Abstract
Purpose
T cells engineered with chimeric antigen receptors (CARs) recognizing CD19 can induce complete remission of B cell malignancies in clinical trials; however, in some disease settings CAR therapy confers only modest clinical benefit due to attenuated persistence of CAR T cells. The purpose of the study was to enhance persistence and augment the antitumor activity of adoptively transferred CD19CAR T cells by re-stimulating CAR+ T cells through an endogenous cytomegalovirus (CMV)-specific T cell receptor.
Experimental design
CMV-specific T cells from CMV seropositive healthy donors were selected after stimulation with pp65 protein and transduced with clincal grade lentivirus expressing the CD19R:CD28:ζ/EGFRt CAR. The resultant bi-specific T cells, targeting CMV and CD19, were expanded via CD19 CAR-mediated signals using CD19-expressing cells.
Results
The bi-specific T cells proliferated vigorously after engagement with either endogenous CMVpp65 T cell receptors or engineered CD19 CARs, exhibiting specific cytolytic activity and IFNγ secretion. Upon adoptive transfer into immunodeficient mice bearing human lymphomas, the bi-specific T cells exhibited proliferative response and enhanced antitumor activity following CMVpp65 peptide vaccine administration.
Conclusions
We have redirected CMV-specific T cells to recognize and lyse tumor cells via CD19CARs, while maintaining their ability to proliferate in response to CMV antigen stimulation. These results illustrate the clinical applications of CMV vaccine to augment the antitumor activity of adoptively transferred CD19CAR T cells in patients with B cell malignancies.
Introduction
Human studies of cancer and infectious diseases demonstrate that adoptive transfer of T cells of defined antigen specificity can establish or augment immunity to eradicate targeted malignant or infected cells. Adoptive transfer of in vitro expanded, chimeric antigen receptor (CAR)-redirected CD19-specific T cells can induce dramatic disease regression in patients with leukemia and lymphoma (1–4). However, the full potential of this emerging modality is hampered in some cancer settings by a significant rate of therapeutic failure arising from the attenuated engraftment and persistence of CAR-redirected T cells following adoptive transfer. In contrast, the adoptive transfer of native virus-specific T cells efficiently prevents progressive viral infections and exhibits longer-term persistence in patients (5–7).
The mechanisms for the differential persistence of adoptively transferred virus-specific T cells in hematopoietic cell transplantation (HCT) recipients versus tumor-reactive T cells in cancer patients is not fully understood, but possibly reflects both the environment into which the T cells are infused and qualitative attributes of the T cells that are isolated and expanded for adoptive transfer. In attempts to improve the efficacy of CAR T cells for tumor eradication, adoptive T cells with dual specificity have been created: isolated Epstein-Barr virus (EBV)-specific T cells modified to express GD2 or CD30 CARs recognizing tumors of neural crest origin (8–10), and isolated influenza A matrix protein 1 (MP1)-specific T cells modified to express CD19 CARs recognizing B cell malignancies (11). These virus and CAR bi-specific T cells demonstrate superior survival and anti-tumor activity compared to CAR T cells alone, possibly due to a more potent co-stimulation of virus-specific T cells after engagement of their native receptors. Recent studies demonstrate that adoptively transferred EBV × CMV × CD19CAR bi (tri)-specific T cells proliferate in patients as a result of CMV reactivation (12).
Cytomegalovirus (CMV) is a common virus for which 75% of adults in the United States test positive (13, 14) and was the first virus targeted by adoptive transfer strategies. Pioneering immunotherapy trials by Riddell and others show that adoptive transfer of virus-specific T cells is sufficient to reduce the incidence of CMV disease without toxicity (including GVHD) (5–7). Phase I studies conducted at City of Hope demonstrate the safety and effectiveness of two different formulations of CMV vaccine for eliciting vaccine-driven expansion of pp65 specific T cells in healthy volunteers and transplant recipients (15).
Based on the clinical observation that enhanced antiviral efficacy can be achieved using a vaccine recognized by an endogenous TCR, we have transduced native CMV-specific T cells with a CD19CAR lentivirus to determine whether CD19CAR-redirected CMV-specific T cells can respond to a CMV vaccine with rapid expansion and enhanced antitumor activity.
Materials and Methods
Antibodies and Flow Cytometry
Fluorochrome-conjugated isotype controls, anti-CD3, anti-CD4, anti-CD8, anti-CD28, anti-CD45, anti-CD27, anti-CD62L, anti-CD127, anti-IFNγ, and streptavidin were obtained from BD Biosciences. Biotinylated cetuximab was generated from cetuximab purchased from the City of Hope pharmacy. The IFN-γ Secretion Assay – Cell Enrichment and Detection Kit and CMVpp65 protein were purchased from Miltenyi Biotec (Miltenyi Biotec, Germany). Phycoerythrin (PE)-conjugated CMV pp65 (NLVPMVATV)–HLA-A2*0201 iTAg MHC tetramer, PE-conjugated multi-allele negative tetramer was obtained from Beckman Coulter (Fullerton, CA). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Invitrogen (Carlsbad, CA). All monoclonal antibodies, tetramers and CFSE were used according to the manufacturer’s instructions. Flow cytometry data acquisition was performed on a MACSQuant (Miltenyi Biotec, Germany) or FACScalibur (BD Biosciences), and the percentage of cells in a region of analysis was calculated using FCS Express V3 (De Novo Software).
Cell lines
EBV-transformed lymphoblastoid cell lines (LCLs) were made from peripheral blood mononuclear cells (PBMC) as previously described (16). To generate LCL-OKT3, allogeneic LCLs were resuspended in nucleofection solution using the Amaxa Nucleofector kit T, OKT3-2A-Hygromycin_pEK plasmid was added to 5µg/107 cells, the cells were electroporated using the Amaxa Nucleofector I, and the resulting cells were grown in RPMI 1640 with 10% FCS containing 0.4mg/ml hygromycin. To generate firefly luciferase+ GFP+ LCLs (fflucGFPLCLs), LCLs were transduced with lentiviral vector encoding eGFP-ffluc. Initial transduction efficiency was 48.5%, so the GFP+ cells were sorted by FACS for >98% purity. To generate CD19 NIH3T3 cells, parental NIH3T3 cells (ATCC) were transduced with a retrovirus encoding CD80, CD54 and CD58 (17). The established cell line was further engineered to express CD19GFP by lentiviral transduction. GFP+ cells were purified by FACS sorting and expanded for the use of stimulation of bi-specific T cells. To generate pp65 stimulator cells, U251T cells derived from human glioblastoma cells from an HLA A2 donor (ATCC) were transduced with a lentiviral vector encoding full length pp65 fused to green fluorescent protein (GFP). pp65U251T cells were purified by GFP expression using flow cytometry. Banks of all cell lines were authenticated for the desired antigen/marker expression by flow cytometry prior to cryopreservation, and thawed cells were cultured for less than 6 months prior to use in assays.
Peptides
The pp65 peptide NLVPMVATV (HLA-A 0201 CMVpp65) at >90% purity was synthesized using automated solid phase peptide synthesis in the TVR (Beckman Research Institute of City of Hope). MP1 GIGFVFTL peptide (HLA-A 0201 influenza) was synthesized at the City of Hope DNA/RNA Peptide Synthesis Facility, (Duarte, CA). pepMix HCMVA (pp65) (pp65pepmix) was purchased from JPT peptide Technologies (GmbH, Berlin Germany). All peptides were used according to the manufacturer’s instructions.
