Abstract
Purpose
Fludarabine monophosphate (fludarabine) is an integral component of many reduced-intensity conditioning regimens for hematopoietic cell transplantation (HCT). Fludarabine’s metabolite, 9-β-D-arabinofuranosyl-2-fluoroadenine (F-ara-A), undergoes cellular uptake and activation to form the active cytotoxic metabolite fludarabine triphosphate (F-ara-ATP), which inhibits cellular DNA synthesis in CD4+ and CD8+ cells. In this study, we evaluated whether fludarabine-based pharmacologic biomarkers were associated with clinical outcomes in HCT recipients.
Methods
Participants with hematologic diseases were conditioned with fludarabine and low-dose total body irradiation (TBI) followed by allogeneic HCT and post-grafting immunosuppression. After fludarabine administration, we evaluated pharmacological biomarkers for fludarabine – F-ara-A area under the curve (AUC) and the ratio of circulating CD4+ and CD8+ cells (CD4+/CD8+ ratio) after fludarabine administration – in 102 patients; F-ara-ATP accumulation rate in enriched CD4+ and CD8+ cells was evaluated in 34 and 36 patients, respectively.
Results
Interpatient variability in the pharmacological biomarkers was high, ranging from 3.7-fold (F-ara-A AUC) to 39-fold (F-ara-ATP in CD8+ cells). Circulating CD8+ cells were more sensitive to fludarabine administration. A population pharmacokinetic-based sampling schedule successfully allowed for estimation of F-ara-A AUC in this outpatient population. There was poor correlation between the F-ara-AUC and the F-ara-ATP accumulation rate in CD4+ (R2=0.01) and CD8+ cells (R2=0.00). No associations were seen between the four biomarkers and clinical outcomes (day +28 donor T-cell chimerism, acute graft-versus-host disease (GVHD), neutrophil nadirs, cytomegalovirus reactivation, chronic GVHD, relapse, non-relapse mortality, or overall mortality).
Conclusions
Considerable interpatient variability exists in pharmacokinetic and fludarabine-based biomarkers, but these biomarkers are not associated with clinical outcomes in fludarabine/TBI-conditioned patients.
Keywords: Fludarabine, biomarkers, nucleoside analogs, pharmacokinetics, pharmacodynamics, hematopoietic cell transplantation
INTRODUCTION
Fludarabine monophosphate (fludarabine) is an essential component of many reduced-intensity hematopoietic cell transplantation (HCT) conditioning regimens. [1] One of the least immunosuppressive conditioning regimens is the nonmyeloablative regimen of 90 mg/m2 fludarabine plus 2 to 4.5 Gy total body irradiation (TBI) developed by the Seattle group. [2,3] Initially, patients were conditioned with TBI only. Engraftment difficulties in TBI-only patients led to the addition of fludarabine to the conditioning regimen and engraftment rates increased. [1,2,4] In recipients of a peripheral blood stem cell graft obtained from a human leukocyte antigen (HLA) matched related donor, a prospective randomized trial revealed that fludarabine/TBI resulted in higher median T-cell and natural killer cell chimerism on day +28, higher progression-free survival, and improved overall survival compared to TBI alone. [3] Thus, fludarabine is important for enhancing the graft-versus-tumor effect by ensuring more rapid and sustained donor engraftment shortly after transplantation. Fludarabine use, however, was also associated with a higher risk of grades 3–4 acute graft-versus-host disease (GVHD), lower CD4+ counts, and a higher rate of bacterial infections. [5–8] Thus, identifying an optimal fludarabine dose, one that maintains high engraftment rates, improves graft-versus-tumor responses, and minimizes the risks of acute GVHD and infections, could be of significant benefit to nonmyeloablative HCT recipients.
After administration, fludarabine is rapidly dephosphorylated by nucleotidases to 9-β-D-arabinofuranosyl-2-fluoroadenine (F-ara-A), [9–11] which is subsequently transported into the cell. In the cell, F-ara-A is sequentially phosphorylated, resulting in the active metabolite fludarabine triphosphate (F-ara-ATP, Supplemental Figure 1).[10] F-ara-ATP inhibits ribonucleotide reductase and DNA polymerase and ultimately leads to cellular apoptosis in both actively dividing and resting cells. [10] Individual patients can have substantially different rates of F-ara-ATP accumulation, which could affect the extent of their T-cell suppression and clinical outcomes. [12] To date, it is not feasible to reliably quantify F-ara-ATP concentrations in lymphocytes isolated from plasma samples obtained from HCT recipients after fludarabine administration. [13] Therefore, we pursued evaluating F-ara-A area under the concentration-time curve (AUC), F-ara-ATP accumulation in CD4+ cells, F-ara-ATP accumulation in CD8+ cells, [12] and the after fludarabine CD4+/CD8+ ratio as potential biomarkers of response to fludarabine/TBI conditioning. Both F-ara-A AUC[14] and F-ara-ATP accumulation rate in CD4+ and CD8+[12] cells are highly variable in HCT recipients, but the clinical relevance of this variability has yet to be examined in fludarabine/TBI conditioned patients. It is essential, however, that any pharmacokinetic/dynamic study of F-ara-A AUC in fludarabine/TBI conditioned patients use a sampling schedule that maximizes compliance for these patients, who undergo HCT in the ambulatory clinic. Therefore, we developed a F-ara-A population pharmacokinetic model and used it to identify a limited sampling schedule to maximize participant compliance. [14] Furthermore, we evaluated the novel phenotypic biomarker of ex vivo accumulation rate of the active metabolite F-ara-ATP in two separate cell populations: CD4+ and CD8+ cells. [12] Finally, we also evaluated CD4+ and CD8+ cell counts circulating in peripheral blood before and after fludarabine administration. Fludarabine administration in patients with chronic lymphocytic leukemia leads to a marked, prolonged reduction in circulating CD4+ and CD8+ cells, [15–17] but the immediate effects of its administration upon these cells has yet to be described. In vitro data regarding the effects of fludarabine on the proportion of CD4+ vs. CD8+ cells undergoing apoptosis are contradictory and suggest that CD4+ cells are similarly[18] or less susceptible[19] to fludarabine than CD8+ cells. The immediate effects of fludarabine upon recipients’ circulating CD4+ and CD8+ counts in the peripheral blood may influence the CD4+/CD8+ ratio. The in vitro CD4+/CD8+ ratio shows an inverse relationship with the amount of apoptosis, both with and without radiation, of CD4+ cells but not CD8+ cells. [20] Therefore, we hypothesized that fludarabine-induced changes in the CD4+/CD8+ ratio could influence sensitivity to subsequent TBI in vivo. We measured circulating CD4+ and CD8+ counts in the peripheral blood before and immediately after fludarabine administration and evaluated possible correlations with F-ara-A AUC and intracellular F-ara-ATP.
