-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Burkhalter A. Stream-Related Preferences of Inputs to the Superior Colliculus from Areas of Dorsal and Ventral Streams of Mouse Visual Cortex. Journal of Neuroscience. 2013;33:1696–1705. doi: 10.1523/JNEUROSCI.3067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth MM, Helmchen F, Kampa BM. Distinct Functional Properties of Primary and Posteromedial Visual Area of Mouse Neocortex. Journal of Neuroscience. 2012;32:9716–9726. doi: 10.1523/JNEUROSCI.0110-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett ME, Nauhaus I, Marshel JH, Callaway EM. Topography and areal organization of mouse visual cortex. Journal of Neuroscience. 2014;34:12587–12600. doi: 10.1523/JNEUROSCI.1124-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack PO, Contreras D. Long-Range Parallel Processing and Local Recurrent Activity in the Visual Cortex of the Mouse. Journal of Neuroscience. 2012;32:11120–11131. doi: 10.1523/JNEUROSCI.6304-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo L, Callaway EM, Svoboda K. Genetic Dissection of Neural Circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends in Neurosciences. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Movshon JA, Adelson EH, Gizzi MS, Newsome WT. The Analysis of Moving Visual Patterns. In: Chagas C, Gattass R, Gross C, editors. Pattern Recognition Mechanisms. Academia Scientiarum Scripta; 1985. [Google Scholar]
- 18.Tinsley CJ, Webb BS, Barraclough NE, Vincent CJ, Parker A, Derrington AM. The nature of V1 neural responses to 2D moving patterns depends on receptive-field structure in the marmoset monkey. Journal of Neurophysiology. 2003;90:930–937. doi: 10.1152/jn.00708.2002. [DOI] [PubMed] [Google Scholar]
- 19.Khawaja FA, Tsui JMG, Pack CC. Pattern Motion Selectivity of Spiking Outputs and Local Field Potentials in Macaque Visual Cortex. Journal of Neuroscience. 2009;29:13702–13709. doi: 10.1523/JNEUROSCI.2844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khawaja FA, Liu LD, Pack CC. Responses of MST neurons to plaid stimuli. Journal of Neurophysiology. 2013;110:63–74. doi: 10.1152/jn.00338.2012. [DOI] [PubMed] [Google Scholar]
- 21.Schuett S, Bonhoeffer T, Hübener M. Mapping retinotopic structure in mouse visual cortex with optical imaging. Journal of Neuroscience. 2002;22:6549–6559. doi: 10.1523/JNEUROSCI.22-15-06549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 23.Adelson EH, Movshon JA. Phenomenal coherence of moving visual patterns. Nature. 1982;300:523–525. doi: 10.1038/300523a0. [DOI] [PubMed] [Google Scholar]
- 24.Smith MA, Majaj NJ, Movshon JA. Dynamics of motion signaling by neurons in macaque area MT. Nat Neurosci. 2005;8:220–228. doi: 10.1038/nn1382. [DOI] [PubMed] [Google Scholar]
- 25.Maunsell JH, Newsome WT. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- 26.Olcese U, Iurilli G, Medini P. Cellular and Synaptic Architecture of Multisensory Integration in the Mouse Neocortex. Neuron. 2013:1–15. doi: 10.1016/j.neuron.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. Journal of Neurophysiology. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- 28.Dubner R, Zeki SM. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Research. 1971;35:528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- 29.Douglas RM, Neve A, Quittenbaum JP, Alam NM, Prusky GT. Perception of visual motion coherence by rats and mice. Vision Research. 2006;46:2842–2847. doi: 10.1016/j.visres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Research. 2000;40:2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, King JA, Burgess N, O’Keefe J. How vision and movement combine in the hippocampal place code. Proceedings of the National Academy of Sciences. 2013;110:378–383. doi: 10.1073/pnas.1215834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoccolan D, Oertelt N, DiCarlo JJ, Cox DD. A rodent model for the study of invariant visual object recognition. Proceedings of the National Academy of Sciences. 2009;106:8748–8753. doi: 10.1073/pnas.0811583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glickfeld LL, Histed MH, Maunsell JHR. Mouse Primary Visual Cortex Is Used to Detect Both Orientation and Contrast Changes. 2013;33:19416–19422. doi: 10.1523/JNEUROSCI.3560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- 35.Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? Trends in Neurosciences. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- 36.Niell CM, Stryker MP. Modulation of Visual Responses by Behavioral State in Mouse Visual Cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A Cortical Circuit for Gain Control by Behavioral State. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pack CC, Berezovskii VK, Born RT. Dynamic properties of neurons in cortical area MT in alert and anaesthetized macaque monkeys. Nature. 2001;414:905–908. doi: 10.1038/414905a. [DOI] [PubMed] [Google Scholar]
- 39.Movshon JA, Albright TD, Stoner GR, Majaj NJ. Cortical responses to visual motion in alert and anesthetized monkeys. Nature. 2003 doi: 10.1038/nn0103-3a. [DOI] [PubMed] [Google Scholar]
- 40.Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann KK, Young JAT, Callaway EM. Monosynaptic Restriction of Transsynaptic Tracing from Single, Genetically Targeted Neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshel JH, Mori T, Nielsen KJ, Callaway EM. Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron. 2010;67:562–574. doi: 10.1016/j.neuron.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiele A, Stoner G. Neuronal synchrony does not correlate with motion coherence in cortical area MT. Nature. 2003;421:366–370. doi: 10.1038/nature01285. [DOI] [PubMed] [Google Scholar]
- 43.Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J Neurosci. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nienborg H, Hasenstaub A, Nauhaus I, Taniguchi H, Huang ZJ, Callaway EM. Contrast dependence and differential contributions from somatostatin- and parvalbumin-expressing neurons to spatial integration in mouse V1. Journal of Neuroscience. 2013;33:11145–11154. doi: 10.1523/JNEUROSCI.5320-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature. 2015;507:358–361. doi: 10.1038/nature12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lien AD, Scanziani M. Tuned thalamic excitation is amplified by visual cortical circuits. Nat Neurosci. 2013;16:1315–1323. doi: 10.1038/nn.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.