Lentivirus vector construction
The lentivirus CAR construct was modified from the previously described CD19-specific scFvFc:ζ chimeric immunoreceptor (18), to create a third-generation vector. The CD19CAR containing a CD28ζ co-stimulatory domain carries mutations at two sites (L235E; N297Q) within the CH2 region on the IgG4-Fc spacers to ensure enhanced potency and persistence after adoptive transfer (Supplemental Figure 1).The lentiviral vector also expressed a truncated human epidermal growth factor receptor (huEGFRt), which includes a cetuximab (Erbitux™) binding domain but excludes the EGF-ligand binding and cytoplasmic signaling domains. A T2A ribosome skip sequence links the codon-optimized CD19R:CD28: ζ sequence to the huEGFRt sequence, resulting in coordinate expression of both CD19R:CD28: ζ and EGFRt from a single transcript (CD19CARCD28EGFRt) (19). The CD19RCD28EGFRt DNA sequence (optimized by GeneArt) was then cloned into a self-inactivating (SIN) lentiviral vector pHIV7 (gift from Jiing-Kuan Yee, Beckman Research Institute of City of Hope) in which the CMV promoter was replaced by the EF-1α promoter.
Enrichment of CMV-specific T cells after CMVpp65 protein stimulation
PBMCs were isolated by density gradient centrifugation over Ficoll-Paque (Pharmacia Biotech, Piscataway, NJ) from peripheral blood of consented healthy, HLA-A2 CMV-immune donors under a City of Hope Internal Review Board-approved protocol. PBMC were frozen for later use. After overnight rest in RPMI medium containing 5% Human AB serum (Gemini Bio Products) without cytokine, the PBMC were stimulated with current good manufacturing practice (cGMP) grade CMVpp65 protein (Miltenyi Biotec, Germany) at 10ul/10×106 cells for 16 hours in RPMI 1640 (Irvine Scientific, Santa Ana, CA) supplemented with 2 mM L-glutamine (Irvine Scientific), 25 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES, Irvine Scientific), 100 U/mL penicillin, 0.1 mg/mL streptomycin (Irvine Scientific) in the presence of 5U/ml IL-2 and 10% human AB serum. CMV-specific T cells were selected using the IFNγ capture (Miltenyi Biotec, Germany) technique according to the manufacturer’s instructions.
Derivation and expansion of bi-specific T cells
The selected CMV-specific T cells were transduced on day 2 post IFNγ capture with lentiviral vector expressing CD19CARCD28EGFRt at MOI 3. Seven to ten days after lenti-transduction, the bi-specific T cells were expanded by stimulation through CAR-mediated activation signals using 8000 cGy-irradiated CD19-expressing NIH 3T3 cells at a 10:1 ratio (T cells:CD19 NIH3T3) once a week as described (17) in the presence of IL-2 50U/ml and IL-15 1ng/ml. After 2 rounds of expansion, the growth and functionality of the bi-specific T cells was evaluated in vitro and in vivo.
Intracellular cytokine staining
Bi-specific T cells (105) were activated overnight with 105 LCL-OKT3, LCL, or KG1a cells in 96-well tissue culture plates, and with 105 U251T and engineered pp65-expressing U251T cells (pp65U251T) in 24-well tissue culture plates in the presence of Brefeldin A (BD Biosciences). The cell mixture was then stained using anti-CD8, cetuximab and streptavidin, and pp65Tetramer to analyze surface co-expression of CD8, CAR and CMV-specific TCR, respectively. Cells were then fixed and permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences). After fixation, the T cells were stained with an anti-IFNγ.
CFSE Proliferation assays
Bi-specific T cells were labeled with 0.5µM CFSE and co-cultured with stimulator cells LCL-OKT3, LCLs, and pp65 U251T for 8 days. Co-cultures with U251T and KG1a cells were used as negative controls. Proliferation of CD3- and CAR-positive populations was determined using multicolor flow cytometry.
Cytokine production assays
T cells (105) were co-cultured overnight in 96-well tissue culture plates with 105 LCL-OKT3, LCL, or KG1a cells and in 24-well tissue culture plates with 105 U251T and engineered pp65-expressing U251T cells. Supernatants were then analyzed by cytometric bead array using the Bio-Plex Human Cytokine 17-Plex Panel (Bio-Rad Laboratories) according to the manufacturer's instructions.
Cytotoxicity assays
4-hour chromium-release assays (CRA) were performed as previously described (20) using effector cells that had been harvested directly after 2 rounds of CD19 Ag stimulations.
Xenograft models
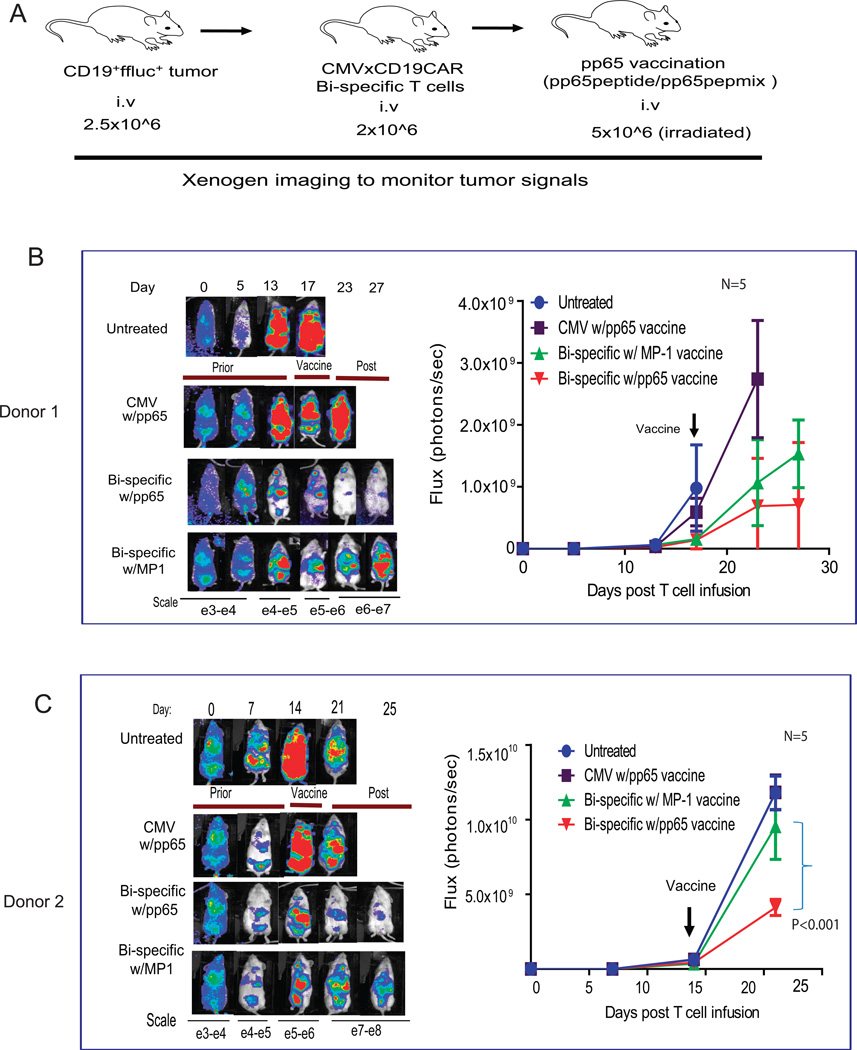
All mouse experiments were approved by the City of Hope Institutional Animal Care and Use Committee. Six- to ten-week old NOD/Scid IL-2RγC null (NSG) mice were injected intravenously (i.v.) on day 0 with 2.5×106 fflucGFPLCLs cells. Three days after tumor inoculation, recipient mice were injected i.v. with 2×106 bi-specific T cells that had undergone 2 rounds of CD19 stimulation. To generate antigen-presenting T cells (T-APC) for vaccine, REM-expanded T cells from the autologous donor were pulsed (2h at 37°C in CM) with 10µg/mL of either HLA-A2 restricted pp65 peptide (NLVPMVATV), 1ug/mL pp65 pepmix depending on whether bi-specific T cell products are pp65 tetramer dominant (GIGFVFTL, donor 2) or not (pp65 pepmix donor 1) or 10µg/mL HLA-A2 restricted control peptide specific for MP1 (GIGFVFTL). Following one wash with phosphate buffered saline (PBS), 5×106 T-APC that had been irradiated with 3700 cGy were injected i.v into the T-cell-treated mice. Tumor burden was monitored with Xenogen® imaging twice a week. Human T cell engraftment in peripheral blood, bone marrow and spleen was determined by flow cytometry.