In this study, we evaluated the association of clinical outcomes with four fludarabine-specific pharmacological biomarkers: F-ara-A area under the curve (AUC) and the ratio of circulating CD4+ and CD8+ cells (CD4+/CD8+ ratio) after fludarabine administration in 102 patients, and F-ara-ATP accumulation rate in enriched CD4+ and CD8+ cells in 34 and 36 patients, respectively. The long-range goal of this work is to determine whether overall survival could be improved by personalized dosing of fludarabine in nonmyeloablative HCT recipients. Interpatient variability in the pharmacological biomarkers was high. However, no associations were seen between the four biomarkers and clinical outcomes (day +28 donor T-cell chimerism, acute graft-versus-host disease (GVHD), neutrophil nadirs, cytomegalovirus reactivation, chronic GVHD, relapse, non-relapse mortality, or overall mortality). Thus, our results suggest a minimal impact of fludarabine-based pharmacological biomarkers (i.e., F-ara-A AUC, F-ara-ATP accumulation rate in CD4+ and CD8+ cells) and T-cell suppression (i.e., CD4+/CD8+ ratio) upon clinical outcomes.
MATERIALS AND METHODS
Participant characteristics
Between May 2008 to February 2012, 102 patients with hematologic diseases participated in this prospective ancillary biomarker study, which was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board (Clinicaltrials.gov identifier NCT00764829). All participants provided written informed consent prior to study procedures. Table 1 summarizes the participant characteristics. The conditioning regimen and postgrafting immunosuppression were not affected by participation in this study.
Table 1.
Participant characteristicsa
| Donor Type | |||
|---|---|---|---|
| Related | Unrelated | All participants | |
| Total number | 24 | 78 | 102 |
| Sex, female/male (% female) | 9/15 (38%) | 28/50 (36%) | 37/65 (36%) |
| Actual body weight (kg) | 80 (50 – 119) | 83 (45 – 142) | 82 (45 – 142) |
| HCT-CI[40] | |||
| 0 | 1 (4%) | 8 (10%) | 9 (9%) |
| 1–2 | 3 (13%) | 17 (22%) | 20 (20%) |
| 3–4 | 11 (46%) | 30 (38%) | 41 (40%) |
| ≥5 | 9 (38%) | 23 (29%) | 32 (32%) |
| Recipients’ ages, years | 59 (20 – 69) | 62 (28 – 75) | 62 (20 – 75) |
| CMV positive recipient or donor | 16 (67%) | 51 (65%) | 67 (66%) |
| Kahl Disease risk[27] | |||
| Low | 4 (17%) | 29 (37%) | 33 (32%) |
| Standard | 16 (67%) | 33 (42%) | 49 (48%) |
| High | 4 (17%) | 16 (21%) | 20 (20%) |
| Diagnosis | |||
| Non-Hodgkin lymphoma | 7 (29%) | 27 (35%) | 34 (33%) |
| Chronic lymphocytic leukemia | 8 (33%) | 14 (18%) | 22 (22%) |
| Acute myelogenous leukemia | 4 (17%) | 11 (14%) | 15 (15%) |
| Myelodysplastic syndrome | 1 (4%) | 9 (12%) | 10 (10%) |
| Multiple myeloma | 1 (4%) | 8 (10%) | 9 (9%) |
| Acute lymphocytic leukemia | 1 (4%) | 3 (4%) | 4 (4%) |
| Myelofibrosis / Myeloproliferative disorder | 0 | 3 (4%) | 3 (3%) |
| Aplastic anemia | 0 | 2 (3%) | 2 (2%) |
| Hodgkin disease | 2 (8%) | 0 | 2 (2%) |
| Paroxysmal nocturnal hemoglobinuria | 0 | 1 (1%) | 1 (1%) |
| Female donor to male recipient | 10 (42%) | 19 (24%) | 29 (28%) |
| Donors’ ages, years | 55 (23 – 73) | 31 (20 – 58) | 35 (20 – 73) |
| HLA-mismatched graft | 1 (4%) | 2 (3%) | 3 (3%) |
| Conditioning regimen | |||
| 2Gy TBI + FLU ± auto | 11 (46%) | 38 (49%) | 49 (48%) |
| 2Gy TBI + FLU + rituximab ± autob | 11 (46%) | 20 (26%) | 31 (30%) |
| 3Gy TBI + FLU ± rituximabb | 2 (8%) | 13 (17%) | 15 (15%) |
| 4–4.5 Gy TBI + FLU | 0 | 7 (9%) | 7 (7%) |
| Post-grafting immunosuppression | |||
| MMF Q8h | 1 (4%) | 78 (100%) | 79 (77%) |
| MMF Q12h | 23 (96%) | 0 | 23 (23%) |
| Cyclosporine+MMF±sirolimusc | 14 (58%) | 54 (69%) | 68 (67%) |
| Tacrolimus+MMF±sirolimusd | 10 (42%) | 24 (31%) | 34 (33%) |
Data shown as median (range) or as number (%).
Rituximab given on days −3, +10, +24, and +38 relative to transplant;
Ten participants received cyclosporine + sirolimus, one with a matched donor and nine with unrelated donors;
Five participants received tacrolimus + sirolimus, all with unrelated donors.
Abbreviations: auto: autologous transplant; CMV: cytomegalovirus; FLU: fludarabine monophosphate; HCT-CI: HCT comorbidity index; HLA: human leukocyte antigen; MMF: mycophenolate mofetil; TBI: total body irradiation.
Participants received a conditioning regimen (summarized in Supplemental Figure 2) of fludarabine (30 mg/m2/day intravenously) from day −4 to day −2 (cumulative dose 90 mg/m2) followed by a single fraction of 2 to 4.5 Gy TBI on day 0.[2] The fludarabine dose was based on body surface area using actual body weight and was not adjusted for renal function, which is supported by our population pharmacokinetic model that revealed that body surface area (and not body weight) was a covariate for F-ara-A clearance. [14] Post-grafting immunosuppression consisted of a calcineurin inhibitor (CNI), either cyclosporine or tacrolimus, starting on Day −3 and mycophenolate mofetil (MMF), starting on Day 0 after the graft infusion. MMF at a dose of 15mg/kg, was given twice a day (Q12h) to recipients of HLA-matched related grafts and three times a day (Q8h) to recipients of unrelated grafts. In general, MMF was continued until Day +27 (related donor) or Day +40 (unrelated donor) at which time, the MMF dose was reduced by 10% weekly in the absence of GVHD. Some participants also received sirolimus in addition to MMF and a CNI, as determined by their HCT treatment protocol. [21] Donor grafts were matched for HLA-A, -B, -C, and -DRB1 by high resolution DNA typing and HLA-DQB1 by intermediate-resolution techniques, with the following exceptions: one related and two unrelated donor grafts with an antigen mismatch and nine unrelated donor grafts with an allelic mismatch. The median time of last follow-up among participants was 1.9 years (range: 0.6 – 3.8 years).