Results
Enrichment of CMV-specific T cells from PBMC of healthy donors after stimulation with cGMP grade CMVpp65 protein
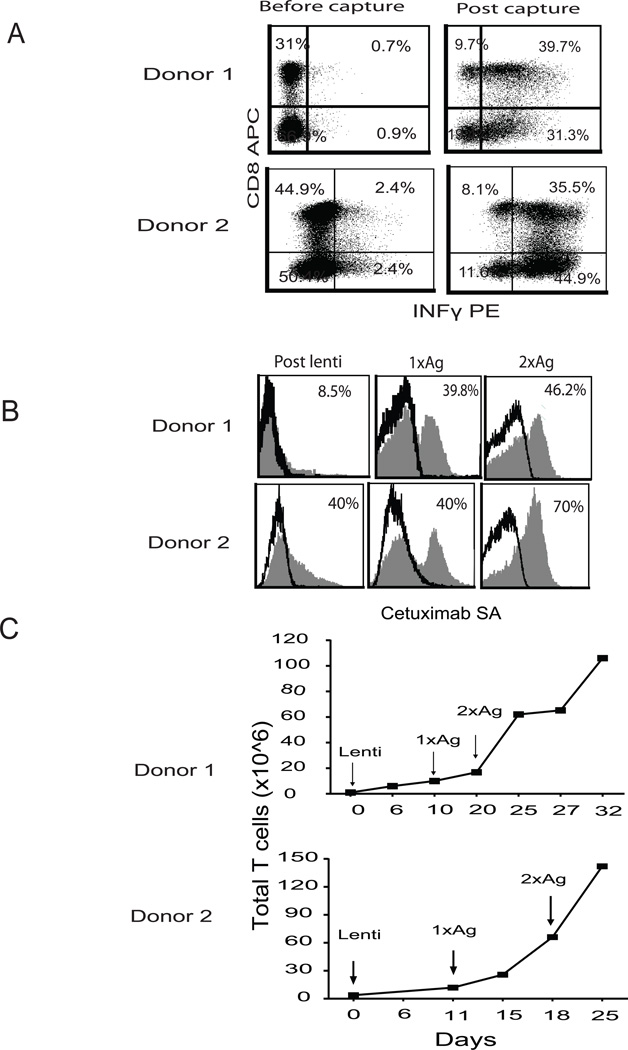
To demonstrate the consistency of this clinically feasible process, the selection was repeated five times using PBMC from three different donors. IFNγ-positive T cells were consistently enriched from a baseline mean of 3.8% (range 1.8–5.6) to a post-capture mean of 71.8% (range 61–81) and contained polyclonal CD8+(34%) and CD4+ T cells (37%) after selection (Supplemental Table 1/Figure 1A). Moreover, the selected CMV-specific T cells included both CD4 and CD8 subsets and represented the entire spectrum of CMV-specificity, showing responsiveness to CMVpp65 pepmix stimulation with broad recognition.
Genetic modification of enriched CMV-specific T cells to express CD19 CAR and in vitro expansion of the bi-specific T cells
In the clinically adaptable procedure, IFNγ-captured CMV-specific T cells were transduced 2 days after the selection, without OKT3 activation, using the second generation CD19RCD28EGFRt lentiviral construct containing the IgG4 Fc hinge region mutations (L235E; N297Q) that we have determined to improve potency due to distortion of the FcR binding domain (21, 22). Starting seven days post lenti-transduction, the cells were stimulated on a weekly basis with 8000 cGy-irradiated, CD19-expressing NIH3T3 cells at a 1:10 ratio (T cells: CD19NIH 3T3). The percentage of CAR+ cells detected by cetuximab increased from 8% post transduction to 46% after 2 rounds of stimulation with a 120-150-fold total cell increase (Figure 1B&C).
Figure 1. Development of clinically feasible platform for derivation of bi-specific T cells.
(A) CMV-specific T cells from CMV immune HLA A2 donors were selected using IFNγ capture after overnight stimulation with cGMP grade CMVpp65 protein. After selection, the cells were stained with antibodies specific to IFNγ, CD4, and CD8. The frequency of each population is presented after exclusion of dead cells with DAPI. (B) The selected cells were transduced with the second generation CD19CAR with a double mutation in the spacer, 24 hours after the IFNγ capture. 7–10 days later, the transduced cells were stimulated with irradiated CD19 expressing NIH3T3 cells at 10:1 ratio (3T3: T cells) and the stimulation was repeated 7 days post the first stimulation. CAR expression was defined by cetuximab-biotin and streptavidin (SA) APC-Cy7 staining. Percentages of CAR+ cells are indicated in each histogram (filled gray), and based on subtraction of that stained with SA- APC-Cy7 alone (black line). (C) Growth of total cell number was determined by Guava Viacount at different time points.
Bi-specific T cells exhibited specific effector function after stimulation through pre-defined viral TCR and CD19CAR
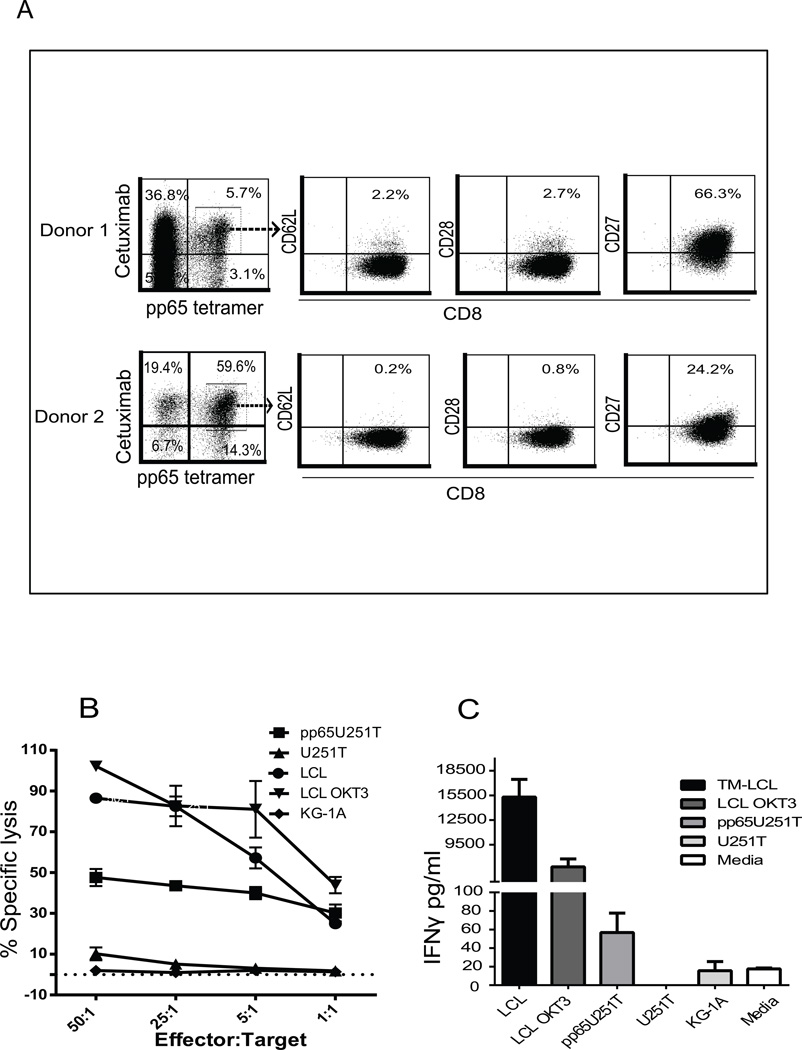
Recapitulating our previous studies (23), the ex vivo expanded CMV-specific T cells possessed an effector phenotype and no longer expressed the central memory markers of the originally selected cells, such as CD62L, CD28, and IL-7Ra (Figure 2A and Supplemental Figure 2). However, levels of CD27 remained high, suggesting a greater proliferative potential that has been associated with greater clinical efficacy (24). To investigate bi-specific T cell effector function via signaling by both the endogenous CMV-specific TCR and the introduced CD19CAR, we evaluated response to engineered pp65-expressing U251T cells from HLA-A2 donors, and also allogeneic CD19+LCLs, based on cytotoxicity, cytokine production and proliferation profiles. As expected, the expanded bi-specific T cells specifically lysed CD19+LCLs with the same maximum killing levels as the OKT3-expressing LCL used as positive controls. Likewise, specific killing was also observed when pp65U251T cells were used as targets as compared to parental U251T cells (Figure 2B). Accordingly, after overnight stimulation, elevated IFNγ secretion was observed after either CD19 or pp65 antigen stimulation as compared to antigen-negative stimulators such as KG1a and U251T parental cells (Figure 2C).
Figure 2. Bi-specific T cells exhibit specific effector function after engagement with CD19+ and CMVpp65+ tumors.
(A) 7 days after the second CD19 Ag stimulation, T cells were stained with HLA A2 restricted pp65 tetramer, cetuximab-biotin, anti-CD8 and antibodies specific to central memory T cell surface markers. Percent positive cells are indicated after dead cell exclusion with DAPI, gating based on pp65 tetramer and cetuximab double-positivity, and isotype-matched stained samples. (B) Four-hour 51Cr release assays were performed using the bi-specific T cells and indicated 51Cr-labeled target cells at different effector : target (E:T) ratios. OKT3-expressing LCLs were used as positive controls, KG1A and U251T as negative controls. CD19+ LCL and engineered pp65U251T cells were used as target for CD19 and CMV-specific T cells, respectively. Data from a representative donor is presented. (C) Bi-specific T cells (105) were activated overnight with 105 LCL-OKT3, LCL, or KG1a in 96-well tissue culture plates and 105 U251T and engineered pp65 expressing U251T cells (pp65U251T) in 24-well tissue culture plates. Supernatants were collected after overnight co-incubation of bi-specific T cells and stimulators. Cytokine levels with indicated stimulators (means ±SEM of triplicate wells) were determined using cytometric bead array.
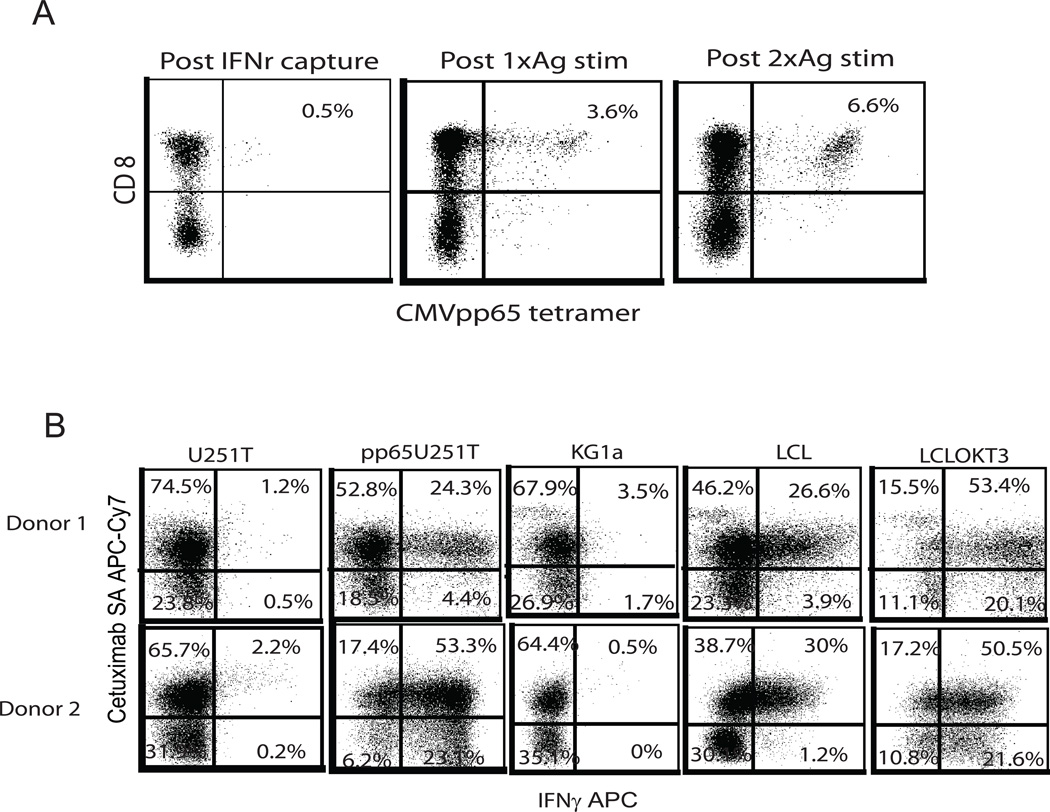
Although CMV-specific T cells were enriched prior to lentiviral transduction, the T cell population is mixed, including CMV-specific T cells, CD19CAR+ T cells, bi-specific T cells, and possibly a small percentage of T cells that are neither CMV-specific nor CD19CAR+. T cell expansion following lentiviral transduction is predominantly CD19-driven through CAR stimulation, so we next investigated how CAR stimulation affects the composition of CMV-specific T cells. Using pp65 tetramer as an indicator of the CMV-specific population, we found that the percentage of CMVpp65 tetramer-positive cells increased from 0.5% to 6.6% by the end of the second CD19 stimulation, indicating bi-specific T cells proliferated strongly with CD19 stimulation (Figure 3A).