Fludarabine-based biomarkers
A full description of the fludarabine-based biomarkers is included in the Supplemental Methods.
F-ara-A pharmacokinetic sampling and quantification
The F-ara-A pharmacokinetic sampling schedules and their respective participant compliance rates are summarized in Supplemental Table 1. The F-ara-A AUC for an individual participant was determined via maximum a posteriori (MAP) Bayesian estimation, where the population prior (from the population pharmacokinetic analysis) may potentially offset the loss of individual data due to the use of the limited sampling schedule. [14] The F-ara-A pharmacokinetic sampling schedule with the highest (98%) compliance rate had samples drawn immediately at the end of the 30-minute infusion, 5 minutes after the end of the infusion and then 1.5h and 24h from the start of the infusion. The F-ara-A plasma concentrations were quantified as previously described. [22] The F-ara-A pharmacokinetic parameters and AUC from each participant were calculated as empirical Bayes estimates using the POSTHOC option in the nonlinear mixed-effects modeling software NONMEM (version 7.2, Icon Development Solutions, Ellicott City, MD).
F-ara-ATP accumulation rate in T-cell subsets
The F-ara-ATP accumulation rate in enriched CD4+ and CD8+ T-cell subsets was evaluated for each participant using our previously published method with minor modifications; the F-ara-ATP sampling time was the same as in our prior analysis. [12] Prior to fludarabine administration, a peripheral blood sample (60 mL) was obtained, which subsequently underwent an enrichment and purification procedure for CD4+ and CD8+ cells. [12] The CD4+ and CD8+ T-cells were purified and subsequently underwent an ex vivo incubation with fludarabine. Cells were incubated with fludarabine for 4h, then washed, solubilized in 1 M perchloric acid, and frozen. After thawing, the sample was centrifuged and the supernatant was neutralized prior to F-ara-ATP quantification using the LC-MS method described previously, with modifications as described in the Supplemental Methods. [13]
Immediate suppression of circulating CD4+/CD+ ratio in the peripheral blood
The circulating CD4+ and CD8+ counts in the peripheral blood were assessed immediately before the first fludarabine dose and then one day after the final fludarabine dose. For the before fludarabine sample, the median time between the collection of the circulating CD4+ and CD8+ count sample and the start of the first fludarabine dose was 4 min (range: 0 – 10.0 h). Fludarabine was then administered once daily for three days. For the after fludarabine sample, the median time between the start of the last fludarabine dose and the collection of the circulating CD4+ and CD8+ count sample was 23.8 h (range: 20.4 – 43.9 h). All CD4+ and CD8+ data were obtained before TBI and allogeneic graft infusion. The CD4+ and CD8+ cell counts were quantitated in a College of American Pathologist-certified clinical laboratory using a flow cytometer.
The percent decline in CD4+ or CD8+ counts was calculated by subtracting the after fludarabine count from the before fludarabine count and dividing the result by the before fludarabine count. A 100% decline means that no cells were detectable after fludarabine administration. A negative percent decline indicates that the participant’s cell count was greater after fludarabine administration, which occurred in three of the 102 participants. Of these three participants, one, diagnosed with non-Hodgkin’s lymphoma, had increased CD4+ counts and two, diagnosed with chronic lymphocytic leukemia, had increased CD8+ counts after fludarabine administration.
Clinical outcomes
Day +28 donor T-cell chimerism was the primary endpoint. Additional endpoints of interest were post-HCT neutrophil nadir, cytomegalovirus (CMV) reactivation, acute GVHD, chronic GVHD, relapse, non-relapse mortality (NRM), and overall survival. Neutropenia was analyzed as a binary endpoint (odds ratio, abbreviated OR), and all others as time-to-event endpoints (hazard ratio, abbreviated HR).
Participants’ peripheral blood samples were evaluated as per standard practice on day +28 after HCT, or as clinically indicated, for the percentage of donor CD3+ cells present. CD3+ cells were sorted by flow cytometry and polymerase chain reactions of polymorphic microsatellite regions were used to measure chimerism. [23]
Post-HCT neutropenia was only assessed through day +28 because multiple potential confounding variables (e.g., viral infection or reactivation, corticosteroid therapy) could affect the neutrophil counts subsequently. Complete blood counts with differential and assessment of absolute neutrophil count (ANC) were used to evaluate neutropenia. CMV reactivation was also evaluated as it represents a significant consequence of immunosuppressed status. CMV serological status was assessed in each participant and donor prior to HCT, and all participants underwent weekly testing to detect the CMV pp65 antigen for the first three months following HCT. Acute GVHD and chronic GVHD were graded according to established criteria. [24–26] To evaluate relapse consistently, the Kahl criteria were used to classify hematological diseases as having a low, standard, or high risk of relapse. [27] Disease relapse or disease progression was defined as disease recurrence after complete remission or progression of persistent disease.
Statistical analysis
Fludarabine-based biomarkers (i.e., F-ara-A dose 1 AUC, F-ara-ATP accumulation rate in CD4+ cells, F-ara-ATP accumulation rate in CD8+ cells, or after fludarabine CD4+/CD8+ ratio) were treated as fixed covariates. The effects of fludarabine-based biomarkers on HRs and ORs were expressed as the effect per doubling of each biomarker. The clinical outcomes evaluated were post-HCT neutrophil nadir (dichotomized into below or above the median ANC nadir of 165 cells/103 μL), Day +28 T-cell chimerism, grades 2–4 acute GVHD, grades 3–4 acute GVHD, chronic GVHD, relapse, CMV reactivation, non-relapse mortality, and overall mortality were evaluated. Cumulative incidence curves for acute GVHD and relapse were estimated using methods previously described. [28] Cox regression analysis was used to model the impact of recipient fludarabine-based biomarkers on time-to-event endpoints. Death and relapse were treated as competing risks for analysis of acute and chronic GVHD. Relapse was treated as a competing risk for the analysis of NRM. Logistic regression was used to evaluate the relationship between the fludarabine-based biomarkers and the post-transplant neutrophil nadir.
All analyses were adjusted for Kahl risk category (low, standard, high), donor-recipient gender (female to male, other), and donor type (related, unrelated). All statistical tests were two-tailed with the threshold for statistical significance set at 0.005 to adjust for multiple comparisons. P-values estimated from regression models were derived from the Wald test. Statistical analysis was performed using SAS 9.3 (Cary, NC, U.S.A.).