Figure 3. Bi-specific T cells exhibit bi-effector function after stimulation through TCR and CAR.
(A) pp65 tetramer analysis of expanded bi-specific T cells was performed before and after each CD19 Ag stimulation by flow cytometry. Percentages of pp65 tetramer and CD8 double-positive cells are indicated based on negative tetramer and isotype gating. (B) Bi-specific T cells (105) were activated overnight with 105 of LCL-OKT3, LCL, KG1a in 96-well tissue culture plates and 105 U251T and engineered pp65 expressing U251T cells (pp65U251T) in 24-well tissue culture plates. Co-cultures were fixed and permeabilized using the BD Cytofix/Cytoperm kit according to manufacturer’s instructions. After fixation and permeabilization, the T cells were stained with anti-IFNγ. Before fixation, anti-cetuximab-biotin and anti-CD3 staining was used to analyze surface expression of CAR and T cells. Percentages of positive cells on gated CD3 T cells are presented based on that stained with isotype antibodies.
To further investigate that these effector functions were attributable to bi-specific T cells rather than distinct CD19CAR+ and CMV-specific T cell subsets in the population, we performed intracellular cytokine (ICC) assays. In response to pp65 antigen stimulation, 24 – 53 % of the T cells in the population were CAR+ and able to secret IFNγ (Figure 3B). ~30% of T cells exhibited IFNγ secretion upon stimulation with CD19+ LCL cells.
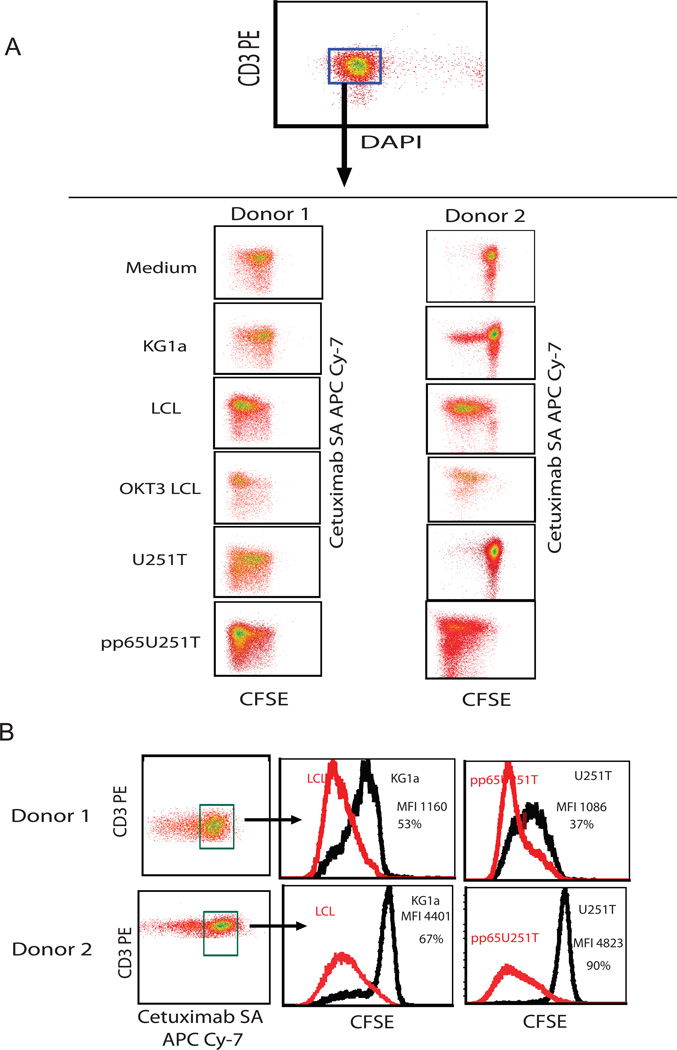
To assess the ability of bi-specific T cells to proliferate in response to CD19 or pp65 antigen stimulation, T cells were labeled with CFSE and co-cultured with different stimulators, and then evaluated for CFSE dilution 8 days later. Unlike the cultures stimulated with CD19-negative KG1a and U251T cells, cell division was more robust after stimulation through either the CD19 CAR+ (LCL cells) or the CMV-specific TCR (pp65U251T cells) (Figure 4). Building on these findings, we next performed in vivo experiments to examine the effects of CMV peptide vaccine on the expansion and anti-lymphoma efficacy of adoptively transferred bi-specific T cells.
Figure 4. Bi-specific T cells proliferate after re-stimulation through TCR and CAR.
Bi-specific T cells isolated by IFNγ capture and stimulated with two cycles of CD19 Ag were labeled with CFSE and co-cultured with indicated stimulators for 8 days. (A) CFSE retention on gated live T cells is shown. (B) Quantification of CFSE retention of CAR+ T cells. Subtractions of percentages and mean fluorescence intensity (MFI) of CFSE expression of negative control KG1a to LCL and U251T to pp65U251T are depicted.
Anti-lymphoma activity of adoptively transferred bi-specific T cells was augmented in vivo by vaccination with CMVpp65 peptide antigen
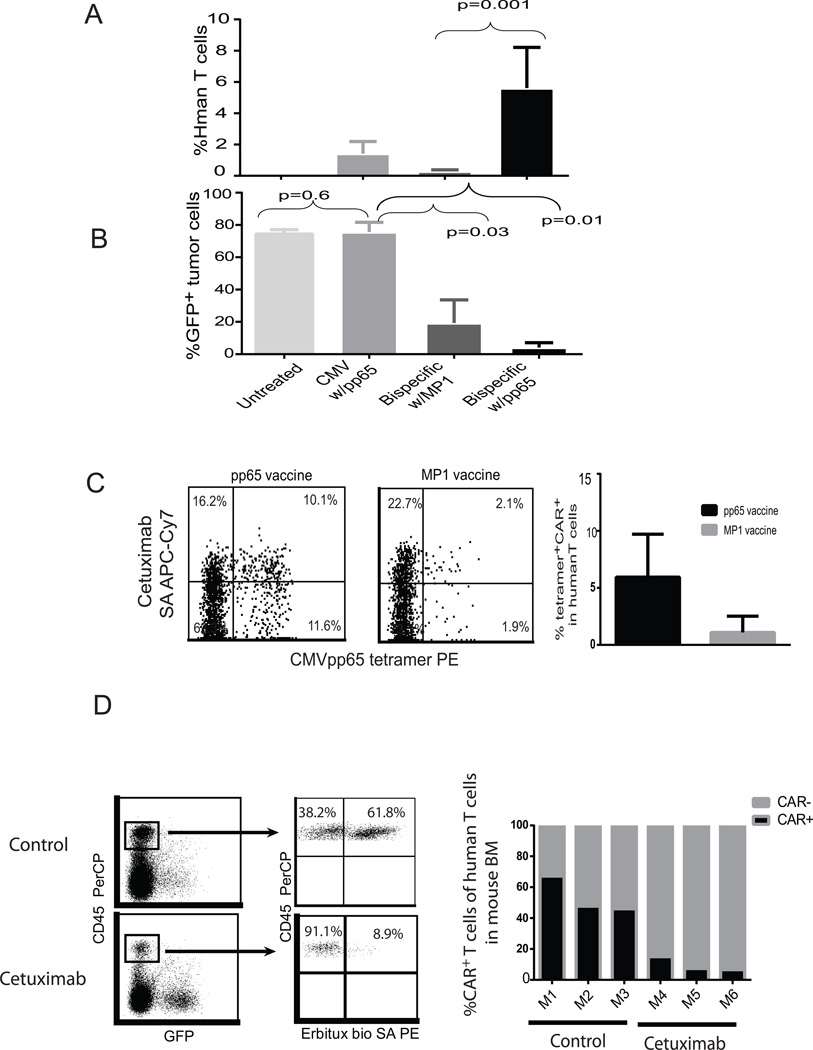
Our preliminary studies have demonstrated that engineered CD19CAR T cells can target and lyse CD19 positive lymphoma in vivo. However, the antitumor efficacy is suboptimal and tumor reduction represents a transient event followed by eventual tumor progression (data not shown) unless high doses of CAR T cells were infused (21). In this study, we wanted to tease out the differences between the targeted and control vaccines. Therefore we chose a suboptimal T cell dose (1×106 CAR T cells), which is 10 times lower than the curative dose we used previously (10×106) (21). We attempted to augment antitumor efficacy using a CMV peptide vaccine boost (Figure 5A). As expected, as few as 2×106 bi-specific T cells were able to induce a specific tumor reduction as compared to untreated and CMV-mono-specific T cell treated mice. We observed augmented anti-tumor activity after vaccination with pp65-peptide-pulsed T-APC of two different formulations [pp65pepmix (Figure 5B) and HLA A2-restricted CMVpp65 peptide (Figure 5C)], but not in mice that were vaccinated using T-APC loaded with the irrelevant peptide MP1 (HLA A2 restricted). Interestingly, mice that received bi-specific T cell treatment had to be euthanized around the same time as control mice even though the tumor signals were dramatically lower (Figure 5). Our further studies indicated that there were highly elevated levels of human specific IFNγ and IL-6 in the mouse serum (Supplemental Figure S3) and it is probable that the mice died of cytokine release syndrome (25) rather than tumor. More interestingly, augmented anti-tumor efficacy induced by pp65 vaccine was supported in a relapsed tumor model (Supplemental Figure S4). To further demonstrate that the enhanced anti-lymphoma activity is attributable to expansion of bi-specific T cells in response to CMVpp65 stimulation, human T cells and CAR+Tetramer+ T cells harvested from mice were analyzed 10–14 days after vaccination. As expected, human T cells in the mice treated with bi-specific T cells were significantly higher in the pp65-challenged mice (5.6 ± 2.6%) than in MP1 controls (0.3 ± 0.1%)(Figure 6A). The levels of human T cells and bi-specific T cells were well correlated with the tumor reduction based on GFP expression by FACS analysis (Figure 6B). Further, CAR+ and CMVpp65 tetramer+ bi-specific T cells harvested from mice were more abundant in the pp65 peptide-challenged mice than in MP1 controls (Figure 6C). However, pp65 Tetramer/CAR+ double positive cells were only detected in the spleen, possibly indicating a unique homing characteristic of the population of bi-specific T cells. In addition to the pre-defined viral TCR that can be used to boost antitumor activity in vivo through peptide vaccine, functional bi-specific T cells are also expected to proliferate upon exposure to CD19 antigen in vivo. This was supported by the finding that there were lower levels of engraftment of CMV-specific T cells as compared to bi-specific T cells in tumor-bearing mice, even though the same pp65 peptide vaccine was used to stimulate both types of T cells (Figure 6A). These data suggested that bi-specific T cells were able to proliferate and expand in vivo in response to stimulation of the TCR as well as the CD19 CAR.
Figure 5. Anti-tumor activity of adoptively transferred bi-specific T cells is enhanced by CMVpp65 vaccination.
(A) NSG mice were injected i.v. on day 0 with 2.5×106 GFPffluc+ LCL cells. Three days after tumor inoculation, recipient mice were injected i.v. with 2×106 bi-specific cells that underwent 2 rounds of CD19 stimulation. Vaccine was given by i.v. injection of peptide pulsed autologous T cells. Fourteen to seventeen days post T cell infusion, 5×106 pp65pepmix (B) or pp65 peptide (C) (or MP1) loaded autologous T cells were irradiated and injected (iv) into T-cell-engrafted mice as vaccine. pp65 vaccine was also supplemented to the mice that were treated with 10×10^6 CMV-specific T cells from the same donor and untreated mice were used as another type of control. Tumor growth was evaluated by Xenogen® imaging. N=5 for each group in the experiments. The Mann Whitney test was used for statistical analysis.
Figure 6. Adoptively transferred bi-specific T cells can be expanded via CMVpp65 vaccine and ablated by cetuximab.
2×106 CMV-specific or bi-specific T cells from the same donor were adoptively transferred into CD19 tumor-bearing NSG mice. 2 weeks post T cell infusion, mice received either pp65 vaccine or MP1 vaccine. (A) Percentages of human T cells pooled from blood, bone marrow and spleen from multiple mice (N=4) and (B) GFP+ tumor cells in the mouse spleen were determined by flow cytometry. The Mann Whitney test was used for statistical analysis. (C) CMVpp65 tetramer and CAR double positive cells in the spleen of mice were analyzed by flow cytometry after labeling with antibodies specific to human CD45, pp65 tetramer and EGFR, 28 days post bi-specific T cell infusion. The percentages of CMVpp65 tetramer+ CAR+ T cells in the human T cell population of a representative mouse are presented. (D) 1×106 bi-specific T cells were adoptively transferred into CD19 tumor-bearing NSG mice. 2 weeks post T cell engraftment, mice received cetuximab (Erbitux™) 1mg/day i.p. injection for 4 days. One day after the last injection, CD45+GFP− human T cells and CD45+CAR+ T cells in the bone marrow were analyzed by flow cytometry after staining with antibodies specific to human CD45 and cetuximab-biotin. Representative FACS data from cetuximab-treated and untreated mice are depicted on the left and percentages of CAR+ T cells in the mouse bone marrow from multiple mice are presented on the right.