RESULTS
Fludarabine-based pharmacologic biomarkers
Patient characteristics are listed in Table 1 and pharmacological biomarker results in Tables 2 and 3. All biomarkers showed considerable interpatient variability (given by fold variability, maximum/minimum). There was a 3.7-fold variability in F-ara-A AUC with a mean of 19.6 μM×h (range: 9.96 – 36.4). The mean F-ara-A clearance was 5.71 L/h/m2 (range: 2.87 – 10.63). Among participants with more than one 24h (trough) concentration, the mean within-patient coefficient of variation (standard deviation/mean) was 28% (range: 0.5 – 63%), and the mean within-patient fold variability was 1.8 (range: 1.0 – 3.7). The mean (range) accumulation rate of F-ara-ATP in CD4+ cells was 4.6 pmol/106 cells/4h (range: 0.7 – 9.4) and in CD8+ cells was 4.0 pmol/106 cells/4h (range: 0.3 – 11.6). There was 13-fold and 39-fold variability in intracellular F-ara-ATP accumulation rate in CD4+ and CD8+ cells, respectively. This variability is greater than our previous observations in HCT patients (10.5- and 12.5-fold variability in CD4+ and CD8+ cells, respectively).[12] Notably, there were no obvious differences in the pharmacological biomarkers between the three most common diagnoses: acute myeloid leukemia, chronic lymphocytic leukemia, and non-Hodgkin’s lymphoma (data not shown).
Table 2.
Variability in F-ara-A AUC and F-ara-ATP accumulation rate
| Biomarker | N | Mean ± SD (range) |
|---|---|---|
| F-ara-A AUC (μM×h) dose 1a | 100 | 19.6 ± 4.80 (9.96 – 36.4) |
| Variability in 24hr F-ara-A concentration | ||
| Within-patient CV | 88a | 28 ± 13% (0.5 – 63) |
| Within-patient maximum/minimum | 88a | 1.8 ± 0.4 (1.0 – 3.7) |
| F-ara-ATP accumulation rate (pmol/106 cells/4h) | ||
| in CD4+ cells | 36 | 4.6 ± 2.1 (0.7 – 9.4) |
| in CD8+ cells | 34 | 4.0 ± 2.4 (0.3 – 11.6) |
Abbreviations: AUC: Area under the concentration-time curve; CV: coefficient of variation; F-ara-A: 9-β-D-arabinofuranosyl-2-fluoroadenine; F-ara-ATP: fludarabine triphosphate.
Dose 1 administered on HCT day −4.
Table 3.
Immediate effects of fludarabine 90 mg/m2 upon circulating lymphocyte counts
| Before fludarabinea | After fludarabineb | |
|---|---|---|
| Fludarabine dose number | Dose 1c | Day after dose 3c |
| Time between lymphocyte count and fludarabine dose | 4 min (0 – 10.0 h) | 23.8 h (20.4 – 43.9 h) |
| Circulating CD4+ (103 cells/μL) | 0.25 ± 0.19 (0.004 – 0.97) | 0.08 ± 0.07d (0 – 0.30) |
| % decline in circulating CD4+ | N/A | 68 ± 21e (−47 – 100) |
| Circulating CD8+ (103 cells/μL) | 0.31 ± 0.31 (0 – 1.4) | 0.05 ± 0.09d (0 – 0.76) |
| % decline in circulating CD8+ | N/A | 82 ± 22f (−20 – 100) |
| CD4+/CD8+ ratio | 1.65 ± 1.99 (0.09 – 15) | 3.08 ± 2.59d (0 – 11) |
Data presented as mean ± standard deviation;
N=100;
N=99;
Doses 1, 2, 3 administered on HCT days −4, −3, −2, respectively.
P<0.001 using paired t-test of before and after fludarabine data;
One participant’s CD4+ count and
two participants’ CD8+ counts increased after fludarabine
Immediate suppression of circulating CD4+/CD8+ ratio in the peripheral blood
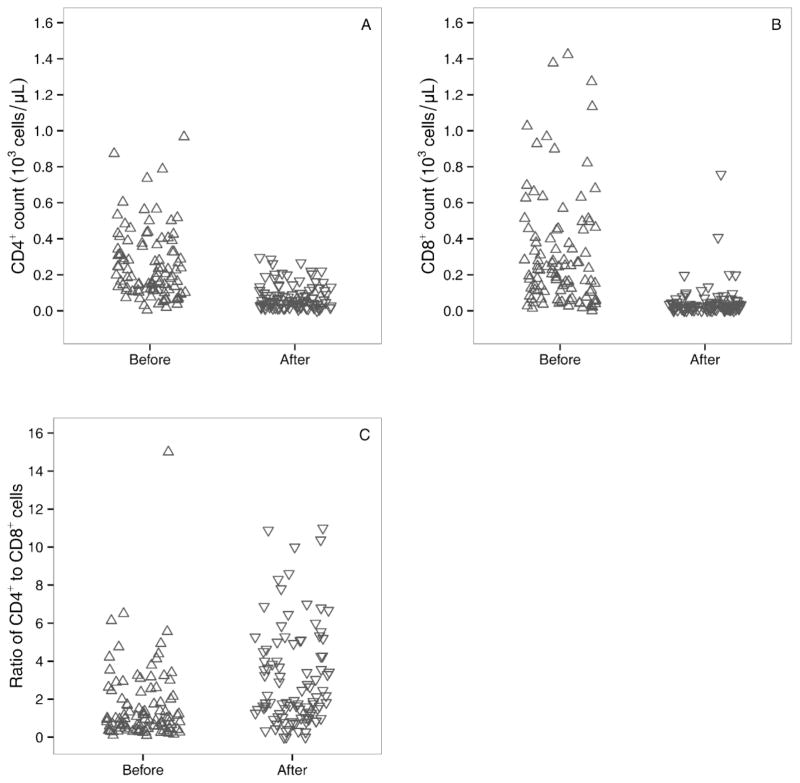
At the time of study initiation, most participants had low blood counts, most likely due to the underlying disease and previous treatments. The majority of participants experienced declines in their CD4+ and CD8+ counts after fludarabine administration (Figure 1 and Table 3). For CD4+ cells, the mean cell count before fludarabine was 0.25 × 103 cells/μL (range: 0.004 – 0.97); after fludarabine the mean CD4+ count dropped to 0.08 × 103 cells/μL (range: 0 – 0.30). For CD8+ cells, the mean cell count before fludarabine was 0.31 × 103 cells/μL (range: 0 – 1.4); after fludarabine, the mean CD8+ count dropped to 0.05 × 103 cells/μL (range: 0 – 0.76). The mean (range) percent decline in CD4+ counts was 68% (−47 – 100) and the mean (range) percent decline in CD8+ counts was 82% (−20 – 100). Before fludarabine, the mean (range) CD4+/CD8+ ratio cells was 1.65 (0.09 – 15) and after fludarabine, the mean (range) of this ratio was 3.08 (0 – 11).
Figure 1.
Effect of fludarabine administration on circulating CD4+ counts (A), circulating CD8+ counts (B), and ratio of circulating CD4+ to CD8+ cells (C). Samples obtained within 12h of first fludarabine dose (labeled “before”) and within 24h after the last fludarabine dose (labeled “after”).
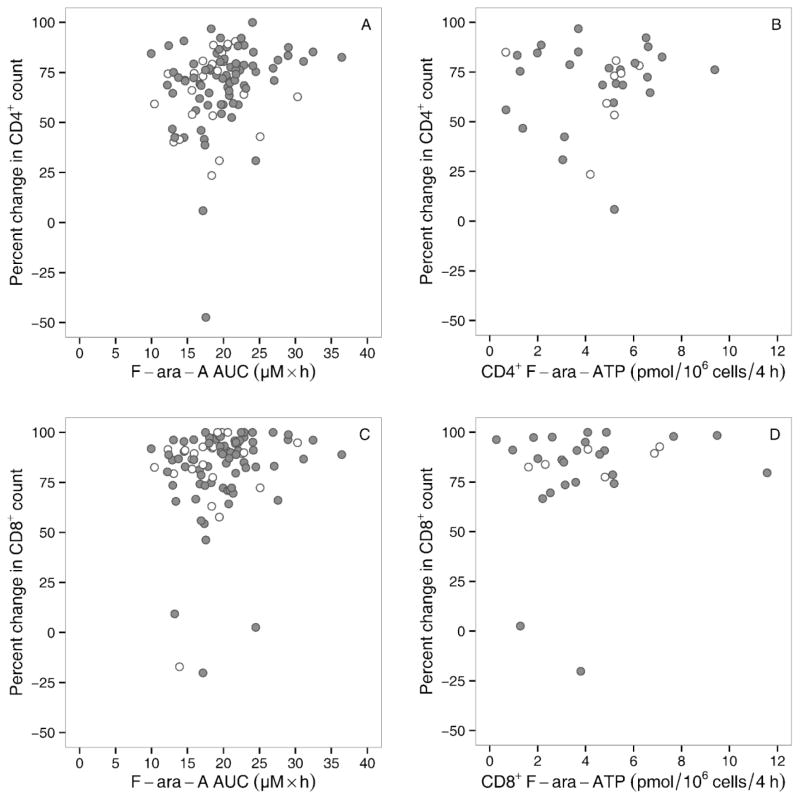
The percent decline of CD4+ cells correlated poorly with F-ara-A AUC (Figure 2A, R2=0.06, P=0.01) and with the F-ara-ATP accumulation rate in CD4+ cells (Figure 2B, R2=0.01, P=0.64). Similarly, the percent decline in CD8+ cells had a low correlation with F-ara-A AUC (Figure 2C, R2=0.03, P=0.07) and with the F-ara-ATP accumulation rate in CD8+ cells (Figure 2D, R2=0.03, P=0.36). F-ara-A AUC was also not correlated with the accumulation rate of F-ara-ATP in CD4+ cells (Supplemental Figure 3A, R2=0.01, P=0.63) or in CD8+ cells (Supplemental Figure 3B, R2=0.00, P=0.97). The F-ara-ATP accumulation rate in CD4+ cells had a low correlation of that in CD8+ cells (R2=0.194, Supplemental Figure 3C).
Figure 2.
Association of F-ara-A AUC or F-ara-ATP accumulation rate with lymphosuppression: Percent decline in related (white) or unrelated (grey) donor grafts in CD4+ cells with F-ara-A AUC (A) and accumulated F-ara-ATP (B). Percent decline in CD8+ cells with F-ara-A AUC (C) and accumulated F-ara-ATP (D).
Association with clinical outcomes
None of the fludarabine-based pharmacological biomarkers achieved the 0.005 threshold for a statistically significant association with clinical outcomes. The mean (range) donor chimerism on Day +28 was 83% (35 – 100%). Two participants experienced graft failure, and one participant died of relapse before engraftment could be determined. Thus, graft failure could not be evaluated as an endpoint. None of the associations between F-ara-A AUC (N=103) and the various clinical outcomes were statistically significant, with p-values ranging from 0.12 to 0.96. Similarly, clinical outcomes were not associated with F-ara-ATP accumulation rate in CD4+ cells (N=36) or in CD8+ cells (N=34), with p-values ranging from 0.02 to 0.95. Similarly, the after fludarabine CD4+/CD8+ (N=99) ratio was not associated with clinical outcomes, with p-values ranging from 0.01 to 0.96. Although not statistically significant after consideration of multiple comparisons, two associations were notable. One was an inverse association between CD4+ F-ara-ATP accumulation rates and chronic GVHD (p=0.02). The hazard ratio was 0.53 (range: 0.3–0.9), so the hazard of chronic GVHD decreased by 47% with each doubling of F-ara-ATP accumulation rate in CD4+ cells. The other was an inverse association between the CD4+/CD8+ ratio and grades 2–4 acute GVHD (p=0.01). The hazard ratio was 0.41 (0.2–0.8), so the hazard of developing grades 2–4 acute GVHD lowered by 59% with a log increase in the CD4+/CD8+ ratio.
DISCUSSION
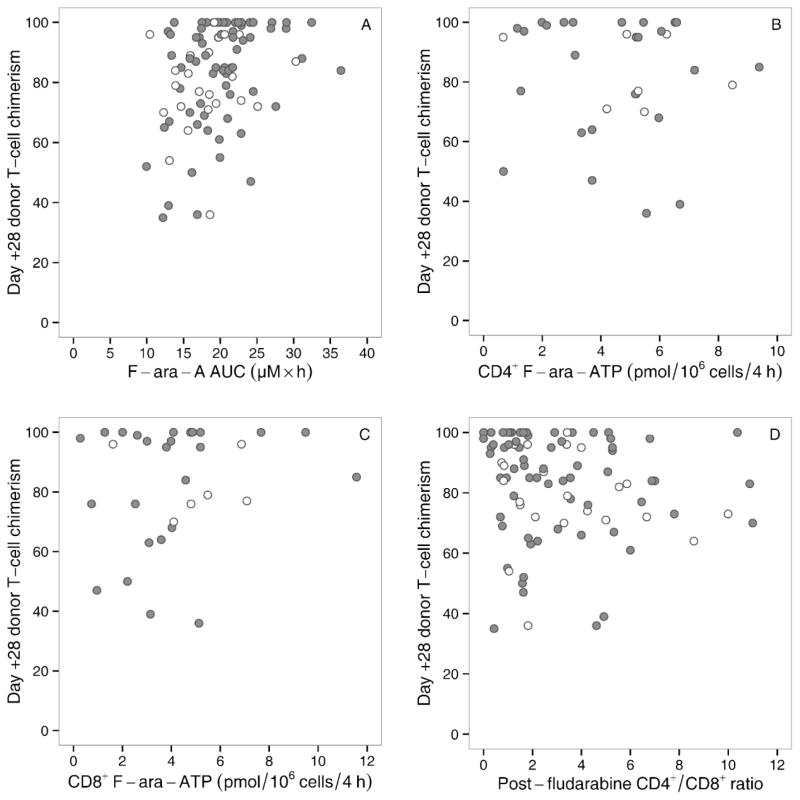
The key findings of this study are the striking interpatient variability in the fludarabine-based pharmacological biomarkers and the lack of a pharmacodynamic relationship between these biomarkers and clinical outcomes following the fludarabine/TBI regimen. Fludarabine is an essential component of reduced-intensity HCT conditioning regimens, the goal of which is to achieve acceptably low rates of GVHD and graft rejection and to maximize the graft-versus-tumor effect. [1] In fludarabine/TBI recipients, the level and rate of change in donor T-cell chimerism has been correlated with several clinical outcomes such as graft rejection, GVHD, disease relapse/progression (via a graft-versus-tumor effect), and progression-free survival. [29] Some of the observed associations between donor T-cell chimerism levels and subsequent clinical responses could, in part, reflect differences in each recipient’s sensitivity to the conditioning regimen. Day +28 T-cell chimerism was not, however, associated with the evaluated biomarkers of F-ara-A AUC, F-ara-ATP accumulation rate in CD4+ or in CD8+ cells, or the CD4+/CD8+ ratio after fludarabine administration and immediately before TBI administration (Figure 3A–D).