Adoptively transferred bi-specific T cells are efficiently ablated by cetuximab-mediated antibody dependent cell mediated cytotoxicity (ADCC) in vivo
The impressive clinical efficacy of CAR T cell therapy and the frequently associated on/off-target toxicities such as cytokine release syndrome (CRS), have highlighted the need for T cell ablation strategies (1, 3, 4, 26). Taking advantage of the properties of the EGFRt receptor translated from the same transcript as the CD19CAR, we tested the anti-EGFR monoclonal antibody cetuximab for its ability to ablate CAR+ T cells. Fourteen days after engrafting mice with bi-specific T cells, cetuximab was administered intraperitoneally at 1mg/day for 4 consecutive days. CAR+ cells in the bone marrow were significantly decreased as compared to untreated mice. 50–60% of human T cells are CAR+ in the bone marrow of untreated controls, however, less than 10% of the human T cells in cetuximab treated mice are CAR+ (Figure 6D), suggesting successful ablation (68% CAR T cell elimination) based on antibody binding to the EGFRt.
Discussion
Adoptively transferred CMV-specific T cells can control latent CMV infection during HCT, are efficiently expanded in response to vaccine, and are able to control viral reactivation in immunocompromised patients (5–7). CAR-engineered adoptive T cell therapy is a developing area in cancer immunotherapy, with the potential for augmentation of antitumor efficacy by manipulating the processes prior to (proximal) and post (distal) T cell infusion. One strategy to improve the persistence of in vitro expanded effector cells is to isolate defined T cell subtypes with an intrinsic capacity to persist during in vitro manipulation. Our studies have shown that central-memory-derived viral- or tumor-specific T cells exhibit superior persistence upon adoptive transfer compared to effector-memory-derived T cells(23, 27). We now seek approaches to magnify the potency of tumor-specific T cells after adoptive transfer.
Initially the major obstacle to the clinical application of CMV-specific T cells was the lengthy process required for their generation and selection (28, 29). In this study, our use of IFNγ capture of CMV-specific T cells consistently and efficiently enriched CMV-specific T cells while preserving the broad spectrum of CMV repertoires. Moreover, these cells remained amenable to gene modification after a brief CMVpp65 stimulation, avoiding the need for CD3/CD28 bead activation prior to transduction, which can cause activation-induced cell death (AICD) of CMV-specific T cells (30). Engineering the bulk IFNγ-captured T cells with the CD19CAR lentivirus followed by stimulation with CD19 antigen resulted in 50 to 70% of the CAR+ T cells responding to pp65 stimulation, representing the subset of functional bi-specific T cells. The bi-specific T cells exhibited specific cytolytic activity and secreted IFNγ, as well as proliferating vigorously after engagement of endogenous CMVpp65 T cell receptors or engineered CD19 CARs. Upon transfer into tumor bearing mice, the bi-specific T cells mediated cytokine released syndrome (CRS), which has been found to correlate with anti-tumor efficacy in the clinic (2, 31). We have demonstrated the IFNγ-capture platform for derivation of bi-specific T cells to be clinically feasible, and able to generate therapeutic doses of functional bi-specific T cells within 3–4 weeks, ensuring timely production for clinical application.
Efficient in vivo activation of virus-specific T cells through the TCR demands that viral antigens are processed and presented in a human leukocyte antigen (HLA)-dependent manner. In mouse models, we generated APC by loading autologous T cells with either pp65 peptide or a full-length pp65pepmix. The effects of vaccination were indistinguishable whether using pp65 peptide or pp65 pepmix. Both approaches elicited bi-specific T cell responses and induced enhanced antitumor activity compared with irrelevant MP1 challenge. We anticipate that the response of bi-specific T cells to vaccine will be even more efficient in immunocompetent patients, where more professional APC are present than in these immunocompromised mouse studies. To further study the extent to which bi-specific T cells eradicate tumors in NSG mice, we performed a population analysis of tumor cells remaining in the peripheral blood, bone marrow, and spleen using flow cytometry. A few tumor cells remained after mice were treated with bi-specific T cells and pp65 vaccine; in contrast, many more tumor cells were detected in the mice receiving only un-engineered CMV-specific T cells – the same percentage as was seen in untreated controls, and in the mice that received bi-specific T cells without pp65 vaccine (Figure 6B). Consistently, expansion of bi-specific T cells was much lower in mice that received an irrelevant MP1vaccine compared to those that received pp65 vaccine (2% vs 10%, Figure 6C), further demonstrating the specificity of the response to vaccination in bi-specific T cell-treated animals. Meanwhile, we noticed that the percentage of pp65+/CAR+ double-positive human cells harvested from mice were much decreased compared to the input human T cell population. We speculate that the tetramer-negative population has disproportionately expanded in vivo compared to tetramer-positive cells, since this subset includes cells expressing mouse xeno-reactive native T cell receptors. It is also possible that another contribution to the decline in the proportion of pp65+/CAR+ cells from the input population could be a result of these double-positive cells undergoing activation-induced cell death (AICD) after killing tumor cells, due to their effector T cell characteristics (Figure 2A). AICD could be thought of as a deleterious effect of the vaccine on pp65+/CAR+, but could actually be a measure of effectiveness as demonstrated by decreased tumor burden (Figure 6B). Ongoing studies on the functional responses to CMV vaccine of the different T cell subsets of the infused product will further reveal the mechanisms of the enhanced antitumor activity.
Pre-clinical studies with engineered CAR T cells in different xenotransplant tumor models have demonstrated variable potency with some showing tumor eradication in the short window tested and some reporting eventual tumor relapse (17, 22, 32, 33). Several variables of these artificial systems, such as the aggressiveness of the tumor cell line, tumor burden at the time of CAR T cell infusion, dose of CAR T cells may account for perceived differences in CAR potency, making it difficult to compare between xenograft models. Optimal growth signals are required for efficient and sustained expansion of transfused effector T cells in vivo. These signals encompass T-helper cell interactions, native TCR/CD3 complex signaling, and the activation of costimulatory signals. Although the CAR is designed to mimic the TCR and transmit activation signaling, the lack of in vivo persistence of some CAR T cells has been attributed to incomplete stimulation after engagement of the CAR (8, 10). This study suggests that the interaction of CAR T cells with tumor cells is inadequate to completely eradicate the transplanted tumor. This could be a result of insufficient growth signal transmission through the CAR for T cell expansion and activation, or insufficient cytolytic activatation of T cells to kill tumor targets. T cell activation through viral TCRs has several advantages over self antigen TCR in promoting robust T cell expansion. Signaling through a viral TCR is generally far more robust than through a self-antigen specific TCR, since the viral-specific TCR affinity to antigen has not been dampened by the effects of tolerance and negative selection (34). A recent study is emblematic of the contrast in T cell activation caused by stimulation through a self antigen such as p53 and the immune response to antigens expressed from a viral vector (35). Since the viral TCR is expressed from the same cell as the CAR, the robust T cell activation caused by an antiviral TCR could lead to enhanced antitumor activity as a consequence of the expansion of CMV-specific CAR T cells.
Efficiently controlling proliferation to avoid cytokine storm and off-target toxicity is an important hurdle for the success of T cell immunotherapy. The EGFRt incorporated in the CD19CAR lentiviral vector will serve not only as a marker for detection and selection of CAR T cells, but may also act as suicide gene to ablate the CAR+ T cells in cases of treatment-related toxicity. In this study, bi-specific T cell engrafted mice were treated with cetuximab daily for 4 days. Consequently, more than 68% of the persistent CAR+ T cells were ablated in NSG mice as a result of ADCC, CDC and direct killing by cetuximab (36), despite the lack of professional ADCC effectors such as NK and B cells in the NSG mouse model. More efficient ablation is expected in humans, in the presence of a full panel of effector cells.