Figure 3.
Day +28 T-cell chimerism and fludarabine-related biomarkers in related (white) or unrelated (grey) donor grafts: F-ara-A AUC (A), F-ara-ATP accumulation rate in CD4+ cells (B), and F-ara-ATP accumulation rate in CD8+ cells (C), and fludarabine CD4+/CD8+ ratio from the day after the last dose of fludarabine (D).
After administration, fludarabine is dephosphorylated to F-ara-A, which is subsequently transported intracellularly and phosphorylated to F-ara-ATP, the active metabolite. Day +28 T-cell chimerism and subsequent clinical outcomes were not associated with F-ara-A AUC. Of note, this pharmacokinetic study was conducted in outpatients due to the creation of a clinic-friendly limited sampling schedule, which allowed for fewer pharmacokinetic samples from each participant, and by using the prior F-ara-A population pharmacokinetic model to predict the MAP Bayesian estimates of each individual AUC. [14] These data suggest that the pharmacodynamic relationship between F-ara-A AUC and clinical outcomes differs by conditioning regimen, since prior pharmacodynamic studies have reported an association between F-ara-A AUC and outcomes in patients receiving fludarabine-based myeloablative conditioning regimens. [30,31] The design of new fludarabine-based conditioning regimens may benefit from the use of a population pharmacodynamic model that characterizes the time course and variability of absolute lymphocyte count suppression – which affects a mixed population of T cells, B cells, and natural killer cells – by plasma F-ara-A. [32]
F-ara-ATP is the major cytotoxic metabolite of fludarabine. Unfortunately, quantification of the F-ara-ATP accumulation rate in T-cells from pharmacokinetic samples obtained from patients receiving fludarabine has not been feasible to date. We developed a novel phenotypic method to assess F-ara-ATP accumulation rate in CD4+, CD8+, or natural killer cells isolated from a peripheral blood sample obtained from HCT recipients. [12,33] In this study, the average (± standard deviation) F-ara-ATP accumulation rate in CD4+ cells and in CD8+ cells was lower than our previous results in 34 myeloablative HCT recipients. [12] Due to the considerable interpatient variability in F-ara-ATP accumulation rate, however, there was overlap between the two cohorts. The underlying reason for this discrepancy is most likely differences in the patient population, because the same blood draw timing (i.e., before conditioning was administered), cell isolation, incubation, and F-ara-ATP quantitation methods were used. Notably, there was a low correlation between F-ara-ATP accumulation rate between CD4+ and CD8+ cells (R2=0.194, Supplemental Figure 3C), which is worse than our previous findings (R2=0.553) in myeloablative HCT recipients. [12] The clinical significance of these differences, however, is unclear since F-ara-ATP accumulation rate was not associated with clinical outcomes (Figure 3). One limitation is that F-ara-ATP accumulation rate in CD4+ and CD8+ cells was assessed in only 36 and 34 participants, respectively. This was, in part, due to the logistical challenges detailed in the Supplemental Methods (F-ara-ATP accumulation rate in T-cell subsets). A further improvement in analytical sensitivity (current limit of detection of 50 fmol F-ara-ATP) is also desirable since F-ara-ATP concentrations could be quantified in CD4+ and CD8+ cells from 80% and 76% participants, respectively, with a minimum of 1.25×105 cells. We suggest using F-ara-ATP accumulation rate as a biomarker for fludarabine sensitivity be revisited after these logistical and analytical barriers are overcome. The benefit of pharmacogenomic studies of genes involved in F-ara-A pharmacokinetics, using DNA from the HCT recipient, are unclear since fludarabine is mainly renally eliminated. [34,16,35] Alternatively, proteomic[36] or metabolomic[37] signatures after fludarabine administration could be worthy of investigation.
The complexity of the pharmacodynamic effects of fludarabine upon lymphocytes makes it unsurprising that there is poor correlation of F-ara-A AUC with F-ara-ATP accumulation rate (Supplemental Figure 3) and with the decline in circulating CD4+ and CD8+ counts (Figures 2B and 2D, respectively). After ex vivo exposure to pharmacologically-relevant fludarabine concentrations, apoptosis of human CD4+ and CD8+ cells occurs after 24h. [12] We also characterized the immediate effects of fludarabine administration on circulating CD4+ and CD8+ cells (Figure 1 and Table 3). In vitro data regarding the effects of fludarabine on the proportion of CD4+ vs. CD8+ cells undergoing apoptosis are contradictory and suggest that CD4+ cells are similarly[18] or less susceptible[19] to fludarabine than CD8+ cells. On average, our data shows CD4+ cells are less susceptible to fludarabine than CD8+ cells in vivo (Figure 1 and Table 3). We recently reported that fludarabine results in a concentration-dependent and time-dependent decrease in viable CD4+ and CD8+ T-cells after 24h exposure to concentrations of fludarabine between 5 and 25 μM. [12] While no apoptosis had occurred after 4h (the duration of our ex vivo incubation procedure to evaluate F-ara-ATP), very few viable cells remained after 48h. [12] In vivo, CD8+ cells seem to have more short-term sensitivity after fludarabine administration, but with considerable variability (Table 3 and Figure 2). Three participants had aberrant responses of their circulating CD4+ or CD8+ counts in the peripheral blood after fludarabine administration. Two of these patients had chronic lymphocytic leukemia, and the other had anaplastic large cell non-Hodgkin’s lymphoma. Their F-ara-A AUCs were not aberrant (3.41, 7.67, and 13.8 μM×h); none had F-ara-ATP accumulation rates available. None of these patients rejected their graft, and all had grade 2 acute GVHD (days +27, +41, and +85).
The immediate effects of fludarabine upon recipients’ circulating CD4+ and CD8+ cell counts may influence their CD4+/CD8+ ratios, which in turn could affect their sensitivity to TBI. [20] This issue cannot be evaluated in preclinical HCT models because human lymphocytes are more sensitive to the cytotoxicity of fludarabine than those from mice, rats, or dogs. [38] Using pooled lymphocytes from four individuals, Wilkins et al. observed ex vivo that the CD4+/CD8+ ratio has an inverse relationship with the amount of apoptosis of lymphocytes and CD4+ cells, but not CD8+ cells, with or without radiation. [20] In our study, the average CD4+/CD8+ ratio rose from 1.65 before fludarabine administration to 3.08 afterwards (Table 3). No association was observed, however, between CD4+/CD8+ ratios and clinical outcomes. In HCT recipients, there is increasing interest in the influence of peritransplant T-cell and neutrophil counts with clinical outcomes. In a separate analysis in 459 nonmyeloablative HCT recipients, [39] Storb et al. found that high lymphocyte counts immediately before HCT had significant associations with reduced risks of relapse and overall mortality, but no association with the risks of GVHD or non-relapse mortality. Further research is required to identify sources of the patient heterogeneity in peritransplant T-cell counts.
We conclude that F-ara-A AUC and circulating CD4+/CD8+ ratio are not associated with clinical outcomes in fludarabine/TBI conditioned patients. Furthermore, technological improvements (e.g., T-cells from a lower blood volume or improved analytic sensitivity for F-ara-ATP) are needed before further evaluating this potential biomarker. Future work should focus on alternative biomarkers (e.g., peritransplant T-cell counts, proteomics or metabolomics) to see if fludarabine/TBI conditioning can be improved.
Supplementary Material
Acknowledgments
Financial disclosure statement: This work is supported in part by the following grants from the National Institutes of Health: HL091744, HL091744S1, CA018029, CA078902, HL036444, HL093294, CA015704, DK056465.
The authors are very grateful to the patients who participated in this study. The authors also wish to thank all physicians, nurses, and support personnel for their care of patients on this study. The efforts of Ms. Linda Risler and Mr. Brian Phillips for F-ara-A and F-ara-ATP quantification are acknowledged, as are the efforts of Dr. Brent Wood and the University of Washington Hematopathology Laboratory for the quantitation of circulating CD4+ and CD8+ cells.
Footnotes
Disclsoures: None.
COMPLIANCE WITH ETHICAL STANDARDS
Disclosure of potential conflicts of interest
This work was supported by the following grants from the National Institutes of Health: NHLBI (HL91744, HL36444), NCI (CA15704 (core), CA18029, CA78902), NIBIB (EB001975), and NIDDK (DK56465). The authors declare that they have no conflicts of interest.
Research involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Deeg HJ, Maris MB, Scott BL, Warren EH. Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia. 2006;20(10):1701–1705. doi: 10.1038/sj.leu.2404327. [DOI] [PubMed] [Google Scholar]
- 2.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, Petersdorf E, McSweeney P, Pulsipher M, Woolfrey A, Chauncey T, Agura E, Heimfeld S, Slattery J, Hegenbart U, Anasetti C, Blume K, Storb R. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 3.Kornblit B, Maloney DG, Storb R, Storek J, Hari P, Vucinic V, Maziarz RT, Chauncey TR, Pulsipher MA, Bruno B, Petersen FB, Bethge WA, Hubel K, Bouvier ME, Fukuda T, Storer BE, Sandmaier BM. Fludarabine and 2-Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: a phase III randomized trial. Biol Blood Marrow Transplant. 2013;19(9):1340–1347. doi: 10.1016/j.bbmt.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mielcarek M, Burroughs L, Leisenring W, Diaconescu R, Martin PJ, Sandmaier BM, Maloney DG, Maris MB, Chauncey TR, Shizuru JA, Blume KG, Hegenbart U, Niederwieser D, Forman S, Bruno B, Woolfrey A, Storb R. Prognostic relevance of ‘early-onset’ graft-versus-host disease following non-myeloablative haematopoietic cell transplantation. Br J Haematol. 2005;129(3):381–391. doi: 10.1111/j.1365-2141.2005.05458.x. [DOI] [PubMed] [Google Scholar]
- 5.Burroughs L, Mielcarek M, Leisenring W, Sandmaier BM, Maloney DG, Baron F, Martin PJ, Flowers ME, Forman SJ, Chauncey TR, Bruno B, Storb R. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81(6):818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 6.Baron F, Storer B, Maris MB, Storek J, Piette F, Metcalf M, White K, Sandmaier BM, Maloney DG, Storb R, Boeckh M. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12(11):1176–1187. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, Sandmaier B, Maloney D, Storb R, Storek J. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol. 2003;31(10):941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 8.Maris MB, Sandmaier BM, Storer BE, Maloney DG, Shizuru JA, Agura E, Kliem C, Pulsipher M, Maziarz RT, McSweeney PA, Wade J, Langston AA, Chauncey TR, Bruno B, Blume KG, Storb R. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12(4):454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Danhauser L, Plunkett W, Liliemark J, Gandhi V, Iacoboni S, Keating M. Comparison between the plasma and intracellular pharmacology of 1-beta-D-arabinofuranosylcytosine and 9-beta-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate in patients with relapsed leukemia. Leukemia. 1987;1(9):638–643. [PubMed] [Google Scholar]
- 10.Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41(2):93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Robak T, Lech-Maranda E, Korycka A, Robak E. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity. Current medicinal chemistry. 2006;13(26):3165–3189. doi: 10.2174/092986706778742918. [DOI] [PubMed] [Google Scholar]
- 12.Woodahl EL, Wang J, Heimfeld S, Sandmaier BM, O’Donnell PV, Phillips B, Risler L, Blough DK, McCune JS. A novel phenotypic method to determine fludarabine triphosphate accumulation in T-lymphocytes from hematopoietic cell transplantation patients. Cancer Chemother Pharmacol. 2009;63(3):391–401. doi: 10.1007/s00280-008-0748-0. Epub 2008 Apr2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalhorn TF, Ren AG, Slattery JT, McCune JS, Wang J. A highly sensitive high-performance liquid chromatography-mass spectrometry method for quantification of fludarabine triphosphate in leukemic cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820(2):243–250. doi: 10.1016/j.jchromb.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Salinger DH, Blough DK, Vicini P, Anasetti C, O’Donnell PV, Sandmaier BM, McCune JS. A limited sampling schedule to estimate individual pharmacokinetic parameters of fludarabine in hematopoietic cell transplant patients. Clin Cancer Res. 2009;15(16):5280–5287. doi: 10.1158/1078-0432.CCR-09-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson LE, Denny AW, Huh YO, Plunkett W, Keating MJ, Nelson JA. Natural killer cell activity in chronic lymphocytic leukemia patients treated with fludarabine. Cancer Chemother Pharmacol. 1996;37(5):445–450. doi: 10.1007/s002800050410. [DOI] [PubMed] [Google Scholar]