This study has demonstrated that the antitumor activity of bi-specific CMV/CD19 T cells can be enhanced as a consequence of proliferation following CMV peptide vaccination. We hypothesize that the cell dose of bi-specific T cells could be significantly decreased as compared to conventional CD19CAR T cells, due to their potential to proliferate in vivo in response to vaccine, avoiding prolonged culture times and the risk of terminal differentiation. Potential on/off-target toxicity can potentially be controlled by ablation of infused CAR T cells using cetuximab. These results illustrate the clinical applications of CMV vaccine to augment the antitumor activity of adoptively transferred CD19CAR T cells in several scenarios: 1) to salvage patients not achieving complete remission or relapsing after CAR T cell therapy, 2) vaccine boost when CD19 CAR T cells are failing to persist regardless of tumor responses at that time, 3) planned vaccination on days 28 and 58 post-CD19 CAR T cells, which has been shown an effective immune-stimulation in our CMV peptide vaccine. There is also potential benefit of using the bi-specific T cells pre-emptively post-allogeneic HCT, both to eliminate minimal residual disease (MRD) and control CMV, potentially preventing reactivation of virus or undergoing expansion in response to latent CMV re-activation. City of Hope is currently accruing patients on clinical trials of two CMV vaccines (NCT01588015 and NCT01941056) (15), enabling us to advance this combined therapy to the clinic rapidly, since we already hold INDs for both the CD19CAR T cell vector and the CMV vaccines.
Moreover, this CMV vaccine strategy has the potential to profoundly impact the general field of adoptive T cell therapy, since by transducing a variety of tumor-directed CARs into our CMV-specific T cells, we have the potential to tailor this strategy to a wide range of malignancies and tumor targets.
Supplementary Material
Translational Reference.
Adoptive transfer of chimeric antigen receptor (CAR)-redirected CD19-specific T cells can induce durable regression in patients with leukemia and lymphoma. However, in some disease settings CAR therapy confers only modest clinical benefit due to attenuated persistence of CAR T cells. We have improved the existing CAR technology by engrafting CD19CAR into CMV-specific T cells and investigating the effects of re-stimulating CAR+ T cells through an endogenous cytomegalovirus (CMV)-specific T cell receptor. This study has demonstrated that the antitumor activity of CMV/CD19 bi-specific T cells can be enhanced as a consequence of proliferation following CMV peptide vaccination. These results illustrate the clinical applications of CMV vaccine to augment the antitumor activity of adoptively transferred CD19CAR T cells in patients with B cell malignancies. This CMV vaccine strategy has the potential to profoundly impact the general field of T cell therapy by substituting a CAR specific to any individual tumor.
Acknowledgments
Grant Support
This work was supported by NIH grants P50 CA107399, P30 CA033572, P01 CA030206, 2R01 CA077544, 1R01 CA181045, the Lymphoma Research Foundation, the Marcus Foundation, the Skirball Foundation, the Tim Lindenfelser Lymphoma Research Fund, and the Tim Nesvig Lymphoma Research Foundation.
Footnotes
Disclosure of Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Authors’ Contributions
Conception and design: S.J. Forman, X. Wang
Development of methodology: S.J. Forman, X. Wang, C.W. Wong, R. Urak
Acquisition of data: X. Wang, C.W. Wong, R. Urak, W. Chang,
Analysis and interpretation of data: S.J. Forman, X. Wang, C.W. Wong, R. Urak
Writing, review, and/or revision of the manuscript: S.J. Forman, X. Wang, A. Mardiros, L.
E. Budde, S.H. Thomas, C.E. Brown, C. La Rosa, D.J. Diamond, M.C. Jensen,
R. Nakamura, J.A. Zaia
References
- 1.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 6.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollard CM, Kuehnle I, Leen A, Rooney CM, Heslop HE. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant. 2004;10:143–155. doi: 10.1016/j.bbmt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 11.Cooper LJ, Al-Kadhimi Z, Serrano LM, Pfeiffer T, Olivares S, Castro A, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005;105:1622–1631. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 12.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Bij W, Speich R. Management of cytomegalovirus infection and disease after solid-organ transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;33(Suppl 1):S32–S37. doi: 10.1086/320902. [DOI] [PubMed] [Google Scholar]
- 14.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? Journal of internal medicine. 2006;259:219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 15.La Rosa C, Longmate J, Lacey SF, Kaltcheva T, Sharan R, Marsano D, et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. The Journal of infectious diseases. 2012;205:1294–1304. doi: 10.1093/infdis/jis107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelloquin F, Lamelin JP, Lenoir GM. Human B lymphocytes immortalization by Epstein-Barr virus in the presence of cyclosporin A. In Vitro Cell Dev Biol. 1986;22:689–694. doi: 10.1007/BF02621085. [DOI] [PubMed] [Google Scholar]
- 17.Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One. 2013;8:e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stastny MJ, Brown CE, Ruel C, Jensen MC. Medulloblastomas expressing IL13Ralpha2 are targets for IL13-zetakine+ cytolytic T cells. J Pediatr Hematol Oncol. 2007;29:669–677. doi: 10.1097/MPH.0b013e3181468c68. [DOI] [PubMed] [Google Scholar]
- 21.Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, et al. Chimeric Antigen Receptors with Mutated IgG4 Fc Spacer Avoid Fc Receptor Binding and Improve T cell Persistence and Anti-Tumor Efficacy. Mol Ther. 2014 doi: 10.1038/mt.2014.208. 10.1038/mt.2014.208. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer immunology research. 2014 doi: 10.1158/2326-6066.CIR-14-0127. 10.1158/2326-6066.cir-14-0127. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchi LF, Sesti-Costa R, Ignacchiti MD, Chedraoui-Silva S, Mantovani B. In vitro activation of mouse neutrophils by recombinant human interferon-gamma: increased phagocytosis and release of reactive oxygen species and pro-inflammatory cytokines. International immunopharmacology. 2014;18:228–235. doi: 10.1016/j.intimp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8 T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Burton RL, Dai LJ, Britt WJ, Lucas KG. B lymphoblastoid cell lines as efficient APC to elicit CD8+ T cell responses against a cytomegalovirus antigen. J Immunol. 2000;165:4105–4111. doi: 10.4049/jimmunol.165.7.4105. [DOI] [PubMed] [Google Scholar]
- 29.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 30.Kalamasz D, Long SA, Taniguchi R, Buckner JH, Berenson RJ, Bonyhadi M. Optimization of human T-cell expansion ex vivo using magnetic beads conjugated with anti-CD3 and Anti-CD28 antibodies. J Immunother. 2004;27:405–418. doi: 10.1097/00002371-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, et al. Determinants of successful CD8+ T cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aleksic M, Liddy N, Molloy PE, Pumphrey N, Vuidepot A, Chang KM, et al. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur J Immunol. 2012;42:3174–3179. doi: 10.1002/eji.201242606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardwick NR, Carroll M, Kaltcheva T, Qian D, Lim D, Leong L, et al. p53MVA therapy in patients with refractory gastrointestinal malignancies elevates p53-specific CD8+ T-cell responses. Clin Cancer Res. 2014;20:4459–4470. doi: 10.1158/1078-0432.CCR-13-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.