- 16.Plunkett W, Gandhi V, Huang P, Robertson LE, Yang LY, Gregoire V, Estey E, Keating MJ. Fludarabine: pharmacokinetics, mechanisms of action, and rationales for combination therapies. Semin Oncol. 1993;20(5 Suppl 7):2–12. [PubMed] [Google Scholar]
- 17.Keating MJ, O’Brien S, Lerner S, Koller C, Beran M, Robertson LE, Freireich EJ, Estey E, Kantarjian H. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92(4):1165–1171. [PubMed] [Google Scholar]
- 18.Consoli U, El-Tounsi I, Sandoval A, Snell V, Kleine HD, Brown W, Robinson JR, DiRaimondo F, Plunkett W, Andreeff M. Differential induction of apoptosis by fludarabine monophosphate in leukemic B and normal T cells in chronic lymphocytic leukemia. Blood. 1998;91(5):1742–1748. [PubMed] [Google Scholar]
- 19.Gamberale R, Galmarini CM, Fernandez-Calotti P, Jordheim L, Sanchez-Avalos J, Dumontet C, Geffner J, Giordano M. In vitro susceptibility of CD4+ and CD8+ T cell subsets to fludarabine. Biochem Pharmacol. 2003;66(11):2185–2191. doi: 10.1016/j.bcp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins RC, Kutzner BC, Truong M, McLean JR. The effect of the ratio of CD4+ to CD8+ T-cells on radiation-induced apoptosis in human lymphocyte subpopulations. International journal of radiation biology. 2002;78(8):681–688. doi: 10.1080/09553000210144475. [DOI] [PubMed] [Google Scholar]
- 21.Kornblit B, Maloney DG, Storer BE, Maris MB, Vindelov L, Hari P, Langston AA, Pulsipher MA, Bethge WA, Chauncey TR, Lange T, Petersen FB, Hubel K, Woolfrey AE, Flowers ME, Storb R, Sandmaier BM. A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after nonmyeloablative unrelated donor transplantation. Haematologica. 2014 doi: 10.3324/haematol.2014.108340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, Martin PJ, McDonald GB, Nichols WG, Radich J, Woolfrey A, Jenke A, Schleyer E, Thiede C, Ehninger G, Anasetti C. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102(3):820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 23.Bryant E, Martin PJ. Documentation of Engraftment and Characterization of Chimerism Following Hematopoietic Cell Transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 2. Blackwell Science, Inc; Malden, MA: 1999. pp. 197–206. [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 25.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, Erickson K, Flowers M, Hansen J, Loughran T, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250–259. [PubMed] [Google Scholar]
- 26.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kahl C, Storer BE, Sandmaier BM, Mielcarek M, Maris MB, Blume KG, Niederwieser D, Chauncey TR, Forman SJ, Agura E, Leis JF, Bruno B, Langston A, Pulsipher MA, McSweeney PA, Wade JC, Epner E, Bo Petersen F, Bethge WA, Maloney DG, Storb R. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110(7):2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Baron F, Sandmaier BM. Oct) Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20(10):1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 30.Long-Boyle JR, Green KG, Brunstein CG, Cao Q, Rogosheske J, Weisdorf DJ, Miller JS, Wagner JE, McGlave PB, Jacobson PA. High fludarabine exposure and relationship with treatment-related mortality after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(1):20–26. doi: 10.1038/bmt.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCune JS, Woodahl EL, Furlong T, Storer B, Wang J, Heimfeld S, Deeg HJ, O’Donnell PV. A pilot pharmacologic biomarker study of busulfan and fludarabine in hematopoietic cell transplant recipients. Cancer Chemother Pharmacol. 2012;69(1):263–272. doi: 10.1007/s00280-011-1736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCune JS, Vicini P, Salinger DH, O’Donnell PV, Sandmaier BM, Anasetti C, Mager DE. Population pharmacokinetic/dynamic model of lymphosuppression after fludarabine administration. Cancer Chemother Pharmacol. 2014 doi: 10.1007/s00280-014-2618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodahl EL, Wang J, Heimfeld S, Sandmaier BM, McCune JS. Intracellular disposition of fludarabine triphosphate in human natural killer cells. Cancer Chemother Pharmacol. 2009;63(5):959–964. doi: 10.1007/s00280-008-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hersh MR, Kuhn JG, Phillips JL, Clark G, Ludden TM, Von Hoff DD. Pharmacokinetic study of fludarabine phosphate (NSC 312887) Cancer Chemother Pharmacol. 1986;17(3):277–280. doi: 10.1007/BF00256699. [DOI] [PubMed] [Google Scholar]
- 35.Malspeis L, Grever MR, Staubus AE, Young D. Pharmacokinetics of 2-F-ara-A (9-beta-D-arabinofuranosyl-2-fluoroadenine) in cancer patients during the phase I clinical investigation of fludarabine phosphate. Semin Oncol. 1990;17(5 Suppl 8):18–32. [PubMed] [Google Scholar]
- 36.Whiteaker JR, Halusa GN, Hoofnagle AN, Sharma V, MacLean B, Yan P, Wrobel JA, Kennedy J, Mani DR, Zimmerman LJ, Meyer MR, Mesri M, Rodriguez H, Paulovich AG Clinical Proteomic Tumor Analysis C. CPTAC Assay Portal: a repository of targeted proteomic assays. Nature methods. 2014;11(7):703–704. doi: 10.1038/nmeth.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James LP. Metabolomics: integration of a new “omics” with clinical pharmacology. Clin Pharmacol Ther. 2013;94(5):547–551. doi: 10.1038/clpt.2013.166. [DOI] [PubMed] [Google Scholar]
- 38.Hassan SB, Haglund C, Aleskog A, Larsson R, Lindhagen E. Primary lymphocytes as predictors for species differences in cytotoxic drug sensitivity. Toxicol In Vitro. 2007;21(6):1174–1181. doi: 10.1016/j.tiv.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Storb R, Gyurkocza B, Storer BE, Maloney DG, Sorror ML, Mielcarek M, Martin PJ, Sandmaier BM. Allogeneic hematopoietic cell transplantation following minimal intensity conditioning: predicting acute graft-versus-host disease and graft-versus-tumor effects. Biol Blood Marrow Transplant. 2013;19(5):792–798. doi: 10.1016/j.bbmt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, Deeg HJ, Appelbaum FR, Storer B, Storb R. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.